-
PDF
- Split View
-
Views
-
Cite
Cite
Chrysoula Zouraraki, Penny Karamaouna, Leda Karagiannopoulou, Stella G Giakoumaki, Schizotypy-Independent and Schizotypy-Modulated Cognitive Impairments in Unaffected First-Degree Relatives of Schizophrenia-spectrum Patients, Archives of Clinical Neuropsychology, Volume 32, Issue 8, December 2017, Pages 1010–1025, https://doi.org/10.1093/arclin/acx029
Close - Share Icon Share
Abstract
The aim of the study was to compare the neurocognitive profile of unaffected first-degree relatives of schizophrenia patients with control individuals, controlling for different schizotypal traits.
One hundred and fifteen adult unaffected first-degree relatives of schizophrenia-spectrum patients and 122 controls were tested for schizotypy with the Schizotypal Personality Questionnaire. They also underwent a thorough neurocognitive assessment with a range of tasks covering several aspects of executive functioning. Between-group differences in cognition were examined first with multivariate analysis of variance and then with a series of multivariate analyses of covariance, including the schizotypal dimensions as covariates.
The relatives had higher scores on all schizotypal dimensions compared with controls and poorer planning, problem solving, strategy formation and working memory, irrespective of schizotypal traits. They also scored lower in executive working memory and verbal fluency. The difference in executive working memory was sensitive to the effects of paranoid and negative schizotypy (both dimensions abolished the between-group difference) whereas the difference in verbal fluency was sensitive only to the effects of paranoid schizotypy. Neither cognitive-perceptual nor disorganized schizotypy accounted for any differences in neurocognition between relatives and the controls.
Impairments in planning, problem solving, strategy formation and working memory are “core” impairments in the schizophrenia-spectrum, possibly due to high heritability effects in these functions. Impairments in executive working memory and verbal fluency are associated with paranoid and negative schizotypy, possibly due to alterations in a common fronto-temporo-parietal neural network.
Introduction
Schizotypy is a multidimensional personality concept (Vollema & Van Den Bosch, 1995) indicating liability for schizophrenia-spectrum disorders (Lenzenweger & Korfine, 1995). Schizotypal traits are widely organized according to a three-factor model into positive, negative and disorganized factors (Raine et al., 1994) and as schizophrenia and schizotypy share a similar factor structure, schizotypal traits are considered to correspond to the positive, negative, and disorganized symptoms of schizophrenia (Liddle & Barnes, 1990; Liddle, 1987). A more detailed four-factor model for schizotypy, which is suggested to fit to the data more accurately, has also been proposed (Compton, Goulding, Bakeman, & McClure-Tone, 2009; Stefanis, Smyrnis, et al., 2004; Tsaousis, Zouraraki, Karamaouna, Karagiannopoulou, & Giakoumaki, 2015). According to this model, positive schizotypy is sub-divided into a cognitive-perceptual (including traits such as magical ideation and unusual perceptual experiences) and a paranoid (encompassing traits such as suspiciousness and ideas of reference) factor; the negative (referring to traits such as constricted affect and excessive social anxiety) and disorganized (including odd speech and odd/eccentric behavior) factors are maintained as in the three-factor model.
It is thus easily evident that the phenomenology of schizotypy substantially overlaps with the phenomenology of schizophrenia. It is not surprising, therefore, that an overlap between these two conditions is also found in genetic, structural and functional brain mechanisms/pathways (Ettinger et al., 2015; Ettinger, Meyhöfer, Steffens, Wagner, & Koutsouleris, 2014; Mohr & Ettinger, 2014; Walter, Fernandez, Snelling, & Barkus, 2016). In detail, neuroimaging studies have revealed that schizotypy is associated with volumetric reductions in sub-regions of the frontal, temporal and parietal cortices (DeRosse et al. 2015; Ettinger et al., 2012; Kühn, Schubert, & Gallinat, 2012; Modinos et al., 2010; Wiebels, Waldie, Roberts, & Park, 2016), abnormal activation patterns within this circuitry (Aichert, Williams, Möller, Kumari, & Ettinger, 2012; Arzy, Mohr, Michel, & Blanke, 2007; Ettinger, Corr, Mofidi, Williams, & Kumari, 2013; Park, Kirk, & Waldie, 2015) during the performance of tasks requiring activation of these brain areas along with impaired connectivity within the frontal and temporal lobes (Nelson et al., 2011). In analogy, schizophrenia has also been consistently associated with (a) reductions of the frontal, temporal and parietal cortices in both clinical (Bartholomeusz et al., 2016; Shenton, Dickey, Frumin, & McCarley, 2001; Vita, De Peri, Deste, & Sacchetti, 2012) and high-risk populations (Bartholomeusz et al., 2016; Smieskova et al., 2013), (b) altered connectivity in the frontal and temporal lobes in both schizophrenia patients and high-risk individuals (Canu, Agosta, & Filippi, 2015; Kubicki et al., 2007; Wheeler & Voineskos, 2014), and (c) functional brain changes in the frontal, temporal and parietal cortices again both in patients and high-risk individuals (Brown & Thompson, 2010; Kraguljac, Srivastava, & Lahti, 2013; MacDonald, Thermenos, Barch, & Seidman, 2009; Smieskova et al. 2013).
The genetic overlap between schizotypy and schizophrenia is also now well-established. Thus, the val158met functional single nucleotide polymorphism (SNP) in the catechol-O-methyltransferase gene has been consistently implicated in schizophrenia (Glatt, Faraone, & Tsuang, 2003) and also predicts schizotypal traits in healthy individuals (Avramopoulos et al., 2002; de Castro-Catala et al., 2015; Grant et al., 2013; Ma, Sun, et al., 2007; Schürhoff et al., 2007; Smyrnis et al., 2007), schizophrenia patients (Schürhoff et al., 2007) and their unaffected relatives (Docherty & Sponheim, 2008; Schürhoff et al., 2007). Studies in healthy individuals (Roussos et al., 2013; Roussos, Giakoumaki, & Bitsios, 2009; Roussos, Giakoumaki, Georgakopoulos, Robakis, & Bitsios, 2011; Stefanis et al., 2008, 2013; Yasuda et al., 2011) have also reported that increased schizotypy is associated with several SNPs in genes implicated in schizophrenia susceptibility, such as the Proline Dehydrogenase (Li et al., 2004), the CACNA1C (Green et al., 2010), the regulator of the G-protein signaling 4 (Levitt, Ebert, Mirnics, Nimgaonkar, & Lewis, 2006), the Zinc Finger Protein 804A (O'Donovan et al., 2008) and the ERBB4 gene (Silberberg, Darvasi, Pinkas-Kramarski, & Navon, 2006).
As expected, therefore, schizotypal traits are increased in schizophrenia (Cochrane, Petch, & Pickering, 2010; Torti et al., 2013; Vollema & Postma, 2002) and Schizotypal Personality Disorder (SPD) patients (Rosell, Futterman, McMaster, & Siever, 2014), as well as in unaffected first-degree relatives of schizophrenia patients (Bora & Veznedaroglu, 2007; Calkins, Curtis, Grove, & Iacono, 2004; Solanki, Swami, Singh, & Gupta, 2012; Yaralian et al., 2000). The study of the latter high genetic-risk group is a promising approach for the investigation of the underlying liability for the disorder, as it is not compromised by limitations inherent in the study of patient samples (Gruzelier, 2003). Despite the evidence of increased schizotypal traits in the unaffected relatives of schizophrenia patients, though, it is not yet clear which schizotypal dimension prevails in this group of subjects. In a review article, Tarbox and Pogue-Geile (2011) concluded that the relatives of schizophrenia patients are mainly characterized by increased negative schizotypal traits along with small increases in positive and disorganized traits.
