-
PDF
- Split View
-
Views
-
Cite
Cite
Katri Räikkönen, Anu-Katriina Pesonen, Kati Heinonen, Jari Lahti, Niina Komsi, Johan G. Eriksson, Jonathan R. Seckl, Anna-Liisa Järvenpää, Timo E. Strandberg, Maternal Licorice Consumption and Detrimental Cognitive and Psychiatric Outcomes in Children, American Journal of Epidemiology, Volume 170, Issue 9, 1 November 2009, Pages 1137–1146, https://doi.org/10.1093/aje/kwp272
Close - Share Icon Share
Abstract
Overexposure to glucocorticoids may link prenatal adversity with detrimental outcomes in later life. Glycyrrhiza, a natural constituent of licorice, inhibits placental 11-beta-hydroxysteroid dehydrogenase type 2, the feto-placental “barrier” to higher maternal levels of cortisol. The authors studied whether prenatal exposure to glycyrrhiza in licorice exerts detrimental effects on cognitive performance (subtests of the Wechsler Intelligence Scale for Children III as well as the Children's Developmental Neuropsychological Assessment and the Beery Developmental Test of Visual-Motor Integration) and psychiatric symptoms (Child Behavior Checklist) in 321 Finnish children 8.1 years of age born in 1998 as healthy singletons at 35–42 weeks of gestation. In comparison to the group with zero–low glycyrrhiza exposure (0–249 mg/week), those with high exposure (≥500 mg/week) had significant decrements in verbal and visuospatial abilities and in narrative memory (range of mean differences in standard deviation units, −0.31 to −0.41; P < 0.05) and significant increases in externalizing symptoms and in attention, rule-breaking, and aggression problems (range of odds ratios, 2.15 to 3.43; P < 0.05). The effects on cognitive performance appeared dose related. Data are compatible with adverse fetal “programming” by overexposure to glucocorticoids and caution against excessive intake of licorice-containing foodstuffs during pregnancy.
Although glucocorticoids are essential for brain development, elevated levels are detrimental, affecting neuronal division, maturation, migration, interactions, and apoptosis (1). The limbic system, particularly the hippocampus, a key locus for cognition and behavior, is sensitive to glucocorticoid manipulations in early life (2). Maintenance of low glucocorticoid exposure during critical periods of brain development is thus crucial.
Under normal circumstances, fetal cortisol levels are 2–10 times lower than maternal levels (3). This difference is ensured by the placental enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which catalyzes the rapid inactivation of cortisol to its inert 11-keto form cortisone, thus acting as a physiologic placental barrier to maternal cortisol (4). 11β-HSD2 is also highly expressed in the human fetal brain until mid-gestation but appears to be silenced between gestational weeks 19 and 26 (3). In animal models, bypass of the placental barrier using poor 11β-HSD2 substrates such as betamethasone or dexamethasone (5), maternal treatment with licorice-derived enzyme inhibitors (6), 11β-HSD2 knockout mice (7), or a low-protein maternal diet that selectively attenuates placental 11β-HSD2 (8) suggest that placental 11β-HSD2 deficiency is associated with defective brain function in later life (4–9).
The extent to which these experimental findings translate to humans remains largely unknown. Consumption of licorice by young Finnish women is common. An exceptional opportunity to shed light on prenatal glucocorticoid “programming” mechanisms in humans exists in a unique Finnish cohort, where maternal consumption of glycyrrhiza (3β-D-diglucuronyl-18β-glycyrrhetinic acid) in licorice confectionery during pregnancy has been determined. Glycyrrhiza inhibits human placental 11β-HSD2 (4). We previously showed that prenatal exposure to a high (≥500 mg/week) compared with a zero–low (0–249 mg/week) or a moderate (250–499 mg/week) level of glycyrrhiza was associated with a slightly shorter (2.4 days) duration of gestation (10) without differences in infant birth weight or in other anthropometry. Here, we report the cognitive and behavioral associations of prenatal exposure to glycyrrhiza in licorice in these children at age 8.1 years (range, 7.4–8.8).
MATERIALS AND METHODS
Study population
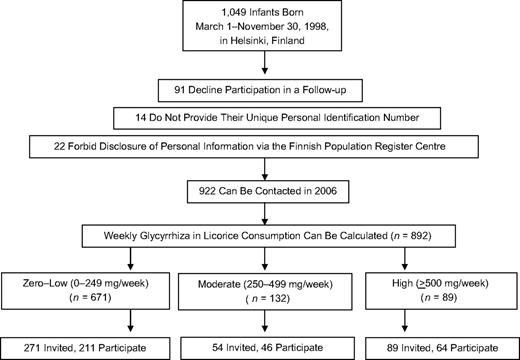
The children were included in a random, population-based urban cohort initially comprising 1,049 infants born between March 1, 1998, and November 30, 1998, in Helsinki, Finland, and their mothers. Infants eligible for our study were born healthy and singleton. The gestational age of the infants fulfilling these criteria ranged from 35 weeks to 42 weeks. Figure 1 presents the study design and selection of the study participants. Details of recruitment of the initial sample can be found elsewhere (10).
Study design and selection of the participants assessed for an association between maternal licorice consumption and detrimental cognitive and psychiatric outcomes in their children at 8.1 years of age, Helsinki, Finland.
In 2006, children and their parents were invited to participate in a follow-up with a focus on individual differences in physical and psychological development. A total of 958 (91.3%) mothers of the initial cohort agreed to be included in the follow-up, and 922 (87.9% of the initial cohort) could be contacted. Of these mothers, a subsample was invited for a follow-up examination. We invited all 89 children belonging to the group prenatally exposed to high levels of glycyrrhiza in licorice, and 64 participated. When inviting the other children, we emphasized asking those still living in or close to the greater Helsinki area to manage travel costs. Of the 271 invited children exposed to zero–low glycyrrhiza levels (0–249 mg/week), 211 participated. Of the 54 invited children exposed to moderate glycyrrhiza levels (250–449 mg/week), 46 participated. Because of cancellations for various reasons, 11 children did not participate in the cognitive testing, and one child's test results were excluded because of a mild fever. An additional 11 mothers did not return the checklist of their child's psychiatric symptoms. For 309 children, cognitive data were available, and data on psychiatric symptoms were available for 298 of them. They formed the analytic sample for the current study (Tables 1 and 2).
