-
PDF
- Split View
-
Views
-
Cite
Cite
Yong Cui, Xiao-Ou Shu, Yu-Tang Gao, Hui Cai, Meng-Hua Tao, Wei Zheng, Association of Ginseng Use with Survival and Quality of Life among Breast Cancer Patients, American Journal of Epidemiology, Volume 163, Issue 7, 1 April 2006, Pages 645–653, https://doi.org/10.1093/aje/kwj087
Close - Share Icon Share
Abstract
The authors evaluated the associations of ginseng use as a complementary therapy with survival and quality of life (QOL) in a cohort of 1,455 breast cancer patients who were recruited to the Shanghai Breast Cancer Study between August 1996 and March 1998 in Shanghai, China. Patients were followed through December 2002. Information on ginseng use before cancer diagnosis was collected at baseline recruitment and was linked to survival. Survivors' ginseng use after cancer diagnosis was obtained at the follow-up survey and was correlated to QOL at the same time. The Kaplan-Meier method and Cox regression models were applied to evaluate the association of ginseng use with overall and disease-free survival. The relation of ginseng use and QOL was evaluated by using multiple linear regression models. Approximately 27% of study participants were regular ginseng users before cancer diagnosis. Compared with patients who never used ginseng, regular users had a significantly reduced risk of death; adjusted hazard ratios associated with ginseng use were 0.71 (95% confidence interval: 0.52, 0.98) for total mortality and 0.70 (95% confidence interval: 0.53, 0.93) for disease-specific mortality/recurrence. Ginseng use after cancer diagnosis, particularly current use, was positively associated with QOL scores, with the strongest effect in the psychological and social well-being domains. Additionally, QOL improved as cumulative ginseng use increased.
In the past decade, interest in complementary/alternative medicine has increased greatly throughout the world, particularly regarding the use of such medicine by patients with cancer (1, 2). Common reasons or expectations for its use by cancer patients are to improve clinical outcomes and enhance quality of life (QOL) (3, 4). The majority of cancer patients have used at least one type of complementary/alternative medicine after cancer diagnosis (4–6), and its use is more prevalent among women with breast cancer than among patients with other types of malignancies (7). Across the broad spectrum of complementary/alternative medicine treatments, herbal medicine is one of the most commonly used modalities (3, 5). However, there is a serious lack of scientific evidence showing that use of complementary/alternative medicine, especially certain popular herbal remedies, in cancer therapy indeed improves clinical outcomes and/or patients' QOL.
Ginseng is one of the most popular herbal medicines and has been used to proactively promote health, vitality, and longevity in Asian countries for more than 2,000 years. Ginseng comprises a number of different species; Asian ginseng (the root of Panax ginseng C.A. Meyer) and American ginseng (the root of Panax quinquefolius L) are most commonly used. In recent years, ginseng has gained significant popularity in Western societies. It was estimated, for instance, that ginseng was the second top-selling herbal supplement in the United States in 2000, with $62 million in annual sales (8). Ginseng has been included in the Pharmacopoeias of several Western countries, including Germany, France, Austria, and the United Kingdom (8). Many studies have reported that ginseng promotes a wide range of pharmacologic activities in the immune, cardiovascular, endocrine, and central nervous systems (9, 10). Recently, evidence from in vitro experiments and animal models has suggested that ginseng and its active constituents have antimutagenic and cancer-inhibitory properties (11, 12). Epidemiologic studies conducted in Korea have reported that ginseng consumption was related to a significantly decreased risk of cancers of the respiratory tract, gastrointestinal tract, liver, pancreas, and ovaries (13). In Asian countries, cancer patients commonly use ginseng as a complementary therapy for cancer and cancer-related symptoms (5, 11). However, the effect of ginseng in cancer treatment has not been adequately examined in human studies.
The purpose of this study was to evaluate the effects of ginseng use on breast cancer survival and survivors' QOL. We used data collected in a large, population-based epidemiologic study of breast cancer in Shanghai, China.
MATERIALS AND METHODS
Study population
Subjects were breast cancer patients enrolled as part of the Shanghai Breast Cancer Study, a large, population-based case-control study in China. Details of this study have been described elsewhere (14). In brief, through a rapid case-ascertainment system, supplemented by the population-based Shanghai Cancer Registry, 1,602 women aged 25–64 years with breast cancer newly diagnosed between August 1996 and March 1998 were identified. In-person interviews were completed for 1,459 of these women (91.1 percent) an average of 66 days postdiagnosis. The initial diagnoses of breast cancer were confirmed by two senior pathologists through independent review of pathology slides. In this study, the consistency rate between the two pathologists' diagnoses was 98 percent. Inconsistent diagnoses were resolved by joint review of diagnostic slides by both pathologists.
All of the 1,459 breast cancer patients were followed through December 2002 with a combination of active follow-up and record linkage to the death certificates kept by the Vital Statistics Unit of the Shanghai Center for Disease Control and Prevention. Of these patients, 1,290 (88.4 percent) were successfully contacted either in person (n = 1,241, 85.0 percent) or by telephone (n = 49, 3.4 percent) between March 1, 2000, and December 31, 2002; 200 patients were deceased. Through interview of patients or next of kin for deceased patients, we obtained information on disease progress, recurrence, and cause of death (if deceased). Survival status for the remaining 169 participants who could not be contacted in person or by telephone was established on June 30, 2003, by linkage to the death registry. Through the linkage, 40 deaths were identified; information on date of death and cause of death was obtained. One hundred twenty-six subjects had no match in the death registry and were assumed to still be living on December 31, 2002, 6 months prior to our search of the vital statistics registry, to allow for a possible delay of entry of the death certificates into the registry. For four subjects, information for the record linkage was insufficient, and these subjects were excluded from the current analysis.
During the in-person follow-up interviews from March 2000 through June 2002, QOL was assessed for 1,065 of the 1,248 (85.3 percent) surviving patients using the General Quality of Life Inventory-74, an instrument that has been demonstrated to have a satisfactory level of reliability, validity, and sensitivity in the Chinese population (15–17). QOL was not evaluated for 183 (14.7 percent) survivors, and the reasons were refusal (n = 43, 3.4 percent), critical illness at the time of the survey (n = 6, 0.5 percent), inability to locate or other miscellaneous reasons (n = 60, 4.8 percent), or budget constraints (n = 74, 5.9 percent). With the exception of slightly higher educational attainment among nonparticipants, there were no significant differences between participants and nonparticipants in other demographic characteristics, including age, marital status, and income (15). This study was approved by the institutional review boards of all participating institutions.
Data collection
Information on the use of ginseng (ginseng root) or a ginseng product was elicited via in-person interviews conducted by trained interviewers during subject recruitment (an average of 66 days postdiagnosis) for prediagnosis use and during the follow-up survey for postdiagnosis use. Information collected included the type of ginseng used (white or red Asian ginseng, American ginseng, and ginseng products (extract, powder, tablet, capsule, etc.)), the duration (years) of use, and the frequency (times/month) of use. Patients' sociodemographic and medical information, including age at diagnosis, marital status, annual household income, education, menopausal status, stage of breast cancer (tumor-node-metastasis status), recurrence/metastasis of disease, estrogen and progesterone receptor status, and mainstream oncology care for breast cancer (surgery, chemotherapy, and radiotherapy) was also collected via in-person interviews and medical chart abstraction using structured questionnaires.
Patients' QOL was assessed at the follow-up survey by using General Quality of Life Inventory-74. The inventory comprises a total of 74 items that can be grouped into 20 facets and covers the following four domains: 1) physical well-being (sleep and energy, pain and physical discomfort, eating function, sexual function, daily living capability), 2) psychological well-being (psychological distress, negative feelings, positive feelings, cognitive function, body/self-image), 3) social well-being (social support, interpersonal relationships, work and study capacity, recreational and leisure activities, marriage and family), and 4) material well-being (housing situation, community services, living environment, financial situation). Participants' responses were converted to a score with 0–100 scales for each domain and facet, with higher scores reflecting better QOL. In addition, information on use of other complementary/alternative medicine, such as Chinese herbal medicine and supplements (omega-3 fatty acid, ganoderma, shark cartilage, lecithin, metatonin, honey tonic, etc.) was also collected at the follow-up survey. Among cancer survivors, patients who used ginseng after diagnosis appeared to be more likely than nonusers to have used other forms of complementary/alternative medicine (88.5 percent vs. 83.6 percent; p = 0.02).