In addition to the above, schizophrenia (Green, 2006; Kar & Jain, 2016), SPD (Dickey et al., 2005; Mitropoulou et al., 2002) and schizotypy have been associated with cognitive deficits (Giakoumaki, 2012, 2016). Interestingly, the unaffected relatives of schizophrenia patients present with a similar profile of cognitive deficits with their probands but to a milder degree (for a meta-analysis see Snitz, MacDonald, & Carter, 2006). In three meta-analyses comparing cognitive functions between schizophrenia relatives and controls, the largest between-group differences were found in verbal memory and processing speed (Sitskoorn, Aleman, Ebisch, Appels, & Kahn, 2004), executive functions (Szöke et al., 2005) and attention (Snitz et al., 2006) with effect sizes ranging between small to high. As regards the effects of schizotypy on cognition, several schizotypal dimensions/traits have been associated with poorer performance in a range of tasks in the relatives’ group (studies summarized in Supplementary material online, Supplementary Table 1) although non-significant associations have also been reported (Conklin, Curtis, Calkins, & Iacono, 2005; Laurent et al., 1999; Vollema & Postma, 2002).
Within this framework, the aim of this study was to compare the neurocognitive profile of unaffected first-degree relatives of schizophrenia patients with control individuals, controlling for schizotypal traits. We employed the more detailed four-factor model of schizotypy described earlier (e.g., Tsaousis et al., 2015), as in addition to its psychometric advantages compared with the original three-factor model, it has also been reported to provide a differential profile of associations with cognitive functions in the general population (Karagiannopoulou et al., 2016). Thus, we hypothesized that (a) the unaffected first-degree relatives of schizophrenia-spectrum patients would have poorer cognition and higher schizotypal traits compared with controls and (b) the schizotypal dimensions would differentially “affect” these between-group differences.
Methods
Participants
The initial sample consisted of one hundred and thirty-nine unaffected first-degree relatives (offspring, siblings and parents; parents were included only if they had at least one sibling diagnosed with a schizophrenia-spectrum disorder) of patients who had a diagnosis of a schizophrenia-spectrum disorder according to the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV; American Psychiatric Association, 1994). They were recruited via the local psychiatric services and via advertisements in local media and were assessed with the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Exclusion criteria were (i) personal history of head trauma, medical or neurological conditions, (ii) current use of prescribed or recreational drugs, and (iii) personal history of DSM-IV Axis I disorders. Nineteen subjects did not fulfill the criteria for participation (7 due to Axis I pathology, 6 reported personal history of head trauma, medical or neurological conditions, 6 were excluded due to self-reported current use of recreational or prescribed drugs and another 5 participants dropped-out); therefore, the final sample consisted of 115 unaffected first-degree relatives of schizophrenia-spectrum patients (age mean ± SD: 35.42 ± 12.02; years of education mean ± SD: 14.37 ± 3.51; gender: 50 females/65 males; for a detailed analysis of their demographic characteristics; see Supplementary material online, Supplementary Table 2). As regards the diagnoses of the patients, 75 were diagnosed with schizophrenia and 40 were diagnosed with schizoaffective disorder. One hundred and twenty-two community participants (matched for gender, age, years of education and smoking habits with the relatives; age mean ± SD: 33.10 ± 10.16; years of education mean ± SD: 14.94 ± 2.14; gender: 64 females/58 males) were also included in the study. This group also underwent psychiatric evaluation using the MINI (Sheehan et al., 1998) and had identical exclusion criteria with the relatives, with the additional exclusion criterion of family (up to second-degree) history of DSM-IV Axis I disorders. The present study was part of the Prefrontally-Mediated Endophenotypes in the Schizophrenia Spectrum (PreMES) study, which took place in Rethymno and Heraklion, Crete, Greece. The study was approved by the Research Ethics Committee of the Department of Psychology in the University of Crete, the central Research Ethics Committee of the University of Crete and the Bureau for the Protection of Personal Data of the Greek State. All participants gave written informed consent, after full presentation of the study's aims and procedures and prior to their inclusion in the study and their anonymity has been preserved throughout the study.
Assessment of Schizotypy
Schizotypy was assessed with the Greek version of the Schizotypal Personality Questionnaire (SPQ) (Tsaousis et al., 2015). The SPQ is a dichotomous self-report, 74-item questionnaire (Raine, 1991); items are organized into nine subscales (ideas of reference, social anxiety, odd beliefs/magical thinking, unusual perceptual experiences, eccentric/odd behavior, no close friends, odd speech, constricted affect, and suspiciousness) which are in turn organized into four schizotypal factors (i.e., Negative [NegS], Paranoid [ParS], Cognitive-Perceptual [CPS] and Disorganized [DiS]). Cronbach's alphas in the present study were 0.862 for NegS, 0.779 for ParS, 0.771 for CPS and 0.721 for DiS.
Neuropsychological Assessment and Subjective Ratings of Mood and Feelings
Participants were assessed with the Iowa Gambling Task (IGT), for emotional decision making (Bechara, Damasio, Damasio, & Anderson, 1994), Stroop color-word test for control inhibition (Golden, 1978), Wisconsin Card Sorting test (WCST) for set-shifting (Nelson, 1976), Letter–Number Sequencing (LNS) for executive working memory (Wechsler, 2008), Trail-Making Test (TMT) for processing speed/set-shifting (Tombaugh, 2004; Zalonis, et al., 2008), Verbal Fluency test (VF) for phonemic/semantic fluency (Kosmidis, Vlahou, Panagiotaki, & Kiosseoglou, 2004) and Raven's Progressive Matrices for abstract reasoning (Raven, Raven, & Court, 2003). They were also examined with two tasks of the Cambridge Neuropsychological Test Automated Battery (CANTAB; Robbins et al., 1998): Stockings of Cambridge (SoC) which evaluates planning/complex problem solving (Owen, Downes, Sahakian, Polkey, & Robbins, 1990) and Spatial Working Memory (SWM) for the assessment of spatial working memory/strategy formation (Owen et al., 1990). We selected these tasks in order to assess executive functions in as much detail as possible (e.g., although strategy formation and planning are closely related, in the present study we aimed to examine each component with more “specialized” tasks). For a detailed description of the tasks and outcome measures, see Supplementary material online.
For the evaluation of their mood and feelings on the day of testing, participants completed a set of 16-item 10-cm visual analog scales (VAS) upon arrival at the testing site (Bond & Lader, 1974). For a detailed description, see Supplementary material online. We administered the VAS in order to control for between-group differences in mood/feelings on the day of testing, which might bias our findings.
Statistical Αnalyses
Between-group differences in demographic variables, VAS and SPQ scores were examined with either parametric or non-parametric analyses, according to normality of the distribution; gender differences were examined with Pearson's χ2. For the sake of data reduction and in order to better streamline the cognitive battery into factor scores for further analyses of their profile and relations to schizotypy, all variables from the cognitive tasks were subjected to a Principal Component Analysis (PCA) using the promax rotation method. Components with Eigenvalues >1.00 and factor loadings >0.5 were accepted. Although the varimax rotation method is the most widely used method in psychological research, we selected the promax oblique rotation method because when we deal with cognitive constructs, there is always a possibility that the factors produced might correlate with each other. Thus, the promax oblique rotation is more accurate and provides more information compared with the varimax rotation (Fabrigar, Wegener, MacCallum, & Strahan, 1999; Kieffer, 1998). Between-group differences in the neurocognitive factors derived by the PCA, were examined first with multivariate analysis of variance (MANOVA) and then with a series of multivariate analyses of covariance (MANCOVA), including the SPQ factor scores separately as covariates. To correct for multiple testing and reduce the probability of type I error, p values were Bonferroni corrected (.05/9 neurocognitive factors = .0055); thus we considered findings with a p value <.0055 as significant but we also present findings with p values <.01 as findings with trends for significance.
Results
Descriptives, Demographics and Schizotypal Traits
There were no significant differences between the two groups in any demographic or VAS-rated variable (all p values >.10). The unaffected relatives scored higher compared with the control group in SPQ total and all factor scores (all p values <.05; Cohen's d values: CPS = 0.259; ParS = 0.631; NegS = 0.507; DiS = 0.344). For a detailed description, see Supplementary material online, Supplementary Table 3.