Characteristics of the Study Sample of Children Born in Helsinki, Finland, in 1998 and Their Mothers, According to Level of Maternal Consumption of Glycyrrhiza in Licorice During Pregnancy
| Consumption of Glycyrrhiza, mg/week | P Value for a Difference Between the 3 Groups | ||||||
| Zero–Low: 0–249 (n = 202) | Moderate: 250–499 (n = 202) | High: ≥500 (n = 62) | |||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Maternal characteristics | |||||||
| Consumption of glycyrrhiza in licorice during pregnancy, mg/week | 133.1 (57.3) | 365.3 (67.6) | 863.9 (408.7) | <0.001 | |||
| Age at delivery, years | 30.5 (4.5) | 29.8 (4.1) | 29.4 (4.3) | 0.19 | |||
| Mode of delivery: cesarean section | 8.4 | 11.1 | 9.7 | 0.84 | |||
| Gestational diabetes | 2.0 | 2.2 | 4.8 | 0.46 | |||
| Gestational hypertension | 4.0 | 2.2 | 1.6 | 0.60 | |||
| Preeclampsia | 0.5 | 0 | 1.6 | 0.53 | |||
| Systolic blood pressure at delivery, mm Hga | 123.2 (13.8) | 120.4 (13.2) | 123.1 (15.5) | 0.50 | |||
| Diastolic blood pressure at delivery, mm Hga | 75.1 (10.6) | 72.5 (10.3) | 74.6 (10.5) | 0.34 | |||
| Occupational status at delivery | 0.77 | ||||||
| Senior clerical | 45.5 | 48.9 | 40.3 | ||||
| Junior clerical | 33.2 | 35.6 | 33.9 | ||||
| Manual worker | 21.3 | 15.6 | 25.8 | ||||
| Occupational status at child's age 8.1 years | 0.31 | ||||||
| Senior clerical | 60.9 | 55.6 | 59.7 | ||||
| Junior clerical | 30.2 | 42.2 | 29.0 | ||||
| Manual worker | 8.9 | 2.2 | 11.3 | ||||
| Educational level at child's age 8.1 years | 0.60 | ||||||
| High school or less | 17.8 | 28.9 | 27.4 | ||||
| Vocational school | 32.2 | 28.9 | 27.4 | ||||
| College graduate | 14.4 | 13.3 | 14.5 | ||||
| Degree beyond college | 35.6 | 28.9 | 30.6 | ||||
| Smoking during pregnancy | 0.45 | ||||||
| Zero | 90.6 | 91.1 | 83.9 | ||||
| 1–10 cigarettes/day | 5.0 | 6.7 | 11.3 | ||||
| >10 cigarettes/day | 4.5 | 2.2 | 4.8 | ||||
| Alcohol consumption during pregnancy | 19.3 | 15.6 | 16.1 | 0.76 | |||
| g/week for those reporting consumption | 12.7 (11.2) | 9.4 (7.6) | 16.8 (11.2) | 0.37 | |||
| Stress during pregnancy, visual analog scale 0–100 mm | 36.4 (25.7) | 40.3 (26.8) | 34.3 (26.1) | 0.50 | |||
| Neonatal characteristics | |||||||
| Sex: male | 49.3 | 42.2 | 51.6 | 0.62 | |||
| Length of gestation, weeks | 40.1 (1.2) | 40.3 (1.1) | 39.9 (1.4) | 0.24 | |||
| Birth weight, kg | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) | 0.67 | |||
| Birth length, cm | 50.3 (1.8) | 50.8 (1.6) | 50.3 (2.0) | 0.20 | |||
| Head circumference, cm | 35.7 (1.5) | 35.7 (1.4) | 35.6 (1.3) | 0.92 | |||
| Ponderal index, kg/m3 | 28.0 (2.0) | 27.5 (2.4) | 27.8 (2.1) | 0.34 | |||
| Birth order ≥2nd | 44.1 | 35.6 | 43.5 | 0.57 | |||
| Perinatal disorders | |||||||
| Asphyxia | 0.5 | 0 | 3.2 | 0.12 | |||
| Neonatal jaundice | 0 | 2.2 | 1.6 | 0.14 | |||
| Infection | 2.5 | 0 | 0 | 0.26 | |||
| Hypoglycemia | 1.0 | 2.2 | 0 | 0.51 | |||
| Child characteristics at 8.1 years of age | |||||||
| Age at testing, years | 8.1 (0.3) | 8.2 (0.3) | 8.2 (0.3) | 0.21 | |||
| Height, cm | 131.3 (5.9) | 130.8 (5.2) | 130.4 (4.5) | 0.49 | |||
| Dysphasia | 1.5 | 2.2 | 1.6 | 0.94 | |||
| Dyslexia | 1.5 | 0 | 0 | 0.45 | |||
| Consumption of Glycyrrhiza, mg/week | P Value for a Difference Between the 3 Groups | ||||||
| Zero–Low: 0–249 (n = 202) | Moderate: 250–499 (n = 202) | High: ≥500 (n = 62) | |||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Maternal characteristics | |||||||
| Consumption of glycyrrhiza in licorice during pregnancy, mg/week | 133.1 (57.3) | 365.3 (67.6) | 863.9 (408.7) | <0.001 | |||
| Age at delivery, years | 30.5 (4.5) | 29.8 (4.1) | 29.4 (4.3) | 0.19 | |||
| Mode of delivery: cesarean section | 8.4 | 11.1 | 9.7 | 0.84 | |||
| Gestational diabetes | 2.0 | 2.2 | 4.8 | 0.46 | |||
| Gestational hypertension | 4.0 | 2.2 | 1.6 | 0.60 | |||
| Preeclampsia | 0.5 | 0 | 1.6 | 0.53 | |||
| Systolic blood pressure at delivery, mm Hga | 123.2 (13.8) | 120.4 (13.2) | 123.1 (15.5) | 0.50 | |||
| Diastolic blood pressure at delivery, mm Hga | 75.1 (10.6) | 72.5 (10.3) | 74.6 (10.5) | 0.34 | |||
| Occupational status at delivery | 0.77 | ||||||
| Senior clerical | 45.5 | 48.9 | 40.3 | ||||
| Junior clerical | 33.2 | 35.6 | 33.9 | ||||
| Manual worker | 21.3 | 15.6 | 25.8 | ||||
| Occupational status at child's age 8.1 years | 0.31 | ||||||
| Senior clerical | 60.9 | 55.6 | 59.7 | ||||
| Junior clerical | 30.2 | 42.2 | 29.0 | ||||
| Manual worker | 8.9 | 2.2 | 11.3 | ||||
| Educational level at child's age 8.1 years | 0.60 | ||||||
| High school or less | 17.8 | 28.9 | 27.4 | ||||
| Vocational school | 32.2 | 28.9 | 27.4 | ||||
| College graduate | 14.4 | 13.3 | 14.5 | ||||
| Degree beyond college | 35.6 | 28.9 | 30.6 | ||||
| Smoking during pregnancy | 0.45 | ||||||
| Zero | 90.6 | 91.1 | 83.9 | ||||
| 1–10 cigarettes/day | 5.0 | 6.7 | 11.3 | ||||
| >10 cigarettes/day | 4.5 | 2.2 | 4.8 | ||||
| Alcohol consumption during pregnancy | 19.3 | 15.6 | 16.1 | 0.76 | |||
| g/week for those reporting consumption | 12.7 (11.2) | 9.4 (7.6) | 16.8 (11.2) | 0.37 | |||
| Stress during pregnancy, visual analog scale 0–100 mm | 36.4 (25.7) | 40.3 (26.8) | 34.3 (26.1) | 0.50 | |||
| Neonatal characteristics | |||||||
| Sex: male | 49.3 | 42.2 | 51.6 | 0.62 | |||
| Length of gestation, weeks | 40.1 (1.2) | 40.3 (1.1) | 39.9 (1.4) | 0.24 | |||
| Birth weight, kg | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) | 0.67 | |||
| Birth length, cm | 50.3 (1.8) | 50.8 (1.6) | 50.3 (2.0) | 0.20 | |||
| Head circumference, cm | 35.7 (1.5) | 35.7 (1.4) | 35.6 (1.3) | 0.92 | |||
| Ponderal index, kg/m3 | 28.0 (2.0) | 27.5 (2.4) | 27.8 (2.1) | 0.34 | |||
| Birth order ≥2nd | 44.1 | 35.6 | 43.5 | 0.57 | |||
| Perinatal disorders | |||||||
| Asphyxia | 0.5 | 0 | 3.2 | 0.12 | |||
| Neonatal jaundice | 0 | 2.2 | 1.6 | 0.14 | |||
| Infection | 2.5 | 0 | 0 | 0.26 | |||
| Hypoglycemia | 1.0 | 2.2 | 0 | 0.51 | |||
| Child characteristics at 8.1 years of age | |||||||
| Age at testing, years | 8.1 (0.3) | 8.2 (0.3) | 8.2 (0.3) | 0.21 | |||
| Height, cm | 131.3 (5.9) | 130.8 (5.2) | 130.4 (4.5) | 0.49 | |||
| Dysphasia | 1.5 | 2.2 | 1.6 | 0.94 | |||
| Dyslexia | 1.5 | 0 | 0 | 0.45 | |||
Abbreviation: SD, standard deviation.
Maternal systolic and diastolic blood pressure values were available for 172, 43, and 56 participants in the zero–low, moderate, and high groups of glycyrrhiza consumption, respectively.
