-
PDF
- Split View
-
Views
-
Cite
Cite
R Marshall Austin, Agnieszka Onisko, Chengquan Zhao, Enhanced Detection of Cervical Cancer and Precancer Through Use of Imaged Liquid-Based Cytology in Routine Cytology and HPV Cotesting, American Journal of Clinical Pathology, Volume 150, Issue 5, November 2018, Pages 385–392, https://doi.org/10.1093/ajcp/aqy114
Close - Share Icon Share
Abstract
Cervical screening strives to prevent cervical cancer (CxCa), minimizing morbidity and mortality. Most large US reports on cytology and human papillomavirus (HPV) cotesting of women aged 30 years and older are from one laboratory, which used conventional Papanicolaou (Pap) smears from 2003 to 2009.
We quantified detection of CxCa and precancer (cervical intraepithelial neoplasia 3/adenocarcinoma in situ [CIN3/AIS]) in 300,800 cotests at Magee Womens Hospital since 2005. Screening histories preceding CxCa and CIN3/AIS diagnoses were examined to assess the contribution of cytology and HPV testing. Cotesting utilized Food and Drug Administration-approved imaged liquid-based cytology (LBC) and from-the-vial HPV tests.
LBC identified more women subsequently diagnosed with CxCa and CIN3/AIS than HPV testing. HPV-negative/cytology-positive results preceded 13.1% of CxCa and 7.2% of CIN3/AIS diagnoses.
LBC enhanced cotesting detection of CxCa and CIN3/AIS to a greater extent than previously reported with conventional Pap smear and HPV cotesting.
Human papillomavirus (HPV) and Papanicolaou (Pap) cotesting is currently the preferred cervical screening method for women 30 years and older in consensus guidelines of the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, the American Society for Clinical Pathology, and the American College of Obstetricians and Gynecologists.1,2 Several very large US clinical datasets have now been published documenting the performance of cotesting in routine cervical screening and assessing the follow-up risk among screened women for histopathologic diagnoses of cervical high-grade intraepithelial lesions and invasive cervical cancer (CxCa).3-6 These large-scale analyses have almost exclusively come from a single large laboratory, Kaiser Permanente Northern California (KPNC). At KPNC, over 1,200,000 women aged 30 years and older have, since 2003, undergone triennial cervical cotesting.3,5,6 This laboratory, however, has followed a number of cervical screening practices that are unusual in the United States. First, in relying exclusively on the conventional Pap smear until 2009, KPNC may have been the last large US system to adopt liquid-based cytology (LBC).7 To the best of our knowledge, only the US prison system now continues to rely largely on the conventional Pap smear. Furthermore, KPNC has collected Pap and HPV specimens as two separate specimens rather than utilizing from-the-vial HPV testing as recommended by the Food and Drug Administration (FDA) in clinical trials.6,8 Apart from the KPNC reports, only a single very large nationwide study from a Quest Diagnostics database of 8.6 million patients has reported cotesting results utilizing FDA-approved LBC and from-the-vial HPV cotesting.4 Because the KPNC studies6 have questioned the contribution of cytology to detection by cotesting of CxCa and precancer, we decided to analyze our own extended clinical experience with cotesting in a large academic women’s hospital laboratory utilizing the most widely employed FDA-approved LBC methods and FDA-approved from-the-vial HPV cotesting.
Materials and Methods
Magee-Womens Hospital Data
This study analyzed cervical screening data collected over a period of almost 13 years, between January 2005 and August 2017, at Magee-Womens Hospital (MWH) of University of Pittsburgh Medical Center (UPMC). This database reflects cervical screening test results generated from widely used FDA-approved cervical screening technologies, including LBC using the ThinPrep Pap test9 (Hologic Corp, Bedford, MA), computer-assisted LBC imaging using the ThinPrep Imaging System10 (Hologic Corp, Bedford, MA), and from-the-PreservCyt vial testing for the presence of high-risk HPV (hrHPV) using either the Digene Hybrid Capture 2 HPV test11 (until May 2013) (Qiagen Corp, Gaithersburg, MD), the Cervista HR HPV test12 (June 2013 to May 2015) (Hologic Corp, Madison, WI), or the Aptima HPV test13 (since June 2015) (Hologic/Gen-Probe, San Diego, CA). Cytology results were reported utilizing the 2001 Bethesda System (TBS).14 Abnormal or “positive” cytology results were considered to be all Pap tests reporting epithelial cell abnormalities at the level of atypical squamous cells of underdetermined significance (ASC-US) or a more severe cytologic abnormality.