Principal Component Analysis of Neurocognitive Variables
First, we ran the PCA analysis separately for the control and the relatives’ groups; as these analyses yielded identical factor solutions (a detailed description is provided in Supplementary material and Supplementary tables 4 and 5 online, respectively) we repeated the analysis in the whole sample. In detail, 32 variables were included in the analysis, and nine factors were extracted (Table 1). Namely, the factors were:
Set-shifting (comprising the total categories achieved, Nelson and Milner-type perseverative errors and Nelson and Milner-type non-perseverative errors of the WCST; Eigenvalue: 6.708, variance explained: 23.96%).
Working memory (comprising the correct responses for sub-blocks 1 to 5 of the LNS; Eigenvalue: 2.525, variance explained: 9.02%).
Emotional decision making (comprising IGT cards selected from the advantageous decks minus cards selected from disadvantageous decks for the second up to the fifth sub-blocks of the task; Eigenvalue: 2.367, variance explained 8.46%).
Control inhibition (comprising the word, color and color-word score of the Stroop test; Eigenvalue: 1.931, variance explained: 6.90%).
Problem solving (comprising the total problems solved with minimum moves and mean moves of SoC; Eigenvalue: 1.552, variance explained: 5.54%).
Strategy formation (comprising the total between and within errors and total strategy score of SWM; Eigenvalue: 1.379, variance explained: 4.93%).
Executive working memory (comprising the correct responses for sub-blocks 6 and 7 of the LNS; Eigenvalue: 1.326, variance explained: 4.74%).
Verbal fluency (comprising the total correct responses in the phonemic and semantic part of the Verbal/Phonemic fluency task; Eigenvalue: 1.200, variance explained: 4.29%).
Planning (comprising the mean initial and subsequent thinking times of SoC; Eigenvalue: 1.040, variance explained: 3.72%).
Principal component analysis factor loadings for both groups
| . | . | Set shifting . | Working memory . | Emotional decision making . | Control inhibition . | Problem solving . | Strategy formation . | Executive WM . | Verbal fluency . | Planning . |
|---|---|---|---|---|---|---|---|---|---|---|
| WCST | Categories achieved | −.800 | .066 | .085 | −.075 | .033 | .068 | .011 | .040 | −.165 |
| WCST | Nelson P.E | .808 | .023 | .003 | .031 | .000 | .104 | −.008 | −.072 | −.136 |
| WCST | Milner P.E | .809 | −.070 | .050 | −.112 | .073 | −.147 | −.023 | −.126 | −.084 |
| WCST | Milner non P.E | .799 | .046 | .014 | .030 | −.051 | .165 | −.007 | .150 | .011 |
| WCST | Nelson non P.E | .898 | .058 | .025 | −.073 | −.026 | −.006 | −.039 | .056 | .043 |
| LNS | Sb1 (2 digits) cr | −.064 | .797 | −.117 | −.073 | .021 | .136 | −.040 | −.028 | .235 |
| LNS | Sb2 (3 digits) cr | .031 | .834 | .026 | .079 | .119 | .080 | −.137 | −.018 | −.034 |
| LNS | Sb3 (4 digits) cr | −.014 | .766 | .103 | .014 | −.029 | −.184 | −.072 | −.023 | −.110 |
| LNS | Sb4 (5 digits) cr | .017 | .563 | −.018 | .080 | .128 | −.052 | .321 | −.043 | −.065 |
| LNS | Sb5 (6 digits) cr | .081 | .531 | .000 | .011 | −.056 | −.107 | .250 | .123 | −.147 |
| LNS | Sb6 (7 digits) cr | .006 | .084 | .061 | −.045 | −.111 | −.010 | .879 | .018 | .025 |
| LNS | Sb7 (8 digits) cr | −.082 | −.106 | −.060 | −.072 | .072 | .087 | .830 | −.008 | .020 |
| IGT | 1_20_CDminusAB | Factor solution <0.5; variable excluded from analysis | ||||||||
| IGT | 2_20_CDminusAB | −.054 | −.065 | .678 | .011 | −.200 | .217 | .045 | .031 | −.176 |
| IGT | 3_20_CDminusAB | .011 | .038 | .816 | .062 | .124 | .114 | −.036 | −.059 | .081 |
| IGT | 4_20_CDminusAB | .069 | .052 | .844 | −.025 | .020 | −.042 | .002 | −.004 | .029 |
| IGT | 5_20_CDminusAB | −.037 | −.051 | .740 | −.065 | .016 | −.173 | −.007 | .075 | .148 |
| STROOP | Word score | .083 | −.002 | −.111 | .828 | −.015 | .099 | .023 | .191 | .086 |
| STROOP | Color score | −.050 | −.007 | .024 | .901 | −.010 | −.066 | −.084 | −.052 | −.057 |
| STROOP | Color-Word score | −.084 | .079 | .076 | .838 | −.052 | −.029 | −.063 | −.084 | −.043 |
| SOC | Problems solved | −.030 | .057 | −.024 | −.015 | .978 | .067 | .007 | −.017 | .081 |
| SOC | M.M | −.001 | −.086 | −.035 | .037 | −.935 | −.012 | .021 | .009 | −.076 |
| SOC | ITT average | −.012 | −.040 | .066 | −.020 | .160 | −.039 | .016 | .039 | .936 |
| SOC | STT average | .012 | .120 | .020 | .043 | −.152 | .029 | .049 | −.110 | .596 |
| SWM | Between errors total | .000 | .065 | −.026 | −.113 | −.140 | .737 | .006 | −.131 | −.101 |
| SWM | Within errors total | .085 | −.182 | .074 | .201 | .202 | .829 | .195 | −.046 | .047 |
| SWM | Strategy score | −.058 | .166 | .018 | −.186 | −.042 | .644 | −.211 | .153 | −.014 |
| VF | Phonemic correct responses | .004 | .005 | −.038 | .078 | .000 | .021 | −.039 | .860 | −.003 |
| VF | Semantic correct responses | −.023 | −.025 | .064 | −.035 | −.001 | −.071 | .047 | .833 | .019 |
| TMT | Part A | Factor solution <0.5; variable excluded from analysis | ||||||||
| TMT | Part B | Factor solution <0.5; variable excluded from analysis | ||||||||
| Raven | Total score | Factor solution <0.5; variable excluded from analysis | ||||||||
| . | . | Set shifting . | Working memory . | Emotional decision making . | Control inhibition . | Problem solving . | Strategy formation . | Executive WM . | Verbal fluency . | Planning . |
|---|---|---|---|---|---|---|---|---|---|---|
| WCST | Categories achieved | −.800 | .066 | .085 | −.075 | .033 | .068 | .011 | .040 | −.165 |
| WCST | Nelson P.E | .808 | .023 | .003 | .031 | .000 | .104 | −.008 | −.072 | −.136 |
| WCST | Milner P.E | .809 | −.070 | .050 | −.112 | .073 | −.147 | −.023 | −.126 | −.084 |
| WCST | Milner non P.E | .799 | .046 | .014 | .030 | −.051 | .165 | −.007 | .150 | .011 |
| WCST | Nelson non P.E | .898 | .058 | .025 | −.073 | −.026 | −.006 | −.039 | .056 | .043 |
| LNS | Sb1 (2 digits) cr | −.064 | .797 | −.117 | −.073 | .021 | .136 | −.040 | −.028 | .235 |
| LNS | Sb2 (3 digits) cr | .031 | .834 | .026 | .079 | .119 | .080 | −.137 | −.018 | −.034 |
| LNS | Sb3 (4 digits) cr | −.014 | .766 | .103 | .014 | −.029 | −.184 | −.072 | −.023 | −.110 |
| LNS | Sb4 (5 digits) cr | .017 | .563 | −.018 | .080 | .128 | −.052 | .321 | −.043 | −.065 |
| LNS | Sb5 (6 digits) cr | .081 | .531 | .000 | .011 | −.056 | −.107 | .250 | .123 | −.147 |
| LNS | Sb6 (7 digits) cr | .006 | .084 | .061 | −.045 | −.111 | −.010 | .879 | .018 | .025 |
| LNS | Sb7 (8 digits) cr | −.082 | −.106 | −.060 | −.072 | .072 | .087 | .830 | −.008 | .020 |