Statistical analyses
Statistical analyses were performed by using Statistical Analysis Software (version 9.1; SAS Institute, Inc., Cary, North Carolina). The endpoints for the analyses of overall survival and disease-free survival were any death and death related to breast cancer/cancer recurrence, respectively. Survival time was calculated as the time from cancer diagnosis to the endpoints of the study, censoring at the date of last contact or noncancer death (for disease-free survival only). For subjects who had died without information on date of breast cancer recurrence (i.e., the deceased subjects identified from the death registry), we defined disease-free survival time by substituting the total survival time for the disease-free survival time. Cox regression models were applied to evaluate the effect of ginseng use on overall survival and disease-free survival with adjustments for age, education, income, and the known prognostic factors for breast cancer, including stage of disease, estrogen and progesterone receptor status, and standard cancer treatments. The Kaplan-Meier method was used to compute 5-year survival rates, and the log-rank test was applied to test the differences in survival between regular ginseng users and nonusers. Multiple linear regression models were used to evaluate the effect of ginseng use on survivors' QOL, adjusting for potential confounding factors, including age, marital status, education, income, menopausal status, recurrence of disease, time since cancer diagnosis, and use of other complementary/alternative medicine. Tests for trend were performed by entering the categorical variables as continuous parameters in the models. All tests were two sided.
RESULTS
Table 1 summarizes the sociodemographic and medical characteristics of the 1,455 breast cancer patients included in the survival study. At the baseline enrollment, 398 patients (27.4 percent) reported having used ginseng on a regular basis (regular ginseng users), and 1,057 patients reported never having used ginseng (nonusers) before cancer diagnosis. Regarding the former, American ginseng and white ginseng were the types used most commonly, accounting for approximately 98 percent of ginseng use. All of the 398 ginseng users had received at least one type of mainstream treatment for breast cancer (surgery, chemotherapy, or radiotherapy) after cancer diagnosis. Compared with nonusers, regular ginseng users were 2.5 years older (p < 0.01), were more likely to have received a better education (p < 0.01), and were more likely to have received tamoxifen treatment (p = 0.02).
Sociodemographic and medical characteristics of women breast cancer patients in the Shanghai Breast Cancer Study, Shanghai, China, 1996–2002
Characteristic . | % of all cases (n = 1,455) . | All cases (n = 1,455) . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | % of regular ginseng users (n = 398) . | % of ginseng nonusers (n = 1,057) . | p value . | ||
| Age (years) at diagnosis (mean (standard deviation)) | 48.2 (8.0) | 50.7 (8.2) | 47.2 (7.6) | <0.01 | ||
| Marital status | ||||||
| Married/living with partner | 92.2 | 92.5 | 92.1 | |||
| Unmarried | 7.8 | 7.5 | 7.9 | 0.80 | ||
| Education | ||||||
| <Middle school | 12.2 | 15.3 | 11.0 | |||
| Middle school | 42.7 | 36.9 | 44.9 | <0.01 | ||
| >Middle school | 45.1 | 47.8 | 44.1 | |||
| Income (yuan*/year) | ||||||
| <10,000 | 11.5 | 9.3 | 12.3 | |||
| 10,000–19,999 | 40.7 | 38.7 | 41.5 | 0.09 | ||
| ≥20,000 | 47.8 | 52.0 | 46.2 | |||
| Tumor-node metastasis | ||||||
| 0–I | 24.6 | 24.1 | 25.9 | |||
| IIa | 34.9 | 34.5 | 36.2 | |||
| IIb | 22.0 | 22.2 | 21.3 | 0.77 | ||
| III–IV | 11.3 | 11.7 | 10.3 | |||
| Unknown | 7.2 | 7.5 | 6.3 | |||
| Estrogen receptor status | ||||||
| Positive | 44.5 | 45.5 | 44.1 | |||
| Negative | 25.5 | 22.4 | 26.7 | 0.22 | ||
| Unknown | 30.0 | 32.1 | 29.2 | |||
| Progesterone receptor status | ||||||
| Positive | 43.6 | 45.0 | 43.1 | |||
| Negative | 25.3 | 21.9 | 26.6 | 0.17 | ||
| Unknown | 31.1 | 33.1 | 30.3 | |||
| Treatment received | ||||||
| Surgery for breast cancer | 99.4 | 99.5 | 99.3 | 0.82 | ||
| Chemotherapy | 94.6 | 94.0 | 93.9 | 0.99 | ||
| Radiotherapy | 38.9 | 41.2 | 38.0 | 0.32 | ||
| Tamoxifen use | 63.3 | 69.1 | 61.1 | 0.02 | ||
Characteristic . | % of all cases (n = 1,455) . | All cases (n = 1,455) . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | % of regular ginseng users (n = 398) . | % of ginseng nonusers (n = 1,057) . | p value . | ||
| Age (years) at diagnosis (mean (standard deviation)) | 48.2 (8.0) | 50.7 (8.2) | 47.2 (7.6) | <0.01 | ||
| Marital status | ||||||
| Married/living with partner | 92.2 | 92.5 | 92.1 | |||
| Unmarried | 7.8 | 7.5 | 7.9 | 0.80 | ||
| Education | ||||||
| <Middle school | 12.2 | 15.3 | 11.0 | |||
| Middle school | 42.7 | 36.9 | 44.9 | <0.01 | ||
| >Middle school | 45.1 | 47.8 | 44.1 | |||
| Income (yuan*/year) | ||||||
| <10,000 | 11.5 | 9.3 | 12.3 | |||
| 10,000–19,999 | 40.7 | 38.7 | 41.5 | 0.09 | ||
| ≥20,000 | 47.8 | 52.0 | 46.2 | |||
| Tumor-node metastasis | ||||||
| 0–I | 24.6 | 24.1 | 25.9 | |||
| IIa | 34.9 | 34.5 | 36.2 | |||
| IIb | 22.0 | 22.2 | 21.3 | 0.77 | ||
| III–IV | 11.3 | 11.7 | 10.3 | |||
| Unknown | 7.2 | 7.5 | 6.3 | |||
| Estrogen receptor status | ||||||
| Positive | 44.5 | 45.5 | 44.1 | |||
| Negative | 25.5 | 22.4 | 26.7 | 0.22 | ||
| Unknown | 30.0 | 32.1 | 29.2 | |||
| Progesterone receptor status | ||||||
| Positive | 43.6 | 45.0 | 43.1 | |||
| Negative | 25.3 | 21.9 | 26.6 | 0.17 | ||
| Unknown | 31.1 | 33.1 | 30.3 | |||
| Treatment received | ||||||
| Surgery for breast cancer | 99.4 | 99.5 | 99.3 | 0.82 | ||
| Chemotherapy | 94.6 | 94.0 | 93.9 | 0.99 | ||
| Radiotherapy | 38.9 | 41.2 | 38.0 | 0.32 | ||
| Tamoxifen use | 63.3 | 69.1 | 61.1 | 0.02 | ||
1 yuan = approximately US $0.125.
