-
PDF
- Split View
-
Views
-
Cite
Cite
D L Frank, T C Leskey, J C Bergh, Morphological Characterization of Antennal Sensilla of the Dogwood Borer (Lepidoptera: Sesiidae), Annals of the Entomological Society of America, Volume 103, Issue 6, 1 November 2010, Pages 993–1002, https://doi.org/10.1603/AN09182
Close - Share Icon Share
Abstract
The external morphology of the dogwood borer, Synanthedon scitula (Harris) (Lepidoptera: Sesiidae), antennae and their sensilla was investigated using light and scanning electron microscopy. Male and female antennae were clavate before tapering to an apical point and consisted of three main segments; scape, pedicel, and flagellum. Although there was no significant difference in the length of the flagellum between genders, the number of flagellomeres was significantly greater in females than in males and the length and width of individual flagellomeres was significantly greater in males than in females, except near the proximal and distal end of the antennae. The antennal flagellum of both male and female dogwood borer contained seven sensillum types: auricillica, basiconica, chaetica, coeloconica, squamiformia, styloconica, and trichoidea (three subtypes). The mean number of sensilla basiconica did not differ between female and male antennae, but all other sensillum types were significantly more abundant on female antennae. The morphology and purported function of each sensillum type are discussed in relation to the host and oviposition site finding and acceptance behaviors exhibited by dogwood borer.
Defining plant—insect interactions related to the use of host plant compounds for host and oviposition site location, identification, and acceptance has become an important topic of entomological research (Renwick 1989, Bernays and Chapman 1994). Host plant compounds can play an important role in host selection processes of female insects, thus affecting the survival and distribution of their offspring (Mayhew 1997). Although investigation of the behavioral responses of insects to host plant compounds for development of monitoring or management strategies has often been difficult because of the numerous signals used by insects in host location and mate finding and the potential synergistic interactions between them (Viser 1986, Landolt and Phillips 1997), some success in the integration of these chemical stimuli in behaviorally based management strategies has been documented (Van Steenwyk and Barnett 1987, Prokopy et al. 1992, Yang et al. 2004, Leskey et al. 2008).
Females of many species of Sesiidae seem to respond to particular host plant odors, because they often oviposit preferentially near damaged host tissues (Solomon 1995). For example, oleoresin exudates from pruning wounds served as attractive oviposition stimuli for Synanthedon novaroensis (Henry Edwards) in managed Douglas-fir (Pseudotsuga Carrière) stands (Johnson 1993). Rocchini et al. (1999) suggested that the occurrence of fungal-induced cankers and galls in lodgepole pine (Pinus contorta Douglas) influenced the susceptibility of trees to attack by S. novaroensis. Cottrell et al. (2008) showed that Synanthedon pictipes (Grote & Robinson) deposited significantly more eggs on peach limbs that were mechanically damaged or possessed fungal-induced wounds compared with nondamaged limbs. In addition, Derksen et al. (2007) showed that volatile compounds emanating from the gum and frass of infested peach trees induced oviposition by Synanthedon exitiosa (Say).
The dogwood borer, Synanthedon scitula (Harris) (Lepidoptera: Sesiidae), is a polyphagous clearwing moth that occurs throughout much of eastern North America (Eichlin and Duckworth 1988) and is much more abundant in commercial apple (Malus spp.) orchards than in forest or managed urban landscapes (Bergh et al. 2009). In apple orchards, females preferentially oviposit on or nearby burr knot tissue on host trees, which seem to be an important resource for developing larvae (Riedl et al. 1985, Kain and Straub 2001, Leskey and Bergh 2005). Frank et al. (2009) documented that mated female dogwood borer frequently engaged in casting flight toward, and oviposition near, sites below the graft union of apple trees where there were burr knots and cracks in the bark. Furthermore, both virgin and mated female dogwood borer responded electrophysiologically to volatile compounds collected from this tissue (Frank et al. 2007). Although the response of male dogwood borer to its sex pheromone (Zhang et al. 2005, Leskey et al. 2006) has been examined, the investigation of the role of host plant compounds on the behavioral responses of females and males has largely been neglected.
