-
PDF
- Split View
-
Views
-
Cite
Cite
Margaret S. Smith, Andrew Shirley, Michael R. Strand, Copidosoma floridanum (Hymenoptera: Encyrtidae) Rapidly Alters Production of Soldier Embryos in Response to Competition, Annals of the Entomological Society of America, Volume 110, Issue 5, September 2017, Pages 501–505, https://doi.org/10.1093/aesa/sax056
Close - Share Icon Share
Abstract
Most social insects are free living and produce castes that develop in response to extrinsic environmental cues. Caste-forming polyembryonic insects, in contrast, are all endoparasitoids that form social groups inside the bodies of host insects. The best studied polyembryonic wasp is Copidosoma floridanum (Ashmead), which produces ∼3,000 clonal offspring that develop into two castes named reproductive and soldier larvae. Caste determination in this species is mediated by whether or not embryos inherit primary germ cells (PGCs). Prior results showed that C. floridanum increases the proportion of female soldier larvae it produces per host in response to other parasitoids like Microplitis demolitor. Here we show that caste ratio shifts occur through increased formation of embryos lacking PGCs. Our results further indicated that increased soldier production was a specific response to multiparasitism elicited by the chorion of M. demolitor eggs.
Polyphenism, the ability of a single genome to produce two or more alternative morphologies in response to an environmental cue, is an ecologically important and phylogenetically widespread feature of plants and animals (Schlichting and Smith 2002, West-Eberhard 2003). Among the best examples of polyphenisms occur in social insects like bees, ants, and termites that form colonies composed of castes. Castes have also evolved in less studied insect groups, including social aphids, thrips, and polyembryonic wasps (Zablotny 2003, Strand 2003). Many extrinsic factors have been implicated in regulating caste formation, including diet, temperature, density, and pheromones (Wheeler 1986, Evans and Wheeler 2001, Nijhout 2003). Using extrinsic factors as cues in regulating caste differentiation allows colonies to adapt to changing environmental conditions by producing different numbers of individuals in each caste, representing a colony-level, plastic response to environmental conditions (Passera etal. 1996).
Social bees and ants reside in the order Hymenoptera, but the majority of species in this order are parasitoids that develop by feeding in or on the bodies of other insects (Owen and Owen 1974, Strand 2003, Veijalainen etal. 2012). Polyembryony is defined as the clonal production of multiple embryos from a single egg. Polyembryony has evolved in four families of Hymenoptera that are composed exclusively of parasitoids (Strand 2003). Select species of wasps in the family Encyrtidae form the only group of Hymenoptera that is polyembryonic and has also evolved a caste system (Ivanova-Kasas 1972, Cruz 1981, Strand and Grbic 1997, Strand 2003). The best studied of these species is Copidosoma floridanum (Ashmead). Copidosoma floridanum females lay their eggs in the egg stage of select moth species in the subfamily Plusiinae (Lepidoptera). Each C. floridanum egg initially develops into an embryo called the primary morula, which consists of embryonic cells surrounded by extracellular membranes. Upon hatching of the host egg, the primary morula enters a proliferation stage of development that is characterized by rapid division of embryonic cells and repeated invagination of the surrounding extracellular membranes. These events partition the embryonic cells into multiple daughter embryos. Collectively the assemblage of embryos produced are referred to as the polygerm. Each C. floridanum egg produces a polygerm that is composed of up to 3,000 embryos (Ode and Strand, 1995). Most of these embryos develop into reproductive caste larvae that synchronously emerge during the host’s last instar, consume the host, and metamorphose into adult wasps (Grbic etal. 1998, Gordon and Strand 2009). The remaining embryos develop into soldier caste larvae, which emerge during the host’s first through fourth instars. Soldier caste larvae never molt and die when their reproductive caste siblings consume the host and pupate (Grbic etal. 1992).