Characteristics of the Study Sample of Children Born in Helsinki, Finland, in 1998 and Their Mothers, According to Level of Maternal Consumption of Glycyrrhiza in Licorice During Pregnancy
| Consumption of Glycyrrhiza, mg/week | P Value for a Difference Between the 3 Groups | ||||||
| Zero–Low: 0–249 (n = 202) | Moderate: 250–499 (n = 202) | High: ≥500 (n = 62) | |||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Maternal characteristics | |||||||
| Consumption of glycyrrhiza in licorice during pregnancy, mg/week | 133.1 (57.3) | 365.3 (67.6) | 863.9 (408.7) | <0.001 | |||
| Age at delivery, years | 30.5 (4.5) | 29.8 (4.1) | 29.4 (4.3) | 0.19 | |||
| Mode of delivery: cesarean section | 8.4 | 11.1 | 9.7 | 0.84 | |||
| Gestational diabetes | 2.0 | 2.2 | 4.8 | 0.46 | |||
| Gestational hypertension | 4.0 | 2.2 | 1.6 | 0.60 | |||
| Preeclampsia | 0.5 | 0 | 1.6 | 0.53 | |||
| Systolic blood pressure at delivery, mm Hga | 123.2 (13.8) | 120.4 (13.2) | 123.1 (15.5) | 0.50 | |||
| Diastolic blood pressure at delivery, mm Hga | 75.1 (10.6) | 72.5 (10.3) | 74.6 (10.5) | 0.34 | |||
| Occupational status at delivery | 0.77 | ||||||
| Senior clerical | 45.5 | 48.9 | 40.3 | ||||
| Junior clerical | 33.2 | 35.6 | 33.9 | ||||
| Manual worker | 21.3 | 15.6 | 25.8 | ||||
| Occupational status at child's age 8.1 years | 0.31 | ||||||
| Senior clerical | 60.9 | 55.6 | 59.7 | ||||
| Junior clerical | 30.2 | 42.2 | 29.0 | ||||
| Manual worker | 8.9 | 2.2 | 11.3 | ||||
| Educational level at child's age 8.1 years | 0.60 | ||||||
| High school or less | 17.8 | 28.9 | 27.4 | ||||
| Vocational school | 32.2 | 28.9 | 27.4 | ||||
| College graduate | 14.4 | 13.3 | 14.5 | ||||
| Degree beyond college | 35.6 | 28.9 | 30.6 | ||||
| Smoking during pregnancy | 0.45 | ||||||
| Zero | 90.6 | 91.1 | 83.9 | ||||
| 1–10 cigarettes/day | 5.0 | 6.7 | 11.3 | ||||
| >10 cigarettes/day | 4.5 | 2.2 | 4.8 | ||||
| Alcohol consumption during pregnancy | 19.3 | 15.6 | 16.1 | 0.76 | |||
| g/week for those reporting consumption | 12.7 (11.2) | 9.4 (7.6) | 16.8 (11.2) | 0.37 | |||
| Stress during pregnancy, visual analog scale 0–100 mm | 36.4 (25.7) | 40.3 (26.8) | 34.3 (26.1) | 0.50 | |||
| Neonatal characteristics | |||||||
| Sex: male | 49.3 | 42.2 | 51.6 | 0.62 | |||
| Length of gestation, weeks | 40.1 (1.2) | 40.3 (1.1) | 39.9 (1.4) | 0.24 | |||
| Birth weight, kg | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) | 0.67 | |||
| Birth length, cm | 50.3 (1.8) | 50.8 (1.6) | 50.3 (2.0) | 0.20 | |||
| Head circumference, cm | 35.7 (1.5) | 35.7 (1.4) | 35.6 (1.3) | 0.92 | |||
| Ponderal index, kg/m3 | 28.0 (2.0) | 27.5 (2.4) | 27.8 (2.1) | 0.34 | |||
| Birth order ≥2nd | 44.1 | 35.6 | 43.5 | 0.57 | |||
| Perinatal disorders | |||||||
| Asphyxia | 0.5 | 0 | 3.2 | 0.12 | |||
| Neonatal jaundice | 0 | 2.2 | 1.6 | 0.14 | |||
| Infection | 2.5 | 0 | 0 | 0.26 | |||
| Hypoglycemia | 1.0 | 2.2 | 0 | 0.51 | |||
| Child characteristics at 8.1 years of age | |||||||
| Age at testing, years | 8.1 (0.3) | 8.2 (0.3) | 8.2 (0.3) | 0.21 | |||
| Height, cm | 131.3 (5.9) | 130.8 (5.2) | 130.4 (4.5) | 0.49 | |||
| Dysphasia | 1.5 | 2.2 | 1.6 | 0.94 | |||
| Dyslexia | 1.5 | 0 | 0 | 0.45 | |||
| Consumption of Glycyrrhiza, mg/week | P Value for a Difference Between the 3 Groups | ||||||
| Zero–Low: 0–249 (n = 202) | Moderate: 250–499 (n = 202) | High: ≥500 (n = 62) | |||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | ||
| Maternal characteristics | |||||||
| Consumption of glycyrrhiza in licorice during pregnancy, mg/week | 133.1 (57.3) | 365.3 (67.6) | 863.9 (408.7) | <0.001 | |||
| Age at delivery, years | 30.5 (4.5) | 29.8 (4.1) | 29.4 (4.3) | 0.19 | |||
| Mode of delivery: cesarean section | 8.4 | 11.1 | 9.7 | 0.84 | |||
| Gestational diabetes | 2.0 | 2.2 | 4.8 | 0.46 | |||
| Gestational hypertension | 4.0 | 2.2 | 1.6 | 0.60 | |||
| Preeclampsia | 0.5 | 0 | 1.6 | 0.53 | |||
| Systolic blood pressure at delivery, mm Hga | 123.2 (13.8) | 120.4 (13.2) | 123.1 (15.5) | 0.50 | |||
| Diastolic blood pressure at delivery, mm Hga | 75.1 (10.6) | 72.5 (10.3) | 74.6 (10.5) | 0.34 | |||
| Occupational status at delivery | 0.77 | ||||||
| Senior clerical | 45.5 | 48.9 | 40.3 | ||||
| Junior clerical | 33.2 | 35.6 | 33.9 | ||||
| Manual worker | 21.3 | 15.6 | 25.8 | ||||
| Occupational status at child's age 8.1 years | 0.31 | ||||||
| Senior clerical | 60.9 | 55.6 | 59.7 | ||||
| Junior clerical | 30.2 | 42.2 | 29.0 | ||||
| Manual worker | 8.9 | 2.2 | 11.3 | ||||
| Educational level at child's age 8.1 years | 0.60 | ||||||
| High school or less | 17.8 | 28.9 | 27.4 | ||||
| Vocational school | 32.2 | 28.9 | 27.4 | ||||
| College graduate | 14.4 | 13.3 | 14.5 | ||||
| Degree beyond college | 35.6 | 28.9 | 30.6 | ||||
| Smoking during pregnancy | 0.45 | ||||||
| Zero | 90.6 | 91.1 | 83.9 | ||||
| 1–10 cigarettes/day | 5.0 | 6.7 | 11.3 | ||||
| >10 cigarettes/day | 4.5 | 2.2 | 4.8 | ||||
| Alcohol consumption during pregnancy | 19.3 | 15.6 | 16.1 | 0.76 | |||
| g/week for those reporting consumption | 12.7 (11.2) | 9.4 (7.6) | 16.8 (11.2) | 0.37 | |||
| Stress during pregnancy, visual analog scale 0–100 mm | 36.4 (25.7) | 40.3 (26.8) | 34.3 (26.1) | 0.50 | |||
| Neonatal characteristics | |||||||
| Sex: male | 49.3 | 42.2 | 51.6 | 0.62 | |||
| Length of gestation, weeks | 40.1 (1.2) | 40.3 (1.1) | 39.9 (1.4) | 0.24 | |||
| Birth weight, kg | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) | 0.67 | |||
| Birth length, cm | 50.3 (1.8) | 50.8 (1.6) | 50.3 (2.0) | 0.20 | |||
| Head circumference, cm | 35.7 (1.5) | 35.7 (1.4) | 35.6 (1.3) | 0.92 | |||
| Ponderal index, kg/m3 | 28.0 (2.0) | 27.5 (2.4) | 27.8 (2.1) | 0.34 | |||
| Birth order ≥2nd | 44.1 | 35.6 | 43.5 | 0.57 | |||
| Perinatal disorders | |||||||
| Asphyxia | 0.5 | 0 | 3.2 | 0.12 | |||
| Neonatal jaundice | 0 | 2.2 | 1.6 | 0.14 | |||
| Infection | 2.5 | 0 | 0 | 0.26 | |||
| Hypoglycemia | 1.0 | 2.2 | 0 | 0.51 | |||
| Child characteristics at 8.1 years of age | |||||||
| Age at testing, years | 8.1 (0.3) | 8.2 (0.3) | 8.2 (0.3) | 0.21 | |||
| Height, cm | 131.3 (5.9) | 130.8 (5.2) | 130.4 (4.5) | 0.49 | |||
| Dysphasia | 1.5 | 2.2 | 1.6 | 0.94 | |||
| Dyslexia | 1.5 | 0 | 0 | 0.45 | |||
Abbreviation: SD, standard deviation.
Maternal systolic and diastolic blood pressure values were available for 172, 43, and 56 participants in the zero–low, moderate, and high groups of glycyrrhiza consumption, respectively.