As a fundamental companion study to our ongoing research on the use of plant-derived semiochemicals for host finding and oviposition by female dogwood borer, we examined the antennal morphology of male and female dogwood borer and compared the types, number, and distribution of antennal sensilla of both genders.
Materials and Methods
Insects. In May 2009, a laboratory colony of S. scitula was initiated with late instar larvae from commercial apple orchards in Frederick Co., VA, and then propagated and maintained on green thinning apples as described by Frank et al. (2010), with periodic introductions of field collected larvae. Adults (1 to 2 d old) from the colony were frozen until prepared for examination.
Measurements of Antennae. Excised heads of 35 male and 35 female dogwood borer were placed in a 10% potassium hydroxide solution at 80°C for 1 h until the scales were removed and the antennae cleared. The heads were transferred to a bath of distilled water at 80°C for 1 h and then dehydrated in 70 and 100% alcohol for 30 min each. Antennae were excised from the heads and photographed at 20× magnification using a Paxcam (MIS Inc., Villa Park, IL) digital camera mounted on an SMZ1500 (Nikon Instruments Inc., Melville, NY) microscope. Pax-it version 7.0 (MIS Inc.) imaging software was used to examine the images for measurements of the length of the antennal flagellum; the number of antennal flagellomeres; and the length and width of the scape, pedicel, and selected flagellomeres.
Measurements of Sensilla. Excised heads of eight male and eight female dogwood borer were placed in a 10% potassium hydroxide solution at 100°C for ≈2 min until the antennal scales were removed. The heads were rinsed with distilled water, then dehydrated through a graded series of 70, 80, 90, and 100% ethanol for 30 min each. Antennae were removed from the heads and mounted on specimen stubs with adhesive, so that either the ventral (five antennae per gender) or dorsal (three antennae/gender) surface was visible. After air drying for 5 min, the antennae were sputter coated with gold /palladium in a Hummer VI (Anatech Ltd., Alexandria, VA) sputtering system followed by examination under a Stereoscan 120 (Cambridge Scientific Instruments Ltd., Cambridge, United Kingdom) scanning electron microscope (SEM) at 10 kV. SEM images of each antennal flagellomere were captured using an Orion 5.0 (E.L.I. Microscopy, Brussels, Belgium) imaging system from which the types and number of sensilla from each gender was determined. All SEM digital images were subsequently analyzed using Pax-it version 7.0 (MIS Inc.) imaging software to determine the length and basal diameter of each type of sensillum. A stage micrometer was used to calibrate the Pax-It measurement module. Then measurements of each type of sensillum were taken using the line function tool which allowed us to draw point-to-point line measurements along the entire length and for the basal diameter of each sensillum type. For each sensillum type, 30 measurements were taken along the length of the ventral surface of the antennae from five individuals of each gender.
Mean ± SE length of the pedicel, scape, and selected antennal flagella of dogwood borer
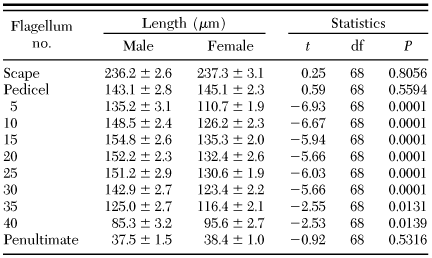
Mean ± SE length of the pedicel, scape, and selected antennal flagella of dogwood borer

Statistical Analysis. All data were analyzed using an unpaired t-test (SAS Institute 2003). When necessary, the results were square root transformed to meet the assumptions of normality and homogeneity of variances. Results from all tests were considered statistically different at P < 0.05.
