-
PDF
- Split View
-
Views
-
Cite
Cite
Emmanuel Stamatakis, I -Min Lee, Jason Bennie, Jonathan Freeston, Mark Hamer, Gary O’Donovan, Ding Ding, Adrian Bauman, Yorgi Mavros, Does Strength-Promoting Exercise Confer Unique Health Benefits? A Pooled Analysis of Data on 11 Population Cohorts With All-Cause, Cancer, and Cardiovascular Mortality Endpoints, American Journal of Epidemiology, Volume 187, Issue 5, May 2018, Pages 1102–1112, https://doi.org/10.1093/aje/kwx345
Close - Share Icon Share
Abstract
Public health guidance includes recommendations to engage in strength-promoting exercise (SPE), but there is little evidence on its links with mortality. Using data from the Health Survey for England and the Scottish Health Survey from 1994–2008, we examined the associations between SPE (gym-based and own-body-weight strength activities) and all-cause, cancer, and cardiovascular disease mortality. Multivariable-adjusted Cox regression was used to examine the associations between SPE (any, low-/high-volume, and adherence to the SPE guideline (≥2 sessions/week)) and mortality. The core sample comprised 80,306 adults aged ≥30 years, corresponding to 5,763 any-cause deaths (736,463 person-years). Following exclusions for prevalent disease/events occurring in the first 24 months, participation in any SPE was favorably associated with all-cause (hazard ratio (HR) = 0.77, 95% confidence interval (CI): 0.69, 0.87) and cancer (HR = 0.69, 95% CI: 0.56, 0.86) mortality. Adhering only to the SPE guideline was associated with all-cause (HR = 0.79, 95% CI: 0.66, 0.94) and cancer (HR = 0.66, 95% CI: 0.48, 0.92) mortality; adhering only to the aerobic activity guideline (equivalent to 150 minutes/week of moderate-intensity activity) was associated with all-cause (HR = 0.84, 95% CI: 0.78, 0.90) and cardiovascular disease (HR = 0.78, 95% CI: 0.68, 0.90) mortality. Adherence to both guidelines was associated with all-cause (HR = 0.71, 95% CI: 0.57, 0.87) and cancer (HR = 0.70, 95% CI: 0.50, 0.98) mortality. Our results support promoting adherence to the strength exercise guidelines over and above the generic physical activity targets.
There is a well-established association between participation in regular physical activity and reductions in all-cause, cardiovascular disease (CVD), diabetes, and cancer-related mortality (1, 2). In the last decade, strength-promoting exercise (SPE) has become an integral component of physical activity guidelines around the world (3, 4), with the World Health Organization recommending at least 2 sessions per week.
Current SPE guidelines are primarily intended to increase strength and function, and there are few data on associations with chronic disease and mortality. Participation in strengthening exercise has been associated with reduced risk of type 2 diabetes in men (ages 40–75 years) (5), women (ages 36–81 years) (6), and working-age populations (ages 30–64 years) (7). These associations were independent of aerobic exercise, conferred greater benefit when combined with aerobic exercise (5, 6), and were more pronounced in older adults (7). Compared with aerobic forms of physical activity, SPE is unique in its ability to promote increases in muscle size and strength, with higher muscle mass (8, 9) and strength (10) being found to be associated with a lower mortality risk. Thus, SPE may be promising for reducing premature mortality and chronic disease risk.
However, few studies have explored associations between SPE and cause-specific mortality. SPE has been shown to be associated with reduced risk of fatal and nonfatal myocardial infarction among adult men (11) and reduced risk of all-cause mortality in cancer survivors (12), and recent studies have also shown reductions in all-cause mortality among adults who meet the guidelines of 2 sessions per week (13–15). However, limited conclusions can be drawn, and the few studies that have been published have usually been limited to older adults residing in the United States (13) and small cohorts (12, 14, 15), with no measures being taken to account for reverse causality by removing prevalent cases (13–15) or by excluding events taking place during the first few months or years of follow-up (11, 13–15).
Our aim in this study was to examine the associations between SPE and all-cause, CVD, and cancer mortality and to compare the SPE and aerobic activity guidelines in terms of their associations with mortality outcomes.
METHODS
Sample
The Health Survey for England (16) and the Scottish Health Survey (17) are established household-based population surveillance studies that have been conducted annually since 1991 and 1995, respectively. Each year, samples of the general population are selected using a multistage, stratified probability design aimed at recruiting a nationally representative sample. Trained interviewers visit the selected households, and the recruited participants are administered the study questionnaires. All survey participants give written consent to have their deaths flagged in the National Health Service Central Register.
The current study included persons aged ≥30 years from the 1994, 1997, 1998, 1999, 2003, 2004, 2006, and 2008 administrations of the Health Survey for England and the 1995, 1998, and 2003 administrations of the Scottish Health Survey, with the corresponding linkage to mortality data. Each baseline survey was approved by the relevant Research Ethics Committees in England and Scotland.
Mortality outcomes
Participants were followed up for mortality until December 31, 2009 (Scottish Health Survey) or March 31, 2011 (Health Survey for England). Diagnoses for primary causes of death were recorded according to the International Classification of Diseases, Ninth Revision (ICD-9) or the International Classification of Diseases, Tenth Revision (ICD-10). Cancer deaths were identified using ICD-9 codes 140.0–239.9 and ICD-10 codes C00.0–D48.9; CVD deaths were identified using ICD-9 codes 390.0–459.9 and ICD-10 codes I01.0–I99.
Assessment of SPE and other physical activity
Physical activity was assessed using a questionnaire (18) that inquired about participation in sports and exercises during the 4 weeks prior to the interview. Participants were shown a card (see the Web Appendix, available at https://academic.oup.com/aje) with 10 exercise groupings, including working out at a gym/weight training/exercise biking, which we labeled “gym-based” SPE, and exercises such as press-ups and sit-ups, which we labeled “own-body-weight” SPE. For each positive response, participants were asked whether they had participated in the activity for at least 15 minutes, the frequency of activity (number of occasions), and the duration of activity per occasion. “All strength exercise” (total SPE) was defined as the sum of gym-based SPE and own-body-weight SPE. The questionnaire also included items on domestic physical activity and walking that have been described in detail elsewhere (19, 20). All physical activity variables were summarized to reflect weekly averages. In a large validation study, the Spearman correlation coefficients for correlation between accelerometry counts and self-reported activity (converted to weekly metabolic equivalent of task (MET)-minutes) were 0.41 (95% confidence interval (CI): 0.36, 0.46) for women and 0.32 (95% CI: 0.26, 0.38) for men (18).
To minimize misclassification arising from likely inclusion of aerobic exercise, the volume of gym-based workouts was weighted using age- and sex-specific proportions of total gym-based activity that was reported on the questionnaire to be “strength workout at a gym using machines or free weights.” These proportions were derived using the pooled samples from the Health Survey for England 2008 (21) (n = 12,360) and the Health Survey for England 2012 (22) (n = 6,883), which included additional questions specifying the nature of the gym-based activity (Web Table 1). On average, 63% of the gym-based activity during those 2 years was SPE, with a tendency toward a decrease by age group, from 86% in respondents aged 30–35 years to 61% among those aged 75 years or older. The Compendium of Physical Activities (23) was used to assign MET values for all physical activities for calculation of total MET-hours/week. As we did previously (24), we estimated adherence to the aerobic guideline as 150 minutes/week of moderate-intensity activity or 75 minutes/week of vigorous-intensity activity or equivalent combinations of moderate and vigorous non-SPE/nondomestic physical activity (4). We also computed an alternative interpretation of the aerobic guideline defined as accumulating at least 7.5 MET-hours/week (25) of any type and intensity (26) of non-SPE physical activity. Adherence to the SPE guideline was defined as reporting participation in at least 2 sessions per week, on average.
Covariates
Height and weight were measured by the interviewers using standard protocols (16, 17); body mass index was calculated as weight (in kilograms) divided by height (in meters) squared. Additional questions assessed age, educational attainment (age at completion of full-time education), presence of long-standing illness, weekly frequency of alcohol consumption, smoking habits (never smoker, ex-smoker, currently smoking 1–9 cigarettes/day, currently smoking 10–19 cigarettes/day, or currently smoking ≥20 cigarettes/day), psychological distress/depression (12-point General Health Questionnaire score), and number of servings of fruit and vegetables consumed on the day prior to the interview.