In the MWH database there were 270,263 women aged 30 years and older with 748,947 LBC test results, and 300,800 (40.2%) of these LBC specimens also had from-the-vial hrHPV test results. Around 8.8% of the LBC test results (66,045) were followed by gynecological histopathologic evaluations of cervical tissue specimens. The MWH database also included clinical information such as history of infections, cancers, use of contraception, menstrual history, and HPV vaccine status. In this study we focused our analysis on women aged 30 years and older who had at least one available Pap and HPV cotest result. There were 300,800 cotest results from 186,000 patients. These screening test results preceded histopathologic diagnoses of 129 invasive CxCas and 632 histopathologic diagnoses of high-grade cervical intraepithelial lesions at the level of either cervical intraepithelial neoplasia 3 (CIN3) or adenocarcinoma in situ (AIS). To facilitate comparison, we have reported our cotesting data in a format similar to that presented recently in the largest and most recent cotesting publication from KPNC.6
MWH cervical screening cotesting data were also analyzed using the Pittsburgh Cervical Cancer Screening Model (PCCSM).15-18 This model is a dynamic Bayesian network constructed in GeNIe and SMILE (Structural Modeling, Inference, and Learning Engine), a development environment for creating and reasoning in graphical probabilistic models developed at the Decision Systems Laboratory, University of Pittsburgh (http://www.bayesfusion.com). The PCCSM was built based on expert knowledge and the ongoing collection of follow-up data from over 13 years of practice experience at MWH-UPMC. The continuously updated model includes CxCa screening test results, history of contraception, menstrual history, demographics, and the results of gynecological surgical procedures. The PCCSM model allows for quantitative risk stratification of patients for development over varying periods of follow-up time for selected diagnostic endpoints such as biopsy-proven cervical precancer or cervical invasive cancer, all based on MWH-UPMC system data.
Results
Cotesting Data Preceding Cervical Invasive Cancer and Cervical Precancer Diagnoses
We first analyzed cotesting data preceding invasive CxCa and CIN3/AIS diagnoses. Table 1 shows all HPV and cytology cotest results preceding invasive CxCa diagnoses, both overall and broken down according to specific tumor histopathology, ie, squamous carcinomas, adenocarcinoma or adenosquamous carcinoma, and other cervical carcinomas. There were 198 cotest results preceding 129 CxCas; 76.3% of these preceding cotest results were HPV positive and 83.3% were cytology positive (ASC-US positive); 89.4% of all cotest results preceding CxCa diagnoses were either HPV positive or cytology positive.
High-Risk Human Papilloma Virus and Cytology Cotesting Results Preceding Invasive Cervical Cancer Diagnoses, Both Overall and by Specific Histopathology: SCC, ADC, and Other Cervical Carcinomas
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| All cancers | 198 (100) | 151 (76.3) | 165 (83.3) | 177 (89.4) | 139 (70.2) | 12 (6.1) | 26 (13.1) | 21 (10.6) | .0828 |
| SCC | 96 (100) | 74 (77.1) | 89 (92.7) | 90 (93.8) | 73 (76.0) | 1 (1.0) | 16 (16.7) | 6 (6.3) | .0025 |
| ADC | 93 (100) | 71 (76.3) | 68 (73.1) | 79 (84.9) | 60 (64.5) | 11 (11.8) | 8 (8.6) | 14 (15.2) | .6157 |
| Other | 9 (100) | 6 (66.7) | 8 (88.9) | 8 (88.9) | 6 (66.7) | 0 (0.0) | 2 (22.2) | 1 (11.1) | |
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| All cancers | 198 (100) | 151 (76.3) | 165 (83.3) | 177 (89.4) | 139 (70.2) | 12 (6.1) | 26 (13.1) | 21 (10.6) | .0828 |
| SCC | 96 (100) | 74 (77.1) | 89 (92.7) | 90 (93.8) | 73 (76.0) | 1 (1.0) | 16 (16.7) | 6 (6.3) | .0025 |
| ADC | 93 (100) | 71 (76.3) | 68 (73.1) | 79 (84.9) | 60 (64.5) | 11 (11.8) | 8 (8.6) | 14 (15.2) | .6157 |
| Other | 9 (100) | 6 (66.7) | 8 (88.9) | 8 (88.9) | 6 (66.7) | 0 (0.0) | 2 (22.2) | 1 (11.1) | |
ADC, adenocarcinoma or adenosquamous carcinoma; HPV, human papillomavirus; Pap, Papanicolaou test; SCC, squamous cell carcinoma.