Prior studies show that caste is determined in C. floridanum by differential parceling of primary germ cells (PGCs) during the proliferation phase of development (Donnell etal. 2004, Zhurov etal. 2004, Gordon and Strand 2009). Like many insects, C. floridanum specifies its germline by localizing determinants to particular cells that form during early embryogenesis. One of these determinants is a conserved germline component in metazoans named Vasa (Donnell etal. 2004, Zhurov etal. 2004). Copidosoma floridanum specifies its germline at the four-cell stage of embryogenesis which results in one PGC that contains Vasa and three somatic cells that do not (Donnell etal. 2004, Zhurov etal. 2004). Further division of these cells results in the presence of four to six PGCs and ∼200 somatic cells at the primary morula stage, while during the proliferation stage, invagination of the surrounding extraembryonic membranes produces daughter embryos that contain multiple somatic cells and at least one PGC (Gordon and Strand 2009). However, a small proportion of daughter embryos that form during proliferation inherit no PGCs (Gordon and Strand 2009). Embryos without PGCs develop into soldier caste larvae, whereas embryos with PGCs develop into reproductive caste larvae (Donnell etal. 2004, Zhurov etal. 2004, Gordon and Strand 2009).
Like most Hymenoptera, polyembryonic wasps are haplodiploid with females developing from fertilized (diploid) eggs and males developing from unfertilized (haploid) eggs. In the case of C. floridanum, adult females lay either one (female or male) or two eggs (usually one female and one male) per host that produce broods of all females, all males, or both sexes (mixed; Strand 1989). Female eggs produce more soldier caste larvae than male eggs (Grbic etal. 1992). Behavioral assays further show that a function of soldier caste larvae is defense against other parasitoids that attempt to develop in the same host (Strand 2003, Gardner etal. 2007). Female soldier larvae rapidly attack both con- and heterospecific competitors, whereas male soldiers are much less aggressive (Giron etal. 2004, Giron etal. 2007, Uka etal. 2013). In the case of conspecific competitors, female soldiers exhibit kin recognition, killing nonrelatives but only partially eliminating brothers. The latter results in mixed broods with strongly female-biased sex ratios (Strand 1989, Grbic etal. 1992, Giron etal. 2007, Bowker etal. 2014). Female soldiers likewise kill heterospecific competitors. The presence of a heterospecific competitor in the host also induces female broods to produce more soldier larvae (Harvey etal. 2000). Caste ratio shifts toward more soldiers is consistent with the hypothesis that C. floridanum increases investment in defense under conditions where host resources could be coopted by a competitor. In contrast, it is unknown how shifts in caste ratio occur, whether caste ratio shifts occur in response to other factors besides competitor wasps, and what factor(s) C. floridanum broods perceive that stimulates increased soldier formation.
The goal of this study was to address these issues. We first tested the hypothesis that increased soldier production is due to formation of more daughter embryos that lack PGCs. This was accomplished by allowing host larvae containing C. floridanum to be multiparasitized by Microplitis demolitor (Hymenoptera: Braconidae), which is the endoparasitoid that was previously shown to induce caste ratio shifts (Harvey etal. 2000). Second, we examined whether caste ratio shifts occur in response to other stress factors besides multiparasitism. Third, we examined whether increased soldier production occurred in response to particular factors M. demolitor injects into hosts during parasitism. Results indicated that multiparasitism rapidly increased the proportion of embryos in female broods with no PGCs. Our results also show that increased soldier production is not a general stress response and is induced by the chorion of M. demolitor eggs.
Materials and Methods
Insect Rearing
Copidosoma floridanum was reared in Trichoplusia ni (Strand 2003). Trichoplusia ni larvae were reared on a pinto bean diet at 27 °C with a photoperiod of 16:8 (L:D) h while C. floridanum was reared on T. ni as previously described (Strand 1989).
Multiparasitism
Mated C. floridanum females were allowed to singly parasitize T. ni eggs. Host eggs containing female C. floridanum broods were identified by observing oviposition behavior while unmated females were used to produce host eggs containing male broods (Strand 1989). Upon hatching, parasitized host larvae were held on artificial pinto bean diet until the last day of the second instar. At this point, hosts were individually multiparasitized by M. demolitor. Hosts parasitized by C. floridanum alone were held as controls. All hosts were reared for an additional 0, 16, 24, 66, or 120 h and dissected in physiological saline. The polygerm was collected while the total number of soldier caste larvae in each host was counted. Six to ten hosts were dissected for each time point and treatment. The entire experiment was also independently replicated two to three times for each time point.