| IGT | 1_20_CDminusAB | Factor solution <0.5; variable excluded from analysis | ||||||||
| IGT | 2_20_CDminusAB | −.054 | −.065 | .678 | .011 | −.200 | .217 | .045 | .031 | −.176 |
| IGT | 3_20_CDminusAB | .011 | .038 | .816 | .062 | .124 | .114 | −.036 | −.059 | .081 |
| IGT | 4_20_CDminusAB | .069 | .052 | .844 | −.025 | .020 | −.042 | .002 | −.004 | .029 |
| IGT | 5_20_CDminusAB | −.037 | −.051 | .740 | −.065 | .016 | −.173 | −.007 | .075 | .148 |
| STROOP | Word score | .083 | −.002 | −.111 | .828 | −.015 | .099 | .023 | .191 | .086 |
| STROOP | Color score | −.050 | −.007 | .024 | .901 | −.010 | −.066 | −.084 | −.052 | −.057 |
| STROOP | Color-Word score | −.084 | .079 | .076 | .838 | −.052 | −.029 | −.063 | −.084 | −.043 |
| SOC | Problems solved | −.030 | .057 | −.024 | −.015 | .978 | .067 | .007 | −.017 | .081 |
| SOC | M.M | −.001 | −.086 | −.035 | .037 | −.935 | −.012 | .021 | .009 | −.076 |
| SOC | ITT average | −.012 | −.040 | .066 | −.020 | .160 | −.039 | .016 | .039 | .936 |
| SOC | STT average | .012 | .120 | .020 | .043 | −.152 | .029 | .049 | −.110 | .596 |
| SWM | Between errors total | .000 | .065 | −.026 | −.113 | −.140 | .737 | .006 | −.131 | −.101 |
| SWM | Within errors total | .085 | −.182 | .074 | .201 | .202 | .829 | .195 | −.046 | .047 |
| SWM | Strategy score | −.058 | .166 | .018 | −.186 | −.042 | .644 | −.211 | .153 | −.014 |
| VF | Phonemic correct responses | .004 | .005 | −.038 | .078 | .000 | .021 | −.039 | .860 | −.003 |
| VF | Semantic correct responses | −.023 | −.025 | .064 | −.035 | −.001 | −.071 | .047 | .833 | .019 |
| TMT | Part A | Factor solution <0.5; variable excluded from analysis | ||||||||
| TMT | Part B | Factor solution <0.5; variable excluded from analysis | ||||||||
| Raven | Total score | Factor solution <0.5; variable excluded from analysis | ||||||||
Note: WM = working memory; SWM = spatial working memory task; WCST = Wisconsin card sorting test; P.E = persevarative errors; VF = verbal fluency task; LNS = letter number sequencing task; Sb = sub-block; cr = correct responses; IGT = Iowa gambling task; SOC = Stockings of Cambridge task; MM = mean moves; ITT = initial thinking time-Stockings of Cambridge task; STT = subsequent thinking time-Stockings of Cambridge task; CD = advantageous card decks of Iowa gambling task; AB = disadvantageous card decks of Iowa gambling task; TMT = trail making task. Significant loadings are bolded.
Principal component analysis factor loadings for both groups
| . | . | Set shifting . | Working memory . | Emotional decision making . | Control inhibition . | Problem solving . | Strategy formation . | Executive WM . | Verbal fluency . | Planning . |
|---|---|---|---|---|---|---|---|---|---|---|
| WCST | Categories achieved | −.800 | .066 | .085 | −.075 | .033 | .068 | .011 | .040 | −.165 |
| WCST | Nelson P.E | .808 | .023 | .003 | .031 | .000 | .104 | −.008 | −.072 | −.136 |
| WCST | Milner P.E | .809 | −.070 | .050 | −.112 | .073 | −.147 | −.023 | −.126 | −.084 |
| WCST | Milner non P.E | .799 | .046 | .014 | .030 | −.051 | .165 | −.007 | .150 | .011 |
| WCST | Nelson non P.E | .898 | .058 | .025 | −.073 | −.026 | −.006 | −.039 | .056 | .043 |
| LNS | Sb1 (2 digits) cr | −.064 | .797 | −.117 | −.073 | .021 | .136 | −.040 | −.028 | .235 |
| LNS | Sb2 (3 digits) cr | .031 | .834 | .026 | .079 | .119 | .080 | −.137 | −.018 | −.034 |
| LNS | Sb3 (4 digits) cr | −.014 | .766 | .103 | .014 | −.029 | −.184 | −.072 | −.023 | −.110 |
| LNS | Sb4 (5 digits) cr | .017 | .563 | −.018 | .080 | .128 | −.052 | .321 | −.043 | −.065 |
| LNS | Sb5 (6 digits) cr | .081 | .531 | .000 | .011 | −.056 | −.107 | .250 | .123 | −.147 |
| LNS | Sb6 (7 digits) cr | .006 | .084 | .061 | −.045 | −.111 | −.010 | .879 | .018 | .025 |
| LNS | Sb7 (8 digits) cr | −.082 | −.106 | −.060 | −.072 | .072 | .087 | .830 | −.008 | .020 |
| IGT | 1_20_CDminusAB | Factor solution <0.5; variable excluded from analysis | ||||||||
| IGT | 2_20_CDminusAB | −.054 | −.065 | .678 | .011 | −.200 | .217 | .045 | .031 | −.176 |
| IGT | 3_20_CDminusAB | .011 | .038 | .816 | .062 | .124 | .114 | −.036 | −.059 | .081 |
| IGT | 4_20_CDminusAB | .069 | .052 | .844 | −.025 | .020 | −.042 | .002 | −.004 | .029 |
| IGT | 5_20_CDminusAB | −.037 | −.051 | .740 | −.065 | .016 | −.173 | −.007 | .075 | .148 |
| STROOP | Word score | .083 | −.002 | −.111 | .828 | −.015 | .099 | .023 | .191 | .086 |
| STROOP | Color score | −.050 | −.007 | .024 | .901 | −.010 | −.066 | −.084 | −.052 | −.057 |
| STROOP | Color-Word score | −.084 | .079 | .076 | .838 | −.052 | −.029 | −.063 | −.084 | −.043 |
| SOC | Problems solved | −.030 | .057 | −.024 | −.015 | .978 | .067 | .007 | −.017 | .081 |
| SOC | M.M | −.001 | −.086 | −.035 | .037 | −.935 | −.012 | .021 | .009 | −.076 |
| SOC | ITT average | −.012 | −.040 | .066 | −.020 | .160 | −.039 | .016 | .039 | .936 |
| SOC | STT average | .012 | .120 | .020 | .043 | −.152 | .029 | .049 | −.110 | .596 |
| SWM | Between errors total | .000 | .065 | −.026 | −.113 | −.140 | .737 | .006 | −.131 | −.101 |
| SWM | Within errors total | .085 | −.182 | .074 | .201 | .202 | .829 | .195 | −.046 | .047 |
| SWM | Strategy score | −.058 | .166 | .018 | −.186 | −.042 | .644 | −.211 | .153 | −.014 |
| VF | Phonemic correct responses | .004 | .005 | −.038 | .078 | .000 | .021 | −.039 | .860 | −.003 |
| VF | Semantic correct responses | −.023 | −.025 | .064 | −.035 | −.001 | −.071 | .047 | .833 | .019 |
| TMT | Part A | Factor solution <0.5; variable excluded from analysis | ||||||||
| TMT | Part B | Factor solution <0.5; variable excluded from analysis | ||||||||
| Raven | Total score | Factor solution <0.5; variable excluded from analysis | ||||||||
| . | . | Set shifting . | Working memory . | Emotional decision making . | Control inhibition . | Problem solving . | Strategy formation . | Executive WM . | Verbal fluency . | Planning . |
|---|---|---|---|---|---|---|---|---|---|---|
| WCST | Categories achieved | −.800 | .066 | .085 | −.075 | .033 | .068 | .011 | .040 | −.165 |
| WCST | Nelson P.E | .808 | .023 | .003 | .031 | .000 | .104 | −.008 | −.072 | −.136 |
| WCST | Milner P.E | .809 | −.070 | .050 | −.112 | .073 | −.147 | −.023 | −.126 | −.084 |
| WCST | Milner non P.E | .799 | .046 | .014 | .030 | −.051 | .165 | −.007 | .150 | .011 |
| WCST | Nelson non P.E | .898 | .058 | .025 | −.073 | −.026 | −.006 | −.039 | .056 | .043 |
| LNS | Sb1 (2 digits) cr | −.064 | .797 | −.117 | −.073 | .021 | .136 | −.040 | −.028 | .235 |