aTwo-sided P values comparing HPV tests with cytology were based on Z scores.6
High-Risk Human Papilloma Virus and Cytology Cotesting Results Preceding Invasive Cervical Cancer Diagnoses, Both Overall and by Specific Histopathology: SCC, ADC, and Other Cervical Carcinomas
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| All cancers | 198 (100) | 151 (76.3) | 165 (83.3) | 177 (89.4) | 139 (70.2) | 12 (6.1) | 26 (13.1) | 21 (10.6) | .0828 |
| SCC | 96 (100) | 74 (77.1) | 89 (92.7) | 90 (93.8) | 73 (76.0) | 1 (1.0) | 16 (16.7) | 6 (6.3) | .0025 |
| ADC | 93 (100) | 71 (76.3) | 68 (73.1) | 79 (84.9) | 60 (64.5) | 11 (11.8) | 8 (8.6) | 14 (15.2) | .6157 |
| Other | 9 (100) | 6 (66.7) | 8 (88.9) | 8 (88.9) | 6 (66.7) | 0 (0.0) | 2 (22.2) | 1 (11.1) | |
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| All cancers | 198 (100) | 151 (76.3) | 165 (83.3) | 177 (89.4) | 139 (70.2) | 12 (6.1) | 26 (13.1) | 21 (10.6) | .0828 |
| SCC | 96 (100) | 74 (77.1) | 89 (92.7) | 90 (93.8) | 73 (76.0) | 1 (1.0) | 16 (16.7) | 6 (6.3) | .0025 |
| ADC | 93 (100) | 71 (76.3) | 68 (73.1) | 79 (84.9) | 60 (64.5) | 11 (11.8) | 8 (8.6) | 14 (15.2) | .6157 |
| Other | 9 (100) | 6 (66.7) | 8 (88.9) | 8 (88.9) | 6 (66.7) | 0 (0.0) | 2 (22.2) | 1 (11.1) | |
ADC, adenocarcinoma or adenosquamous carcinoma; HPV, human papillomavirus; Pap, Papanicolaou test; SCC, squamous cell carcinoma.
aTwo-sided P values comparing HPV tests with cytology were based on Z scores.6
Table 2 shows all HPV and cytology cotest results preceding cervical precancer diagnoses of CIN3 and/or AIS, both overall and broken down according to specific histopathology, ie, CIN3 and AIS. There were 1,000 cotest results preceding 632 CIN3/AIS diagnoses; 86.7% of these results were HPV positive and 91.0% were cytology positive (ASC-US positive); 93.9% of all cotest results preceding CIN3/AIS diagnoses were either HPV positive or cytology positive.
High-Risk Human Papilloma Virus and Cytology Cotesting Results Preceding Precancer Diagnoses, Both Overall and by Specific Histopathology: CIN3 and AIS
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| All precancers | 1,000 (100) | 867 (86.7) | 910 (91.0) | 939 (93.9) | 838 (83.8) | 29 (2.9) | 72 (7.2) | 61 (6.1) | .0023 |
| CIN3 | 845 (100) | 747 (88.4) | 785 (92.9) | 803 (95.0) | 729 (86.3) | 18 (2.1) | 56 (6.6) | 42 (5.0) | .0015 |
| AIS | 155 (100) | 120 (77.4) | 125 (80.6) | 136 (87.7) | 109 (70.3) | 11 (7.1) | 16 (10.3) | 19 (12.3) | .4892 |
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| All precancers | 1,000 (100) | 867 (86.7) | 910 (91.0) | 939 (93.9) | 838 (83.8) | 29 (2.9) | 72 (7.2) | 61 (6.1) | .0023 |
| CIN3 | 845 (100) | 747 (88.4) | 785 (92.9) | 803 (95.0) | 729 (86.3) | 18 (2.1) | 56 (6.6) | 42 (5.0) | .0015 |
| AIS | 155 (100) | 120 (77.4) | 125 (80.6) | 136 (87.7) | 109 (70.3) | 11 (7.1) | 16 (10.3) | 19 (12.3) | .4892 |
AIS, adenocarcinoma in situ; CIN3, cervical intraepithelial neoplasia 3; HPV, human papillomavirus; Pap, Papanicolaou test.