Unadjusted Differences in Cognitive Abilities and “Broad-Band” Psychiatric Symptoms in Children Born in 1998 in Helsinki, Finland, According to Level of Maternal Consumption of Glycyrrhiza in Licorice During Pregnancya
| Cognitive Abilities and Psychiatric Symptoms Test | Consumption of Glycyrrhiza, mg/week | P Value for Unadjusted Differences Between the 3 Groups | ||
| Zero–Low: 0–249 (n = 202) | Moderate: 250–499 (n = 45) | High: ≥500 (n = 62) | ||
| Wechsler Intelligence Scale for Children III | ||||
| Vocabulary | 11.6 (3.0) | 11.4 (3.5) | 10.4 (2.8) | 0.02 |
| Similarities | 11.6 (3.1) | 11.5 (3.8) | 10.2 (3.3) | 0.01 |
| Block design | 10.9 (2.9) | 11.1 (2.8) | 9.8 (3.1) | 0.04 |
| Symbol search | 10.8 (3.1) | 10.8 (3.2) | 10.3 (3.6) | 0.46 |
| Beery Developmental Test of Visual-Motor Integration | 101.9 (12.3) | 102.2 (15.9) | 98.7 (14.9) | 0.23 |
| Developmental Neuropsychological Assessment | ||||
| Narrative memory | 10.4 (3.1) | 10.6 (3.5) | 9.3 (3.4) | 0.04 |
| Child Behavior Checklistb | ||||
| Internalizing symptoms | 50.4 (9.9) | 52.8 (9.2) | 52.7 (9.5) | 0.13 |
| Externalizing symptoms | 50.1 (8.4) | 51.2 (9.7) | 53.6 (8.8) | 0.03 |
| Total behavior problems | 49.1 (9.1) | 50.5 (9.7) | 53.2 (8.7) | 0.01 |
| Cognitive Abilities and Psychiatric Symptoms Test | Consumption of Glycyrrhiza, mg/week | P Value for Unadjusted Differences Between the 3 Groups | ||
| Zero–Low: 0–249 (n = 202) | Moderate: 250–499 (n = 45) | High: ≥500 (n = 62) | ||
| Wechsler Intelligence Scale for Children III | ||||
| Vocabulary | 11.6 (3.0) | 11.4 (3.5) | 10.4 (2.8) | 0.02 |
| Similarities | 11.6 (3.1) | 11.5 (3.8) | 10.2 (3.3) | 0.01 |
| Block design | 10.9 (2.9) | 11.1 (2.8) | 9.8 (3.1) | 0.04 |
| Symbol search | 10.8 (3.1) | 10.8 (3.2) | 10.3 (3.6) | 0.46 |
| Beery Developmental Test of Visual-Motor Integration | 101.9 (12.3) | 102.2 (15.9) | 98.7 (14.9) | 0.23 |
| Developmental Neuropsychological Assessment | ||||
| Narrative memory | 10.4 (3.1) | 10.6 (3.5) | 9.3 (3.4) | 0.04 |
| Child Behavior Checklistb | ||||
| Internalizing symptoms | 50.4 (9.9) | 52.8 (9.2) | 52.7 (9.5) | 0.13 |
| Externalizing symptoms | 50.1 (8.4) | 51.2 (9.7) | 53.6 (8.8) | 0.03 |
| Total behavior problems | 49.1 (9.1) | 50.5 (9.7) | 53.2 (8.7) | 0.01 |
All consumption values are expressed as mean (standard deviation).
Values were available for 195, 45, and 58 participants in the zero–low, moderate, and high groups of glycyrrhiza consumption, respectively.
Unadjusted Differences in Cognitive Abilities and “Broad-Band” Psychiatric Symptoms in Children Born in 1998 in Helsinki, Finland, According to Level of Maternal Consumption of Glycyrrhiza in Licorice During Pregnancya
| Cognitive Abilities and Psychiatric Symptoms Test | Consumption of Glycyrrhiza, mg/week | P Value for Unadjusted Differences Between the 3 Groups | ||
| Zero–Low: 0–249 (n = 202) | Moderate: 250–499 (n = 45) | High: ≥500 (n = 62) | ||
| Wechsler Intelligence Scale for Children III | ||||
| Vocabulary | 11.6 (3.0) | 11.4 (3.5) | 10.4 (2.8) | 0.02 |
| Similarities | 11.6 (3.1) | 11.5 (3.8) | 10.2 (3.3) | 0.01 |
| Block design | 10.9 (2.9) | 11.1 (2.8) | 9.8 (3.1) | 0.04 |
| Symbol search | 10.8 (3.1) | 10.8 (3.2) | 10.3 (3.6) | 0.46 |
| Beery Developmental Test of Visual-Motor Integration | 101.9 (12.3) | 102.2 (15.9) | 98.7 (14.9) | 0.23 |
| Developmental Neuropsychological Assessment | ||||
| Narrative memory | 10.4 (3.1) | 10.6 (3.5) | 9.3 (3.4) | 0.04 |
| Child Behavior Checklistb | ||||
| Internalizing symptoms | 50.4 (9.9) | 52.8 (9.2) | 52.7 (9.5) | 0.13 |
| Externalizing symptoms | 50.1 (8.4) | 51.2 (9.7) | 53.6 (8.8) | 0.03 |
| Total behavior problems | 49.1 (9.1) | 50.5 (9.7) | 53.2 (8.7) | 0.01 |
| Cognitive Abilities and Psychiatric Symptoms Test | Consumption of Glycyrrhiza, mg/week | P Value for Unadjusted Differences Between the 3 Groups | ||
| Zero–Low: 0–249 (n = 202) | Moderate: 250–499 (n = 45) | High: ≥500 (n = 62) | ||
| Wechsler Intelligence Scale for Children III | ||||
| Vocabulary | 11.6 (3.0) | 11.4 (3.5) | 10.4 (2.8) | 0.02 |
| Similarities | 11.6 (3.1) | 11.5 (3.8) | 10.2 (3.3) | 0.01 |
| Block design | 10.9 (2.9) | 11.1 (2.8) | 9.8 (3.1) | 0.04 |
| Symbol search | 10.8 (3.1) | 10.8 (3.2) | 10.3 (3.6) | 0.46 |
| Beery Developmental Test of Visual-Motor Integration | 101.9 (12.3) | 102.2 (15.9) | 98.7 (14.9) | 0.23 |
| Developmental Neuropsychological Assessment | ||||
| Narrative memory | 10.4 (3.1) | 10.6 (3.5) | 9.3 (3.4) | 0.04 |
| Child Behavior Checklistb | ||||
| Internalizing symptoms | 50.4 (9.9) | 52.8 (9.2) | 52.7 (9.5) | 0.13 |
| Externalizing symptoms | 50.1 (8.4) | 51.2 (9.7) | 53.6 (8.8) | 0.03 |
| Total behavior problems | 49.1 (9.1) | 50.5 (9.7) | 53.2 (8.7) | 0.01 |
All consumption values are expressed as mean (standard deviation).
Values were available for 195, 45, and 58 participants in the zero–low, moderate, and high groups of glycyrrhiza consumption, respectively.
Nonparticipation did not relate to maternal licorice use; to child's sex, birth date, weight, length, head circumference, or ponderal index (kg/m3) at birth, or birth order; or to mother's mode of delivery, gestational diabetes, gestational hypertension, preeclampsia, age, height, weight, body mass index, occupational status, or blood pressures at delivery; alcohol consumption; or stress during pregnancy (P > 0.10). Nonparticipation was related to more frequent maternal smoking during pregnancy (P = 0.02). Of the participating children exposed to high glycyrrhiza levels, 6 were living in rural areas in 2006 (none of the participating children exposed to zero–low or moderate glycyrrhiza levels lived in rural areas). Thus, we examined whether children living in rural and urban areas who were exposed to high glycyrrhiza levels differed in cognitive performance and psychiatric symptoms, but we found no significant differences (P > 0.27).
The Ethical Committee of the City of Helsinki Health Department and the Ethical Committee of the Helsinki University Hospital of Children and Adolescents at Helsinki and the Uusimaa Hospital District approved the project. Each child's parent gave written informed consent.
Maternal licorice consumption
While in the maternity ward, mothers were given a list including all brands of licorice-containing confectionery available in Finland in 1998. The list was prepared by the National Food Administration in 1993 and was updated with information from the manufacturers. The mothers reported the brand(s) and frequency of weekly consumption, and glycyrrhiza intake in grams per week was calculated and categorized into groups of zero–low (0–249 mg/week), moderate (250–499 mg/week), and high (≥500 mg/week). These categories represented 75%, 14%, and 11% of births of the initial cohort, respectively (10).
Main outcome measures
A standardized and validated neuropsychological test battery included vocabulary (word knowledge, verbal fluency), similarities (abstract reasoning, categories, relationships), block design (spatial, visual abstract problem solving) and symbol search (speed of processing novel information) subtests of the Wechsler Intelligence Scale for Children III (11) (these subtests display the highest correlations with verbal and performance intelligence quotients, with symbol search also uniquely measuring processing speed) (12, 13), the Beery Developmental Test of Visual-Motor Integration (14), and the narrative memory subtest of A Developmental Neuropsychological Assessment for children (15). Mothers completed the Child Behavior Checklist, a standardized and validated rating scale that screens for emotional, social, and behavioral problems (16).
Potential confounders
The variables described in the next 2 paragraphs were treated as confounders because they are implicated as risks for pregnancy and/or cognitive and psychiatric outcomes. Gestational age (week), confirmed by ultrasound before 20 weeks’ gestation; weight (kilograms) and head circumference (centimeters) at birth; birth order; and mode of delivery (vaginal/cesarean) were derived from birth records. Birth anthropometry was transformed into standard deviation units according to Finnish growth charts. Pregnancy disorders were derived from hospital records (gestational diabetes: plasma glucose >5.0 (0 hours), 10.0 (1 hour), 7.0 (2 hours) mmol/L during at least 2 time points in a 75-g oral glucose tolerance test; gestational hypertension: systolic and/or diastolic blood pressure ≥140/90 mm Hg; and preeclampsia: systolic and/or diastolic blood pressure ≥140/90 mm Hg on more than one occasion after midpregnancy, together with proteinuria (0.3 g/24 hours or a positive dipstick) in women without a history of hypertension treated before pregnancy or during the first trimester).