Antibody Staining and Embryo Quantification
Primary germ cells were identified as previously described by collecting polygerms from hosts, fixing them in paraformaldehyde, and then labeling with an anti-Vasa primary antibody and a goat anti-rabbit Alexa-fluor 488 secondary antibody (Thermofisher, Molecular Probes; Donnell etal. 2004, Gordon and Strand 2009). Polygerms were counterstained with Alexaflour 488 phalloidin (Thermofisher, Molecular Probes; Gordon and Strand 2009) and then examined using a Leica DM-IRE2 inverted, epifluorescent microscope (Leica, Buffalo Grove, IL). Embryos containing Vasa stained cells were counted as having PGCs, while embryos with no Vasa stained cells were counted as lacking PGCs. Embryos in the process of dividing were considered a single embryo while soldier embryos undergoing morphogenesis were omitted from the analysis. The proportion of embryos lacking PGCs was calculated as the number of embryos lacking PGCs divided by the total number of embryos counted. The proportion of embryos without PGCs were compared among treatments using a Pearson’s chi-square test of independence. All statistical analysis was done in SPSSv.16 (SPSS Inc 2007).
Heat Shock and Infection
To determine if increased soldier production was specific to multiparasitism, host larvae containing female or male C. floridanum broods were heat shocked or infected with heat-killed bacteria. Host larvae were reared as described above until the first day of the third instar. Larvae were then either heat shocked for 4 h at 42 °C, injected with ∼10,000 Escherichia coli which had been inactivated by heat treatment at 70°C for 45 min, or held as controls. Treated and untreated (control) hosts were then reared for an additional 120 h, followed by dissection and counting the number of soldier larvae present as described above.
Injections
Microplitis demolitor parasitizes hosts by injecting one to three eggs and a viral symbiont named M. demolitor bracovirus (MdBV; Burke etal. 2014). To determine whether factors associated with M. demolitor eggs induced C. floridanum to produce more soldiers, host larvae containing female broods were injected with the content of eggs (slurry) or egg chorions. Microplitis demolitor eggs were dissected out of female ovaries, washed multiple times in physiological saline to remove MdBV, and then sonicated in a 1.5-ml microfuge tube in PBS for 1 min. Samples were then gently centrifuged which resulted in the content of eggs (slurry) forming the supernatant and chorions forming a pellet. After collecting the slurry, chorions were washed with physiological saline three times. The equivalent of 2–3 eggs/chorions were injected into each host using previously described methods (Harvey etal. 2000). Hosts multiparasitized by M. demolitor served as a positive control while hosts containing a C. floridanum female brood and injected with physiological saline alone served as the negative control.
Results
Multiparasitism Increases the Proportion of Embryos Lacking PGCs in Female Broods
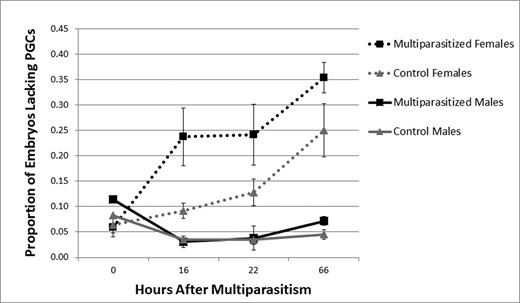
Prior results showed that hosts containing female broods produce significantly more soldier larvae when a host is multiparasitized by M. demolitor than control hosts containing only C. floridanum (Harvey etal. 2000). We, therefore, began this study by examining how this increase in solider number occurs. First, we labeled C. floridanum embryos dissected out at different times postmultiparastism with an anti-Vasa antibody to determine whether multiparasitism affected the proportion of embryos in female and male broods without PGCs. Second, we counted the number of soldiers present in hosts to verify that the number of soldier larvae actually increased in response to multiparasitism as previously reported by Harvey etal. (2000). Results showed that female broods from multiparasitized hosts contained a significantly higher proportion of embryos without PGCs than control hosts (χ2 = 174.727; df = 1; P < 0.001). This effect was most pronounced 16 and 22 h after M. demolitor oviposition (16 h: χ2 = 50.655; df = 1; P < .0001, 22 h: χ2 = 28.876; df = 1; P < .0001; Fig. 1). However, by 66 h, when host larvae were fourth instars, we no longer detected a difference in the proportion of C. floridanum embryos without PGCs between multiparasitized and control hosts (66 h: χ2 = 0.656; df = 1; P = 0.418). Consistent with Harvey et al (2000), soldier number also increased in female broods over time. For males, we detected no significant difference between the proportion of embryos lacking PGCs from multiparasitized and control hosts at any time point (0 h: χ2 = 2.11; df = 1; P = 0.146, 16 h: χ2 = 0.338; df = 1; P = 0.561, 22 h: χ2 = 5.966; df = 1; P = 0.015, 66 h: χ2 = 0.008; df = 1; P = 0.930; Fig. 1). We also detected no differences in the number of soldiers produced by male broods in multiparasitized and control hosts.