Sociodemographic and medical characteristics of women breast cancer patients in the Shanghai Breast Cancer Study, Shanghai, China, 1996–2002
Characteristic . | % of all cases (n = 1,455) . | All cases (n = 1,455) . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | % of regular ginseng users (n = 398) . | % of ginseng nonusers (n = 1,057) . | p value . | ||
| Age (years) at diagnosis (mean (standard deviation)) | 48.2 (8.0) | 50.7 (8.2) | 47.2 (7.6) | <0.01 | ||
| Marital status | ||||||
| Married/living with partner | 92.2 | 92.5 | 92.1 | |||
| Unmarried | 7.8 | 7.5 | 7.9 | 0.80 | ||
| Education | ||||||
| <Middle school | 12.2 | 15.3 | 11.0 | |||
| Middle school | 42.7 | 36.9 | 44.9 | <0.01 | ||
| >Middle school | 45.1 | 47.8 | 44.1 | |||
| Income (yuan*/year) | ||||||
| <10,000 | 11.5 | 9.3 | 12.3 | |||
| 10,000–19,999 | 40.7 | 38.7 | 41.5 | 0.09 | ||
| ≥20,000 | 47.8 | 52.0 | 46.2 | |||
| Tumor-node metastasis | ||||||
| 0–I | 24.6 | 24.1 | 25.9 | |||
| IIa | 34.9 | 34.5 | 36.2 | |||
| IIb | 22.0 | 22.2 | 21.3 | 0.77 | ||
| III–IV | 11.3 | 11.7 | 10.3 | |||
| Unknown | 7.2 | 7.5 | 6.3 | |||
| Estrogen receptor status | ||||||
| Positive | 44.5 | 45.5 | 44.1 | |||
| Negative | 25.5 | 22.4 | 26.7 | 0.22 | ||
| Unknown | 30.0 | 32.1 | 29.2 | |||
| Progesterone receptor status | ||||||
| Positive | 43.6 | 45.0 | 43.1 | |||
| Negative | 25.3 | 21.9 | 26.6 | 0.17 | ||
| Unknown | 31.1 | 33.1 | 30.3 | |||
| Treatment received | ||||||
| Surgery for breast cancer | 99.4 | 99.5 | 99.3 | 0.82 | ||
| Chemotherapy | 94.6 | 94.0 | 93.9 | 0.99 | ||
| Radiotherapy | 38.9 | 41.2 | 38.0 | 0.32 | ||
| Tamoxifen use | 63.3 | 69.1 | 61.1 | 0.02 | ||
Characteristic . | % of all cases (n = 1,455) . | All cases (n = 1,455) . | . | . | ||
|---|---|---|---|---|---|---|
| . | . | % of regular ginseng users (n = 398) . | % of ginseng nonusers (n = 1,057) . | p value . | ||
| Age (years) at diagnosis (mean (standard deviation)) | 48.2 (8.0) | 50.7 (8.2) | 47.2 (7.6) | <0.01 | ||
| Marital status | ||||||
| Married/living with partner | 92.2 | 92.5 | 92.1 | |||
| Unmarried | 7.8 | 7.5 | 7.9 | 0.80 | ||
| Education | ||||||
| <Middle school | 12.2 | 15.3 | 11.0 | |||
| Middle school | 42.7 | 36.9 | 44.9 | <0.01 | ||
| >Middle school | 45.1 | 47.8 | 44.1 | |||
| Income (yuan*/year) | ||||||
| <10,000 | 11.5 | 9.3 | 12.3 | |||
| 10,000–19,999 | 40.7 | 38.7 | 41.5 | 0.09 | ||
| ≥20,000 | 47.8 | 52.0 | 46.2 | |||
| Tumor-node metastasis | ||||||
| 0–I | 24.6 | 24.1 | 25.9 | |||
| IIa | 34.9 | 34.5 | 36.2 | |||
| IIb | 22.0 | 22.2 | 21.3 | 0.77 | ||
| III–IV | 11.3 | 11.7 | 10.3 | |||
| Unknown | 7.2 | 7.5 | 6.3 | |||
| Estrogen receptor status | ||||||
| Positive | 44.5 | 45.5 | 44.1 | |||
| Negative | 25.5 | 22.4 | 26.7 | 0.22 | ||
| Unknown | 30.0 | 32.1 | 29.2 | |||
| Progesterone receptor status | ||||||
| Positive | 43.6 | 45.0 | 43.1 | |||
| Negative | 25.3 | 21.9 | 26.6 | 0.17 | ||
| Unknown | 31.1 | 33.1 | 30.3 | |||
| Treatment received | ||||||
| Surgery for breast cancer | 99.4 | 99.5 | 99.3 | 0.82 | ||
| Chemotherapy | 94.6 | 94.0 | 93.9 | 0.99 | ||
| Radiotherapy | 38.9 | 41.2 | 38.0 | 0.32 | ||
| Tamoxifen use | 63.3 | 69.1 | 61.1 | 0.02 | ||
1 yuan = approximately US $0.125.
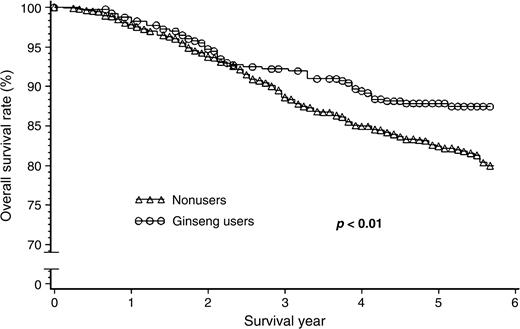
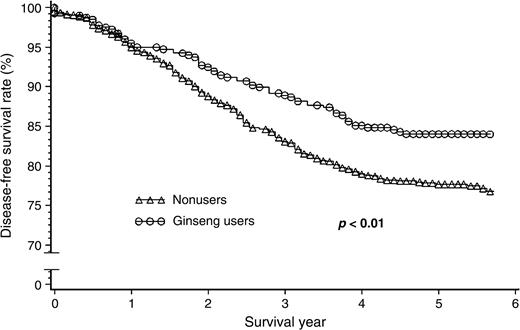
This cohort of 1,455 patients was followed for survival, with an average follow-up time of 4.8 years. The Kaplan-Meier survival curve showed that ginseng users had better overall and disease-free breast cancer survival rates after diagnosis (figures 1 and 2). Table 2 presents the association between regular use of ginseng before cancer diagnosis and breast cancer survival. After adjustment for age at diagnosis, education, income, tumor-node metastasis, estrogen and progesterone receptor status, surgery, chemotherapy, radiotherapy, and tamoxifen treatment, use of any type of ginseng was consistently associated with total survival and disease-free survival. Hazard ratios for total mortality and disease-specific mortality/recurrence were, respectively, 0.43 (95 percent confidence interval (CI): 0.23, 0.80) and 0.53 (95 percent CI: 0.33, 0.87) for white ginseng users, 0.68 (95 percent CI: 0.16, 2.79) and 0.64 (95 percent CI: 0.16, 2.59) for red ginseng users, 0.77 (95 percent CI: 0.55, 1.08) and 0.74 (95 percent CI: 0.55, 1.00) for American ginseng users, and 0.80 (95 percent CI: 0.11, 5.81) and 0.55 (95 percent CI: 0.08, 3.95) for users of ginseng extracts. The hazard ratios for total mortality and disease-specific mortality/recurrence were 0.68 (95 percent CI: 0.30, 1.57) and 0.78 (95 percent CI: 0.41, 1.48), respectively, for ever having used more than one type of ginseng (separately or in combination) before diagnosis. Because in vitro studies have shown that white or red Asian ginseng and American ginseng all have anticarcinogenic effects (13, 18–21), and because we observed that all types of ginseng were positively associated with overall and disease-free survival, use of different types of ginseng was combined to increase the study's statistical power. The hazard ratios associated with any type of ginseng use as a whole were 0.71 (95 percent CI: 0.52, 0.98) for total mortality and 0.70 (95 percent CI: 0.53, 0.93) for disease-specific mortality/recurrence after the adjustment. Although the tests for trend on the amount of ginseng used prior to diagnosis were significant, the risk reduction with increasing amounts of ginseng used for both total mortality and disease-specific mortality/recurrence appeared to be small.
Survival curves for breast cancer patients, by ginseng use before diagnosis, showing that regular users had a better overall survival rate compared with nonusers and that the beneficial effect of ginseng use started 2.5 years after cancer diagnosis, Shanghai Breast Cancer Study, Shanghai, China, 1996–2002.