As proxies of socioeconomic status, we used mother's occupational status (manual worker, junior clerical, senior clerical), which was derived from hospital records at delivery and self-reported in conjunction with the child's cognitive testing at age 8.1 years, and mother's level of education (high school or less, vocational education, college graduate, and degree beyond college), which was self-reported in conjunction with the child's cognitive testing at age 8.1 years. Mother's smoking (zero, 1–10, >10 cigarettes/day), alcohol consumption (grams/week), and stress during pregnancy (visual analog scale of 0–100 mm) were self-reported while in the maternity ward after delivery. Child's height at age 8.1 years (centimeters) was measured in the clinic.
Statistical analyses
Continuous outcome variables (cognitive outcomes and broad-band psychiatric symptoms; if skewed, log-transformed to attain normality; all converted to z scores, mean = 0, standard deviation, 1) were analyzed by univariate analysis of variance and multiple linear regression analysis and are presented as mean differences and 95 percent confidence intervals in this paper. P values are 2-sided. Binary outcome variables (narrow-band, broad-band, and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition–based psychiatric symptoms >82nd percentile, indicating borderline clinically significant psychiatric symptoms) (16) were analyzed by logistic regression analyses and are presented as odds ratios and 95% confidence intervals. In analysis of variance, the 3 groups of children whose mothers consumed zero–low, moderate, and high glycyrrhiza levels in licorice represented the between-groups factor. In regression analyses, the 3 groups were dummy coded and were contrasted against the low-consumption group as the referent. In regression analyses, maternal consumption of glycyrrhiza in licorice was also treated as a continuous variable, which enabled us to test whether the associations reflected a continuous relation or a threshold exposure. Analyses of maternal licorice consumption as a continuous variable were restricted to those mothers consuming at least some glycyrrhiza in licorice during pregnancy (n = 164; mean = 473.9, standard deviation, 407.4; range = 36–2,464 mg/week).
In the first step, the analyses were adjusted for the child's sex, age at testing, length of gestation, birth weight and head circumference in standard deviation units according to Finnish growth charts, and birth order and for the mother's age and occupational status at delivery, for smoking, alcohol consumption, and psychological stress during pregnancy, and for mode of delivery, gestational diabetes, gestational hypertension, and preeclampsia. In the second and third steps, the mother's occupational status at delivery was replaced by the mother's occupational status and the mother's level of education, respectively, at the child's age of 8.1 years. In the final step, the child's height at 8.1 years of age in relation to birth length was added to the regression equation as an additional covariate.
RESULTS
We found no differences between the 3 glycyrrhiza exposure-level groups regarding any prenatal, perinatal, or maternal characteristics and, notably, no differences in birth weight, birth anthropometry, or gestation length (Table 1). Children of mothers who consumed high amounts of glycyrrhiza in licorice performed significantly more poorly in the cognitive tests than offspring of mothers consuming zero–low amounts (Table 2). The former scored −0.38, −0.41, −0.31, and −0.34 standard deviations lower in vocabulary, similarities, block design, and narrative memory tests, respectively (P < 0.03; Table 3); The moderate-exposure group did not differ from the zero–low group regarding any cognitive test result (P > 0.52; data not shown). The unadjusted and fully adjusted associations were virtually identical (Tables 2 and 3).
Fully Adjusteda Mean Differences in Cognitive Abilities of Children 8.1 Years of Age Born in 1998 in Helsinki, Finland, Whose Mothers Consumed Zero–Low (0–249 mg/week) and High (≥500 mg/week) Levels of Glycyrrhiza in Licorice During Pregnancy
| Cognitive Abilities Test | Fully Adjusted Mean Difference in Standard Deviation Units | 95% CI | P Value |
| Wechsler Intelligence Scale for Children III | |||
| Vocabulary | −0.38 | −0.66, −0.09 | 0.009 |
| Similarities | −0.41 | −0.70, −0.12 | 0.006 |
| Block design | −0.31 | −0.60, −0.03 | 0.03 |
| Symbol search | −0.15 | −0.44, 0.13 | 0.28 |
| Beery Developmental Test of Visual-Motor Integration | −0.19 | −0.47, 0.09 | 0.19 |
| Developmental Neuropsychological Assessment | |||
| Narrative memory | −0.34 | −0.62, −0.05 | 0.02 |
| Cognitive Abilities Test | Fully Adjusted Mean Difference in Standard Deviation Units | 95% CI | P Value |
| Wechsler Intelligence Scale for Children III | |||
| Vocabulary | −0.38 | −0.66, −0.09 | 0.009 |
| Similarities | −0.41 | −0.70, −0.12 | 0.006 |
| Block design | −0.31 | −0.60, −0.03 | 0.03 |
| Symbol search | −0.15 | −0.44, 0.13 | 0.28 |
| Beery Developmental Test of Visual-Motor Integration | −0.19 | −0.47, 0.09 | 0.19 |
| Developmental Neuropsychological Assessment | |||
| Narrative memory | −0.34 | −0.62, −0.05 | 0.02 |
Abbreviation: CI, confidence interval.
Fully adjusted refers to a model adjusted for the child's sex, age at testing, length of gestation, birth weight and head circumference in standard deviation units according to Finnish growth charts, and birth order and for the mother's age and occupational status at delivery, for smoking, alcohol consumption, and psychological stress during pregnancy, and for mode of delivery, gestational diabetes, gestational hypertension, and preeclampsia. The analyses were rerun by replacing mother's occupational status at delivery by her occupational status when the child was 8.1 years of age. The significant differences between high and zero–low exposure groups remained: 0.007, 0.005, 0.02, and 0.02 for P values indicating significant mean differences in vocabulary, similarities, block design, and narrative memory, respectively. The analyses were rerun again by replacing mother's occupational status at delivery by her level of education when the child was 8.1 years of age. The significant differences between high and zero–low exposure groups remained: 0.01, 0.008, 0.03, and 0.03 for P values indicating significant mean differences in vocabulary, similarities, block design, and narrative memory, respectively.
Fully Adjusteda Mean Differences in Cognitive Abilities of Children 8.1 Years of Age Born in 1998 in Helsinki, Finland, Whose Mothers Consumed Zero–Low (0–249 mg/week) and High (≥500 mg/week) Levels of Glycyrrhiza in Licorice During Pregnancy
| Cognitive Abilities Test | Fully Adjusted Mean Difference in Standard Deviation Units | 95% CI | P Value |
| Wechsler Intelligence Scale for Children III | |||
| Vocabulary | −0.38 | −0.66, −0.09 | 0.009 |
| Similarities | −0.41 | −0.70, −0.12 | 0.006 |
| Block design | −0.31 | −0.60, −0.03 | 0.03 |
| Symbol search | −0.15 | −0.44, 0.13 | 0.28 |
| Beery Developmental Test of Visual-Motor Integration | −0.19 | −0.47, 0.09 | 0.19 |
| Developmental Neuropsychological Assessment | |||
| Narrative memory | −0.34 | −0.62, −0.05 | 0.02 |
| Cognitive Abilities Test | Fully Adjusted Mean Difference in Standard Deviation Units | 95% CI | P Value |
| Wechsler Intelligence Scale for Children III | |||
| Vocabulary | −0.38 | −0.66, −0.09 | 0.009 |
| Similarities | −0.41 | −0.70, −0.12 | 0.006 |
| Block design | −0.31 | −0.60, −0.03 | 0.03 |
| Symbol search | −0.15 | −0.44, 0.13 | 0.28 |
| Beery Developmental Test of Visual-Motor Integration | −0.19 | −0.47, 0.09 | 0.19 |
| Developmental Neuropsychological Assessment | |||
| Narrative memory | −0.34 | −0.62, −0.05 | 0.02 |
Abbreviation: CI, confidence interval.
Fully adjusted refers to a model adjusted for the child's sex, age at testing, length of gestation, birth weight and head circumference in standard deviation units according to Finnish growth charts, and birth order and for the mother's age and occupational status at delivery, for smoking, alcohol consumption, and psychological stress during pregnancy, and for mode of delivery, gestational diabetes, gestational hypertension, and preeclampsia. The analyses were rerun by replacing mother's occupational status at delivery by her occupational status when the child was 8.1 years of age. The significant differences between high and zero–low exposure groups remained: 0.007, 0.005, 0.02, and 0.02 for P values indicating significant mean differences in vocabulary, similarities, block design, and narrative memory, respectively. The analyses were rerun again by replacing mother's occupational status at delivery by her level of education when the child was 8.1 years of age. The significant differences between high and zero–low exposure groups remained: 0.01, 0.008, 0.03, and 0.03 for P values indicating significant mean differences in vocabulary, similarities, block design, and narrative memory, respectively.