Mean proportion of embryos lacking PGCs (± SE) in male (solid lines) and female broods (dashed lines). Multiparasitized hosts (black) were parasitized by C. floridanum and M. demolitor while control hosts (gray) were parasitized by C. floridanum alone.
Heat Shock and Infection Do Not Impact Soldier Number
To assess whether increased soldier formation in C. floridanum female broods is a specific response to multiparasitism or a more general response to environmental stress, hosts were heat shocked or infected with heat-killed E. coli and compared to control hosts that were reared under standard conditions. Soldier numbers were slightly lower in both heat shock and infection treatments when compared to control larvae, although neither effect was significant (F=3.11; df=2, 74; P=0.51, Table 1). In contrast, the average number of soldiers per host at 120h posttreatment was, as expected, significantly higher in multiparasitized hosts when compared to control hosts (Table 2).
Mean number (± SE) of C. floridanum soldiers produced from female broods when exposed to heat shock or infection with E. coli
| Treatment . | Avg soldier no. (±SE) . |
|---|---|
| Control (n = 41) | 33.59 (±2.18) |
| Heat shock (n = 6) | 20.33 (±6.05) |
| Infection (n = 30) | 28.37 (±2.33) |
| Treatment . | Avg soldier no. (±SE) . |
|---|---|
| Control (n = 41) | 33.59 (±2.18) |
| Heat shock (n = 6) | 20.33 (±6.05) |
| Infection (n = 30) | 28.37 (±2.33) |
Control female broods were subjected to no treatment. Sample sizes per treatment are indicated by n.
Mean number (± SE) of C. floridanum soldiers produced from female broods when exposed to heat shock or infection with E. coli
| Treatment . | Avg soldier no. (±SE) . |
|---|---|
| Control (n = 41) | 33.59 (±2.18) |
| Heat shock (n = 6) | 20.33 (±6.05) |
| Infection (n = 30) | 28.37 (±2.33) |
| Treatment . | Avg soldier no. (±SE) . |
|---|---|
| Control (n = 41) | 33.59 (±2.18) |
| Heat shock (n = 6) | 20.33 (±6.05) |
| Infection (n = 30) | 28.37 (±2.33) |
Control female broods were subjected to no treatment. Sample sizes per treatment are indicated by n.
Mean number (± SE) of C. floridanum soldiers produced by female broods after multiparasitism by M. demolitor, injection of egg slurry, or injection of washed chorions
| Treatment . | Avg soldier no. (±SE) . |
|---|---|
| Negative control (n = 15) | 25 (±2.32) |
| Multiparasitized (n = 15) | 36 (±3.38) |
| Egg slurry (n = 11) | 34 (±3.71) |
| Chorion alone (n = 13) | 35 (±2.36) |
| Treatment . | Avg soldier no. (±SE) . |
|---|---|
| Negative control (n = 15) | 25 (±2.32) |
| Multiparasitized (n = 15) | 36 (±3.38) |
| Egg slurry (n = 11) | 34 (±3.71) |
| Chorion alone (n = 13) | 35 (±2.36) |
Negative control female broods were injected with saline only. Sample sizes per treatment are indicated by n.