aTwo-sided P values comparing HPV tests with cytology test were based on Z scores.6
High-Risk Human Papilloma Virus and Cytology Cotesting Results Preceding Precancer Diagnoses, Both Overall and by Specific Histopathology: CIN3 and AIS
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| All precancers | 1,000 (100) | 867 (86.7) | 910 (91.0) | 939 (93.9) | 838 (83.8) | 29 (2.9) | 72 (7.2) | 61 (6.1) | .0023 |
| CIN3 | 845 (100) | 747 (88.4) | 785 (92.9) | 803 (95.0) | 729 (86.3) | 18 (2.1) | 56 (6.6) | 42 (5.0) | .0015 |
| AIS | 155 (100) | 120 (77.4) | 125 (80.6) | 136 (87.7) | 109 (70.3) | 11 (7.1) | 16 (10.3) | 19 (12.3) | .4892 |
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| All precancers | 1,000 (100) | 867 (86.7) | 910 (91.0) | 939 (93.9) | 838 (83.8) | 29 (2.9) | 72 (7.2) | 61 (6.1) | .0023 |
| CIN3 | 845 (100) | 747 (88.4) | 785 (92.9) | 803 (95.0) | 729 (86.3) | 18 (2.1) | 56 (6.6) | 42 (5.0) | .0015 |
| AIS | 155 (100) | 120 (77.4) | 125 (80.6) | 136 (87.7) | 109 (70.3) | 11 (7.1) | 16 (10.3) | 19 (12.3) | .4892 |
AIS, adenocarcinoma in situ; CIN3, cervical intraepithelial neoplasia 3; HPV, human papillomavirus; Pap, Papanicolaou test.
aTwo-sided P values comparing HPV tests with cytology test were based on Z scores.6
Table 3 shows HPV and cytology cotest results performed less than 12 months preceding histopathologic invasive CxCa or cervical precancer (CIN3/AIS) diagnoses compared to HPV and Pap cotest results performed more than 12 months prior to the same histopathologic diagnoses. Cotesting results performed less than 12 months prior to CxCa or CIN3/AIS diagnoses were more likely to be positive (both HPV positive and/or cytology positive) than cotest results performed 12 or more months before the same histopathologic diagnoses. Abnormal cytology results were more likely than HPV-positive results to be recorded prior to either CxCa (90.8% vs 84.4%) or cervical precancer (97.9% vs 95.9%) diagnoses when cotesting was performed less than 12 months prior to diagnosis. When cotesting was performed 12 or more months before the CxCa or CIN3/AIS diagnoses, abnormal cytology results were also more likely than HPV-positive results prior to either CxCa (74.2% vs 66.3%) or cervical precancer (81.3% vs 73.8%).
High-Risk Human Papilloma Virus and Cytology Cotesting Results Less Than 12 Months vs 12 or More Months Preceding Invasive Cervical Cancer or Cervical Precancer Diagnoses
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| CxCa | |||||||||
| <12 mo | 109 (100) | 92 (84.4) | 99 (90.8) | 103 (94.5) | 88 (80.7) | 4 (3.7) | 11 (10.1) | 6 (5.5) | .1517 |
| ≥12 mo | 89 (100) | 59 (66.3) | 66 (74.2) | 74 (83.1) | 51 (57.3) | 8 (9.0) | 15 (16.9) | 15 (16.9) | .249 |
| CIN3/AIS | |||||||||
| <12 mo | 584 (100) | 560 (95.9) | 572 (97.9) | 581 (99.5) | 551 (94.3) | 9 (1.5) | 21 (3.6) | 3 (0.5) | .0486 |
| ≥12 mo | 416 (100) | 307 (73.8) | 338 (81.3) | 358 (86.1) | 287 (69.0) | 20 (4.8) | 51 (12.3) | 58 (13.9) | .0095 |
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| CxCa | |||||||||
| <12 mo | 109 (100) | 92 (84.4) | 99 (90.8) | 103 (94.5) | 88 (80.7) | 4 (3.7) | 11 (10.1) | 6 (5.5) | .1517 |
| ≥12 mo | 89 (100) | 59 (66.3) | 66 (74.2) | 74 (83.1) | 51 (57.3) | 8 (9.0) | 15 (16.9) | 15 (16.9) | .249 |
| CIN3/AIS | |||||||||
| <12 mo | 584 (100) | 560 (95.9) | 572 (97.9) | 581 (99.5) | 551 (94.3) | 9 (1.5) | 21 (3.6) | 3 (0.5) | .0486 |
| ≥12 mo | 416 (100) | 307 (73.8) | 338 (81.3) | 358 (86.1) | 287 (69.0) | 20 (4.8) | 51 (12.3) | 58 (13.9) | .0095 |
AIS, adenocarcinoma in situ; CIN3, cervical intraepithelial neoplasia 3; CxCa, cervical cancer; Pap, Papanicolaou test; HPV, human papillomavirus.