Statistical analysis
Analyses were carried out using SPSS, version 22 (SPSS Inc., Chicago, Illinois). Cox proportional hazards models were used to examine the associations between total and type-specific SPE and all-cause, cancer, and CVD mortality, with “no participation” set as the reference category. Log-minus-log plots were used to examine the proportional hazards assumption, and no violations were observed. The results of the analyses were adjusted for age, sex, all of the covariates listed above, and weekly MET-hours of non-SPE activity. We examined associations of overall participation in activity (none/any) and volume of activity (none/low/high) with mortality outcomes. High and low weekly volumes were classified using the sex-specific median values of the corresponding variable (Web Table 2). We examined the association between meeting the strength-promoting activity guideline (≥2 sessions/week) (4) and mortality, and we compared associations with meeting the general (aerobic) activity guideline using a 4-level variable: meeting neither of the 2 recommendations (referent), meeting the SPE recommendation only, meeting the aerobic recommendation only, and meeting both recommendations.
To minimize the possibility of spurious associations due to occult disease, we excluded participants who died during the first 24 months of follow-up. We excluded persons with prevalent cancer at baseline from the cancer mortality analyses; persons with prevalent CVD (angina, stroke, or ischemic heart disease) from the CVD mortality analyses; and both persons with prevalent CVD and persons with prevalent cancer from the all-cause mortality analyses. Unless otherwise stated in the Results section, the own-body-weight and gym-based SPE categories were not mutually exclusive.
We performed a series of sensitivity analyses to minimize bias and enable a more robust interpretation of the results. These analyses are listed and explained in Web Table 3.
RESULTS
Sample characteristics
The core sample comprised 80,306 participants corresponding to 736,463 person-years and a mean follow-up period of 9.2 (standard deviation, 4.5) years. Among them, 36.2% met only the aerobic guidelines, 3.4% met only the SPE guidelines, and 5.5% met both. Characteristics of the core sample by overall SPE participation are presented in Table 1 (which includes all participants who were eligible prior to the exclusions described below). Compared with nonparticipants, SPE participants were younger; had a slightly lower body mass index; were less likely to have long-standing illness, to be current smokers, to be depressed, or to meet only the aerobic physical activity guideline; and more likely to have finished full-time education at age ≥19 years. In total, 1,891 participants had cancer at baseline and 5,292 had major CVD at baseline and were excluded from the corresponding analyses. Another 938 participants died during the first 24 months of follow-up and were excluded from all further prospective analyses. The main analyses included 72,459 participants for the analysis of all-cause mortality, 73,937 participants for the analysis of CVD mortality, and 77,195 participants for the analysis of cancer mortality.
Baseline Characteristics of Adults Aged 30 Years or More (n = 80,306) According to Participation in Strength-Promoting Exercise, Health Survey for England (1994–2008) and Scottish Health Survey (1995–2003)
| Variable . | Overall Participation in SPE, %a . | P Valueb . | |
|---|---|---|---|
| Did Not Participate (n = 68,222) . | Participated (n = 12,084) . | ||
| Age, yearsc | 53.0 (14.5) | 45.6 (12.4) | <0.001 |
| Female sex | 54.9 | 51.8 | <0.001 |
| Body mass indexc,d | 27.3 (4.9) | 26.6 (4.2) | <0.001 |
| Long-standing illnesse | 49.4 | 38.2 | <0.001 |
| Current smokerf | 26.1 | 17.8 | <0.001 |
| Frequent alcohol consumption (≥5 times/week)g | 19.4 | 19.8 | 0.341 |
| Psychological distress (GHQ score ≥4)h | 15.3 | 12.2 | <0.001 |
| Finished education at age ≥19 years | 16.2 | 28.9 | <0.001 |
| Physical activity recommendation(s) met | |||
| Met aerobic guideline onlyi | 38.4 | 24.0 | <0.001 |
| Met SPE guideline onlyj | N/A | 22.5 | |
| Met both guidelinesi,j | N/A | 36.2 | |
| Variable . | Overall Participation in SPE, %a . | P Valueb . | |
|---|---|---|---|
| Did Not Participate (n = 68,222) . | Participated (n = 12,084) . | ||
| Age, yearsc | 53.0 (14.5) | 45.6 (12.4) | <0.001 |
| Female sex | 54.9 | 51.8 | <0.001 |
| Body mass indexc,d | 27.3 (4.9) | 26.6 (4.2) | <0.001 |
| Long-standing illnesse | 49.4 | 38.2 | <0.001 |
| Current smokerf | 26.1 | 17.8 | <0.001 |
| Frequent alcohol consumption (≥5 times/week)g | 19.4 | 19.8 | 0.341 |
| Psychological distress (GHQ score ≥4)h | 15.3 | 12.2 | <0.001 |
| Finished education at age ≥19 years | 16.2 | 28.9 | <0.001 |
| Physical activity recommendation(s) met | |||
| Met aerobic guideline onlyi | 38.4 | 24.0 | <0.001 |
| Met SPE guideline onlyj | N/A | 22.5 | |
| Met both guidelinesi,j | N/A | 36.2 | |
Abbreviations: GHQ, General Health Questionnaire; N/A, not applicable; SPE, strength-promoting exercise.
a Defined as participation at least once during the 4 weeks prior to the interview.
bP values were calculated using the Mann-Whitney U test for continuous variables and the likelihood ratio χ2 test for categorical variables.
c Values are expressed as mean (standard deviation).
d Weight (kg)/height (m)2.
e Dichotomous variable derived from responses to a series of questions (yes/no) on illness within 8 listed body systems (nervous system, digestive system, heart and circulatory system, etc.). Having at least 1 illness was required in order to meet the definition of long-standing illness.
f Based on 1 question about smoking status, with the response options never smoker, ex-smoker, currently smoking 1–9 cigarettes/day, currently smoking 10–19 cigarettes/day, and currently smoking ≥20 cigarettes/day.
g Derived from the question, “On how many days in the last 7 days did you have an alcoholic drink?”.
h The GHQ comprises 12 questions related to psychological health (concentration, feeling depressed, etc.); the response categories were 0, 1–3, and ≥4.
i Reflecting moderate-to-vigorous physical activity only: at least 150 minutes/week of moderate-intensity activity or 75 minutes/week of vigorous-intensity activity or equivalent combinations of moderate and vigorous non–strength-promoting/nondomestic physical activity.
j Participation in at least 2 sessions of SPE per week.
Baseline Characteristics of Adults Aged 30 Years or More (n = 80,306) According to Participation in Strength-Promoting Exercise, Health Survey for England (1994–2008) and Scottish Health Survey (1995–2003)
| Variable . | Overall Participation in SPE, %a . | P Valueb . | |
|---|---|---|---|
| Did Not Participate (n = 68,222) . | Participated (n = 12,084) . | ||
| Age, yearsc | 53.0 (14.5) | 45.6 (12.4) | <0.001 |
| Female sex | 54.9 | 51.8 | <0.001 |
| Body mass indexc,d | 27.3 (4.9) | 26.6 (4.2) | <0.001 |
| Long-standing illnesse | 49.4 | 38.2 | <0.001 |
| Current smokerf | 26.1 | 17.8 | <0.001 |
| Frequent alcohol consumption (≥5 times/week)g | 19.4 | 19.8 | 0.341 |
| Psychological distress (GHQ score ≥4)h | 15.3 | 12.2 | <0.001 |
| Finished education at age ≥19 years | 16.2 | 28.9 | <0.001 |
| Physical activity recommendation(s) met | |||
| Met aerobic guideline onlyi | 38.4 | 24.0 | <0.001 |
| Met SPE guideline onlyj | N/A | 22.5 | |
| Met both guidelinesi,j | N/A | 36.2 | |
| Variable . | Overall Participation in SPE, %a . | P Valueb . | |
|---|---|---|---|
| Did Not Participate (n = 68,222) . | Participated (n = 12,084) . | ||
| Age, yearsc | 53.0 (14.5) | 45.6 (12.4) | <0.001 |
| Female sex | 54.9 | 51.8 | <0.001 |
| Body mass indexc,d | 27.3 (4.9) | 26.6 (4.2) | <0.001 |
| Long-standing illnesse | 49.4 | 38.2 | <0.001 |
| Current smokerf | 26.1 | 17.8 | <0.001 |
| Frequent alcohol consumption (≥5 times/week)g | 19.4 | 19.8 | 0.341 |
| Psychological distress (GHQ score ≥4)h | 15.3 | 12.2 | <0.001 |
| Finished education at age ≥19 years | 16.2 | 28.9 | <0.001 |
| Physical activity recommendation(s) met | |||
| Met aerobic guideline onlyi | 38.4 | 24.0 | <0.001 |
| Met SPE guideline onlyj | N/A | 22.5 | |
| Met both guidelinesi,j | N/A | 36.2 | |
Abbreviations: GHQ, General Health Questionnaire; N/A, not applicable; SPE, strength-promoting exercise.