| LNS | Sb2 (3 digits) cr | .031 | .834 | .026 | .079 | .119 | .080 | −.137 | −.018 | −.034 |
| LNS | Sb3 (4 digits) cr | −.014 | .766 | .103 | .014 | −.029 | −.184 | −.072 | −.023 | −.110 |
| LNS | Sb4 (5 digits) cr | .017 | .563 | −.018 | .080 | .128 | −.052 | .321 | −.043 | −.065 |
| LNS | Sb5 (6 digits) cr | .081 | .531 | .000 | .011 | −.056 | −.107 | .250 | .123 | −.147 |
| LNS | Sb6 (7 digits) cr | .006 | .084 | .061 | −.045 | −.111 | −.010 | .879 | .018 | .025 |
| LNS | Sb7 (8 digits) cr | −.082 | −.106 | −.060 | −.072 | .072 | .087 | .830 | −.008 | .020 |
| IGT | 1_20_CDminusAB | Factor solution <0.5; variable excluded from analysis | ||||||||
| IGT | 2_20_CDminusAB | −.054 | −.065 | .678 | .011 | −.200 | .217 | .045 | .031 | −.176 |
| IGT | 3_20_CDminusAB | .011 | .038 | .816 | .062 | .124 | .114 | −.036 | −.059 | .081 |
| IGT | 4_20_CDminusAB | .069 | .052 | .844 | −.025 | .020 | −.042 | .002 | −.004 | .029 |
| IGT | 5_20_CDminusAB | −.037 | −.051 | .740 | −.065 | .016 | −.173 | −.007 | .075 | .148 |
| STROOP | Word score | .083 | −.002 | −.111 | .828 | −.015 | .099 | .023 | .191 | .086 |
| STROOP | Color score | −.050 | −.007 | .024 | .901 | −.010 | −.066 | −.084 | −.052 | −.057 |
| STROOP | Color-Word score | −.084 | .079 | .076 | .838 | −.052 | −.029 | −.063 | −.084 | −.043 |
| SOC | Problems solved | −.030 | .057 | −.024 | −.015 | .978 | .067 | .007 | −.017 | .081 |
| SOC | M.M | −.001 | −.086 | −.035 | .037 | −.935 | −.012 | .021 | .009 | −.076 |
| SOC | ITT average | −.012 | −.040 | .066 | −.020 | .160 | −.039 | .016 | .039 | .936 |
| SOC | STT average | .012 | .120 | .020 | .043 | −.152 | .029 | .049 | −.110 | .596 |
| SWM | Between errors total | .000 | .065 | −.026 | −.113 | −.140 | .737 | .006 | −.131 | −.101 |
| SWM | Within errors total | .085 | −.182 | .074 | .201 | .202 | .829 | .195 | −.046 | .047 |
| SWM | Strategy score | −.058 | .166 | .018 | −.186 | −.042 | .644 | −.211 | .153 | −.014 |
| VF | Phonemic correct responses | .004 | .005 | −.038 | .078 | .000 | .021 | −.039 | .860 | −.003 |
| VF | Semantic correct responses | −.023 | −.025 | .064 | −.035 | −.001 | −.071 | .047 | .833 | .019 |
| TMT | Part A | Factor solution <0.5; variable excluded from analysis | ||||||||
| TMT | Part B | Factor solution <0.5; variable excluded from analysis | ||||||||
| Raven | Total score | Factor solution <0.5; variable excluded from analysis | ||||||||
Note: WM = working memory; SWM = spatial working memory task; WCST = Wisconsin card sorting test; P.E = persevarative errors; VF = verbal fluency task; LNS = letter number sequencing task; Sb = sub-block; cr = correct responses; IGT = Iowa gambling task; SOC = Stockings of Cambridge task; MM = mean moves; ITT = initial thinking time-Stockings of Cambridge task; STT = subsequent thinking time-Stockings of Cambridge task; CD = advantageous card decks of Iowa gambling task; AB = disadvantageous card decks of Iowa gambling task; TMT = trail making task. Significant loadings are bolded.
For this model, the KMO = .731, p < .001 and the total variance explained was 71.53%. Eight unaffected relatives and five control participants were identified as outliers and the analysis was repeated excluding these subjects. As no statistically significant differences were found, these subjects were included in the final analysis.
Neurocognitive Performance
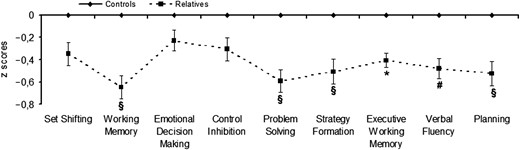
Table 2 presents the scores (mean ± SD) of the two groups in the neurocognitive factors derived by the PCA and the between-group differences in the MANOVA and MANCOVAs analyses. Fig. 1 also presents the group differences in the neurocognitive factors with the mean of the control group set to 0, as described in Bozikas, Kosmidis, Kiosseoglou, and Karavatos, (2006). The raw scores (mean ± SD) of the two groups in the individual measures of the cognitive tasks are presented in Supplementary material online, Supplementary Table 6.
Scores (mean [±SD]) of the two groups in the neurocognitive factors derived by the PCA and between-group differences
| Neurocognitive factors . | Controls (n = 122) . | Relatives (n = 115) . | p Valuea . | p Value (CPs covariate) . | p Value (ParS covariate) . | p Value (NegS covariate) . | p Value (DiS covariate) . |
|---|---|---|---|---|---|---|---|
| Set shifting | −0.17 (0.83) | 0.18 (1.13) | =.007 | = .009 | >.02 | >.01 | =.009 |
| Working memory | 0.32 (0.80) | −0.33 (1.08) | <.001 | <.001b | <.001b | <.001b | <.001 |
| Emotional decision making | 0.11 (0.96) | −0.12 (1.03) | >.08 | >.90 | >.20 | >.08 | >.08 |
| Control inhibition | 0.15 (0.84) | −0.16 (1.12) | >.01 | >.01 | >.02 | >.010 | >.03 |
| Problem solving | 0.29 (0.83) | −0.30 (1.07) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Strategy formation | −0.25 (0.74) | 0.26 (1.16) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Executive working memory | 0.20 (1.19) | −0.21 (0.70) | =.002 | =.003 | =.01 | =.007 | =.002 |
| Verbal fluency | 0.24 (0.98) | −0.24 (0.97) | <.001 | =.001 | >.01b | =.004b | =.001 |
| Planning | −0.26 (0.75) | 0.27 (1.15) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Neurocognitive factors . | Controls (n = 122) . | Relatives (n = 115) . | p Valuea . | p Value (CPs covariate) . | p Value (ParS covariate) . | p Value (NegS covariate) . | p Value (DiS covariate) . |
|---|---|---|---|---|---|---|---|
| Set shifting | −0.17 (0.83) | 0.18 (1.13) | =.007 | = .009 | >.02 | >.01 | =.009 |
| Working memory | 0.32 (0.80) | −0.33 (1.08) | <.001 | <.001b | <.001b | <.001b | <.001 |
| Emotional decision making | 0.11 (0.96) | −0.12 (1.03) | >.08 | >.90 | >.20 | >.08 | >.08 |
| Control inhibition | 0.15 (0.84) | −0.16 (1.12) | >.01 | >.01 | >.02 | >.010 | >.03 |
| Problem solving | 0.29 (0.83) | −0.30 (1.07) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Strategy formation | −0.25 (0.74) | 0.26 (1.16) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Executive working memory | 0.20 (1.19) | −0.21 (0.70) | =.002 | =.003 | =.01 | =.007 | =.002 |
| Verbal fluency | 0.24 (0.98) | −0.24 (0.97) | <.001 | =.001 | >.01b | =.004b | =.001 |
| Planning | −0.26 (0.75) | 0.27 (1.15) | <.001 | <.001 | <.001 | <.001 | <.001 |
Note: CPs = cognitive perceptual schizotypy; ParS = paranoid schizotypy; NegS = negative schizotypy; DiS = disorganized schizotypy. Significant between group differences after the Bonferroni correction (p ≤ .0055) are marked in bold. Underlined are p values <.01, indicating trends for significance.
ap Value in the MANOVA analysis.
bsignificant main effect of the covariate.
