-
PDF
- Split View
-
Views
-
Cite
Cite
HOWARD C. BECKER, HUGH MYRICK, LYNN M. VEATCH, PREGABALIN IS EFFECTIVE AGAINST BEHAVIORAL AND ELECTROGRAPHIC SEIZURES DURING ALCOHOL WITHDRAWAL, Alcohol and Alcoholism, Volume 41, Issue 4, July/August 2006, Pages 399–406, https://doi.org/10.1093/alcalc/agl029
Close - Share Icon Share
Abstract
Aims: Pregabalin has been shown to possess anticonvulsant, analgesic, and anxiolytic properties in a variety of testing situations. This study was designed to evaluate the ability of pregabalin to exert its anticonvulsant effects against behavioral and electrographic measures of CNS hyperexcitability associated with alcohol withdrawal in a mouse model of ethanol dependence. Methods: Adult mice were chronically exposed to ethanol and, upon withdrawal, were tested for behavioral signs of seizure activity (handling-induced convulsions) or abnormalities in spontaneous EEG activity recorded from cortical and subcortical sites. Results: Pregabalin (50–200 mg/kg) administered 1 and 4 h into withdrawal dose dependently reduced severity of handling-induced convulsions in comparison to vehicle-treated mice. Similarly, pregabalin reduced the frequency in which EEG activity was interrupted by trains of high-voltage synchronous activity in a dose-related fashion. Finally, pregabalin treatment of repeated withdrawals was effective in blocking the development of withdrawal sensitization observed in vehicle-treated mice. Conclusions: Collectively, these results suggest that pregabalin may be an effective therapeutic agent for medical management of alcohol detoxification.
(Received 25 July 2005; first review notified 23 August 2005; in revised form 21 March 2006; accepted 21 March 2006)
INTRODUCTION
Abrupt reduction or cessation of chronic alcohol intake has long been known to lead to a characteristic withdrawal syndrome. While the alcohol withdrawal syndrome is typically characterized by benign symptoms such as tremor, agitation, and diaphoresis, potentially severe symptoms such as seizure activity and delirium tremens may occur (Myrick and Anton, 2000). Given the life-threatening nature of alcohol withdrawal, it is usually treated with medication to avoid more severe medical complications.
Benzodiazepines are considered by research studies and consensus reports to be the medications of choice to treat alcohol withdrawal (Griffiths, 1990). Despite their well-recognized therapeutic efficacy and relative safety, some potential drawbacks associated with use of benzodiazepines for treatment of alcohol detoxification have been noted. These include tolerance, dependence, and abuse liabilities, potential interactive effects with alcohol, and cognitive impairing side effects that may interfere with psychosocial/motivational intervention efforts to prevent relapse (Griffiths and Sannerud, 1987). Thus, alternative medications that afford similar therapeutic efficacy, but with less side effects, are clearly desirable for treatment of alcohol withdrawal. Recently, a number of anticonvulsant agents have shown promise in clinical studies for their use in medical management of alcohol detoxification (Malcolm et al., 2000, 2001; Myrick et al., 2001).
There are several potential advantages in the use of anticonvulsants to treat alcohol withdrawal. First, seizures are one of the most serious complications of alcohol withdrawal, and use of an anticonvulsant medication should decrease the probability of experiencing a seizure. Second, anticonvulsants have been shown to be effective in blocking kindling, a phenomenon thought to underlie, at least in part, the progressive worsening of alcohol withdrawal symptoms over multiple detoxification episodes (Ballenger and Post, 1978; Becker and Littleton, 1996; Becker, 1998). Third, anticonvulsants have not been shown to have abuse potential. This is especially important given that a majority of alcohol detoxifications occur on an outpatient basis. Fourth, these agents have been found useful in affective and anxiety disorders, which share symptomatology such as depression, irritability, and anxiety with alcohol withdrawal (Myrick et al., 2003). Finally, anticonvulsants have typically not shown the cognitive impairing effects of benzodiazepines, thereby allowing quicker integration into recovery programs. This is especially important in an era of reduced hospital stays.