a Defined as participation at least once during the 4 weeks prior to the interview.
bP values were calculated using the Mann-Whitney U test for continuous variables and the likelihood ratio χ2 test for categorical variables.
c Values are expressed as mean (standard deviation).
d Weight (kg)/height (m)2.
e Dichotomous variable derived from responses to a series of questions (yes/no) on illness within 8 listed body systems (nervous system, digestive system, heart and circulatory system, etc.). Having at least 1 illness was required in order to meet the definition of long-standing illness.
f Based on 1 question about smoking status, with the response options never smoker, ex-smoker, currently smoking 1–9 cigarettes/day, currently smoking 10–19 cigarettes/day, and currently smoking ≥20 cigarettes/day.
g Derived from the question, “On how many days in the last 7 days did you have an alcoholic drink?”.
h The GHQ comprises 12 questions related to psychological health (concentration, feeling depressed, etc.); the response categories were 0, 1–3, and ≥4.
i Reflecting moderate-to-vigorous physical activity only: at least 150 minutes/week of moderate-intensity activity or 75 minutes/week of vigorous-intensity activity or equivalent combinations of moderate and vigorous non–strength-promoting/nondomestic physical activity.
j Participation in at least 2 sessions of SPE per week.
Association between SPE and mortality
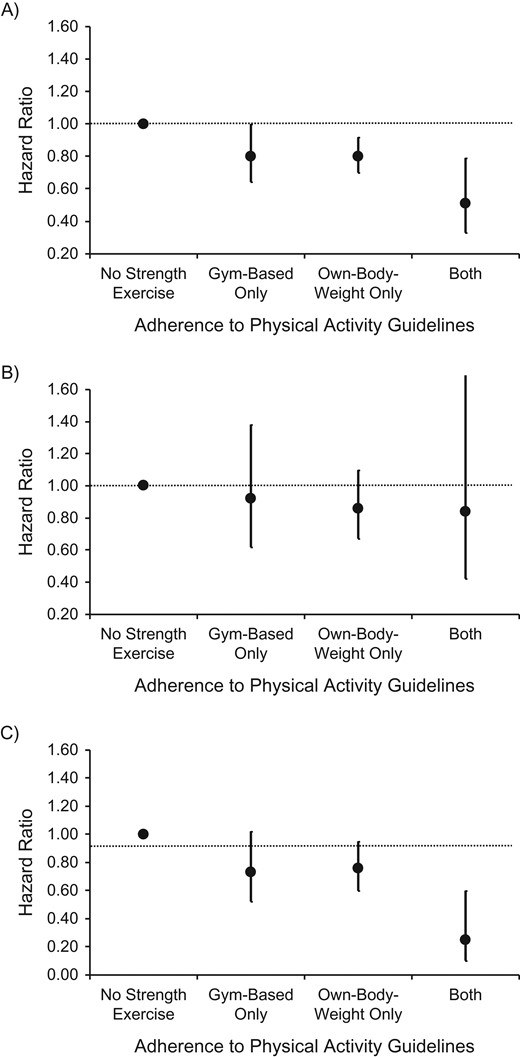
Figure 1 shows the fully adjusted associations between mutually exclusive categories of SPE and mortality. Own-body-weight SPE showed clearer associations than gym-based SPE in terms of all-cause and cancer mortality; compared with no SPE participation, participation in both types of activity was linked with the largest reductions in risk of all-cause (hazard ratio (HR) = 0.51, 95% CI: 0.33, 0.79) and cancer (HR = 0.25, 95% CI: 0.10, 0.60) mortality.
Hazard ratios for the associations between different types of strength-promoting exercise (mutually exclusive categories) and all-cause mortality (A), cardiovascular disease mortality (B), and cancer mortality (C) in the Health Survey for England (1994–2011) and the Scottish Health Survey (1995–2009). Results were adjusted for age, body mass index, educational attainment, presence of long-standing illness, weekly frequency of alcohol consumption, smoking habits, and psychological distress/depression and were mutually adjusted for volume of all other (non–strength-promoting) types of physical activity. Sample sizes (number of cases/total number) by type of activity—all-cause mortality: no strength exercise (5,435/60,937), gym-based only (83/4,440), own-body-weight only (223/4,822), both (20/2,224); cardiovascular disease mortality: no strength exercise (1,623/62,252), gym-based only (25/4,498), own-body-weight only (67/4,902), both (8/2,249); cancer mortality: no strength exercise (1,969/65,347), gym-based only (35/4,564), own-body-weight only (79/5,000), both (5/2,247).
Table 2 presents the associations of own-body-weight SPE, gym-based SPE, and total SPE with all-cause mortality. Participation in both types of SPE was consistently associated with lower risk of all-cause mortality in both partially adjusted and fully adjusted models, with evidence for a modest dose-response association with higher volumes. Similarly, in fully adjusted models, the hazard ratio was 0.81 (95% CI: 0.69, 0.95) for a low weekly volume of total SPE and 0.75 (95% CI: 0.64, 0.88) for a higher weekly volume of SPE.