Mean number (± SE) of C. floridanum soldiers produced by female broods after multiparasitism by M. demolitor, injection of egg slurry, or injection of washed chorions
| Treatment . | Avg soldier no. (±SE) . |
|---|---|
| Negative control (n = 15) | 25 (±2.32) |
| Multiparasitized (n = 15) | 36 (±3.38) |
| Egg slurry (n = 11) | 34 (±3.71) |
| Chorion alone (n = 13) | 35 (±2.36) |
| Treatment . | Avg soldier no. (±SE) . |
|---|---|
| Negative control (n = 15) | 25 (±2.32) |
| Multiparasitized (n = 15) | 36 (±3.38) |
| Egg slurry (n = 11) | 34 (±3.71) |
| Chorion alone (n = 13) | 35 (±2.36) |
Negative control female broods were injected with saline only. Sample sizes per treatment are indicated by n.
Microplitis demolitor Egg Chorions Stimulate Soldier Production
Given evidence that increased solider production occurred only in response to multiparasitism, we assessed whether particular factors that M. demolitor injects into hosts trigger increased soldier production. Normally, M. demolitor injects one to three eggs that are surrounded by a chorion plus MdBV. We therefore compared soldier production by C. floridanum female broods in hosts that were: 1) naturally multiparasitized by M. demolitor, 2) injected with a slurry of homogenized M. demolitor eggs that had been washed free of MdBV, or 3) injected with washed chorions alone from M. demolitor eggs. Results showed that hosts injected with chorions alone induced the same increase in soldier production as observed in multiparasitized hosts (F = 3.44, df = 3, P = 0.02; Table 2).
Discussion
Prior studies indicate that soldier larvae from several species of polyembryonic encyrtid wasps defend against conspecific and heterospecific competitor parasitoids (Cruz 1981, Grbic etal. 1992, Keasar etal. 2006, Giron etal. 2007, Smith etal. 2010, Uka etal. 2013). Prior results also indicate that C. floridanum female broods shift caste ratios in response to multiparasitism while also showing that soldier larvae develop from embryos that have no PGCs (Harvey etal. 2000, Donnell etal. 2004, Zhurov etal. 2004, Gordon and Strand 2009). This study provides new data showing that female, but not male, broods shift caste ratios by rapidly increasing the proportion of embryos that lack PGCs. The lag time between when daughter embryos lacking PGCs are formed and soldier numbers increase reflects the time it takes for embryos to develop once the cascade of events triggered by the absence of PGCs begins.
In contrast, we observed no increase in soldier larvae following heat shock or bacterial challenge. The lack of effect these challenges have on soldier production suggests caste ratio shifts are not a general response to stress but rather a direct response to multiparasitism by M. demolitor. However, we also recognize that the small number of heat shock replicates we were able to conduct relative to other treatments warrant caution in interpreting these results. Whether other parasitoid species whose host ranges overlap those of C. floridanum induce increased soldier production as observed for M. demolitor is also unknown. On the other hand, that washed chorions from M. demolitor induce a similar increase in soldier production as multiparasitism suggests several endoparasitoids could trigger increased soldier production given most species lay eggs that are also surrounded by a chorion. However, how C. floridanum perceives the chorions of other wasp species is unclear. One possibility is that embryos directly or indirectly perceive soluble factors that are released from the chorion into the hemocoel while another is that insoluble factors are perceived if chorions contact the polygerm. It is also possible that already formed soldier larvae perceive chorions and release signals that alter polygerm growth given evidence that soldier larvae perceive surface cues that allow them to discriminate kin from nonkin in conspecific competition (Giron and Strand 2004).
The regulatory events that control the parceling of PGCs to embryos during the proliferation phase of development are unknown. As a result we currently have no insights into how perception of competitor wasp eggs alters the parceling of PGCs to daughter embryos to increase soldier production. Future work in this area may yield fruitful insights into the underlying mechanistic basis by which soldier production is regulated.
Acknowledgments
We would like to thank Ian Milton, Jena Johnson, and Erin Barding for their help with this project. This work was supported by a Ruth Kirschstein Fellowship Award (F32GM087106) by the National Institute of Health (NIH) to M.S.S.
References Cited
Author notes
Subject Editor: Nelson Thompson