Survival curves for breast cancer patients, by ginseng use before diagnosis, showing that regular users had a higher disease-free survival rate compared with nonusers and that the beneficial effect of ginseng use started 1.2 years after cancer diagnosis, Shanghai Breast Cancer Study, Shanghai, China, 1996–2002.
Association of regular ginseng use before cancer diagnosis with breast cancer survival for women in the Shanghai Breast Cancer Study, Shanghai, China, 1996–2002
Ginseng use . | Total mortality . | . | . | . | Disease-specific mortality/recurrence . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Total no. . | No. of events . | Adjusted* HR† . | 95% CI† . | Total no. . | No. of events . | Adjusted* HR . | 95% CI . | ||||||
| Never use before diagnosis | 1,057 | 191 | 1.00 (Ref†) | 1,057 | 235 | 1.00 (Ref) | ||||||||
| Regular use before diagnosis | ||||||||||||||
| White Asian ginseng | 134 | 11 | 0.43 | 0.23, 0.80 | 134 | 18 | 0.53 | 0.33, 0.87 | ||||||
| Red ginseng | 11 | 2 | 0.68 | 0.16, 2.79 | 11 | 2 | 0.64 | 0.16, 2.59 | ||||||
| American ginseng | 331 | 43 | 0.77 | 0.55, 1.08 | 331 | 54 | 0.74 | 0.55, 1.00 | ||||||
| Ginseng product | 10 | 1 | 0.80 | 0.11, 5.81 | 10 | 1 | 0.55 | 0.08, 3.95 | ||||||
| Ever use of more than one type of ginseng | 87 | 7 | 0.68 | 0.30, 1.57 | 87 | 11 | 0.78 | 0.41, 1.48 | ||||||
| Any type of ginseng | 398 | 49 | 0.71 | 0.52, 0.98 | 398 | 63 | 0.70 | 0.53, 0.93 | ||||||
| Cumulative use (any type of ginseng) | ||||||||||||||
| None | 1,057 | 191 | 1.00 (Ref) | 1,057 | 235 | 1.00 (Ref) | ||||||||
| <Median | 192 | 23 | 0.72 | 0.46, 1.11 | 192 | 29 | 0.70 | 0.48, 1.04 | ||||||
| ≥Median | 201 | 25 | 0.69 | 0.45, 1.06 | 201 | 34 | 0.71 | 0.49, 1.02 | ||||||
| p for trend | 0.04 | 0.03 | ||||||||||||
Ginseng use . | Total mortality . | . | . | . | Disease-specific mortality/recurrence . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Total no. . | No. of events . | Adjusted* HR† . | 95% CI† . | Total no. . | No. of events . | Adjusted* HR . | 95% CI . | ||||||
| Never use before diagnosis | 1,057 | 191 | 1.00 (Ref†) | 1,057 | 235 | 1.00 (Ref) | ||||||||
| Regular use before diagnosis | ||||||||||||||
| White Asian ginseng | 134 | 11 | 0.43 | 0.23, 0.80 | 134 | 18 | 0.53 | 0.33, 0.87 | ||||||
| Red ginseng | 11 | 2 | 0.68 | 0.16, 2.79 | 11 | 2 | 0.64 | 0.16, 2.59 | ||||||
| American ginseng | 331 | 43 | 0.77 | 0.55, 1.08 | 331 | 54 | 0.74 | 0.55, 1.00 | ||||||
| Ginseng product | 10 | 1 | 0.80 | 0.11, 5.81 | 10 | 1 | 0.55 | 0.08, 3.95 | ||||||
| Ever use of more than one type of ginseng | 87 | 7 | 0.68 | 0.30, 1.57 | 87 | 11 | 0.78 | 0.41, 1.48 | ||||||
| Any type of ginseng | 398 | 49 | 0.71 | 0.52, 0.98 | 398 | 63 | 0.70 | 0.53, 0.93 | ||||||
| Cumulative use (any type of ginseng) | ||||||||||||||
| None | 1,057 | 191 | 1.00 (Ref) | 1,057 | 235 | 1.00 (Ref) | ||||||||
| <Median | 192 | 23 | 0.72 | 0.46, 1.11 | 192 | 29 | 0.70 | 0.48, 1.04 | ||||||
| ≥Median | 201 | 25 | 0.69 | 0.45, 1.06 | 201 | 34 | 0.71 | 0.49, 1.02 | ||||||
| p for trend | 0.04 | 0.03 | ||||||||||||
Adjusted for age at diagnosis, marital status, education, income, tumor-node metastasis, estrogen and progesterone receptor status, surgery, chemotherapy, radiotherapy, and tamoxifen use.
HR, hazard ratio (obtained from Cox regression models); CI, confidence interval; Ref, reference.
Association of regular ginseng use before cancer diagnosis with breast cancer survival for women in the Shanghai Breast Cancer Study, Shanghai, China, 1996–2002
Ginseng use . | Total mortality . | . | . | . | Disease-specific mortality/recurrence . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Total no. . | No. of events . | Adjusted* HR† . | 95% CI† . | Total no. . | No. of events . | Adjusted* HR . | 95% CI . | ||||||
| Never use before diagnosis | 1,057 | 191 | 1.00 (Ref†) | 1,057 | 235 | 1.00 (Ref) | ||||||||
| Regular use before diagnosis | ||||||||||||||
| White Asian ginseng | 134 | 11 | 0.43 | 0.23, 0.80 | 134 | 18 | 0.53 | 0.33, 0.87 | ||||||
| Red ginseng | 11 | 2 | 0.68 | 0.16, 2.79 | 11 | 2 | 0.64 | 0.16, 2.59 | ||||||
| American ginseng | 331 | 43 | 0.77 | 0.55, 1.08 | 331 | 54 | 0.74 | 0.55, 1.00 | ||||||
| Ginseng product | 10 | 1 | 0.80 | 0.11, 5.81 | 10 | 1 | 0.55 | 0.08, 3.95 | ||||||
| Ever use of more than one type of ginseng | 87 | 7 | 0.68 | 0.30, 1.57 | 87 | 11 | 0.78 | 0.41, 1.48 | ||||||
| Any type of ginseng | 398 | 49 | 0.71 | 0.52, 0.98 | 398 | 63 | 0.70 | 0.53, 0.93 | ||||||
| Cumulative use (any type of ginseng) | ||||||||||||||
| None | 1,057 | 191 | 1.00 (Ref) | 1,057 | 235 | 1.00 (Ref) | ||||||||
| <Median | 192 | 23 | 0.72 | 0.46, 1.11 | 192 | 29 | 0.70 | 0.48, 1.04 | ||||||
| ≥Median | 201 | 25 | 0.69 | 0.45, 1.06 | 201 | 34 | 0.71 | 0.49, 1.02 | ||||||
| p for trend | 0.04 | 0.03 | ||||||||||||
Ginseng use . | Total mortality . | . | . | . | Disease-specific mortality/recurrence . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Total no. . | No. of events . | Adjusted* HR† . | 95% CI† . | Total no. . | No. of events . | Adjusted* HR . | 95% CI . | ||||||
| Never use before diagnosis | 1,057 | 191 | 1.00 (Ref†) | 1,057 | 235 | 1.00 (Ref) | ||||||||
| Regular use before diagnosis | ||||||||||||||
| White Asian ginseng | 134 | 11 | 0.43 | 0.23, 0.80 | 134 | 18 | 0.53 | 0.33, 0.87 | ||||||
| Red ginseng | 11 | 2 | 0.68 | 0.16, 2.79 | 11 | 2 | 0.64 | 0.16, 2.59 | ||||||
| American ginseng | 331 | 43 | 0.77 | 0.55, 1.08 | 331 | 54 | 0.74 | 0.55, 1.00 | ||||||
| Ginseng product | 10 | 1 | 0.80 | 0.11, 5.81 | 10 | 1 | 0.55 | 0.08, 3.95 | ||||||
| Ever use of more than one type of ginseng | 87 | 7 | 0.68 | 0.30, 1.57 | 87 | 11 | 0.78 | 0.41, 1.48 | ||||||
| Any type of ginseng | 398 | 49 | 0.71 | 0.52, 0.98 | 398 | 63 | 0.70 | 0.53, 0.93 | ||||||
| Cumulative use (any type of ginseng) | ||||||||||||||
| None | 1,057 | 191 | 1.00 (Ref) | 1,057 | 235 | 1.00 (Ref) | ||||||||
| <Median | 192 | 23 | 0.72 | 0.46, 1.11 | 192 | 29 | 0.70 | 0.48, 1.04 | ||||||
| ≥Median | 201 | 25 | 0.69 | 0.45, 1.06 | 201 | 34 | 0.71 | 0.49, 1.02 | ||||||
| p for trend | 0.04 | 0.03 | ||||||||||||
Adjusted for age at diagnosis, marital status, education, income, tumor-node metastasis, estrogen and progesterone receptor status, surgery, chemotherapy, radiotherapy, and tamoxifen use.