Associations Between Strength-Promoting Exercise and All-Cause Mortality Among Adults Aged 30 Years or More With No Cancer or Cardiovascular Diseasea at Baseline Who Survived the First 24 Months of Follow-up (n = 72,459), Health Survey for England (1994–2011) and Scottish Health Survey (1995–2009)
| Type and Level of Strength-Promoting Exercise . | No. of Deaths . | Total No. of Deaths . | Risk of All-Cause Mortality . | |||
|---|---|---|---|---|---|---|
| Model 1b . | Model 2c . | |||||
| HR . | 95% CI . | HR . | 95% CI . | |||
| Own-body-weight activityd | ||||||
| Overall participation | ||||||
| None | 5,518 | 65,383 | 1.00 | 1.00 | ||
| Any | 245 | 7,076 | 0.67 | 0.59, 0.76 | 0.78 | 0.68, 0.88 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumee | ||||||
| None | 5,518 | 65,383 | 1.00 | 1.00 | ||
| Low | 102 | 3,539 | 0.66 | 0.54, 0.81 | 0.76 | 0.63, 0.93 |
| High | 143 | 3,537 | 0.68 | 0.57, 0.80 | 0.79 | 0.67, 0.93 |
| P for trend | <0.001 | 0.033 | ||||
| Gym-based activityf | ||||||
| Overall participation | ||||||
| None | 5,658 | 65,769 | 1.00 | 1.00 | ||
| Any | 105 | 6,690 | 0.60 | 0.49, 0.73 | 0.75 | 0.62, 0.91 |
| P for trend | <0.001 | 0.004 | ||||
| Weekly volumeb,f | ||||||
| None | 5,658 | 65,769 | 1.00 | 1.00 | ||
| Low | 30 | 3,284 | 0.63 | 0.49, 0.81 | 0.77 | 0.60, 0.99 |
| High | 41 | 3,406 | 0.56 | 0.41, 0.76 | 0.71 | 0.52, 0.97 |
| P for trend | 0.002 | 0.071 | ||||
| All strength exercise | ||||||
| Overall participation | ||||||
| None | 5,435 | 60,938 | 1.00 | 1.00 | ||
| Any | 326 | 11,521 | 0.66 | 0.59, 0.74 | 0.77 | 0.69, 0.87 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumee,f | ||||||
| None | 5,435 | 60,938 | 1.00 | 1.00 | ||
| Low | 165 | 5,707 | 0.69 | 0.59, 0.81 | 0.81 | 0.69, 0.95 |
| High | 163 | 5,814 | 0.63 | 0.54, 0.74 | 0.75 | 0.64, 0.88 |
| P for trend | <0.001 | 0.002 | ||||
| Adherence to strength exercise guidelineg | ||||||
| Did not meet the guideline | 5,536 | 65,681 | 1.00 | 1.00 | ||
| Met the guideline | 227 | 6,778 | 0.68 | 0.60, 0.78 | 0.80 | 0.70, 0.91 |
| P for trend | <0.001 | 0.001 | ||||
| Type and Level of Strength-Promoting Exercise . | No. of Deaths . | Total No. of Deaths . | Risk of All-Cause Mortality . | |||
|---|---|---|---|---|---|---|
| Model 1b . | Model 2c . | |||||
| HR . | 95% CI . | HR . | 95% CI . | |||
| Own-body-weight activityd | ||||||
| Overall participation | ||||||
| None | 5,518 | 65,383 | 1.00 | 1.00 | ||
| Any | 245 | 7,076 | 0.67 | 0.59, 0.76 | 0.78 | 0.68, 0.88 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumee | ||||||
| None | 5,518 | 65,383 | 1.00 | 1.00 | ||
| Low | 102 | 3,539 | 0.66 | 0.54, 0.81 | 0.76 | 0.63, 0.93 |
| High | 143 | 3,537 | 0.68 | 0.57, 0.80 | 0.79 | 0.67, 0.93 |
| P for trend | <0.001 | 0.033 | ||||
| Gym-based activityf | ||||||
| Overall participation | ||||||
| None | 5,658 | 65,769 | 1.00 | 1.00 | ||
| Any | 105 | 6,690 | 0.60 | 0.49, 0.73 | 0.75 | 0.62, 0.91 |
| P for trend | <0.001 | 0.004 | ||||
| Weekly volumeb,f | ||||||
| None | 5,658 | 65,769 | 1.00 | 1.00 | ||
| Low | 30 | 3,284 | 0.63 | 0.49, 0.81 | 0.77 | 0.60, 0.99 |
| High | 41 | 3,406 | 0.56 | 0.41, 0.76 | 0.71 | 0.52, 0.97 |
| P for trend | 0.002 | 0.071 | ||||
| All strength exercise | ||||||
| Overall participation | ||||||
| None | 5,435 | 60,938 | 1.00 | 1.00 | ||
| Any | 326 | 11,521 | 0.66 | 0.59, 0.74 | 0.77 | 0.69, 0.87 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumee,f | ||||||
| None | 5,435 | 60,938 | 1.00 | 1.00 | ||
| Low | 165 | 5,707 | 0.69 | 0.59, 0.81 | 0.81 | 0.69, 0.95 |
| High | 163 | 5,814 | 0.63 | 0.54, 0.74 | 0.75 | 0.64, 0.88 |
| P for trend | <0.001 | 0.002 | ||||
| Adherence to strength exercise guidelineg | ||||||
| Did not meet the guideline | 5,536 | 65,681 | 1.00 | 1.00 | ||
| Met the guideline | 227 | 6,778 | 0.68 | 0.60, 0.78 | 0.80 | 0.70, 0.91 |
| P for trend | <0.001 | 0.001 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Prevalent cardiovascular disease was defined as physician-diagnosed or self-reported (long-standing illness module) ischemic heart disease, angina, or stroke; prevalent cancer was determined through cancer registration records or self-reported (long-standing illness module).
b Model 1 adjusted for age and sex.
c Model 2 also adjusted for long-standing illness, frequency of alcohol consumption, psychological distress, body mass index, smoking status, educational level, and weekly volume of physical activity, excluding the volume of strength-promoting activity that was the main exposure in the corresponding model.
d Own-body-weight and gym-based strength-promoting exercise are not mutually exclusive in this table.
e Groups were defined using the sex-specific median values of the corresponding variable (see Web Table 2).
f Weekly volumes of gym-based exercise were weighted using age- (10-year bands) and sex-specific proportions of total gym-based activity that was designated “strength workout at a gym using machines or free weights,” derived from the Health Survey for England 2008 and 2012 data sets (see Web Appendix).
g Participation in at least 2 sessions of strength-promoting exercise per week. Results of this analysis were adjusted as in footnotes b and c above, including weekly volume of aerobic physical activity.
Associations Between Strength-Promoting Exercise and All-Cause Mortality Among Adults Aged 30 Years or More With No Cancer or Cardiovascular Diseasea at Baseline Who Survived the First 24 Months of Follow-up (n = 72,459), Health Survey for England (1994–2011) and Scottish Health Survey (1995–2009)
| Type and Level of Strength-Promoting Exercise . | No. of Deaths . | Total No. of Deaths . | Risk of All-Cause Mortality . | |||
|---|---|---|---|---|---|---|
| Model 1b . | Model 2c . | |||||
| HR . | 95% CI . | HR . | 95% CI . | |||
| Own-body-weight activityd | ||||||
| Overall participation | ||||||
| None | 5,518 | 65,383 | 1.00 | 1.00 | ||
| Any | 245 | 7,076 | 0.67 | 0.59, 0.76 | 0.78 | 0.68, 0.88 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumee | ||||||
| None | 5,518 | 65,383 | 1.00 | 1.00 | ||
| Low | 102 | 3,539 | 0.66 | 0.54, 0.81 | 0.76 | 0.63, 0.93 |
| High | 143 | 3,537 | 0.68 | 0.57, 0.80 | 0.79 | 0.67, 0.93 |
| P for trend | <0.001 | 0.033 | ||||
| Gym-based activityf | ||||||
| Overall participation | ||||||
| None | 5,658 | 65,769 | 1.00 | 1.00 | ||
| Any | 105 | 6,690 | 0.60 | 0.49, 0.73 | 0.75 | 0.62, 0.91 |
| P for trend | <0.001 | 0.004 | ||||
| Weekly volumeb,f | ||||||
| None | 5,658 | 65,769 | 1.00 | 1.00 | ||
| Low | 30 | 3,284 | 0.63 | 0.49, 0.81 | 0.77 | 0.60, 0.99 |
| High | 41 | 3,406 | 0.56 | 0.41, 0.76 | 0.71 | 0.52, 0.97 |
| P for trend | 0.002 | 0.071 | ||||
| All strength exercise | ||||||
| Overall participation | ||||||
| None | 5,435 | 60,938 | 1.00 | 1.00 | ||
| Any | 326 | 11,521 | 0.66 | 0.59, 0.74 | 0.77 | 0.69, 0.87 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumee,f | ||||||
| None | 5,435 | 60,938 | 1.00 | 1.00 | ||
| Low | 165 | 5,707 | 0.69 | 0.59, 0.81 | 0.81 | 0.69, 0.95 |
| High | 163 | 5,814 | 0.63 | 0.54, 0.74 | 0.75 | 0.64, 0.88 |
| P for trend | <0.001 | 0.002 | ||||
| Adherence to strength exercise guidelineg | ||||||
| Did not meet the guideline | 5,536 | 65,681 | 1.00 | 1.00 | ||
| Met the guideline | 227 | 6,778 | 0.68 | 0.60, 0.78 | 0.80 | 0.70, 0.91 |
| P for trend | <0.001 | 0.001 | ||||
| Type and Level of Strength-Promoting Exercise . | No. of Deaths . | Total No. of Deaths . | Risk of All-Cause Mortality . | |||
|---|---|---|---|---|---|---|