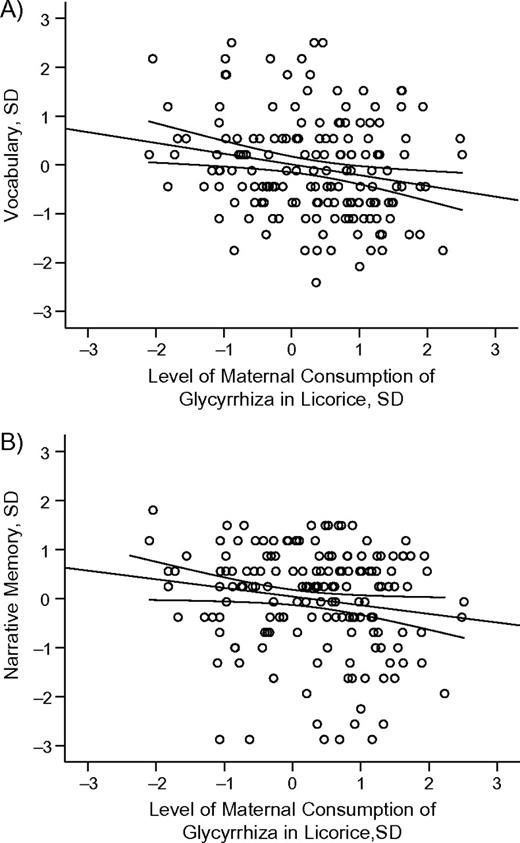
To examine whether the effects of maternal consumption of glycyrrhiza in licorice reflected a continuous relation or a threshold exposure, maternal intake, the sample restricted to those consuming at least some glycyrrhiza in licorice during pregnancy, was regressed on child's cognitive performance. Maternal glycyrrhiza consumption was significantly and linearly associated with poorer performance in the vocabulary and the narrative memory tests, and this association held after adjustments for the covariates. For every 1 standard deviation increase in glycyrrhiza exposure, vocabulary performance decreased by −0.22 (95% confidence interval: −0.38, −0.06, P < 0.007; fully adjusted P < 0.02) (Figure 2, panel A) and narrative memory performance decreased by −0.18 (95% confidence interval: −0.34, −0.01, P < 0.04; fully adjusted P < 0.03) (Figure 2, panel B) standard deviation units.
Association of vocabulary (panel A) and narrative memory (panel B) performance, in standard deviation (SD) units, among children 8.1 years of age born in Helsinki, Finland, in 1998 with level of maternal consumption of glycyrrhiza in licorice during pregnancy, in SD units. The lines represent unadjusted regression coefficients and 95% confidence intervals. Vocabulary and narrative memory decrease −0.22 (P < 0.007) and −0.18 (P < 0.04) SD units, respectively, per each SD unit increase in maternal consumption of licorice.
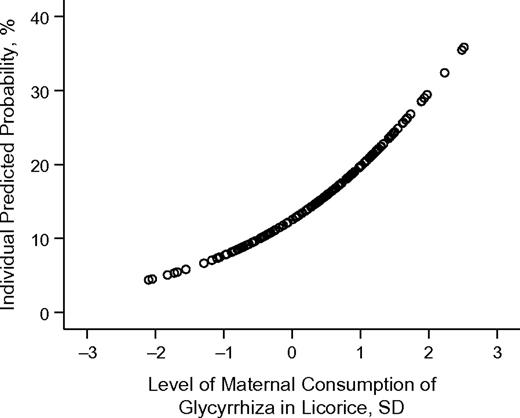
Children of mothers who consumed high amounts of glycyrrhiza in licorice scored significantly higher in externalizing symptoms (0.40 standard deviation, 95% confidence interval: 0.11, 0.69; P = 0.008; fully adjusted P = 0.02) and total behavioral problems (0.45 standard deviation, 95% confidence interval: 0.16, 0.74; P = 0.002; fully adjusted P = 0.003) than offspring of mothers consuming zero–low amounts (Table 2). Table 4 shows that, after adjustment for the covariates, their risk of borderline clinically significant externalizing symptoms, attention problems, rule-breaking behavior problems, aggressive behavior problems, attention deficit hyperactivity disorder, and somatic complaints/problems increased significantly (range of odds ratios, 2.15–3.43; P < 0.05). The moderate-exposure group did not differ from the zero–low group regarding psychiatric symptoms (P > 0.08; data not shown). The unadjusted (unadjusted statistical tests for borderline clinically significant symptoms not shown) and fully adjusted associations (Table 4) were virtually identical except for the following: the risk of attention deficit hyperactivity disorder became significant only after the adjustments (unadjusted P = 0.07), whereas the risks of oppositional defiant disorder (unadjusted P = 0.05) and conduct disorder (unadjusted P = 0.03) were rendered nonsignificant after the adjustments. Analyses testing whether the associations reflected a continuous relation or a threshold exposure revealed that each standard deviation increase in licorice exposure was associated with a 1.72-fold (95% confidence interval: 1.05, 2.81; P = 0.03; fully adjusted P = 0.05) increase in the risk of borderline clinically significant oppositional defiant disorder (Figure 3).
Unadjusted Percentages and Fully Adjusted Differences in Borderline Clinically Significant Psychiatric Symptoms >82nd Percentile in Children 8.1 Years of Age Born in 1998 in Helsinki, Finland, Whose Mothers Consumed Zero–Low (0–249 mg/week) and High (≥500 mg/week) Levels of Glycyrrhiza in Licorice During Pregnancy
| Symptoms >82nd Percentile | Zero–Low, % | High, % | Fully Adjusteda | P Value | |
| OR | 95% CI | ||||
| Narrow-band symptoms | |||||
| Withdrawn | 19.0 | 20.7 | 1.20 | 0.53, 2.72 | 0.66 |
| Anxious/depressed | 12.3 | 17.2 | 1.35 | 0.54, 3.37 | 0.52 |
| Somatic complaints | 17.4 | 29.3 | 2.35 | 1.13, 4.90 | 0.02 |
| Social problems | 15.9 | 24.1 | 1.75 | 0.79, 3.90 | 0.17 |
| Thought problems | 12.3 | 15.5 | 1.69 | 0.69, 4.15 | 0.25 |
| Attention problems | 11.3 | 27.6 | 3.43 | 1.54, 7.62 | 0.003 |
| Rule-breaking behavior | 13.8 | 27.6 | 2.15 | 1.02, 4.52 | 0.05 |
| Aggressive behavior | 12.3 | 25.9 | 2.74 | 1.20, 6.25 | 0.02 |
| Broad-band symptoms (composite of narrow-band symptoms) | |||||
| Internalizing | 13.3 | 20.7 | 1.74 | 0.75, 4.05 | 0.20 |
| Externalizing | 13.8 | 27.6 | 2.23 | 1.05, 4.73 | 0.04 |
| Total problems | 17.4 | 25.9 | 1.78 | 0.84, 3.76 | 0.13 |
| DSM-IV–based symptoms | |||||
| Anxiety disorder | 13.3 | 19.0 | 1.53 | 0.66, 3.58 | 0.32 |
| Affective disorder | 15.9 | 17.2 | 1.07 | 0.45, 2.53 | 0.87 |
| Somatic problems | 12.9 | 24.1 | 2.48 | 1.11, 5.55 | 0.03 |
| Attention deficit hyperactivity disorder | 15.4 | 25.9 | 2.26 | 1.04, 4.91 | 0.04 |
| Oppositional defiant disorder | 13.3 | 24.1 | 2.05 | 0.92, 4.57 | 0.08 |
| Conduct disorder | 14.9 | 27.6 | 2.06 | 0.98, 4.35 | 0.06 |
| Symptoms >82nd Percentile | Zero–Low, % | High, % | Fully Adjusteda | P Value | |
| OR | 95% CI | ||||
| Narrow-band symptoms | |||||
| Withdrawn | 19.0 | 20.7 | 1.20 | 0.53, 2.72 | 0.66 |
| Anxious/depressed | 12.3 | 17.2 | 1.35 | 0.54, 3.37 | 0.52 |
| Somatic complaints | 17.4 | 29.3 | 2.35 | 1.13, 4.90 | 0.02 |
| Social problems | 15.9 | 24.1 | 1.75 | 0.79, 3.90 | 0.17 |
| Thought problems | 12.3 | 15.5 | 1.69 | 0.69, 4.15 | 0.25 |
| Attention problems | 11.3 | 27.6 | 3.43 | 1.54, 7.62 | 0.003 |
| Rule-breaking behavior | 13.8 | 27.6 | 2.15 | 1.02, 4.52 | 0.05 |
| Aggressive behavior | 12.3 | 25.9 | 2.74 | 1.20, 6.25 | 0.02 |
| Broad-band symptoms (composite of narrow-band symptoms) | |||||
| Internalizing | 13.3 | 20.7 | 1.74 | 0.75, 4.05 | 0.20 |
| Externalizing | 13.8 | 27.6 | 2.23 | 1.05, 4.73 | 0.04 |
| Total problems | 17.4 | 25.9 | 1.78 | 0.84, 3.76 | 0.13 |
| DSM-IV–based symptoms | |||||
| Anxiety disorder | 13.3 | 19.0 | 1.53 | 0.66, 3.58 | 0.32 |
| Affective disorder | 15.9 | 17.2 | 1.07 | 0.45, 2.53 | 0.87 |
| Somatic problems | 12.9 | 24.1 | 2.48 | 1.11, 5.55 | 0.03 |
| Attention deficit hyperactivity disorder | 15.4 | 25.9 | 2.26 | 1.04, 4.91 | 0.04 |
| Oppositional defiant disorder | 13.3 | 24.1 | 2.05 | 0.92, 4.57 | 0.08 |
| Conduct disorder | 14.9 | 27.6 | 2.06 | 0.98, 4.35 | 0.06 |
Abbreviations: CI, confidence interval; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; OR, odds ratio.