Results
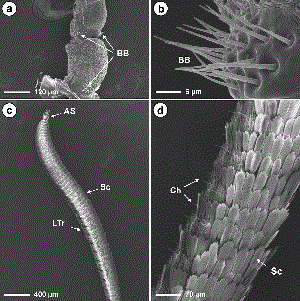
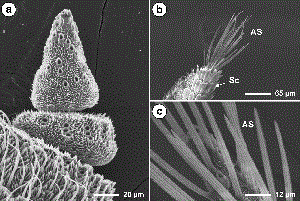
Antennal Morphology. Dogwood borer antennae were situated between the compound eyes and consisted of two basal segments, the scape and pedicel, followed by a distal flagellum consisting of subsegments, or flagellomeres. The scape was the largest part of the antennae (Tables 1 and 2) and was bulbous in form after a constriction distal to its insertion in the antennal socket (Fig. 1a). The pedicel was smaller than the scape and more elongated on the dorsal side compared with the ventral surface (Fig. 1a). Both the scape and pedicel were covered in overlapping scales and possessed numerous spine-like Böhm's bristles near the proximal end of each (Fig. 1a and b). Male and female antennae did not differ significantly in the length of the scape and pedicel (Table 1), although the width of both was significantly larger in males (Table 2). For both genders, the flagellum was clavate before tapering to an apical point (Fig. 1c). Individual flagellomeres were typically cylindrical in shape and could be divided into two main areas, the ventral and dorsal surfaces (Fig. 1c and d). The ventral surface was highly textured with many grooves and ridges and possessed most of the sensilla (Figs. 2 and 3). The dorsal surface was generally smoother in texture (Fig. 4) and possessed numerous rows of overlapping scales along its length (Fig. 1c and d). No sensilla were located on the penultimate flagellomere (Fig. 5a). The most distal, conical-shaped flagellomere was devoid of scales and possessed numerous spike-like apical sensors (Fig. 5a–c). The mean ± SE number of flagellomeres was 46.8 ± 0.3 for males (range, 43–53), and 49.3 ± 0.4 for females (range, 46–55), which was significantly different (t = 5.06, df = 68, P ≤ 0.0001). Although female antennae possessed more flagellomeres, the length of the flagellum was 5,834.8 ± 53.5 and 5,759.0 ± 62.6 µm for males and females, respectively, which was not significantly different (t = -0.92, df = 68, P = 0.359). The length of individual flagellomeres was significantly greater in males than in females except near the distal end (Table 1). Similarly, the width of individual flagellomeres was significantly greater in males than in females except near the proximal and distal ends (Table 2).
Mean ± SE width of the pedicel, scape, and selected antennal flagella of dogwood borer
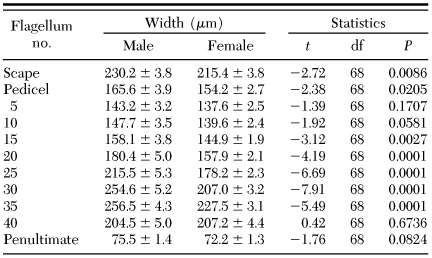
Mean ± SE width of the pedicel, scape, and selected antennal flagella of dogwood borer

SEMs showing scape and pedicel of the male dogwood borer antennae and location of the Böhm's bristles (a); close-up of the Böhm's bristles on the pedicel of the male antennae (b) ; profile of the male dogwood borer antennae possessing numerous overlapping scales on the dorsal surface, large sensilla trichoidea on the ventral surface and apical sensors on the terminal flagellomere (c) ; and close-up profile of the female dogwood borer antennae possessing numerous overlapping scales on the dorsal surface and needle-like sensilla chaetica on the ventral surface (d). AS, apical sensors; BB, Böhm's bristles; Ch, chaetica sensilla; LTr, large trichoidea sensilla; Sc scales; Sq squamiformia sensillum.
Antennal Sensilla. Sensilla morphology was based upon the descriptions and terminology used by Sellier (1977) and Blum (1985). The antennal flagellomeres (except the most distal two) of the dogwood borer possessed seven different sensillum types: auricillica, basiconica, chaetica, coeloconica, squamiformia, styloconica, and trichoidea (three subtypes). The size and structure of each sensillum type was nearly identical between male and female moths. Unless otherwise noted, the following morphological descriptions apply to both genders.