| Model 1b . | Model 2c . | |||||
| HR . | 95% CI . | HR . | 95% CI . | |||
| Own-body-weight activityd | ||||||
| Overall participation | ||||||
| None | 5,518 | 65,383 | 1.00 | 1.00 | ||
| Any | 245 | 7,076 | 0.67 | 0.59, 0.76 | 0.78 | 0.68, 0.88 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumee | ||||||
| None | 5,518 | 65,383 | 1.00 | 1.00 | ||
| Low | 102 | 3,539 | 0.66 | 0.54, 0.81 | 0.76 | 0.63, 0.93 |
| High | 143 | 3,537 | 0.68 | 0.57, 0.80 | 0.79 | 0.67, 0.93 |
| P for trend | <0.001 | 0.033 | ||||
| Gym-based activityf | ||||||
| Overall participation | ||||||
| None | 5,658 | 65,769 | 1.00 | 1.00 | ||
| Any | 105 | 6,690 | 0.60 | 0.49, 0.73 | 0.75 | 0.62, 0.91 |
| P for trend | <0.001 | 0.004 | ||||
| Weekly volumeb,f | ||||||
| None | 5,658 | 65,769 | 1.00 | 1.00 | ||
| Low | 30 | 3,284 | 0.63 | 0.49, 0.81 | 0.77 | 0.60, 0.99 |
| High | 41 | 3,406 | 0.56 | 0.41, 0.76 | 0.71 | 0.52, 0.97 |
| P for trend | 0.002 | 0.071 | ||||
| All strength exercise | ||||||
| Overall participation | ||||||
| None | 5,435 | 60,938 | 1.00 | 1.00 | ||
| Any | 326 | 11,521 | 0.66 | 0.59, 0.74 | 0.77 | 0.69, 0.87 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumee,f | ||||||
| None | 5,435 | 60,938 | 1.00 | 1.00 | ||
| Low | 165 | 5,707 | 0.69 | 0.59, 0.81 | 0.81 | 0.69, 0.95 |
| High | 163 | 5,814 | 0.63 | 0.54, 0.74 | 0.75 | 0.64, 0.88 |
| P for trend | <0.001 | 0.002 | ||||
| Adherence to strength exercise guidelineg | ||||||
| Did not meet the guideline | 5,536 | 65,681 | 1.00 | 1.00 | ||
| Met the guideline | 227 | 6,778 | 0.68 | 0.60, 0.78 | 0.80 | 0.70, 0.91 |
| P for trend | <0.001 | 0.001 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Prevalent cardiovascular disease was defined as physician-diagnosed or self-reported (long-standing illness module) ischemic heart disease, angina, or stroke; prevalent cancer was determined through cancer registration records or self-reported (long-standing illness module).
b Model 1 adjusted for age and sex.
c Model 2 also adjusted for long-standing illness, frequency of alcohol consumption, psychological distress, body mass index, smoking status, educational level, and weekly volume of physical activity, excluding the volume of strength-promoting activity that was the main exposure in the corresponding model.
d Own-body-weight and gym-based strength-promoting exercise are not mutually exclusive in this table.
e Groups were defined using the sex-specific median values of the corresponding variable (see Web Table 2).
f Weekly volumes of gym-based exercise were weighted using age- (10-year bands) and sex-specific proportions of total gym-based activity that was designated “strength workout at a gym using machines or free weights,” derived from the Health Survey for England 2008 and 2012 data sets (see Web Appendix).
g Participation in at least 2 sessions of strength-promoting exercise per week. Results of this analysis were adjusted as in footnotes b and c above, including weekly volume of aerobic physical activity.
All 3 SPE variables were associated with CVD mortality in the partially adjusted models, but further adjustments materially attenuated these associations considerably (Web Table 4).
Table 3 presents the associations between SPE and cancer mortality. Own-body-weight (HR = 0.69, 95% CI: 0.56, 0.86) and gym-based (HR = 0.61, 95% CI: 0.45, 0.84) SPE were both associated with cancer mortality. Participation in any strengthening exercise was associated with cancer mortality in a dose-response manner.
Associations Between Strength-Promoting Exercise and Cancer Mortality Among Adults Aged 30 Years or More With No Cancera at Baseline Who Survived the First 24 Months of Follow-up (n = 77,195), Health Survey for England (1994–2011) and Scottish Health Survey (1995–2009)
| Type and Level of Strength-Promoting Exercise . | No. of Deaths . | Total No. of Deaths . | Risk of Cancer Mortality . | |||
|---|---|---|---|---|---|---|
| Model 1b . | Model 2c . | |||||
| HR . | 95% CI . | HR . | 95% CI . | |||
| Own-body-weight activity | ||||||
| Overall participation | ||||||
| None | 2,004 | 69,917 | 1.00 | 1.00 | ||
| Any | 85 | 7,278 | 0.60 | 0.48, 0.75 | 0.69 | 0.56, 0.86 |
| P for trend | <0.001 | 0.001 | ||||
| Weekly volumed | ||||||
| None | 2,004 | 69,917 | 1.00 | 1.00 | ||
| Low | 36 | 3,622 | 0.57 | 0.41, 0.79 | 0.66 | 0.47, 0.92 |
| High | 49 | 3,656 | 0.63 | 0.47, 0.83 | 0.72 | 0.54, 0.96 |
| P for trend | 0.019 | 0.076 | ||||
| Gym-based activity | ||||||
| Overall participation | ||||||
| None | 2,048 | 70,358 | 1.00 | 1.00 | ||
| Any | 41 | 6,837 | 0.51 | 0.37, 0.69 | 0.61 | 0.45, 0.84 |
| P for trend | <0.001 | 0.002 | ||||
| Weekly volumeb,e | ||||||
| None | 2,048 | 70,358 | 1.00 | 1.00 | ||
| Low | 25 | 3,375 | 0.55 | 0.37, 0.81 | 0.66 | 0.44, 0.98 |
| High | 16 | 3,462 | 0.46 | 0.28, 0.75 | 0.56 | 0.34, 0.91 |
| P for trend | 0.010 | 0.049 | ||||
| All strength exercise | ||||||
| Overall participation | ||||||
| None | 1,969 | 65,348 | 1.00 | 1.00 | ||
| Any | 119 | 11,847 | 0.59 | 0.49, 0.72 | 0.69 | 0.57, 0.84 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumed,e | ||||||
| None | 1,969 | 65,348 | 1.00 | 1.00 | ||
| Low | 62 | 5,884 | 0.62 | 0.48, 0.80 | 0.72 | 0.58, 0.93 |
| High | 58 | 5,963 | 0.58 | 0.44, 0.75 | 0.67 | 0.52, 0.88 |
| P for trend | 0.001 | 0.016 | ||||
| Adherence to strength exercise guidelinef | ||||||
| Did not meet the guideline | 2,012 | 70,230 | 1.00 | 1.00 | ||
| Met the guideline | 77 | 6,965 | 0.59 | 0.47, 0.74 | 0.68 | 0.54, 0.86 |
| P for trend | <0.001 | <0.001 | ||||
| Type and Level of Strength-Promoting Exercise . | No. of Deaths . | Total No. of Deaths . | Risk of Cancer Mortality . | |||
|---|---|---|---|---|---|---|
| Model 1b . | Model 2c . | |||||
| HR . | 95% CI . | HR . | 95% CI . | |||
| Own-body-weight activity | ||||||
| Overall participation | ||||||
| None | 2,004 | 69,917 | 1.00 | 1.00 | ||
| Any | 85 | 7,278 | 0.60 | 0.48, 0.75 | 0.69 | 0.56, 0.86 |
| P for trend | <0.001 | 0.001 | ||||
| Weekly volumed | ||||||
| None | 2,004 | 69,917 | 1.00 | 1.00 | ||
| Low | 36 | 3,622 | 0.57 | 0.41, 0.79 | 0.66 | 0.47, 0.92 |
| High | 49 | 3,656 | 0.63 | 0.47, 0.83 | 0.72 | 0.54, 0.96 |
| P for trend | 0.019 | 0.076 | ||||
| Gym-based activity | ||||||
| Overall participation | ||||||
| None | 2,048 | 70,358 | 1.00 | 1.00 | ||
| Any | 41 | 6,837 | 0.51 | 0.37, 0.69 | 0.61 | 0.45, 0.84 |
| P for trend | <0.001 | 0.002 | ||||
| Weekly volumeb,e | ||||||
| None | 2,048 | 70,358 | 1.00 | 1.00 | ||
| Low | 25 | 3,375 | 0.55 | 0.37, 0.81 | 0.66 | 0.44, 0.98 |
| High | 16 | 3,462 | 0.46 | 0.28, 0.75 | 0.56 | 0.34, 0.91 |
| P for trend | 0.010 | 0.049 | ||||
| All strength exercise | ||||||
| Overall participation | ||||||
| None | 1,969 | 65,348 | 1.00 | 1.00 | ||
| Any | 119 | 11,847 | 0.59 | 0.49, 0.72 | 0.69 | 0.57, 0.84 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumed,e | ||||||
| None | 1,969 | 65,348 | 1.00 | 1.00 | ||
| Low | 62 | 5,884 | 0.62 | 0.48, 0.80 | 0.72 | 0.58, 0.93 |
| High | 58 | 5,963 | 0.58 | 0.44, 0.75 | 0.67 | 0.52, 0.88 |
| P for trend | 0.001 | 0.016 | ||||
| Adherence to strength exercise guidelinef | ||||||
| Did not meet the guideline | 2,012 | 70,230 | 1.00 | 1.00 | ||
| Met the guideline | 77 | 6,965 | 0.59 | 0.47, 0.74 | 0.68 | 0.54, 0.86 |
| P for trend | <0.001 | <0.001 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Determined through cancer registration records or self-reported (using the long-standing illness module).