aTwo-sided P values comparing HPV tests with cytology were based on Z scores6
High-Risk Human Papilloma Virus and Cytology Cotesting Results Less Than 12 Months vs 12 or More Months Preceding Invasive Cervical Cancer or Cervical Precancer Diagnoses
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| CxCa | |||||||||
| <12 mo | 109 (100) | 92 (84.4) | 99 (90.8) | 103 (94.5) | 88 (80.7) | 4 (3.7) | 11 (10.1) | 6 (5.5) | .1517 |
| ≥12 mo | 89 (100) | 59 (66.3) | 66 (74.2) | 74 (83.1) | 51 (57.3) | 8 (9.0) | 15 (16.9) | 15 (16.9) | .249 |
| CIN3/AIS | |||||||||
| <12 mo | 584 (100) | 560 (95.9) | 572 (97.9) | 581 (99.5) | 551 (94.3) | 9 (1.5) | 21 (3.6) | 3 (0.5) | .0486 |
| ≥12 mo | 416 (100) | 307 (73.8) | 338 (81.3) | 358 (86.1) | 287 (69.0) | 20 (4.8) | 51 (12.3) | 58 (13.9) | .0095 |
| Histopathology . | Cotesting Results Prior to Diagnoses, No. (%) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total . | HPV+ . | Pap+ . | Any+ . | HPV+ Pap+ . | HPV+ Pap– . | HPV– Pap+ . | HPV– Pap– . | Pa . | |
| CxCa | |||||||||
| <12 mo | 109 (100) | 92 (84.4) | 99 (90.8) | 103 (94.5) | 88 (80.7) | 4 (3.7) | 11 (10.1) | 6 (5.5) | .1517 |
| ≥12 mo | 89 (100) | 59 (66.3) | 66 (74.2) | 74 (83.1) | 51 (57.3) | 8 (9.0) | 15 (16.9) | 15 (16.9) | .249 |
| CIN3/AIS | |||||||||
| <12 mo | 584 (100) | 560 (95.9) | 572 (97.9) | 581 (99.5) | 551 (94.3) | 9 (1.5) | 21 (3.6) | 3 (0.5) | .0486 |
| ≥12 mo | 416 (100) | 307 (73.8) | 338 (81.3) | 358 (86.1) | 287 (69.0) | 20 (4.8) | 51 (12.3) | 58 (13.9) | .0095 |
AIS, adenocarcinoma in situ; CIN3, cervical intraepithelial neoplasia 3; CxCa, cervical cancer; Pap, Papanicolaou test; HPV, human papillomavirus.
aTwo-sided P values comparing HPV tests with cytology were based on Z scores6
Distribution of Cotesting Results Prior to Cervical Cancer or Cervical Precancer Diagnoses by Time Period
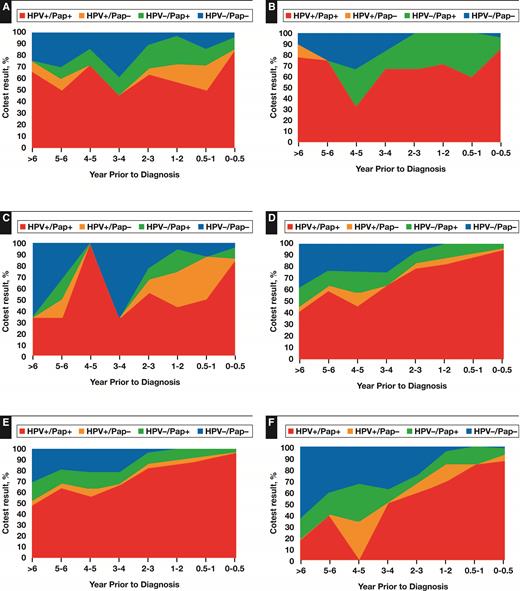
Figure 1 shows cotesting results prior to histopathologic CxCa or CIN3/AIS diagnoses by time period prior to histopathologic diagnosis, 0 to 0.5 years (6 months) prior to diagnosis, 0.5 years to 1 year prior, 1 to 2 years prior, 3 to 4 years prior, 4 to 5 years prior, 5 to 6 years prior, and more than 6 years prior. Prior cotesting results are shown as either HPV positive/Pap positive (red), HPV positive/Pap negative (orange), HPV negative/Pap positive (green), or HPV negative/Pap negative (blue).