Fully adjusted refers to a logistic regression model for the child's sex, age at testing, length of gestation, birth weight and head circumference in standard deviation units according to Finnish growth charts, and birth order and for the mother's age and occupational status at delivery, for smoking, alcohol consumption, and psychological stress during pregnancy, and for mode of delivery, gestational diabetes, gestational hypertension, and preeclampsia. The analyses were rerun by replacing mother's occupational status at delivery by her occupational status when the child was 8.1 years of age. The significant differences between the high and zero–low exposure groups remained: 0.02, 0.002, 0.05, 0.03, 0.05, 0.02, and 0.04 for P values indicating significantly increased risk of somatic complaints, attention problems, rule-breaking behavior, aggressive behavior, externalizing symptoms, somatic problems, and attention deficit hyperactivity disorder, respectively. The analyses were rerun again by replacing mother's occupational status at delivery by her level of education when the child was 8.1 years of age. The significant differences between the high and zero–low exposure groups remained: 0.02, 0.002, 0.04, 0.02, 0.04, 0.03, and 0.04 for P values indicating significantly increased risk of somatic complaints, attention problems, rule-breaking behavior, aggressive behavior, externalizing symptoms, somatic problems, and attention deficit hyperactivity disorder, respectively.
Unadjusted Percentages and Fully Adjusted Differences in Borderline Clinically Significant Psychiatric Symptoms >82nd Percentile in Children 8.1 Years of Age Born in 1998 in Helsinki, Finland, Whose Mothers Consumed Zero–Low (0–249 mg/week) and High (≥500 mg/week) Levels of Glycyrrhiza in Licorice During Pregnancy
| Symptoms >82nd Percentile | Zero–Low, % | High, % | Fully Adjusteda | P Value | |
| OR | 95% CI | ||||
| Narrow-band symptoms | |||||
| Withdrawn | 19.0 | 20.7 | 1.20 | 0.53, 2.72 | 0.66 |
| Anxious/depressed | 12.3 | 17.2 | 1.35 | 0.54, 3.37 | 0.52 |
| Somatic complaints | 17.4 | 29.3 | 2.35 | 1.13, 4.90 | 0.02 |
| Social problems | 15.9 | 24.1 | 1.75 | 0.79, 3.90 | 0.17 |
| Thought problems | 12.3 | 15.5 | 1.69 | 0.69, 4.15 | 0.25 |
| Attention problems | 11.3 | 27.6 | 3.43 | 1.54, 7.62 | 0.003 |
| Rule-breaking behavior | 13.8 | 27.6 | 2.15 | 1.02, 4.52 | 0.05 |
| Aggressive behavior | 12.3 | 25.9 | 2.74 | 1.20, 6.25 | 0.02 |
| Broad-band symptoms (composite of narrow-band symptoms) | |||||
| Internalizing | 13.3 | 20.7 | 1.74 | 0.75, 4.05 | 0.20 |
| Externalizing | 13.8 | 27.6 | 2.23 | 1.05, 4.73 | 0.04 |
| Total problems | 17.4 | 25.9 | 1.78 | 0.84, 3.76 | 0.13 |
| DSM-IV–based symptoms | |||||
| Anxiety disorder | 13.3 | 19.0 | 1.53 | 0.66, 3.58 | 0.32 |
| Affective disorder | 15.9 | 17.2 | 1.07 | 0.45, 2.53 | 0.87 |
| Somatic problems | 12.9 | 24.1 | 2.48 | 1.11, 5.55 | 0.03 |
| Attention deficit hyperactivity disorder | 15.4 | 25.9 | 2.26 | 1.04, 4.91 | 0.04 |
| Oppositional defiant disorder | 13.3 | 24.1 | 2.05 | 0.92, 4.57 | 0.08 |
| Conduct disorder | 14.9 | 27.6 | 2.06 | 0.98, 4.35 | 0.06 |
| Symptoms >82nd Percentile | Zero–Low, % | High, % | Fully Adjusteda | P Value | |
| OR | 95% CI | ||||
| Narrow-band symptoms | |||||
| Withdrawn | 19.0 | 20.7 | 1.20 | 0.53, 2.72 | 0.66 |
| Anxious/depressed | 12.3 | 17.2 | 1.35 | 0.54, 3.37 | 0.52 |
| Somatic complaints | 17.4 | 29.3 | 2.35 | 1.13, 4.90 | 0.02 |
| Social problems | 15.9 | 24.1 | 1.75 | 0.79, 3.90 | 0.17 |
| Thought problems | 12.3 | 15.5 | 1.69 | 0.69, 4.15 | 0.25 |
| Attention problems | 11.3 | 27.6 | 3.43 | 1.54, 7.62 | 0.003 |
| Rule-breaking behavior | 13.8 | 27.6 | 2.15 | 1.02, 4.52 | 0.05 |
| Aggressive behavior | 12.3 | 25.9 | 2.74 | 1.20, 6.25 | 0.02 |
| Broad-band symptoms (composite of narrow-band symptoms) | |||||
| Internalizing | 13.3 | 20.7 | 1.74 | 0.75, 4.05 | 0.20 |
| Externalizing | 13.8 | 27.6 | 2.23 | 1.05, 4.73 | 0.04 |
| Total problems | 17.4 | 25.9 | 1.78 | 0.84, 3.76 | 0.13 |
| DSM-IV–based symptoms | |||||
| Anxiety disorder | 13.3 | 19.0 | 1.53 | 0.66, 3.58 | 0.32 |
| Affective disorder | 15.9 | 17.2 | 1.07 | 0.45, 2.53 | 0.87 |
| Somatic problems | 12.9 | 24.1 | 2.48 | 1.11, 5.55 | 0.03 |
| Attention deficit hyperactivity disorder | 15.4 | 25.9 | 2.26 | 1.04, 4.91 | 0.04 |
| Oppositional defiant disorder | 13.3 | 24.1 | 2.05 | 0.92, 4.57 | 0.08 |
| Conduct disorder | 14.9 | 27.6 | 2.06 | 0.98, 4.35 | 0.06 |
Abbreviations: CI, confidence interval; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; OR, odds ratio.
Fully adjusted refers to a logistic regression model for the child's sex, age at testing, length of gestation, birth weight and head circumference in standard deviation units according to Finnish growth charts, and birth order and for the mother's age and occupational status at delivery, for smoking, alcohol consumption, and psychological stress during pregnancy, and for mode of delivery, gestational diabetes, gestational hypertension, and preeclampsia. The analyses were rerun by replacing mother's occupational status at delivery by her occupational status when the child was 8.1 years of age. The significant differences between the high and zero–low exposure groups remained: 0.02, 0.002, 0.05, 0.03, 0.05, 0.02, and 0.04 for P values indicating significantly increased risk of somatic complaints, attention problems, rule-breaking behavior, aggressive behavior, externalizing symptoms, somatic problems, and attention deficit hyperactivity disorder, respectively. The analyses were rerun again by replacing mother's occupational status at delivery by her level of education when the child was 8.1 years of age. The significant differences between the high and zero–low exposure groups remained: 0.02, 0.002, 0.04, 0.02, 0.04, 0.03, and 0.04 for P values indicating significantly increased risk of somatic complaints, attention problems, rule-breaking behavior, aggressive behavior, externalizing symptoms, somatic problems, and attention deficit hyperactivity disorder, respectively.
Unadjusted individual predicted probability of borderline clinically significant (>82nd percentile) oppositional defiant disorder in children 8.1 years of age born in Helsinki, Finland, in 1998 according to level of maternal consumption of glycyrrhiza in licorice during pregnancy, in standard deviation (SD) units. Risk of borderline clinically significant oppositional defiant disorder increases 1.72-fold (P < 0.03) per each SD unit increase in maternal consumption of licorice.
Tallness and faster growth in height have been related to better cognitive performance in children (17) and may be used as proxies of environmental influences during development (18, 19). The associations were not attenuated after adjustment for current height in relation to length at birth (all P < 0.05). The results were also unaffected after exclusion of children with dyslexia (n = 5), dysphasia (n = 3), or perinatal conditions that may affect cognitive and psychiatric outcomes (asphyxia, n = 3; neonatal jaundice, n = 2; infection, n = 5; hypoglycemia, n = 3); the exceptions were that high licorice exposure was no longer associated with somatic complaints/problems (P > 0.11), and the dose-dependent association of licorice exposure was no longer associated with oppositional defiant disorder (P = 0.07). Finally, decrements in cognitive performance may increase risk of psychiatric symptoms and vice versa, but the associations were independent of the effects on each other (all P < 0.05).