Scores (mean [±SD]) of the two groups in the neurocognitive factors derived by the PCA and between-group differences
| Neurocognitive factors . | Controls (n = 122) . | Relatives (n = 115) . | p Valuea . | p Value (CPs covariate) . | p Value (ParS covariate) . | p Value (NegS covariate) . | p Value (DiS covariate) . |
|---|---|---|---|---|---|---|---|
| Set shifting | −0.17 (0.83) | 0.18 (1.13) | =.007 | = .009 | >.02 | >.01 | =.009 |
| Working memory | 0.32 (0.80) | −0.33 (1.08) | <.001 | <.001b | <.001b | <.001b | <.001 |
| Emotional decision making | 0.11 (0.96) | −0.12 (1.03) | >.08 | >.90 | >.20 | >.08 | >.08 |
| Control inhibition | 0.15 (0.84) | −0.16 (1.12) | >.01 | >.01 | >.02 | >.010 | >.03 |
| Problem solving | 0.29 (0.83) | −0.30 (1.07) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Strategy formation | −0.25 (0.74) | 0.26 (1.16) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Executive working memory | 0.20 (1.19) | −0.21 (0.70) | =.002 | =.003 | =.01 | =.007 | =.002 |
| Verbal fluency | 0.24 (0.98) | −0.24 (0.97) | <.001 | =.001 | >.01b | =.004b | =.001 |
| Planning | −0.26 (0.75) | 0.27 (1.15) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Neurocognitive factors . | Controls (n = 122) . | Relatives (n = 115) . | p Valuea . | p Value (CPs covariate) . | p Value (ParS covariate) . | p Value (NegS covariate) . | p Value (DiS covariate) . |
|---|---|---|---|---|---|---|---|
| Set shifting | −0.17 (0.83) | 0.18 (1.13) | =.007 | = .009 | >.02 | >.01 | =.009 |
| Working memory | 0.32 (0.80) | −0.33 (1.08) | <.001 | <.001b | <.001b | <.001b | <.001 |
| Emotional decision making | 0.11 (0.96) | −0.12 (1.03) | >.08 | >.90 | >.20 | >.08 | >.08 |
| Control inhibition | 0.15 (0.84) | −0.16 (1.12) | >.01 | >.01 | >.02 | >.010 | >.03 |
| Problem solving | 0.29 (0.83) | −0.30 (1.07) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Strategy formation | −0.25 (0.74) | 0.26 (1.16) | <.001 | <.001 | <.001 | <.001 | <.001 |
| Executive working memory | 0.20 (1.19) | −0.21 (0.70) | =.002 | =.003 | =.01 | =.007 | =.002 |
| Verbal fluency | 0.24 (0.98) | −0.24 (0.97) | <.001 | =.001 | >.01b | =.004b | =.001 |
| Planning | −0.26 (0.75) | 0.27 (1.15) | <.001 | <.001 | <.001 | <.001 | <.001 |
Note: CPs = cognitive perceptual schizotypy; ParS = paranoid schizotypy; NegS = negative schizotypy; DiS = disorganized schizotypy. Significant between group differences after the Bonferroni correction (p ≤ .0055) are marked in bold. Underlined are p values <.01, indicating trends for significance.
ap Value in the MANOVA analysis.
bsignificant main effect of the covariate.
Neuropsychological profile of the unaffected relatives’ group. Relatives’ performance is expressed in z scores according to the means/standard deviations of the control group. WM = working memory; VF = verbal fluency. § the two groups differed significantly across MANOVA and MANCOVA analyses when schizotypal traits were included as covariates in the model. * the two groups differed significantly across MANOVA and MANCOVA analyses when CPS and DiS were included as covariates in the model. # the two groups differed significantly across MANOVA and MANCOVA analyses when CPS, NegS, and DiS factors were included as covariates in the model.
Between-group differences without controlling for schizotypy
The MANOVA revealed a significant multivariate main effect for group [Wilks’ λ = 0.786, F(9,224) = 6.795, p < .001, partial η2 = .214]. The unstandardized discriminant function coefficients for the multivariate combination are reported in Supplementary material online, Supplementary Table 7. The coefficients indicated that the groups differed as a function of Working Memory (−0.52), Planning (0.40), Problem Solving (−0.38), Strategy Formation (0.35) and Verbal Fluency (−0.23); the coefficients for Control Inhibition (0.18), Executive Working Memory (−0.13), Set Shifting (−0.09) and Emotional decision making (−0.08) had smaller absolute values. The univariate ANOVAs revealed that controls had superior performance in (a) Working Memory [F(1,232) = 27.176, p < .001, partial η2 = .105]; (b) Problem Solving [F(1,232) = 22.332, p < .001, partial η2 = .088]; (c) Strategy formation [F(1,232) = 16.048, p < .001, partial η2 = .065]; (d) Executive Working Memory [F(1,232) = 10.003, p < .005, partial η2 = .041]; (e) Verbal Fluency [F(1,232) = 14.194, p < .001, partial η2 = .058] and (f) Planning [F(1,232) = 17.214, p < .001, partial η2 = .069]. They also tended to perform better in Set Shifting (F(1,232) = 7.288, p < .01, partial η2 = .030); the remaining main effects and interactions were not significant (all p values > .01).
Between-group differences controlling for cognitive-perceptual schizotypy
The MANCOVA revealed significant multivariate main effects for group [Wilks’ λ = 0.796, F(9,223) = 6.359, p < .001, partial η2 = .204] and CPS [Wilks’ λ = 0.904, F(9,223) = 2.644, p < .01, partial η2 = .096]. When the main effect of CPS was significant [F(1,231) = 8.093, p < .005, partial η2 = .034] the control group had superior performance only in Working Memory [F(1,231) = 24.109, p < .001, partial η2 = .095]. When the main effect of CPS was not significant (all p values > .01), the control group had superior performance in Problem Solving [F(1,231) = 20.564, p < .001, partial η2 = .082], Strategy formation [F(1,231) = 16.096, p < .001, partial η2 = .065], Executive working memory [F(1,231) = 8.879, p < .005, partial η2 = .037], Verbal Fluency [F(1,231) = 12.097, p < .001, partial η2 = .050] and Planning [F(1,231) = 18.136, p < .001, partial η2 = .073]. They also tended to outperform the relatives in Set Shifting [Group main effect: F(1,231) = 6.863, p < .01, partial η2 = .029]. The remaining main effects and interactions did not survive the Bonferroni correction (all p values > .01).