b Model 1 adjusted for age and sex.
c Model 2 also adjusted for long-standing illness, frequency of alcohol consumption, psychological distress, body mass index, smoking status, educational level, and weekly volume of physical activity, excluding the volume of strength-promoting activity that was the main exposure in the corresponding model.
d Groups were defined using the sex-specific median values of the corresponding variable (see Web Table 2).
e Weekly volumes of gym-based exercise were weighted using age- (10-year bands) and sex-specific proportions of total gym-based activity that was designated “strength workout at a gym using machines or free weights,” derived from the Health Survey for England 2008 and 2012 data sets (see Web Appendix).
f Participation in at least 2 sessions of strength-promoting exercise per week. Results of this analysis were adjusted as in footnotes b and c above, including weekly volume of aerobic physical activity.
Associations Between Strength-Promoting Exercise and Cancer Mortality Among Adults Aged 30 Years or More With No Cancera at Baseline Who Survived the First 24 Months of Follow-up (n = 77,195), Health Survey for England (1994–2011) and Scottish Health Survey (1995–2009)
| Type and Level of Strength-Promoting Exercise . | No. of Deaths . | Total No. of Deaths . | Risk of Cancer Mortality . | |||
|---|---|---|---|---|---|---|
| Model 1b . | Model 2c . | |||||
| HR . | 95% CI . | HR . | 95% CI . | |||
| Own-body-weight activity | ||||||
| Overall participation | ||||||
| None | 2,004 | 69,917 | 1.00 | 1.00 | ||
| Any | 85 | 7,278 | 0.60 | 0.48, 0.75 | 0.69 | 0.56, 0.86 |
| P for trend | <0.001 | 0.001 | ||||
| Weekly volumed | ||||||
| None | 2,004 | 69,917 | 1.00 | 1.00 | ||
| Low | 36 | 3,622 | 0.57 | 0.41, 0.79 | 0.66 | 0.47, 0.92 |
| High | 49 | 3,656 | 0.63 | 0.47, 0.83 | 0.72 | 0.54, 0.96 |
| P for trend | 0.019 | 0.076 | ||||
| Gym-based activity | ||||||
| Overall participation | ||||||
| None | 2,048 | 70,358 | 1.00 | 1.00 | ||
| Any | 41 | 6,837 | 0.51 | 0.37, 0.69 | 0.61 | 0.45, 0.84 |
| P for trend | <0.001 | 0.002 | ||||
| Weekly volumeb,e | ||||||
| None | 2,048 | 70,358 | 1.00 | 1.00 | ||
| Low | 25 | 3,375 | 0.55 | 0.37, 0.81 | 0.66 | 0.44, 0.98 |
| High | 16 | 3,462 | 0.46 | 0.28, 0.75 | 0.56 | 0.34, 0.91 |
| P for trend | 0.010 | 0.049 | ||||
| All strength exercise | ||||||
| Overall participation | ||||||
| None | 1,969 | 65,348 | 1.00 | 1.00 | ||
| Any | 119 | 11,847 | 0.59 | 0.49, 0.72 | 0.69 | 0.57, 0.84 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumed,e | ||||||
| None | 1,969 | 65,348 | 1.00 | 1.00 | ||
| Low | 62 | 5,884 | 0.62 | 0.48, 0.80 | 0.72 | 0.58, 0.93 |
| High | 58 | 5,963 | 0.58 | 0.44, 0.75 | 0.67 | 0.52, 0.88 |
| P for trend | 0.001 | 0.016 | ||||
| Adherence to strength exercise guidelinef | ||||||
| Did not meet the guideline | 2,012 | 70,230 | 1.00 | 1.00 | ||
| Met the guideline | 77 | 6,965 | 0.59 | 0.47, 0.74 | 0.68 | 0.54, 0.86 |
| P for trend | <0.001 | <0.001 | ||||
| Type and Level of Strength-Promoting Exercise . | No. of Deaths . | Total No. of Deaths . | Risk of Cancer Mortality . | |||
|---|---|---|---|---|---|---|
| Model 1b . | Model 2c . | |||||
| HR . | 95% CI . | HR . | 95% CI . | |||
| Own-body-weight activity | ||||||
| Overall participation | ||||||
| None | 2,004 | 69,917 | 1.00 | 1.00 | ||
| Any | 85 | 7,278 | 0.60 | 0.48, 0.75 | 0.69 | 0.56, 0.86 |
| P for trend | <0.001 | 0.001 | ||||
| Weekly volumed | ||||||
| None | 2,004 | 69,917 | 1.00 | 1.00 | ||
| Low | 36 | 3,622 | 0.57 | 0.41, 0.79 | 0.66 | 0.47, 0.92 |
| High | 49 | 3,656 | 0.63 | 0.47, 0.83 | 0.72 | 0.54, 0.96 |
| P for trend | 0.019 | 0.076 | ||||
| Gym-based activity | ||||||
| Overall participation | ||||||
| None | 2,048 | 70,358 | 1.00 | 1.00 | ||
| Any | 41 | 6,837 | 0.51 | 0.37, 0.69 | 0.61 | 0.45, 0.84 |
| P for trend | <0.001 | 0.002 | ||||
| Weekly volumeb,e | ||||||
| None | 2,048 | 70,358 | 1.00 | 1.00 | ||
| Low | 25 | 3,375 | 0.55 | 0.37, 0.81 | 0.66 | 0.44, 0.98 |
| High | 16 | 3,462 | 0.46 | 0.28, 0.75 | 0.56 | 0.34, 0.91 |
| P for trend | 0.010 | 0.049 | ||||
| All strength exercise | ||||||
| Overall participation | ||||||
| None | 1,969 | 65,348 | 1.00 | 1.00 | ||
| Any | 119 | 11,847 | 0.59 | 0.49, 0.72 | 0.69 | 0.57, 0.84 |
| P for trend | <0.001 | <0.001 | ||||
| Weekly volumed,e | ||||||
| None | 1,969 | 65,348 | 1.00 | 1.00 | ||
| Low | 62 | 5,884 | 0.62 | 0.48, 0.80 | 0.72 | 0.58, 0.93 |
| High | 58 | 5,963 | 0.58 | 0.44, 0.75 | 0.67 | 0.52, 0.88 |
| P for trend | 0.001 | 0.016 | ||||
| Adherence to strength exercise guidelinef | ||||||
| Did not meet the guideline | 2,012 | 70,230 | 1.00 | 1.00 | ||
| Met the guideline | 77 | 6,965 | 0.59 | 0.47, 0.74 | 0.68 | 0.54, 0.86 |
| P for trend | <0.001 | <0.001 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Determined through cancer registration records or self-reported (using the long-standing illness module).
b Model 1 adjusted for age and sex.
c Model 2 also adjusted for long-standing illness, frequency of alcohol consumption, psychological distress, body mass index, smoking status, educational level, and weekly volume of physical activity, excluding the volume of strength-promoting activity that was the main exposure in the corresponding model.
d Groups were defined using the sex-specific median values of the corresponding variable (see Web Table 2).
e Weekly volumes of gym-based exercise were weighted using age- (10-year bands) and sex-specific proportions of total gym-based activity that was designated “strength workout at a gym using machines or free weights,” derived from the Health Survey for England 2008 and 2012 data sets (see Web Appendix).
f Participation in at least 2 sessions of strength-promoting exercise per week. Results of this analysis were adjusted as in footnotes b and c above, including weekly volume of aerobic physical activity.