Distribution of cotesting results preceding cervical invasive cancer or cervical precancer diagnoses by time period: all cervical cancers (A), squamous cell carcinomas (B), adenocarcinomas (C), all cervical precancers (D), cervical intraepithelial neoplasia 3 (E), and adenocarcinomas in situ (F). HPV, human papillomavirus; Pap, Papanicolaou test.
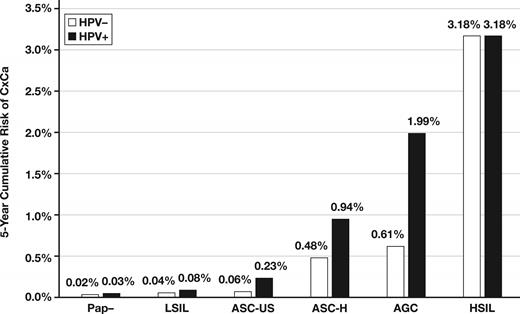
Figure 2 shows PCCSM projections for 5-year cumulative risk of CxCa associated with various MWH cotest results, including each separate TBS cytology category shown along with either HPV-positive or HPV-negative cotest results. HPV-negative cytology-positive (abnormal) document CxCa risk only detectible utilizing the cytology component of cotesting.
The Pittsburgh Cervical Cancer Model risk assessments. AGC, atypical glandular cells; ASC-H, atypical squamous cells-cannot exclude HSIL; ASC-US, atypical squamous cells of undetermined significance; CxCa, cervical cancer; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; Pap, Papanicolaou test.
Discussion
Cytology findings in this study were significantly different from those reported in large cotesting studies from KPNC.3,5-7 We report here that in cotested patients aged 30 years or older, more CxCas and CIN3/AIS diagnoses were detected with LBC than with FDA-approved from-the-LBC vial HPV testing, whereas KPNC investigators reported that HPV cotesting along with large-scale use of conventional Pap smears identified more women subsequently diagnosed with CxCa and precancer.6 As noted above, KPNC has employed several cervical screening practices that are unusual in the United States and which could be related to our different findings in cotested patients. We believe that the exclusive use at KPNC of conventional Pap smears from 2003 to 20097 deserves the most scrutiny. Numerous large datasets comparing high-grade squamous intraepithelial lesion and CxCa detection rates utilizing LBC vs conventional Pap smears have consistently documented increased detection of significant abnormalities with LBC.19-23 Enhanced detection of abnormalities with LBC has been attributed to both increased harvesting of cells from Pap collection devices and the interpretive advantage of immediate wet fixation,24 as well as the opportunity to perform adjunctive molecular testing from residual LBC vial fluids. Although some still question the advantages of LBC based on international trials in two laboratories relatively inexperienced with the LBC method and lacking clearly documented LBC proficiency,25,26 clinical trials in the more tightly quality controlled UK cervical screening system have documented a significantly enhanced performance with LBC compared with conventional cytology.27 Preferential prevention of incident CxCas was also documented with LBC in the UK Artistic trial in which all CxCas diagnosed 2.5 to 8 years after the start of the trial were detected in the HPV arm of the trial compared to no CxCas detected during the same time period in the LBC arm.28 European trials in Holland, Italy, and Sweden using the conventional Pap smear showed the opposite findings, with HPV testing preventing more CxCas than conventional smears.28
This study also highlights the significance of the length of time before CxCa and CIN3/AIS diagnoses in declines in cervical screening test performance (Figure 1). In spite of ongoing controversy about the safety of lengthened screening intervals,29 current guidelines continue to suggest 5-year screening intervals for most patients after “double-negative” cytology and HPV cotest results.1,2 Both this study and KPNC findings6 document that cytology and HPV detection rates prior to CxCa and CIN3/AIS diagnoses decline substantially when testing was more than 12 months before diagnosis. While HPV-positive rates more than 12 months before CxCa diagnoses were similar at KNPC (62.8%)6 and MWH (66.3%) (Table 3), abnormal cytology rates more than 12 months before CxCa were markedly different at KPNC (28.7%)6 and MWH (74.2%) (Table 3). HPV detection rates decline progressively as time before CxCa diagnoses increases,30 most likely due to smaller lesional size and increased difficulties in sampling lesional cells. In European clinical trials, baseline HPV-negative rates in women developing incident CxCa 2.5 to 8 years after the start of the trials rose to 42%.28 These rarely acknowledged data are not reassuring in supporting lengthened screening intervals of 5 to 10 years now being employed in some programs.31 Even KPNC and the National Cancer Institute coinvestigators recently acknowledged “one of the barriers to adopting HPV testing into routine practice is simply a lack of long-term, longitudinal data on safety”.32