Between-group differences controlling for paranoid schizotypy
The MANCOVA revealed significant multivariate main effects for group [Wilks’ λ = 0.829, F(9,223) = 5.097, p < .001, partial η2 = .171] and ParS [Wilks’ λ = 0.898, F(9,223) = 2.830, p < .005, partial η2 = .102]. When the main effect of ParS was significant [F(1,231) = 8.597, p < .005, partial η2 = .036], the control group had superior performance again only in Working Memory [F(1,231) = 17.557, p < .001, partial η2 = .071]. There was also a significant effect of ParS on Verbal Fluency [F(1,231) = 15.504, p < .001, partial η2 = .063] but the main effect of group was not significant (p > .01). When the main effect of ParS was not significant (all p values > .100), the control group had superior performance in Problem solving [F(1,231) = 16.588, p < .001, partial η2 = .067], Strategy Formation [F(1,231) = 15.170, p < .001, partial η2 = .062] and Planning [F(1,231) = 15.652, p < .001, partial η2 = .063]; they also tended to outperform the relatives in Executive working memory [F(1,231) = 6.730, p = .01, partial η2 = .028]. The remaining main effects and interactions did not survive the Bonferroni correction (all p values > .01).
Between-group differences controlling for negative schizotypy
The MANCOVA revealed significant multivariate main effects for group [Wilks’ λ = 0.808, F(9,223) = 5.891, p < .001, partial η2 = .192] and NegS [Wilks’ λ = 0.896, F(9,223) = 2.886, p < .005, partial η2 = .104]. When the main effect of NegS was significant [F(1,231) = 7.882, p < .005, partial η2 = .033 and F(1,231) = 11.156, p < .001, partial η2 = .046, respectively], the control group had superior performance in Working Memory [F(1,231) = 19.824, p < .001, partial η2 = .079] and Verbal Fluency [F(1,231) = 8.559, p < .005, partial η2 = .036]. When the main effect of NegS was not significant (all p values > .100), the control group had superior performance in Problem solving [F(1,231) = 18.929, p < .001, partial η2 = .076], Strategy Formation [F(1,231) = 17.187, p < .001, partial η2 = .069] and Planning [F(1,231) = 19.337, p < .001, partial η2 = .077]. They also tended to outperform the relatives in Executive Working Memory [Group main effect: F(1,231) = 7.402, p < .01, partial η2 = .031]. The remaining main effects and interactions did not survive the Bonferroni correction (all p values > .01).
Between-group differences controlling for disorganized schizotypy
The MANCOVA revealed significant multivariate main effects for group [Wilks’ λ = 0.791, F(9,223) = 6.533, p < .001, partial η2 = .209] but not for DiS (p > .06). Thus, the control group had superior performance in Working memory [F(1,231) = 23.156, p < .001, partial η2 = .091], Problem solving [F(1,231) = 21.476, p < .001, partial η2 = .085], Strategy formation [F(1,231) = 17.907, p < .001, partial η2 = .072], Executive Working Memory [F(1,231) = 10.033, p < .005, partial η2 = .042] and Planning [F(1,231) = 18.749, p < .001, partial η2 = .075]; they also tended to score higher in Set-Shifting [F(1,231) = 6.913, p < .01, partial η2 = .029]. The remaining main effects and interactions did not survive the Bonferroni correction (all p values > .03).
Discussion
Schizotypal Dimensions and Grouping of Neurocognitive Variables into Higher Order Factors
As hypothesized, and in accordance with the literature unaffected first-degree relatives of schizophrenia-spectrum patients had significantly higher scores on all schizotypal factors compared with control individuals (Grove et al., 1991; Squires-Wheeler et al., 1997; Yaralian et al., 2000). The effect sizes ranged from small (Cognitive-perceptual and disorganized factors) to moderate (negative and paranoid factors), in accordance with Tarbox and Pogue-Geile (2011), who reviewed studies assessing schizotypy with self-report questionnaires in unaffected relatives.
The PCA of the cognitive measures revealed a meaningful nine-factor factor solution, which applied to both the relatives’ and the control groups. Importantly, previous studies employing identical/similar tasks have identified analogous factors. Thus, our Set shifting factor, which included measures of the WCST, is comparable to the Executive function/Flexibility factors, which also included measures of this task, in unaffected siblings of schizophrenia patients (Genderson et al., 2007), in first-episode psychotic (Friis, Sundet, Rund, Vaglum, & McGlashan, 2002) and in chronic psychotic patients (Green et al., 2002; Hobart, Goldberg, Bartko, & Gold, 1999; Kremen, Seidman, Faraone, Pepple, & Tsuang, 1992); our Verbal Fluency factor, which comprised the correct responses of the phonemic and semantic fluency task, is similar to the “Elaboration” factor described by Woodward, Mizrahi, Menon, and Christensen, (2009) in psychotic patients; in the latter study, however, the “Elaboration” factor included these measures along with the correct responses in a theory of mind task.
Interestingly, in two tasks (LNS and SoC) the PCA “split” the measures into different factors. Thus, LNS was sub-divided into a Working Memory (consisting of the correct responses for sub-blocks 1 to 5) and an Executive Working Memory (including the correct responses for sub-blocks 6 and 7) factor, possibly due to the processes activated while completing the task. The LNS is a complex test of executive working memory (Twamley, Palmer, Jeste, Taylor, & Heaton, 2006) and as the blocks of the task progress (i.e., from the fifth to the subsequent blocks), the manipulation and recall demands increase (e.g., re-ordering and recall of 7 and 8 digits in the last two blocks, respectively). The measures of SoC were also divided into a Problem solving (including the total problems solved with the minimum moves and the mean moves required) and a Planning factor (comprising the mean initial/planning time and subsequent/execution time). In the same direction with our findings, Robbins et al., (1998) applied factor analysis in a sample of healthy individuals and found that the measures of SoC were divided into different factors, concluding that the task has several cognitive components.
“Schizotypy-independent” Cognitive Impairments
In agreement with the existing literature the group of relatives performed poorly compared with controls in planning, problem solving, strategy formation and working memory, when schizotypal traits were not taken into consideration (Barrantes-Vidal et al., 2007; Conklin et al., 2005; Ma, Wang, et al., 2007; O'Connor et al., 2009). The differences in problem solving, strategy formation and planning remained significant when we controlled for schizotypal traits, which had no significant main effects themselves. Similarly, the difference in working memory remained significant, when we controlled for schizotypy, but this time all schizotypal dimensions apart from disorganized had significant main effects. Taken together, these findings suggest that impairments in these cognitive functions are “core” impairments in unaffected relatives of schizophrenia patients as they are present irrespective of the effects of any schizotypal dimension. One possible explanation for this, is that these impairments reflect strong genetic/familial vulnerability, as supported by studies indicating high genetic effects on working memory (Greenwood et al., 2007; Johnson et al., 2003), strategy formation (Cannon et al., 2000; Glahn et al., 2007) and planning/problem solving (O'Connor et al., 2009).
We failed to find between-group differences in emotional decision making (as measured with the IGT), control Inhibition (including measures of the Stroop task) and set-shifting (including measures of the WCST) factors. This might not be surprising, though, as existing findings on these processes in schizophrenia patients and their relatives are controversial (Henik & Salo, 2004; Sánchez-Torres et al., 2013; Sevy et al., 2007; Yang et al., 2015).
“Schizotypy-modulated” Cognitive Impairments
As previously, the group of relatives had poorer executive working memory compared with controls, when schizotypal traits were not taken into consideration, in agreement with the existing literature (Barrantes-Vidal et al., 2007; Conklin et al., 2000; Delawalla et al., 2006; Horan et al., 2008; Trandafir, Méary, Schürhoff, Leboyer, & Szöke, 2006). However, when we controlled for schizotypal traits, we found that the between-group difference in executive working memory is sensitive to the effects of only paranoid and negative schizotypy: both dimensions abolished the difference between relatives and controls, although their main effect was not statistically significant. The between-group difference was not affected by cognitive-perceptual and disorganized dimensions, though. It is noteworthy, that (a) negative schizotypy has been previously associated with deficits in executive working memory in healthy individuals (Matheson & Langdon, 2008; Park & McTigue, 1997), schizophrenia patients (Orellana & Slachevsky, 2013) and their unaffected relatives (Delawalla et al., 2006); (b) although the role of paranoid schizotypy is less clear (existing studies analyze schizotypy into three instead of four factors), studies assessing patients with paranoid schizophrenia, have reported that they present with worse executive working memory compared with controls (Grover, Kulhara, Bhateja, Nehra, & Kumar, 2011; Schulze-Rauschenbach et al., 2015); and (c) Karagiannopoulou et al. (2016) found poorer executive working memory in community participants, expressing either high paranoid or negative schizotypal traits but not cognitive-perceptual or disorganized.