HR, hazard ratio (obtained from Cox regression models); CI, confidence interval; Ref, reference.
During the follow-up survey, implemented 2.4–5.6 years after cancer diagnosis, 62.8 percent of the 1,065 survivors reported having used ginseng after cancer diagnosis, and 30.6 percent were current users at the time of the follow-up survey. Table 3 shows the mean differences in the QOL scores by ginseng use, adjusted for potential confounding factors including age, marital status, education, income, recurrence of disease, time since diagnosis, menopausal status, and use of other complementary/alternative medicine (Chinese herbal medicine and supplements). The QOL of study participants was first analyzed by stratifying them into four groups: never use of ginseng before or after diagnosis, use of ginseng before but not after, use after but not before, or use both before and after diagnosis. The results showed that ginseng use only before diagnosis had no influence on survivors' current QOL, while a nonsignificant improvement in QOL and a significant improvement in QOL were observed for use of ginseng only after diagnosis and use of ginseng both before and after diagnosis, respectively. Subsequent analyses focused on the association of use of ginseng after diagnosis with survivors' QOL. Compared with those who never used ginseng after cancer diagnosis, patients who had ever used ginseng after cancer diagnosis or were currently using ginseng as a group had significantly better scores in the psychological and social well-being domains, as well as overall QOL.
Status of ginseng use . | No. . | % . | Overall quality of life . | . | . | Physical domain . | . | . | Psychological domain . | . | . | Social domain . | . | . | Material domain . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Score . | AMDS . | 95% CI* . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | ||||||||||
| Pattern of use | |||||||||||||||||||||||||||
| Never before or after cancer diagnosis | 316 | 60.9 | Ref* | 61.1 | Ref | 69.6 | Ref | 66.4 | Ref | 43.7 | Ref | ||||||||||||||||
| Only before cancer diagnosis | 80 | 60.1 | −1.0 | −3.3, 1.4 | 59.0 | −1.0 | −3.6, 1.7 | 68.6 | −1.1 | −4.4, 2.2 | 68.0 | 1.4 | −1.1, 3.9 | 41.5 | −4.2 | −7.2, −1.2 | |||||||||||
| Only after cancer diagnosis | 440 | 62.0 | 0.8 | −0.6, 2.2 | 61.8 | 0.7 | −0.9, 2.4 | 70.9 | 1.1 | −0.9, 2.0 | 68.2 | 1.3 | −0.1, 2.9 | 44.2 | −0.2 | −1.9, 1.6 | |||||||||||
| Before and after cancer diagnosis | 229 | 63.0 | 1.6 | 0, 3.3 | 60.9 | 0.5 | −1.4, 2.5 | 72.4 | 2.5 | 0.2, 4.8 | 69.5 | 2.8 | 1.0, 4.6 | 46.8 | 0.6 | −1.5, 2.7 | |||||||||||
| Use after cancer diagnosis | |||||||||||||||||||||||||||
| No | 396 | 37.2 | 60.7 | Ref | 60.7 | Ref | 69.4 | Ref | 66.8 | Ref | 43.3 | Ref | |||||||||||||||
| Yes | 669 | 62.8 | 62.3 | 1.2 | 0.1, 2.4 | 61.5 | 0.9 | −0.5, 2.3 | 71.4 | 1.8 | 0.1, 3.4 | 68.7 | 1.5 | 0.3, 2.8 | 45.1 | 1.0 | −0.6, 2.5 | ||||||||||
| Ever use | 343 | 32.2 | 61.7 | 0.7 | −0.7, 2.0 | 61.4 | 0.5 | −1.1, 2.1 | 70.9 | 1.2 | −0.7, 3.1 | 68.1 | 0.9 | −0.5, 2.4 | 44.0 | 0.1 | −1.6, 1.9 | ||||||||||
| Current use | 326 | 30.6 | 62.9 | 1.9 | 0.5, 3.3 | 61.6 | 1.3 | −0.3, 3.0 | 72.0 | 2.4 | 0.4, 4.4 | 69.3 | 2.2 | 0.7, 3.7 | 46.2 | 1.8 | 0.1–3.6 | ||||||||||
| Cumulative use by current users | |||||||||||||||||||||||||||
| Never after cancer diagnosis | 396 | 54.9 | 60.7 | Ref | 60.7 | Ref | 69.4 | Ref | 66.8 | Ref | 43.3 | Ref | |||||||||||||||
| <725 times | 112 | 15.5 | 62.0 | 1.2 | −0.8, 3.2 | 61.1 | 0.7 | −1.7, 3.1 | 71.3 | 2.0 | −0.8, 4.8 | 67.5 | 0.7 | −1.5, 2.9 | 45.8 | 1.8 | −0.8, 4.3 | ||||||||||
| 725–1,529 times | 104 | 14.4 | 62.5 | 1.3 | −0.8, 3.4 | 61.5 | 1.0 | −1.5, 3.5 | 71.8 | 1.9 | −1.0, 4.9 | 69.1 | 1.6 | −0.6, 3.9 | 44.9 | 0.6 | −2.0, 3.2 | ||||||||||
| ≥1,530 times | 110 | 15.2 | 64.2 | 3.0 | 1.0, 5.0 | 62.3 | 1.9 | −0.5, 4.3 | 72.8 | 3.0 | 0.2, 5.8 | 71.4 | 4.2 | 2.0, 6.4 | 47.8 | 2.9 | 0.3, 5.5 | ||||||||||
| p for trend | 0.01 | 0.11 | 0.02 | 0.01 | 0.04 | ||||||||||||||||||||||
Status of ginseng use . | No. . | % . | Overall quality of life . | . | . | Physical domain . | . | . | Psychological domain . | . | . | Social domain . | . | . | Material domain . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Score . | AMDS . | 95% CI* . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | ||||||||||
| Pattern of use | |||||||||||||||||||||||||||
| Never before or after cancer diagnosis | 316 | 60.9 | Ref* | 61.1 | Ref | 69.6 | Ref | 66.4 | Ref | 43.7 | Ref | ||||||||||||||||
| Only before cancer diagnosis | 80 | 60.1 | −1.0 | −3.3, 1.4 | 59.0 | −1.0 | −3.6, 1.7 | 68.6 | −1.1 | −4.4, 2.2 | 68.0 | 1.4 | −1.1, 3.9 | 41.5 | −4.2 | −7.2, −1.2 | |||||||||||
| Only after cancer diagnosis | 440 | 62.0 | 0.8 | −0.6, 2.2 | 61.8 | 0.7 | −0.9, 2.4 | 70.9 | 1.1 | −0.9, 2.0 | 68.2 | 1.3 | −0.1, 2.9 | 44.2 | −0.2 | −1.9, 1.6 | |||||||||||
| Before and after cancer diagnosis | 229 | 63.0 | 1.6 | 0, 3.3 | 60.9 | 0.5 | −1.4, 2.5 | 72.4 | 2.5 | 0.2, 4.8 | 69.5 | 2.8 | 1.0, 4.6 | 46.8 | 0.6 | −1.5, 2.7 | |||||||||||
| Use after cancer diagnosis | |||||||||||||||||||||||||||
| No | 396 | 37.2 | 60.7 | Ref | 60.7 | Ref | 69.4 | Ref | 66.8 | Ref | 43.3 | Ref | |||||||||||||||
| Yes | 669 | 62.8 | 62.3 | 1.2 | 0.1, 2.4 | 61.5 | 0.9 | −0.5, 2.3 | 71.4 | 1.8 | 0.1, 3.4 | 68.7 | 1.5 | 0.3, 2.8 | 45.1 | 1.0 | −0.6, 2.5 | ||||||||||
| Ever use | 343 | 32.2 | 61.7 | 0.7 | −0.7, 2.0 | 61.4 | 0.5 | −1.1, 2.1 | 70.9 | 1.2 | −0.7, 3.1 | 68.1 | 0.9 | −0.5, 2.4 | 44.0 | 0.1 | −1.6, 1.9 | ||||||||||
| Current use | 326 | 30.6 | 62.9 | 1.9 | 0.5, 3.3 | 61.6 | 1.3 | −0.3, 3.0 | 72.0 | 2.4 | 0.4, 4.4 | 69.3 | 2.2 | 0.7, 3.7 | 46.2 | 1.8 | 0.1–3.6 | ||||||||||
| Cumulative use by current users | |||||||||||||||||||||||||||