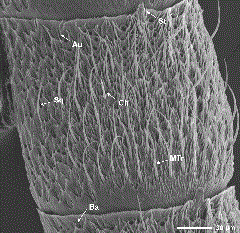
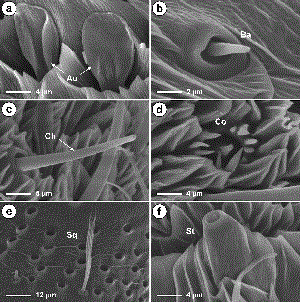
Sensilla Auricillica. These sensilla were the second most abundant of all sensillum types on the antennae of both males and females (Table 3), occurring primarily on the ventral surface along the length of the flagellum (Fig. 2) but also on the dorsal surface among the scales of the distal flagellomeres. Their distribution on each flagellomere was random and their number increased distally along the flagellum, generally reaching their maximum abundance before the 35th flagellomere, at which point the antennae began to taper in size. These sensilla had a rabbit-ear shaped but also could seem dorsoventrally flattened (Fig. 6a). They were 9.1–17.6 µm long and had a basal width of 1.4–3.3 µm. Sensilla auricillica were significantly more abundant on female antennae (t = 8.44, df = 8, P ≤ 0.0001) (Table 3).
SEM of the ventral surface of a female dogwood borer antennal flagellomere showing several sensillum types. Au, auricillica sensillum; Ba, basiconica sensillum; Ch, chaetica sensillum; Sq, squamiformia sensillum; St, styloconica sensillum; MTr, medium trichoidea sensillum.
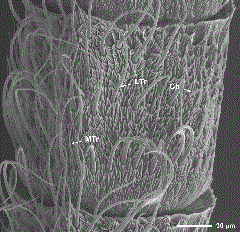
SEM of the ventral surface of a male dogwood borer antennal flagellomere showing several sensillum types. Ch, chaetica sensillum; LTr, large trichoidea sensillum; MTr, medium trichoidea sensillum.
Sensilla Basiconica, These sensilla were the least abundant of the sensillum types on the antennae of both males and females (Table 3). They were found randomly along the length of the flagellum before the 35th flagellomere and were typically located near the distal, lateral region of the ventral surface of the flagellomere (Fig. 2). These sensilla had a conical, peg-like appearance (Fig. 6b) and were 1.9–4.5 µlong and had a basal width of 0.9–1.7 µm. The mean number of sensilla basiconica was similar in both genders (t = 1.50, df = 8, P = 0.173) (Table 3).
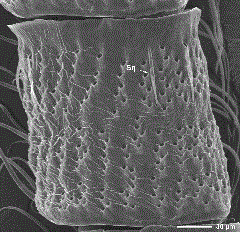
SEM of the dorsal surface of a male dogwood borer antennal flagellomere with the scales removed and showing sensilla squamiformia. Sq, squamiformia sensillum.
SEMs showing details of the most distal region of the male dogwood borer antennae with penultimate flagellomere devoid of sensilla and a conical shaped terminal flagellomere with apical sensors removed (a) ; profile of the most distal region of the female dogwood borer antennae possessing scales on the dorsal surface, and apical sensors on the terminal flagellomere (b) ; and close-up of the apical sensors on the most distal flagellomere of the female antennae (c). AS, apical sensors; Sc scales.
Sensilla Chaetica. These sensilla were distributed evenly along the flagellum, near the center or lateral region of the ventral surface, and typically numbered from one to four per flagellomere (Figs. 1d, 2, and 3). They had a straight, needle-like appearance and arose from a distinctive round collar-like socket (Fig. 6c). They were 12.6–38.3 µm long and had a basal width of 1.5–3.3 µm. Sensilla chaetica were significantly more abundant on the female antennae (t = 3.83, df= 8, P = 0.005) (Table 3).