Data in this study confirm that local cytology performance can significantly impact the contribution of cytology to cotesting and detection of significant cervical lesions. In addition to the advantages of LBC over conventional Pap smear cytology described earlier, acknowledgement needs to be made that LBC can be performed suboptimally as well. We have previously reported verification bias-adjusted (VBA) sensitivity for LBC in the detection of histopathologic CIN2/3/AIS/CxCa lesions as high as 93%.33 In contrast, the four large laboratories participating in the cobas HPV test (Roche Molecular Diagnostics, Pleasanton, CA) ATHENA trials recorded clearly suboptimal cytology performance with VBA CIN3-positive age-stratified sensitivity at only 27% to 42%.30 Even among these four laboratories, VBA-adjusted sensitivity varied significantly.34 MWH has long employed an approach to cervical screening that we refer to as Cervical Cancer Audit-Based Screening,35,36 one in which abnormal immature metaplastic squamous cells and atypical glandular cells, often seen in only small numbers in retrospective audits of CxCa cases, are the specific emphasis of prospective screening. Conservative low-volume screening by cytotechnologists and intensive ongoing staff education are essential parts of this approach.33,35
Data in this study are also relevant to the role of cotesting in detection of difficult-to-prevent cervical adenocarcinomas.37 KPNC data have previously emphasized the significance of HPV-positive/cytology-negative results in detection of endocervical adenocarcinomas.38 In KNPC’s largest cotesting publication,6 160 of 443 (36.1%) cervical adenocarcinomas had prior HPV-positive/cytology-negative cotest results. In contrast, only 11 of 93 (11.8%) of cervical adenocarcinomas diagnosed after MWH cotesting had prior HPV-positive/cytology-negative results (Table 1). Cervical adenocarcinomas diagnosed after cotesting had similar HPV-positive rates at both institutions, KPNC (79.0%) and MWH (76.3%), whereas abnormal cytology rates (cytology positive) were markedly different, at KPNC (45.4%) compared to MWH (73.1%) (Table 1). Once again, local cytology performance significantly impacted the contribution of cytology to cotesting in detection of significant cervical abnormalities.
Clinical trial data exploring cervical screening options have often emphasized detection of more-prevalent CIN3 or CIN2, arguing that “the main goal of cervical screening programs is to detect precancer before cancer develops.”6 However, most CIN2 lesions, particularly in young women, will spontaneously regress.39 Even among women with CIN3, available long-term natural history studies of untreated disease indicate that only 30% of CIN3 lesions will progress to CxCa in 30 years.40 Because most CIN2/3 lesions will not progress to CxCa, detection of these nonprogressive CIN2/3 lesions does not enhance prevention against CxCa and has been termed by some investigators as overdiagnosis.41 Accordingly, it becomes even more important to assess protection against development of the much less-common endpoint of invasive cervical carcinoma. Our Bayesian modeling techniques allow long-term projections of CxCa risk while also directly reflecting local screening system data and treatment methods. Bayesian projections with the PCCSM confirm CxCa risk associated with HPV-negative cytology-positive (abnormal) results, risk only detectible utilizing the imaged LBC cytology component of MWH cotesting (Figure 1).
Acknowledgments
The authors thank Karen Lassige and Lisa Lenik for their invaluable help in retrieving data from the hospital database.