| Never after cancer diagnosis | 396 | 54.9 | 60.7 | Ref | 60.7 | Ref | 69.4 | Ref | 66.8 | Ref | 43.3 | Ref | |||||||||||||||
| <725 times | 112 | 15.5 | 62.0 | 1.2 | −0.8, 3.2 | 61.1 | 0.7 | −1.7, 3.1 | 71.3 | 2.0 | −0.8, 4.8 | 67.5 | 0.7 | −1.5, 2.9 | 45.8 | 1.8 | −0.8, 4.3 | ||||||||||
| 725–1,529 times | 104 | 14.4 | 62.5 | 1.3 | −0.8, 3.4 | 61.5 | 1.0 | −1.5, 3.5 | 71.8 | 1.9 | −1.0, 4.9 | 69.1 | 1.6 | −0.6, 3.9 | 44.9 | 0.6 | −2.0, 3.2 | ||||||||||
| ≥1,530 times | 110 | 15.2 | 64.2 | 3.0 | 1.0, 5.0 | 62.3 | 1.9 | −0.5, 4.3 | 72.8 | 3.0 | 0.2, 5.8 | 71.4 | 4.2 | 2.0, 6.4 | 47.8 | 2.9 | 0.3, 5.5 | ||||||||||
| p for trend | 0.01 | 0.11 | 0.02 | 0.01 | 0.04 | ||||||||||||||||||||||
AMDS, adjusted mean differences in scores; CI, confidence interval; Ref, reference.
Obtained from multiple linear regression models with adjustment for age at diagnosis, marital status, education, income, recurrence of disease, time since diagnosis, menopausal status, and use of other complementary/alternative medicine.
Status of ginseng use . | No. . | % . | Overall quality of life . | . | . | Physical domain . | . | . | Psychological domain . | . | . | Social domain . | . | . | Material domain . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Score . | AMDS . | 95% CI* . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | ||||||||||
| Pattern of use | |||||||||||||||||||||||||||
| Never before or after cancer diagnosis | 316 | 60.9 | Ref* | 61.1 | Ref | 69.6 | Ref | 66.4 | Ref | 43.7 | Ref | ||||||||||||||||
| Only before cancer diagnosis | 80 | 60.1 | −1.0 | −3.3, 1.4 | 59.0 | −1.0 | −3.6, 1.7 | 68.6 | −1.1 | −4.4, 2.2 | 68.0 | 1.4 | −1.1, 3.9 | 41.5 | −4.2 | −7.2, −1.2 | |||||||||||
| Only after cancer diagnosis | 440 | 62.0 | 0.8 | −0.6, 2.2 | 61.8 | 0.7 | −0.9, 2.4 | 70.9 | 1.1 | −0.9, 2.0 | 68.2 | 1.3 | −0.1, 2.9 | 44.2 | −0.2 | −1.9, 1.6 | |||||||||||
| Before and after cancer diagnosis | 229 | 63.0 | 1.6 | 0, 3.3 | 60.9 | 0.5 | −1.4, 2.5 | 72.4 | 2.5 | 0.2, 4.8 | 69.5 | 2.8 | 1.0, 4.6 | 46.8 | 0.6 | −1.5, 2.7 | |||||||||||
| Use after cancer diagnosis | |||||||||||||||||||||||||||
| No | 396 | 37.2 | 60.7 | Ref | 60.7 | Ref | 69.4 | Ref | 66.8 | Ref | 43.3 | Ref | |||||||||||||||
| Yes | 669 | 62.8 | 62.3 | 1.2 | 0.1, 2.4 | 61.5 | 0.9 | −0.5, 2.3 | 71.4 | 1.8 | 0.1, 3.4 | 68.7 | 1.5 | 0.3, 2.8 | 45.1 | 1.0 | −0.6, 2.5 | ||||||||||
| Ever use | 343 | 32.2 | 61.7 | 0.7 | −0.7, 2.0 | 61.4 | 0.5 | −1.1, 2.1 | 70.9 | 1.2 | −0.7, 3.1 | 68.1 | 0.9 | −0.5, 2.4 | 44.0 | 0.1 | −1.6, 1.9 | ||||||||||
| Current use | 326 | 30.6 | 62.9 | 1.9 | 0.5, 3.3 | 61.6 | 1.3 | −0.3, 3.0 | 72.0 | 2.4 | 0.4, 4.4 | 69.3 | 2.2 | 0.7, 3.7 | 46.2 | 1.8 | 0.1–3.6 | ||||||||||
| Cumulative use by current users | |||||||||||||||||||||||||||
| Never after cancer diagnosis | 396 | 54.9 | 60.7 | Ref | 60.7 | Ref | 69.4 | Ref | 66.8 | Ref | 43.3 | Ref | |||||||||||||||
| <725 times | 112 | 15.5 | 62.0 | 1.2 | −0.8, 3.2 | 61.1 | 0.7 | −1.7, 3.1 | 71.3 | 2.0 | −0.8, 4.8 | 67.5 | 0.7 | −1.5, 2.9 | 45.8 | 1.8 | −0.8, 4.3 | ||||||||||
| 725–1,529 times | 104 | 14.4 | 62.5 | 1.3 | −0.8, 3.4 | 61.5 | 1.0 | −1.5, 3.5 | 71.8 | 1.9 | −1.0, 4.9 | 69.1 | 1.6 | −0.6, 3.9 | 44.9 | 0.6 | −2.0, 3.2 | ||||||||||
| ≥1,530 times | 110 | 15.2 | 64.2 | 3.0 | 1.0, 5.0 | 62.3 | 1.9 | −0.5, 4.3 | 72.8 | 3.0 | 0.2, 5.8 | 71.4 | 4.2 | 2.0, 6.4 | 47.8 | 2.9 | 0.3, 5.5 | ||||||||||
| p for trend | 0.01 | 0.11 | 0.02 | 0.01 | 0.04 | ||||||||||||||||||||||
Status of ginseng use . | No. . | % . | Overall quality of life . | . | . | Physical domain . | . | . | Psychological domain . | . | . | Social domain . | . | . | Material domain . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Score . | AMDS . | 95% CI* . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | Score . | AMDS . | 95% CI . | ||||||||||
| Pattern of use | |||||||||||||||||||||||||||
| Never before or after cancer diagnosis | 316 | 60.9 | Ref* | 61.1 | Ref | 69.6 | Ref | 66.4 | Ref | 43.7 | Ref | ||||||||||||||||
| Only before cancer diagnosis | 80 | 60.1 | −1.0 | −3.3, 1.4 | 59.0 | −1.0 | −3.6, 1.7 | 68.6 | −1.1 | −4.4, 2.2 | 68.0 | 1.4 | −1.1, 3.9 | 41.5 | −4.2 | −7.2, −1.2 | |||||||||||
| Only after cancer diagnosis | 440 | 62.0 | 0.8 | −0.6, 2.2 | 61.8 | 0.7 | −0.9, 2.4 | 70.9 | 1.1 | −0.9, 2.0 | 68.2 | 1.3 | −0.1, 2.9 | 44.2 | −0.2 | −1.9, 1.6 | |||||||||||
| Before and after cancer diagnosis | 229 | 63.0 | 1.6 | 0, 3.3 | 60.9 | 0.5 | −1.4, 2.5 | 72.4 | 2.5 | 0.2, 4.8 | 69.5 | 2.8 | 1.0, 4.6 | 46.8 | 0.6 | −1.5, 2.7 | |||||||||||
| Use after cancer diagnosis | |||||||||||||||||||||||||||
| No | 396 | 37.2 | 60.7 | Ref | 60.7 | Ref | 69.4 | Ref | 66.8 | Ref | 43.3 | Ref | |||||||||||||||
| Yes | 669 | 62.8 | 62.3 | 1.2 | 0.1, 2.4 | 61.5 | 0.9 | −0.5, 2.3 | 71.4 | 1.8 | 0.1, 3.4 | 68.7 | 1.5 | 0.3, 2.8 | 45.1 | 1.0 | −0.6, 2.5 | ||||||||||
| Ever use | 343 | 32.2 | 61.7 | 0.7 | −0.7, 2.0 | 61.4 | 0.5 | −1.1, 2.1 | 70.9 | 1.2 | −0.7, 3.1 | 68.1 | 0.9 | −0.5, 2.4 | 44.0 | 0.1 | −1.6, 1.9 | ||||||||||
| Current use | 326 | 30.6 | 62.9 | 1.9 | 0.5, 3.3 | 61.6 | 1.3 | −0.3, 3.0 | 72.0 | 2.4 | 0.4, 4.4 | 69.3 | 2.2 | 0.7, 3.7 | 46.2 | 1.8 | 0.1–3.6 | ||||||||||
| Cumulative use by current users | |||||||||||||||||||||||||||
| Never after cancer diagnosis | 396 | 54.9 | 60.7 | Ref | 60.7 | Ref | 69.4 | Ref | 66.8 | Ref | 43.3 | Ref | |||||||||||||||
| <725 times | 112 | 15.5 | 62.0 | 1.2 | −0.8, 3.2 | 61.1 | 0.7 | −1.7, 3.1 | 71.3 | 2.0 | −0.8, 4.8 | 67.5 | 0.7 | −1.5, 2.9 | 45.8 | 1.8 | −0.8, 4.3 | ||||||||||
| 725–1,529 times | 104 | 14.4 | 62.5 | 1.3 | −0.8, 3.4 | 61.5 | 1.0 | −1.5, 3.5 | 71.8 | 1.9 | −1.0, 4.9 | 69.1 | 1.6 | −0.6, 3.9 | 44.9 | 0.6 | −2.0, 3.2 | ||||||||||
| ≥1,530 times | 110 | 15.2 | 64.2 | 3.0 | 1.0, 5.0 | 62.3 | 1.9 | −0.5, 4.3 | 72.8 | 3.0 | 0.2, 5.8 | 71.4 | 4.2 | 2.0, 6.4 | 47.8 | 2.9 | 0.3, 5.5 | ||||||||||