While a number of anticonvulsants have been studied in the treatment of alcohol withdrawal, no single agent has gained general acceptance (Malcolm et al., 2001). The present study was designed to evaluate the effects of a novel anticonvulsant, pregabalin [(S-(+)-3-isobutyl γ-aminobutyric acid (GABA)] on withdrawal-related seizure activity in a mouse model of alcohol dependence (Becker and Veatch, 2002; Veatch and Becker, 2002). Pregabalin has been found to have anticonvulsant, analgesic, and anxiolytic activity in a variety of animal models (Taylor and Vartanian, 1997; Field et al., 2001; Andre et al., 2003; Chesler et al., 2003). Clinical studies have indicated that pregabalin is effective in treatment of certain types of epilepsy (French et al., 2003; Arroyo et al., 2004; Brodie, 2004), neuropathic pain (Dworkin et al., 2003; Rosenstock et al., 2004; Sabatowski et al., 2004), as well as generalized and social anxiety disorders (Feltner et al., 2003; Pande et al., 2003, 2004). Given this pharmacological profile, pregabalin would appear to be well suited for treatment of alcohol withdrawal seizures and other components of the withdrawal syndrome.
The mechanism of action of pregabalin is not entirely clear. Although the compound was originally synthesized to be a lipophilic analogue of GABA capable of penetrating the blood–brain barrier, pregabalin does not directly interact with GABAA or GABAB receptors, it does not get metabolized into a GABAergic compound, and it does not inhibit GABA uptake or degradation (Ben-Menachem, 2004; Bialer et al., 2004). Rather, studies have shown pregabalin to bind potently to the alpha-2-delta regulatory subunit of voltage-gated calcium channels (VGCC), inhibiting activity-dependent calcium influx in nerve terminals and consequently reducing the release of neurotransmitters such as glutamate, norepinephrine, and Substance P (Dooley et al., 2002; Fink et al., 2002; Fehrenbacher et al., 2003; Cunningham et al., 2004; Stahl, 2004). The interaction of pregabalin with alpha-2-delta binding sites in brain is thought to underlie its therapeutic properties (e.g. anticonvulsant action) (Ben-Menachem, 2004; Bialer et al., 2004).
This study was designed to evaluate the ability of pregabalin to exert its anticonvulsant effects against behavioral and electroencephalographic (EEG) measures of CNS hyperexcitability associated with alcohol withdrawal in a mouse model of ethanol dependence. Additionally, using a model of repeated cycles of chronic ethanol exposure and withdrawal, the ability of pregabalin to reduce or block the development of sensitization (kindling) of alcohol withdrawal seizures was examined.
METHODS
Subjects
Adult male C3H/He mice (80–100 days of age) obtained from Charles River Laboratories (Portage, MI) were used in these experiments. The mice weighed 25–28 g at the start of the experiments. The animals were housed 3–4 per cage in an AAALAC-accredited facility under a 12 h light–dark cycle (lights on at 0600) with ad lib access to food and water throughout the experiments. All procedures were approved by the Institutional Animal Care and Use Committee and followed USDA regulations and the NIH Guide for the Care and Use of Laboratory Animals (1996).
General study design and procedure
Mice were randomly assigned to ethanol treatment conditions and then further separated into pregabalin treatment groups. Details about chronic ethanol exposure procedures and pregabalin treatments are described below.