DISCUSSION
The key findings of this study are that high maternal licorice consumption during pregnancy is associated with poorer cognitive performance and with externalizing symptoms and attention problems in offspring 8.1 years of age. These findings are not interdependent; appear dose related, at least for cognitive effects; and are not obviously confounded by maternal or neonatal parameters. Licorice consumption and other modes of inducing placental 11β-HSD2 deficiency have detrimental affective and cognitive effects in rodent models (6, 9), but rodent placentation and short gestation are different from the human situation. These data are, we believe, the first to translate such preclinical observations to humans.
In principle, consumption of glycyrrhiza in licorice might affect fetal brain development and later neurocognitive performance and pathogenesis by several mechanisms. First, glycyrrhiza also potently inhibits 11-beta-hydroxysteroid dehydrogenase type 1, an oxido-reductase that catalyzes the reverse reaction (cortisone to cortisol). However, adult 11-beta-hydroxysteroid dehydrogenase type 1 knockout mice have improved rather than worsened cognitive performance (20, 21). Higher doses of glycyrrhiza also affect gap junction formation, but such concentrations are unlikely to be achieved with in vivo exposure (22). Second, glycyrrhiza may alter maternal physiology, perhaps via hypertensive effects in a subset of sensitive individuals. However, no differences in maternal blood pressure were found, and the graded dose-response relation does not support a categorical effect. Moreover, breeding male and female mice heterozygous for a null allele of the 11ß-HSD2 gene showed that effects on offspring affective behavior are confined to offspring/placentas lacking 11ß-HSD2 rather than to wild-type littermates of the same dams (7).
Third, in rodents, licorice derivatives or 11ß-HSD2 knockout reduces birth weight (7). However, although we previously demonstrated that maternal consumption of high levels of glycyrrhiza during pregnancy is associated with a subtle, but significant shortening of gestation without changes in birth weight or other anthropometry (10), in the present study glycyrrhiza was linked with extended effects on cognitive performance and psychiatric symptoms independent of length of gestation, birth weight, or head circumference. Likewise, prenatal exposure to the Dutch 1945–1946 famine was associated with adverse effects on adult health without affecting body size at birth (23). Thus, although our findings support the developmental origins of health and disease hypothesis (24, 25), they add credence to the suggestion that many insults in utero that affect offspring biology do not necessarily alter gross birth size (1). Interestingly, in primiparous vervet monkeys, glucocorticoid exposure in doses that did not reduce birth weight or gestation length altered cardiometabolic, neuroendocrine, and behavioral parameters in juvenile offspring (26). In rhesus monkeys, the hippocampus appears structurally and functionally especially vulnerable to antenatal glucocorticoid exposure (27).
Because of glycyrrhiza's sweetening (50–200 times sweeter than refined sugar) and flavoring capacity, it is found in candies and chewing gum, herbal teas, alcoholic and nonalcoholic drinks, tobacco, and traditional (e.g., cough medicine) as well as herbal (to treat stomach ulcers, sore throat, viral infections) medicine. Glycyrrhiza use in foods has been approved by the Council of Europe (28), the joint Food and Agriculture Organization of the United Nations/World Health Organization Expert Committee (29), and the US Food and Drug Administration (30). Although it is generally recognized as safe for use in foods, the European Community's Scientific Committee on Food and the Food and Agriculture Organization of the United Nations/World Health Organization Expert Committee have considered 100 mg/day a reasonable upper limit of consumption for a majority of the population (28, 29), and the Food and Drug Administration assumes that glycyrrhiza levels in foods do not pose a health hazard provided that these foods are not consumed in excess or by individuals sensitive to low levels of glycyrrhiza (30). According to a recent estimate, even in the United States, where licorice confectionery is not popular, daily consumption levels of glycyrrhiza range from 1.6 mg to 215.2 mg (31). Our findings may thus have more widespread implications that extend far beyond the scope of licorice consumed during pregnancy in Finland. We cannot determine whether the amounts in other glycyrrhiza-containing commercial food, tobacco, or pharmaceutical products available globally are sufficient to exert detrimental effects on any developmental outcomes. Yet, it should be borne in mind that we found a graded, linear association between level of exposure and cognitive performance.
There are limitations to our study. We could not determine whether there is any critical timing of prenatal exposure to licorice. In addition, we could not determine whether the mothers in the 3 groups consumed other glycyrrhiza-containing products. However, this possibility would have diminished rather than increased our ability to find associations. We had no information on other nutritional intake of the mothers, for example, protein intake, although hemoglobin levels of all pregnant women in Finland are monitored in maternity clinics routinely (mean = 14 visits during pregnancy) in conjunction with receiving dietary instructions by nurses/physicians if deemed necessary.
Our study sample was composed of singletons born healthy and at 35–42 weeks of gestation. Hence, the occurrence of pregnancy-related and perinatal disorders was lower than the prevalence rates reported in the literature. In addition, very few children suffered from neurocognitive deficits at 8.1 years of age. Thus, our findings may not be generalized to samples with greater variance in health. Furthermore, licorice can cause hypokalemia and salt and water retention, leading to increased blood pressure. Therefore, individuals with a tendency for hypertension are generally advised to avoid licorice. Mothers reporting high use of licorice during pregnancy did not differ significantly from the group reporting zero–low levels of consumption regarding gestational hypertension or blood pressure levels at delivery. Because we could not determine the blood pressure levels of the mothers before their licorice consumption began, a possibility remains that the mothers reporting high licorice consumption might have been initially healthier, although it would not be expected to be associated with worse neurocognitive and psychiatric outcomes in the offspring. Mothers participating in our study were more often nonsmokers during pregnancy. Thus, our findings may not be generalized to samples characterized by less healthier lifestyles during pregnancy.
We had no data available on maternal cognitive abilities or psychiatric symptoms. Although maternal occupational status at delivery and occupational status and level of education at the time of the child's age of 8.1 years can be used as proxies of maternal cognitive abilities and risk of psychiatric symptoms, a possibility remains that a hereditary factor (genetic/epigenetic) determining cognitive abilities and psychiatric symptoms as well as driving dietary licorice preference underlies the associations. Finally, we could not determine the role of environmental factors (apart from occupation and education of the mothers) in contributing to the associations. However, our results were not confounded by height at 8.1 years of age. Slow childhood growth in height is an accepted indicator of adverse childhood socioeconomic conditions (18) reflecting malnutrition, recurrent minor infections that divert nutrition away from growth, or suboptimal parenting (19).
We conclude that prenatal exposure to glycyrrhiza dose-dependently predicts poorer verbal and visuospatial abilities and narrative memory as well as increased risk of externalizing symptoms, attention, rule-breaking, and aggression problems in children aged 8.1 years. Our findings also add to the few, but consistent results showing significantly increased risks of attention problems (32) and distractible, aggression/destructive, and hyperkinetic behavior (33) in early childhood for the children exposed to repeated betamethasone prenatally. Cognitive decrements in childhood predict greater risk of dementia, peripheral disorders, and mortality in subsequent adulthood (34). Childhood psychiatric symptoms, childhood rule-breaking behavior in particular, are related to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition diagnoses in adulthood (35), underlining the potential importance for lifelong mental health. Our findings support adverse fetal “programming” by overexposure to glucocorticoids and counsel concern against consuming excessive amounts of foodstuffs containing glycyrrhiza during pregnancy. Whether other disease outcomes will also become manifest is the subject of ongoing studies.
Abbreviation
Author affiliations: Department of Psychology, University of Helsinki, Helsinki, Finland (Katri Räikkönen, Anu-Katriina Pesonen, Kati Heinonen, Jari Lahti, Niina Komsi); Department of General Practice and Primary Health Care, University of Helsinki, Helsinki, Finland (Johan G. Eriksson); National Institute for Health and Welfare, Helsinki, Finland (Johan G. Eriksson); Department of General Practice and Primary Health Care, Institute of Clinical Medicine, University of Helsinki, Helsinki, Finland (Johan G. Eriksson); Vasa Central Hospital, Vasa, Finland (Johan G. Eriksson); Folkhälsan Research Unit, Helsinki, Finland (Johan G. Eriksson); Helsinki University Contral Hospital, Unit of General Practice, University of Helsinki, Haartmaninkatu, Finland (Johan G. Eriksson); Centre for Cardiovascular Science, The Queen's Medical Research Institute, University of Edinburgh, Edinburgh, United Kingdom (Jonathan R. Seckl); Hospital for Children and Adolescents, University of Helsinki, Helsinki, Finland (Anna-Liisa Järvenpää); Department of Health Sciences/Geriatrics, University of Oulu, Oulu, Finland (Timo E. Strandberg); and Unit of General Practice, Oulu University Hospital, University of Oulu, Oulu, Finland (Timo E. Strandberg).
Sponsored by grants from the Juho Vainio Foundation, the John D. and Catherine T. MacArthur Foundation, the European Science Foundation (EuroSTRESS), and the Finnish Academy.
Conflict of interest: none declared.