There were no significant interactions between total physical activity and SPE participation for any outcomes (all P’s > 0.35). Among participants who did not meet the aerobic physical activity guideline (n = 39,369), participation in any SPE was associated with lower all-cause (fully adjusted HR = 0.76, 95% CI: 0.65, 0.89) and cancer (HR = 0.65, 95% CI: 0.49, 0.87) mortality. Among participants who met the aerobic guideline (n = 33,840), SPE was associated with all-cause (HR = 0.89, 95% CI: 0.77, 1.03) and cancer (HR = 0.75, 95% CI: 0.59, 0.95) mortality. In the subsample with additional adjustment for fruit and vegetable consumption (n = 33,063; 836 deaths/326 cancer deaths), all associations between SPE and mortality outcomes observed in the full sample persisted. For example, the all-cause mortality hazard ratios were 0.44 (95% CI: 0.25, 0.77) for any SPE and 0.60 (95% CI: 0.39, 0.91) for own-body-weight SPE (data available on request).
Adherence to strength exercise and aerobic guidelines
Compared with not meeting the SPE guideline, adherence to the SPE guideline was associated with all-cause (HR = 0.80, 95% CI: 0.70, 0.91) (Table 2) and cancer (HR = 0.68, 95% CI: 0.54, 0.86) (Table 3) mortality. These associations were materially unchanged when adherence to the guideline was calculated from own-body-weight SPE only (e.g., for all-cause and cancer mortality, the hazard ratios were 0.81 (95% CI: 0.70, 0.94) and 0.69 (95% CI: 0.54, 0.90), respectively).
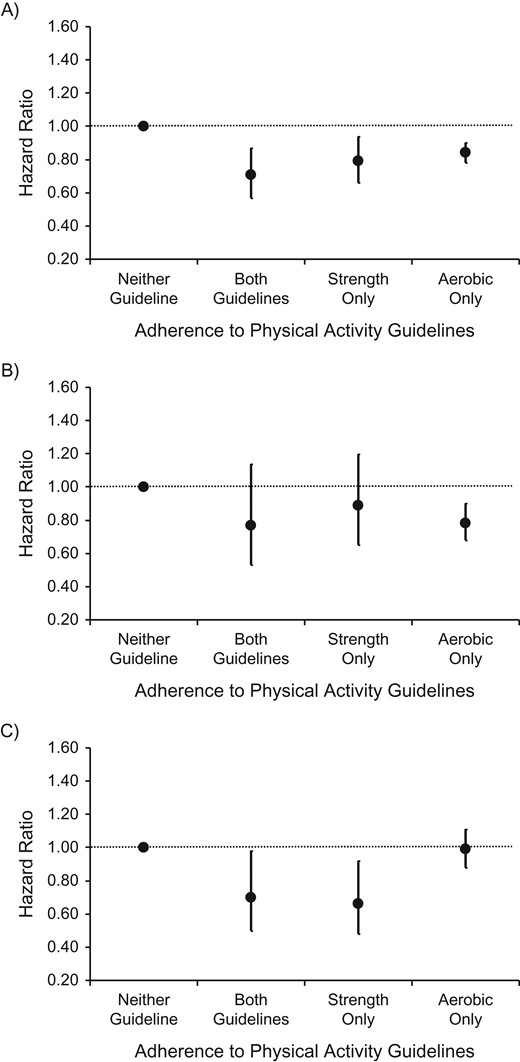
Figure 2 presents results from the fully adjusted comparisons between the aerobic physical activity guideline and the SPE guideline, with persons adhering to neither guideline used as the reference group. Adhering only to the SPE guideline was associated with lower risk of cancer mortality (HR = 0.66, 95% CI: 0.48, 0.92) and to a lesser extent with lower risk of all-cause mortality (HR = 0.79, 95% CI: 0.66, 0.94). Adhering to the aerobic guideline only was associated with lower CVD (HR = 0.78, 95% CI: 0.68, 0.90) and all-cause (HR = 0.84, 95% CI: 0.78, 0.90) mortality. Adhering to both guidelines appeared to elicit risk reduction for all-cause (HR = 0.71, 95% CI: 0.57, 0.87) and cancer (HR = 0.70, 95% CI: 0.50, 0.98) mortality. Results from the analyses that employed the alternative definition of the aerobic guideline (>7.5 MET-hours/week of any type and intensity) were broadly similar but also provided clearer evidence for an association between meeting both guidelines and CVD mortality (Web Figure 1A–1C). When we calculated adherence to the SPE guidelines using own-body-weight exercise only, we observed similar differences between the associations that the SPE and aerobic guidelines exhibited with mortality (Web Figure 2). Among nonsmokers (n = 54,285), the associations between gym-based SPE and all-cause mortality were attenuated compared with the main results presented in Table 2. The associations of all other SPE indicators (including adherence to the SPE guideline and participation in any SPE) with mortality in this subgroup analysis changed little and not in a specific direction (Web Table 5).
Hazard ratios for the associations between adherence to the aerobic physical activity guideline (moderate-to-vigorous physical activity only: achieving at least 150 minutes/week of moderate-intensity activity or 75 minutes/week of vigorous-intensity activity or equivalent combinations of moderate and vigorous non–strength-promoting physical activity) and/or the strength-promoting physical activity guideline and all-cause mortality (A), cardiovascular disease mortality (B), and cancer mortality (C) in the Health Survey for England (1994–2011) and the Scottish Health Survey (1995–2009). Results were adjusted for age, body mass index, educational attainment, presence of long-standing illness, weekly frequency of alcohol consumption, smoking habits, psychological distress/depression, and total volume of physical activity. Sample sizes (number of cases/total number) by type of guideline met—all-cause mortality: neither (4,151/38,208), both (99/4,254), strength only (128/2,524), aerobic only (1,385/27,473); cardiovascular disease mortality: neither (1,280/39,132), both (30/4,311), strength only (42/2,567), aerobic only (371/27,927); cancer mortality: neither (1,407/41,896), both (39/4,320), strength only (38/2,645), aerobic only (605/28,334).
DISCUSSION
The aim of this study was to investigate the association between participation in SPE and all-cause, cancer, and CVD mortality. Participation in any form of SPE was linked with a 23% reduction in all-cause mortality and a 31% reduction in cancer mortality. In addition, there was some relatively modest evidence of a dose-response relationship, with a higher volume of SPE being associated with a slightly greater reduction in all-cause mortality. Adherence to both the SPE and aerobic physical activity guidelines was associated with a greater reduction in mortality risk than adherence to the aerobic guideline alone (Figure 2, Web Figure 1). The lack of association between adherence to the aerobic guideline alone and cancer mortality is surprising, given that previous studies have suggested that the beneficial associations between total physical activity (SPE and aerobic combined) and overall cancer mortality often appear at amounts below the current recommendations (24, 25). One possibility is that in the absence of SPE, amounts of aerobic activity in excess of 150 minutes of moderate-to-vigorous physical activity per week or 7.5 MET-hours/week are needed to reduce cancer mortality risk. However, this interpretation is not supported directly by empirical evidence, as we are not aware of any studies that have specifically assessed associations between adherence to the guidelines through aerobic physical activity only and cancer mortality. It is worth noting that muscle strength, the primary adaptation attributed to SPE, has been associated with reduced cancer mortality independently of aerobic fitness (27).