Mean ± SE number of sensilla on the ventral surface of male and female dogwood borer antennae

Mean ± SE number of sensilla on the ventral surface of male and female dogwood borer antennae

Sensilla Coeloconica. These sensilla were found along the ventral surface of the entire flagellum and typically numbered from zero to six per flagellomere. They consisted of a submerged central peg surrounded by a ring of spine-like cuticular teeth (Fig. 6d). These sensilla were 4.5–12.9 µm in diameter and were significantly more abundant on female antennae (t = 7.05, df = 8, P ≤ 0.0001) (Table 3).
SEM showing close-up of auricillica sensilla, which had either a characteristic rabbit-ear shape or seemed dorsoventrally flattened (a); close-up of the basiconica sensillum (b); details of the chaetica sensillum, which arose from a distinctive collar-like socket (c) ; details of the coeloconica sensillum, characterized by a ring of cuticular teeth surrounding a central peg-like structure (d) ; squamiformia sensillum located on the dorsal surface of an antennal flagellomere (e) ; and close-up of the styloconica sensillum (f). Au, auricillica sensillum; Ba, basiconica sensillum; Ch, chaetica sensillum; Co, coeloconica sensillum; Sq squamiformia sensilla; St, styloconica sensillum.
Sensilla Squamiformia. These sensilla were found along the length of the flagellum near the center or lateral region of the dorsal surface among the scales, near the lateral region of the ventral surface and among the scales on the scape and pedicel (Figs. 2 and 4). They were scale-like in appearance with a distal end tapering to a forked point (Fig. 6e). These sensilla were 29.5–58.1 µm long and had a basal width of 0.9–1.9 µm. The number of sensilla squamiformia was not counted.
Sensilla Styloconica. These sensilla occurred along the entire flagellum near the upper middle region of the ventral surface (Fig. 2). A single sensillum of this type was typically found on each flagellomere, except the most proximal few. They consisted of a solid cylindrical base with an apically protruding conical structure (Fig. 6f) and were 6.5–18.5 µm long, with a basal width of 4.0–10.2 µm. Sensilla styloconica were significantly more abundant on female antennae (t = 4.14, df = 8, P = 0.003) (Table 3).
Sensilla Trichoidea. These sensilla were the most abundant of all sensillum types on the antennae of both males and females (Table 3), occurring along the length of the flagellum on the ventral surface. Their distribution on each flagellomere was random (Figs. 2 and 3) and their number increased distally along the flagellum, generally reaching their maximum abundance before the 35th flagellomere. These sensilla had a hair-like appearance and could be divided into three subtypes (i.e., large, medium, and small) according to their size (Fig. 7a). The size of all sensilla trichoidea decreased distally along the flagellum, which made accurate counts of each subtype difficult; however, the total mean number was significantly higher on female than on male antennae (t = 5.27, df = 8, P = 0.001) (Table 3). Large sensilla trichoidea were 53.2–164.9 µm long, had a basal width of 3.0–5.7 µm, and were present only on male antennae (Figs. 1c, 3, and 7a and b). Medium sensilla trichoidea were 24.7–59.1 µm long and had a basal width of 1.7–3.4 µm, and small sensilla trichoidea were 11.3–29.1 µm long and had a basal width of 0.8–2.3 µm. Large sensilla trichoidea were found along the entire flagellum of male antennae (Fig. 1c). Although medium sensilla trichoidea were found along the entire flagellum of female antennae, they were only located on the middle and distal regions of the flagellum of male antennae (Fig. 3). For both genders, small sensilla trichoidea were found along the entire flagellum except for the most proximal and distal flagellomeres.
SEM showing three distinct subtypes of sensilla trichoidea differentiated according to their size (i.e., large, medium, and small) (a); and close-up of the medium trichoidea sensillum and multiporous large trichoidea sensillum (b). LTr, large trichoidea sensillum; MTr, medium trichoidea sensillum; STr, small trichoidea sensillum; WP, wall pore.