The group of relatives had poorer verbal fluency compared with controls, again in accordance with existing studies (Sitskoorn et al., 2004; Snitz et al., 2006; Szöke et al., 2005). When we controlled for schizotypal traits, a different pattern was observed: paranoid schizotypy had significant main effects and abolished the between-group difference, negative schizotypy also had significant main effects but did not affect the between-group difference, whereas cognitive-perceptual and disorganized dimensions did not have significant main effects and did not affect the between-group difference. Therefore, the difference between relatives and controls in verbal fluency seems to be sensitive only to the effects of paranoid schizotypy, in accordance with findings in patients with paranoid schizophrenia (García-Laredo, Maestú, Castellanos, Molina, & Peréz-Moreno, 2015; Wysokiński et al., 2010) and in individuals with either high paranoid ideation or paranoid schizotypy (Holper et al., 2015; Karagiannopoulou et al., 2016).
Negative and paranoid schizotypal traits have been associated with prefrontal and parietal dysfunction (Kühn et al., 2012; Nenadic et al., 2015; Wang et al., 2015; Yan et al., 2016). It is also of interest, that executive working memory and verbal fluency are subserved by a widely-distributed neural network, including the dorsolateral (DLPFC) and the ventrolateral (VLPFC) prefrontal cortices (Baldo, Schwartz, Wilkins, & Dronkers, 2006; Barbey, Koenigs, & Grafman, 2013; Bunge, Klingberg, Jacobsen, & Gabrieli, 2000; D'Esposito, Postle, Ballard, & Lease, 1999; Haut, Kuwabara, Leach, & Arias, 2000; Klein, Milner, Zatorre, Meyer, & Evans, 1995), as well as the temporal and the parietal cortices (Baldo et al., 2006; Barbey et al., 2013; Bunge et al., 2000; Haut et al., 2000). Functional and anatomical alterations within this network have been well-established in clinical (Smieskova et al., 2013) and genetic high-risk populations (Zhang, Picchioni, Allen, & Toulopoulou, 2016), in recent-onset (Arango et al., 2012; Karlsgodt et al., 2008), first-episode (Guo et al., 2012; Zhang et al., 2015) and chronic schizophrenia-spectrum patients (Baker et al., 2014; White et al., 2011), as well as in SPD (Koenigsberg et al., 2005). Unaffected relatives of schizophrenia patients have also been reported to present with abnormal activation of this neural network while performing executive working memory (Brahmbhatt, Haut, Csernansky, & Barch, 2006; Callicott et al., 2003; Winterer et al., 2004) or verbal fluency tasks (Bhojraj et al., 2009; 2011; Costafreda et al., 2009; Spence et al., 2000). Finally, there is evidence that components of this fronto-temporo-parietal network are associated with negative symptoms in schizophrenia (Nejad et al., 2013) and SPD (Asami et al., 2013) and that they may also be involved in the development of paranoid symptoms (Guo et al., 2012; Zhou et al., 2007). Thus, although highly speculative, based on the above we could hypothesize that paranoid and negative schizotypy are associated with impaired executive working memory and verbal fluency in unaffected relatives of schizophrenia-spectrum patients via a fronto-temporo-parietal link.
The Role of Cognitive-perceptual and Disorganized Schizotypy
In our study neither cognitive-perceptual nor disorganized schizotypy accounted for differences in neurocognition between the unaffected relatives and the controls. Regarding cognitive-perceptual schizotypy, there are studies (Day & Peters, 1999; McCreery & Claridge, 2002; Peters, Day, Mckenna, & Orbach, 1999) indicating that subclinical psychotic experiences similar to those described under cognitive-perceptual factor represent the so-called “healthy-schizotypy” (Mohr & Claridge, 2015). These subclinical psychotic experiences are not associated with the genetic liability for schizophrenia but with psychosocial facets of one's life (Raine, 2006) and this is possibly an explanation for the lack of effects of this schizotypal dimension in the present study.
As regards disorganized schizotypy, conflicting findings have been reported in the literature: although there are studies indicating associations with neurocognitive dysfunction in unaffected relatives of schizophrenia probands, (Chen et al., 1998; Szöke et al., 2009; Vollema & Postma, 2002), other studies report non-significant findings (Alfimova et al., 2009; Conklin et al., 2005). According to Tarbox and Pogue-Geile (2011), self-report questionnaires assessing disorganized schizotypal traits are not as sensitive as the clinical interviews and this could explain, at least to some extent, the lack of findings in the literature and in our study.
Strengths and Limitations of the Study
The main strengths of this study are: (i) the use of an extensive battery of neuropsychological tasks assessing executive functions which have been previously applied in schizophrenia research, (ii) the fact that our findings cannot be attributed to differences in demographic traits or state characteristics on the day of testing between the two groups and (iii) the use of an extensive four-factor model for the assessment of schizotypy.
The limitations of the study include some kind of heterogeneity in the group of relatives (parents, siblings and offspring do not carry the same genetic-risk and schizotypal traits change over the lifespan [Neill, 2014]) and the fact that we did not check for genetic (e.g., carrying risk alleles of the catechol-O-methyltransferase gene [Stefanis, Van Os, et al., 2004]) or environmental (e.g., sub-optimal parenting during childhood [Giakoumaki et al., 2013]) factors implicated in schizotypy. Finally, we administered only a self-report scale for the assessment of schizotypy; self-report scales have already been highlighted as being poorer identifiers of schizotypal traits in relatives compared with structured interviews (Kendler, Thacker, & Walsh, 1996).
Conclusions
To conclude, our findings (i) further support existing studies reporting increased schizotypal traits in unaffected relatives of schizophrenia patients and propose that this is mostly true for negative and paranoid schizotypy, (ii) indicate that impaired planning, problem solving, strategy formation and working memory in the unaffected relatives are not related to schizotypy, and (iii) propose that impairments in executive working memory and verbal fluency are related to paranoid and negative schizotypy, possibly due to alterations in a common fronto-temporo-parietal neural network. However, future larger-scale genetic and neuroimaging studies are required in order to further clarify the topic. As described in the introduction, there is substantial genetic as well as brain structural and functional overlap between schizotypy and schizophrenia. It is therefore essential that future studies apply a more “holistic” approach, employing both genetics and neuroimaging, in order to unveil the mechanisms underlying the findings of the present study.
Finally, the present findings could also have potential clinical implications. Evidence suggests that targeting cognitive impairments in the early course of schizophrenia as well as in individuals at risk for the disorder (Barlati, Deste, De Peri, Ariu, & Vita, 2013; Kar & Jain, 2016; Zaytseva, Korsakova, Agius, & Gurovich, 2013) maximally improves the effects of early intervention programs and eventually the functional outcome of these individuals. Based on the present findings, we could propose that impairments in planning, problem solving, strategy formation and working memory could serve as “first-rank” targets in early intervention/cognitive remediation programs because they are not related to schizotypal traits. Schizotypal traits could confound the effectiveness of such programs (e.g., they could affect an individual's ability to follow instructions) and/or require the application of more personalized interventions for every individual, thus increasing the cost of the overall intervention plan. The latter, however, is the case with impairments in executive working memory and verbal fluency, which were associated with paranoid and negative schizotypy. In accordance to the above, programs targeting impairments in these cognitive domains should take into consideration positive and negative schizotypy in order to obtain the maximum effectiveness of the intervention.
Funding
This work was supported by the “ARISTEIA II” Action of the Operational Programme Education and Lifelong Learning and was co-funded by the European Social Fund (ESF) and National Resources [grant number KA2990]. CANTAB was purchased with a “Start-Up” grant by the Special Account for Research of the University of Crete [grant number KA 3748].
Conflict of Interest
None declared.
Supplementary Material
Supplementary material is available at Archives of Clinical Neuropsychology online.