While the associations of aerobic exercise with morbidity, mortality, and clinical health outcomes are well documented, much less focus has been given to SPE within a public health context (27). Our analysis showed that own-body-weight exercises that can be performed in any setting without equipment yielded results comparable to those of gym-based activities (e.g., see Figure 1). This has practical implications because strength training may be perceived as an activity that is primarily conducted within a gym or clinical setting, where important participation barriers may be present (e.g., social inhibitions, limited access, and financial constraints) (28). Our study also highlights likely gaps in public health practice, since (with very few exceptions (27)) studies estimating the prevalence (29) or burden (30) of physical inactivity as a chronic disease risk factor do not consider strength exercise in its own right. For example, when adherence to the SPE guideline is taken into account, the prevalence of physical inactivity in Australia (27) and the United States (31) increases to approximately 80%–85% (vs. approximately 50% when only the aerobic guideline is taken into account).
Participants who adhered to the World Health Organization guidelines of 2 sessions of SPE per week had a 20% reduction in all-cause mortality. These findings are generally consistent with the 19% and 31% reductions in all-cause mortality reported by Kraschnewski et al. (13) and Dankel et al. (15), respectively. In contrast, we found adherence to the guidelines to be associated with a 32% reduction in cancer mortality, with the study by Kraschnewski et al. (13) showing no significant association. When we compared the 2 World Health Organization guideline components, we observed reductions in cancer mortality only in persons who met the SPE guideline but not the aerobic guideline (Figure 2, Web Figures 1 and 2). Strength training has been shown to lower circulating levels of sex hormones (32), reducing the risks of breast and endometrial cancer in women and prostate cancer in men (33). In addition, strength training has also been shown to be a powerful adjunct therapy in the treatment of cancer, particularly to combat muscle dysfunction and cancer cachexia (34), as well as the side effects of antiandrogenic medication often prescribed in prostate cancer (35). SPE participation has been associated with a 33% reduction in all-cause mortality in cancer survivors (12). Taken together, SPE prior to diagnosis may reduce the risk of cancer mortality, but it may also reduce all-cause mortality risk in cancer survivors. However, observational studies of SPE and cancer mortality in persons free from a cancer diagnosis are lacking; thus, future studies on the associations between this mode of exercise and cancer mortality are warranted.
The present study showed a lack of evidence for an association between SPE and CVD mortality, which is in agreement with previous literature (13, 15). However, participation in at least 30 minutes of SPE per week has been found to confer risk-reduction benefits similar to those of 2.5 hours of brisk walking for fatal and nonfatal myocardial infarction in men (11). Randomized controlled trials of resistance training have been shown to increase arterial stiffness in younger adults (36), with higher arterial stiffness being associated with all-cause and CVD mortality (37). However, recent evidence showed reductions in pulse wave velocity following 12 weeks of high- or low-intensity resistance training in younger men (38). Similarly, aortic reservoir pressure in prehypertensive and hypertensive older men was also shown to decrease following resistance training (39). Thus, the association between SPE and CVD mortality remains unclear and warrants further investigation. The associations between SPE and arterial stiffness remain heterogeneous, but it is possible that increases in arterial stiffness due to SPE may offset any potential beneficial effect on other CVD risk factors, such as reductions in blood pressure (40).
Previously, among 8,772 adults, participation in 8–14 SPE sessions per month was associated with a reduction in all-cause mortality, with no benefit observed at higher frequencies (15). In our data, there was some indication that higher volume and higher perceived intensity of SPE were associated with a greater reduction in all-cause and cancer mortality, respectively. Interestingly, higher muscle strength, as opposed to participation in SPE, was found to be more strongly associated with reductions in mortality (14), suggesting that the strength outcome is more important than the SPE behavior itself. This provides further evidence for a potential dose-response relationship, with experimental data (41) showing that SPE at higher volume and intensity confers greater benefit in muscle strength. Experimental data on the isolated effects of progressive resistance training on mortality are sparse. In a randomized controlled trial of 124 older adults who underwent surgical repair of osteoporotic hip fracture, those who received progressive resistance training had 81% and 84% reductions in mortality and nursing home admission, respectively, compared with those who received standard care (42). The anabolic response to high-intensity progressive resistance training has been associated with improved glucose metabolism (43), reductions in systemic inflammation (44), reductions in depressive symptoms (45), improvements in cognitive function in adults with mild cognitive impairment (46), and improvements in aerobic capacity and functional and mobility outcomes (47)—all of which can collectively reduce mortality risk.
Our study utilized a pooled population sample and (to our knowledge) was one of the largest to date in the field of SPE epidemiology. We took robust approaches to minimize the chances of reverse causality (e.g., our analysis was the only one to exclude both prevalent disease cases and events occurring within the first 2 years) and performed several sensitivity analyses towards the same end, including adjustments for dietary factors in a subsample. A key limitation of this study was the use of self-reported assessments of strength exercise and the use of a 4-week recall time frame. Both of these characteristics of the exposure measurement may have resulted in regression dilution bias and attenuation of the “true” association between strength training and mortality outcomes. At present, there is no feasible substitute for self-report assessments of SPE (48), which is the standard in public health surveillance (27, 31). The question on gym-based exercise inquired about some forms of aerobic exercise, and while we attempted to reduce measurement error by weighting estimation by the volume of gym-based SPE activity, we acknowledge the possibility that some aerobic activity was included. However, own-body-weight SPE showed higher levels of mortality risk reduction (Figure 1) similar to those of the gym-based indicator; and calculating adherence to the SPE guideline using only the own-body-weight indicator did not materially change the results (Web Figure 2). Both of these analyses support the robustness of our overall SPE findings.
Low statistical power may have compromised some of our results. For example, in the combined associations of SPE and aerobic guidelines with CVD mortality, the group adhering to the SPE guideline only had 42 events (event rate 1.6%), although it is notable that in the case of cancer mortality, we did detect an association in the same group despite the low number of events (38 events; event rate 1.4%).
In conclusion, participation in any SPE was associated with a 23% reduction in all-cause mortality and a 31% reduction in cancer mortality. In terms of mortality risk reduction, adherence to the SPE guideline on physical activity appears to be at least as important as adherence to the aerobic guideline. Our results support the value of specifically promoting adherence to the strength exercise guideline over and above the generic physical activity targets.
ACKNOWLEDGMENTS
Author affiliations: Epidemiology Unit, Charles Perkins Centre, University of Sydney, Sydney, New South Wales, Australia (Emmanuel Stamatakis, Ding Ding, Adrian Bauman); Prevention Research Collaboration, Sydney School of Public Health, University of Sydney, Sydney, New South Wales, Australia (Emmanuel Stamatakis, Ding Ding, Adrian Bauman); Institute of Sport, Exercise and Active Living, Victoria University, Melbourne, Victoria, Australia (Jason Bennie); Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts (I.-Min Lee); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (I.-Min Lee); Exercise Health and Performance Faculty Research Group, Faculty of Health Sciences, University of Sydney, Sydney, New South Wales, Australia (Jonathon Freeston, Yorgi Mavros); Research Department of Epidemiology and Public Health, Institute of Epidemiology and Health Care and Faculty of Population Health Sciences, University College London, London, United Kingdom (Mark Hamer); and National Center for Sport and Exercise Medicine–East Midlands, Loughborough University, Loughborough, United Kingdom (Mark Hamer, Gary O’Donovan).
This work was not financially supported directly by any individual, agency, or institution. Harmonization of the pooled data sets used in this analysis was funded by the National Institute for Health Research (United Kingdom) through a grant to E.S. E.S. and D.D. were supported by the National Health and Medical Research Council (Australia) through a Senior Research Fellowship and an Early Career Research Fellowship, respectively. M.H. received support from the National Institute for Health Research Leicester Biomedical Research Center.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Classification of Diseases, Tenth Revision
- MET
metabolic equivalent of task
- SPE
strength-promoting exercise