Discussion
The general shape and structure of the antennae of male and female dogwood borer were similar to that reported for a related species, Synanthedon tipuliformis (Clerck) (Karalius 1994). Although similar in size to male antennae, the antennae of female dogwood borer possessed a greater number of antennal flagellomeres, which were typically smaller in size near the proximal and distal ends compared with males. The antennal flagellomeres of both males and females were cylindrical in shape and possessed a ventral surface on which most of the sensilla were located, and a dorsal surface possessing numerous overlapping scales. This arrangement of a distinct “sensory” and “scale” surface has been reported for several lepidopteran families, including the Sesiidae (Sellier 1977, Karalius 1994), Noctuidae (Mochizuki et al. 1992, Koh et al. 1995), Pyralidae (Hansson et al. 1995, Castrejón-Gómez et al. 2003), and Tortricidae (Castrejón-Gómez and Carrasco 2008). The supposed function of this arrangement may include the ability to detect stimulus direction (Van der Pers et al. 1980) and protection of the antennae and their sensilla from damage (Koh et al. 1995). An additional feature of dogwood borer antennae was the highly textured ventral surface of each flagellomere. The numerous ridge-like processes surrounding individual sensilla were seen in both male and female dogwood borer and may act as a mechanism by which odor molecules are trapped and concentrated along the sensory surface (Wall 1978).
The sensilla auricillica of the dogwood borer were similar in appearance and distribution to those described from S. tipuliformis (Karalius 1994). In Lepidoptera, this sensillum type is generally innervated by two to three sensory neurons and possesses thin multiporous walls (Anderson et al. 2000, Ansebo et al. 2005). Studies of noctuid (Anderson et al. 2000) and tortricid (Den Otter et al. 1978, Ansebo et al. 2005) species showed that these sensilla are involved with plant odor detection, and in Cydia pomonella (L.) the detection of minor sex pheromone components (Ebbinghaus et al. 1998). The large number of sensilla auricillica on the antennae of dogwood borer suggests that these sensilla play an important role in the olfactory response of this species. Because more of these sensilla were located on the individual flagellomeres of female than male antennae, and because females do not respond electrophysiologically to components of their sex pheromone (A. Zhang, personal communication), but do respond to host plant volatiles (Frank et al. 2007), it is probable that they function mainly as olfactory receptors for plant odors.
Sensilla basiconica were the only sensillum type present in roughly equal numbers on the flagellum of male and female dogwood borer and were similar in appearance to those described for Sesia apiformis (Clerck) (Sellier 1977). Studies of other lepidopteran species suggest that these sensilla may function as chemoreceptors because they have multiple sensory neurons and thin multiporous walls (Koh et al. 1995, Anderson et al. 2000). We were unable to confirm the presence of wall pores in the sensilla basiconica of dogwood borer because of poor resolution at higher magnification. Because a small number of these sensilla are located on the antennae of dogwood borer, and they are present in roughly equal numbers on each gender, their role in this species is unclear.
Several studies have suggested that sensilla chaetica serve both a contact chemo- and mechanoreceptive function because they arise from a socket and possess a terminal pore (Altner and Prillinger 1980, Van der Pers et al. 1980). In studies of other sesiid species, the presence of a terminal pore was not documented and the primary function of these sensilla was considered mechanoreceptive (Sellier 1977, Karalius 1994). Although the presence of a terminal pore on the sensilla chaetica of dogwood borer could not be confirmed, the behavior of females suggests that the role of these sensilla may involve both mechano- and contact chemoreception. Female dogwood borer searching for oviposition sites walk on the surface of host plants, probe it with their ovipositor and antennate (Frank et al. 2009). Although more sensilla chaetica were observed on female antennae, we should note that the presence of numerous large sensilla trichoidea located near the proximal end of male antennae may have obstructed the view of additional sensilla chaetica on those flagellomeres, possibly leading to lower counts in males.
In many lepidopteran insects, sensilla coeloconica have been described as possessing sensory neurons receptive to olfactory stimuli (Hansson et al. 1995, Hunger and Steinbrecht 1998) that may be sensitive to plant odors (Altner and Prillinger 1980). However, in the American cockroach, Periplaneta americana. L., these sensilla can function as thermo- or hygrosensory structures (Altner et al. 1977). Although we were unable to confirm the presence of wall pores in the sensilla coeloconica of dogwood borer, more of these sensilla were recorded from individual flagellomeres of female than from male dogwood borer antennae, which suggest that they may function as olfactory receptors for plant odors.