| p for trend | 0.01 | 0.11 | 0.02 | 0.01 | 0.04 | ||||||||||||||||||||||
AMDS, adjusted mean differences in scores; CI, confidence interval; Ref, reference.
Obtained from multiple linear regression models with adjustment for age at diagnosis, marital status, education, income, recurrence of disease, time since diagnosis, menopausal status, and use of other complementary/alternative medicine.
Further stratification and analysis showed that the beneficial effect of ginseng on QOL seemed to come primarily from current use rather than ever use of ginseng. Compared with never users, current users reported having significantly better QOL in almost all domains except physical well-being. Current use of ginseng was positively associated with overall QOL score and the psychological, social, and material domains, and a significant dose-response relation was observed (p for trend: <0.01, 0.02, <0.01, and 0.04, respectively) (table 3). Additional analysis of the facets related to the psychological and social well-being domains showed that current ginseng users were less likely to suffer negative feelings (e.g., depression) and were more likely to report having positive feelings and to be receiving adequate social support (data not shown in table 3).
DISCUSSION
Although many in vitro experiments and in vivo animal studies have investigated various pharmacologic properties of ginseng, the therapeutic potential of ginseng for cancer patients has not been adequately evaluated. To our knowledge, this study is the first to assess the effects of ginseng as a complementary therapy on the survival and QOL of breast cancer patients in a large population-based study. We found that regular use of ginseng before cancer diagnosis was associated with significantly improved overall survival and disease-free survival among Chinese women with breast cancer. Current use of ginseng was also positively associated with QOL scores among breast cancer survivors.
Ginseng is a slow-growing perennial herb. It usually starts flowering in its fourth year, and the roots take 4–6 years to reach maturity. “White” ginseng root (unprocessed) is sometimes bleached and then dried, whereas “red” ginseng is prepared from white ginseng by various processing methods, such as steaming the fresh root before drying it (22). According to traditional Chinese medicine theory, processed ginseng (i.e., red ginseng, the most common Asian type) is considered a “hot” agent and has a strong stimulating and restoring effect, which makes it useful for restoring vitality and energy. Because of its “hot” properties, red ginseng is usually used for a short period to aid disease recovery and during winter to increase energy and build resistance to disease. Overdose or improper use of red ginseng may cause some “hot” symptoms, such as elevated blood pressure and dry eyes and throat. Typical Korean ginsengs are processed and thus belong to the “red” ginseng category. On the other hand, unprocessed ginseng (white Asian ginseng and American ginseng) is considered a “cold” agent and has a much milder effect. It can be used for a longer time period to promote health and enhance resistance to disease without major adverse effects, which may explain why the vast majority of ginseng users in this study used white ginseng and/or American ginseng rather than red ginseng.
Although different types of ginseng have different indications in traditional Chinese medicine, several in vitro studies have shown that white or red Asian ginseng, and American ginseng, all have anticarcinogenic effects (13, 18–21). The major active constituents of both Asian ginseng and American ginseng are a series of 30 triterpene saponins, known as ginsenosides. Ginsenosides can be classified into three groups on the basis of the chemical structure of their sapogenins: the panaxadiol group (e.g., Rb1, Rb2, Rg3, Rh2), the panaxatriol group (e.g., Re, Rf, Rh1), and the oleanolic acid group (e.g., Ro) (10). Several lines of evidence from in vitro experiments and animal models have suggested that ginseng and its active constituents may have anticancer activity that act through multiple pathways. It has been shown that ginseng may inhibit the growth of both estrogen-sensitive (MCF-7) and estrogen-insensitive (MDA-MB-231) breast cancer cells (20, 23), induce apoptosis of breast cancer cells (24, 25), exert synergistically inhibitory effects with chemotherapeutic agents (cyclophosphamide, fluorouracil, methotrexate, doxorubicin, paclitaxel) and endocrine therapy (tamoxifen) on breast cancer cell growth (19, 20), be associated with antineoplastic immunostimulatory activity (26), inhibit cancer cell invasion and metastasis (27, 28), and inhibit tumor angiogenesis (27, 29). Thus, it is possible that the anticancer activities produced by the multiple active constituents of ginseng may work in concert to help improve the survival of breast cancer patients.
Being diagnosed with and living with breast cancer is a stressful experience that can negatively affect QOL, particularly its physical, psychological, and social aspects (15, 30). Ginseng use is believed to maintain natural energy, increase physical and psychomotor performance, improve mood and cognitive function, and thus promote QOL or well-being (31). Several clinical trials have used various instruments to investigate the effect of ginseng or ginseng extracts on QOL and have demonstrated that ginseng may improve overall QOL or certain subscales of QOL in healthy volunteers or patients with certain diseases, such as diabetes (31). However, to our knowledge, no studies have reported the effect of ginseng on QOL among cancer survivors.