The sensilla squamiformia on dogwood borer antennae were similar in appearance to those of S. tipuliformis (Karalius 1994). These sensilla are thought to have a mechanoreceptive function (Schneider 1964, McIver 1975). These sensilla were easily detached from dogwood borer antennae during removal of scales, limiting our ability to make accurate assessments of their number.
The sensilla styloconica on dogwood borer antennae were distributed on the antennae in a similar arrangement as described for several other lepidopteran species (Sellier 1977, Mochizuki et al. 1992, Castrejón-Gómez et al. 2003). These sensilla are considered thermo- and hygrosensitive in Bombyx mori (L.) (Steinbrecht 1989) and the Pyralidae (Hallberg et al. 1994, Hansson et al. 1995); however, the presence of an apical pore in the Tortricidae have suggested a contact chemoreceptive function (Wall 1978, Castrejón-Gómez and Carrasco 2008). An apical pore was not observed on the sensilla styloconica of dogwood borer, perhaps suggesting that they may have a thermo- and hygrosensory function. Although more of these sensilla were observed on female antennae, based on their distribution and number on individual flagellomeres, we believe that the difference between males and females may be due primarily to the greater number of flagellomeres on female antennae.
Three distinct subtypes of sensilla trichoidea, differentiated according to their size, were found on the antennae of dogwood borer. Studies conducted by Sellier (1977) showed that antennae of the sesiid Sesia vespiformis Hüfnagel similarly possessed large, medium, and small sensilla trichoidea. These sensilla were of particular interest because the absence of large sensilla trichoidea on female dogwood borer antennae was the only characteristic that could be used to easily differentiate between male and female antennae under low magnification. Several studies have shown that large-type sensilla trichoidea of male lepidopterans are involved with olfactory reception of pheromone components (Mochizuki et al. 1992, Hansson et al. 1995, Ebbinghaus et al. 1998). The presence of numerous multiporous large sensilla trichoidea on male antennae and their complete absence on the antennae of females suggests that their main role is detection of the female-produced sex pheromone. However, the function of medium and small sensilla trichoidea is unclear due to an inability to obtain better resolution images of these sensilla at higher magnification. Medium sensilla trichoidea were present on both male and female antennae, although female antennae possessed a greater number of this sensillum subtype, which probably lead to significantly greater overall numbers of sensilla trichoidea compared with males. Small sensilla trichoidea seemed to be in roughly equal numbers on the antennae of both males and females. Although it is possible that the sensilla trichoidea on the antennae of female moths can detect their own sex pheromone (Ljungberg et al. 1993, Pearson and Schal 1999), it is more likely that they function as general olfactory receptors as they do for some species in the Noctuidae (Mochizuki et al. 1992, Koh et al. 1995).
Other sensory structures observed on the antennae of dogwood borer but that were not studied in further detail included the Böhm's bristles and apical sensors. Böhm's bristles are mechanoreceptive sensilla (Schneider 1964), and their placement near the proximal end of the scape and pedicel suggests a pro-prioreceptive function (Chapman 1998). Apical sensors were the only sensory structure observed on the terminal flagellomere. They have similarly been observed on the terminal flagellomere of other sesiid species and are thought to function as mechanoreceptors for sensing air currents (Karalius 1994).
This study has provided fundamental background information on the sensory structures present on the antennae of male and female dogwood borer and will enhance our ability to resolve the electrophysiological and behavioral responses of females to host plant volatiles. An understanding of the mechanisms involved with host-finding and oviposition acceptance behaviors will create opportunities to assess the possibility of using plant-derived compounds for the monitoring or management of female dogwood borer in apple orchards or managed urban landscapes.
Acknowledgments
We thank Sharon Jones for technical assistance.