The present study provides evidence that ginseng use may improve QOL, particularly psychological and social well-being, among Chinese breast cancer survivors. Our findings are in line with the known pharmacologic activities of ginseng and are, in general, consistent with the effect of ginseng on QOL reported in previous studies (31–35). However, we did not observe a significant beneficial effect of ginseng in the physical well-being domain among the breast cancer survivors, although ginseng has conventionally been considered an adaptogen. In traditional Chinese medicine, ginseng's ability to restore energy and enhance physical performance is usually considered more beneficial for convalescents or patients with chronic diseases than for physically healthy subjects. Our previous study showed that Chinese breast cancer patients may attain maximum physical recovery within the first 1–2 years of initial treatment, while maximum recovery of psychological and social well-being will take longer to achieve (15). Thus, the lack of ginseng's effect on physical well-being observed in this study may be due to the fact that patients had already fully recovered physically by the time the survey was conducted. To confirm this hypothesis, a further study to measure the effect of ginseng on physical well-being at earlier time points is needed.
Both the World Health Organization and the German Commission E concluded that, at the recommended dose (1–2 g of dry ginseng root or 200–600 mg of standardized ginseng extracts per day), ginseng is safe (12). A recent, systematic review of the adverse effects of ginseng found that ginseng is well tolerated by most users, with the most frequently experienced adverse effects being mild and reversible (8). A concern regarding the use of ginseng by breast cancer patients is that it may have estrogen-like activity because a few isolated instances of vaginal bleeding were reported in postmenopausal women who used Rumanian ginseng or ginseng products of unspecified source or type (36–38). In vitro studies of various ginseng extracts have produced conflicting data about estrogenicity and about the effects of these extracts on the growth of breast cancer cells. On the one hand, certain ginsenosides (Rh1, Rb1, and Rg1) have been reported to act as a weak phytoestrogen activating both α and β estrogen receptors (39, 40). On the other hand, evidence has shown that ginseng can inhibit the growth of both estrogen-sensitive and estrogen-insensitive breast cancer cells (20) and can induce apoptosis of breast cancer cells (23, 25). In our study, the average daily dose of ginseng was 1.3 g of ginseng root material, and the average cumulative duration of use was 4.3 months per year. We found that ginseng use at this level significantly improved breast cancer survival and survivors' QOL.
Noticeable strengths of this study are the large population-based sample, an excellent participation rate, and inclusion of both survival and QOL as study outcomes. More importantly, all patients who used ginseng had received at least one type of mainstream treatment for breast cancer (e.g., surgery, chemotherapy, and radiotherapy), thus providing a well-defined, unique sample to evaluate the effects of ginseng use as a complementary therapy rather than an alternative therapy (41).
However, we also recognize several limitations to this study. First, because of the lack of information on ginseng use after diagnosis for patients who died before a follow-up interview, we could not examine the effect of ginseng use postdiagnosis on breast cancer survival. Second, we were unable to exclude the confounding effect of use of other complementary/alternative medicine on survival because such information was not collected until the follow-up visit that took place 3–5 years after cancer diagnosis. Third, information for this study was based mainly on patients' self-reports; thus, potential recall bias and self-report bias might exist. In addition, we could not evaluate the effect of methods of ginseng use on survival or QOL because of a lack of relevant information. In general, the most popular way to consume ginseng in China is to slice up or cut up ginseng root to make ginseng soup or tea. Sometimes, ginseng root slices are cooked with foods. Currently, we know of no available information on whether the way ginseng is consumed would influence its effects. Thus, caution is required in interpreting the results, and our findings need to be confirmed in more rigorous and randomized clinical trails.
In conclusion, in this large, population-based cohort study, we found that regular use of ginseng at a dose of 1.3 g per day may improve both overall and disease-free survival and enhance the QOL of Chinese women breast cancer survivors.
This study was supported by National Cancer Institute grant USPHS R01CA64277 (Principal Investigator: Dr. Wei Zheng). Dr. Yong Cui is partially supported by the following grants: USPHS R01CA64277, NCMHHD 5 P20 MD000516-03, and 5 U54CA091408.
The authors thank Shanghai Breast Cancer Study staff members for making this study possible. They also thank Bethanie Hull for her assistance with manuscript preparation.
Conflict of interest: none declared.
References
Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey.
Ernst E, Cassileth BR. The prevalence of complementary/alternative medicine in cancer: a systematic review.
DiGianni LM, Garber JE, Winer EP. Complementary and alternative medicine use among women with breast cancer.
Richardson MA, Sanders T, Palmer JL, et al. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology.
Cui Y, Shu XO, Gao Y, et al. Use of complementary and alternative medicine by Chinese women with breast cancer.
Morris KT, Johnson N, Homer L, et al. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites.
Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions.
Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions.
Kennedy DO, Scholey AB. Ginseng: potential for the enhancement of cognitive performance and mood.
Chang YS, Seo EK, Gyllenhaal C, et al. Panax ginseng: a role in cancer therapy?
Yun TK, Choi SY. Non-organ specific cancer prevention of ginseng: a prospective study in Korea.
Gao YT, Shu XO, Dai Q, et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study.
Cui Y, Shu XO, Gao Y, et al. The long-term impact of medical and socio-demographic factors on the quality of life of breast cancer survivors among Chinese women.
Li L, Wei H, Young D. The development of the General Quality of Life Inventory.
Li L, Young D, Wei H, et al The relationship between objective life status and subjective life satisfaction with quality of life.
Yun TK, Lee YS, Lee YH, et al. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds.
Duda RB, Zhong Y, Navas V, et al. American ginseng and breast cancer therapeutic agents synergistically inhibit MCF-7 breast cancer cell growth.
Duda RB, Kang SS, Archer SY, et al. American ginseng transcriptionally activates p21 mRNA in breast cancer cell lines.
Shin HJ, Kim YS, Kwak YS, et al. Enhancement of antitumor effects of paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP).
Ginseng root. In: Blumenthal M, Goldberg A, Brinckmann J, eds. Herbal medicine: expanded Commission E monographs. American Botanical Council. Newton, MA: Integrative Medicine Communications,
Oh M, Choi YH, Choi S, et al. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells.
Jia WW, Bu X, Philips D, et al. Rh2, a compound extracted from ginseng, hypersensitizes multidrug-resistant tumor cells to chemotherapy.
Loo WT, Cheung MN, Chow LW. The inhibitory effect of a herbal formula comprising ginseng and carthamus tinctorius on breast cancer.
Lee YS, Chung IS, Lee IR, et al. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acid polysaccharide ginsan, isolated from Panax ginseng.
Mochizuki M, Yoo YC, Matsuzawa K, et al. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng.
Shinkai K, Akedo H, Mukai M, et al. Inhibition of in vitro tumor cell invasion by ginsenoside Rg3.
Sato K, Mochizuki M, Saiki I, et al. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2.
Ganz PA, Coscarelli A, Fred C, et al L. Breast cancer survivors: psychosocial concerns and quality of life.
Coleman CI, Hebert JH, Reddy P. The effects of Panax ginseng on quality of life.
Bahrke MS, Morgan WR. Evaluation of the ergogenic properties of ginseng.
Caso Marasco A, Vargas Ruiz R, Salas Villagomez A, et al. Double-blind study of a multivitamin complex supplemented with ginseng extract.
Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients.
Wiklund IK, Mattsson LA, Lindgren R, et al. Effects of a standardized ginseng extract on quality of life and physiological parameters in symptomatic postmenopausal women: a double-blind, placebo-controlled trial. Swedish Alternative Medicine Group.
Hopkins MP, Androff L, Benninghoff AS. Ginseng face cream and unexplained vaginal bleeding.
Chan RY, Chen WF, Dong A, et al. Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng.
Cho J, Park W, Lee S, et al. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor-alpha and -beta, independent of ligand binding.
National Center for Complementary and Alternative Medicine. What is complementary and alternative medicine (CAM)? (http://nccam.nih.gov/health/whatiscam). Accessed July 8,