Experiment 1
Mice were continuously exposed for 64 h to ethanol vapor in inhalation chambers, as detailed below. Upon removal from the inhalation chambers, blood samples were collected for blood ethanol concentration (BEC) determination and the mice were individually housed. At 1 and 4 h into withdrawal, separate groups of mice (n = 4–8/group) received injections (i.p.) of pregabalin (0, 50, 100, or 200 mg/kg). The rationale for administering pregabalin at 1 and 4 h into withdrawal was based on published work (Taylor et al., 1992) as well as our pilot work with this compound and a related anticonvulsant, gabapentin, which suggested a relatively short duration of action in mice. Behavioral signs of withdrawal-related CNS hyperexcitability were assessed by scoring handling-induced convulsions (HIC) using a previously described scoring scale (Crabbe and Kosobud, 1990). The HIC response has proven to be a sensitive and reliable index of CNS hyperexcitability associated with ethanol withdrawal (Crabbe and Kosobud, 1990; Becker and Hale, 1993; Becker, 1994; Becker et al., 1997a,b). HIC activity was recorded hourly for the first 10 h of withdrawal, and then at 24, 32, 48, and 72 h post-withdrawal. Data are presented as hourly HIC scores. Additionally, area under the HIC withdrawal curve was calculated using the integrative trapezoidal formula (Ellis and Gulick, 1991).
Experiment 2
Separate groups of mice were used to assess electrographic measures of ethanol withdrawal. Mice were stereotaxically implanted with chronic indwelling electrodes, as previously described (Veatch and Becker, 2002). Briefly, monopolar, stainless steel, semi-micro electrodes (120 µm) were implanted into hippocampus (AP: −1.65 mm; L: 1.5 mm; V: −2.25 mm), amygdala (AP: −0.7 mm; L: −2.25 mm; V: −5.25 mm), and visual cortex (AP: −3.0 mm; L: −2.0 mm),along with a stainless steel screw in the nasal area (AP: +4.0 mm; L: +0.5 mm) for use as a reference electrode. The coordinates are given relative to bregma (Franklin and Paxinos, 1997). Electrodes were connected to a four-pin MicroTech plug and fixed to the skull using dental acrylic and a light-cured resin composite (Groseclose et al., 1998). Following recovery from surgery (3–5 days), baseline EEG activity was recorded from freely moving mice in 30 min samples every 2 h over an 8 h period. The next day, mice were placed in the inhalation chambers and received 64 h continuous exposure to ethanol vapor. At 1 and 4 h into withdrawal, different groups of mice (n = 3–6/group) received injections (i.p.) of pregabalin (0, 100, or 200 mg/kg). EEG data were collected during withdrawal (as described for baseline), with additional samples recorded at 24, 32, 48, and 72 h post-withdrawal. Recording sessions were conducted in electrically-shielded chambers, with electrode cables connected to Grass 7P511 amplifiers. Spontaneous EEG data were digitized by a CED 1401 analog-to-digital converter and trains of high-voltage electrographic activity, known as brief spindling episodes (BSE), were identified by a computer program (Spike2), as previously described (Veatch and Becker, 2002). Briefly, automated analysis entailed identifying and classifying bursts of EEG activity with a frequency between 7 and 9 Hz and duration of at least 1 s as BSE events. Data are presented as percent BSE activity (i.e. cumulative duration of all BSE events relative to entire duration of each recording session).
Experiment 3
Mice were randomly assigned to one of two ethanol treatment conditions. One group received 3 cycles of 16 h exposure to ethanol vapor, each separated by 8 h periods of withdrawal. The remaining mice served as ethanol-naïve controls, receiving similar handling, but maintained in control (air) chambers throughout the experiment. At 1 and 4 h into each of the three withdrawal cycles, chronic ethanol and control groups were injected (i.p.) with pregabalin (0, 100, or 200 mg/kg). Animals (n = 6–8/group) were tested hourly for HIC activity during each withdrawal cycle. Data are presented as hourly HIC scores and area under the 7 h HIC curve for each withdrawal cycle.
Chronic ethanol exposure
Mice were chronically exposed to ethanol vapor in Plexiglas inhalation chambers (60 × 36 × 60 cm3), slightly modified after that previously described (Becker and Hale, 1993). Briefly, ethanol (95%) was volatilized by passing air through an air stone submerged in the ethanol. The ethanol vapor was mixed with fresh air and delivered to the chambers at a rate of 10 l/min, which maintained the ethanol concentration in the chamber in the range of 10–13 mg/l air. Prior to entry into the ethanol chambers, intoxication was initiated by administration of ethanol (1.6 g/kg; 8% w/v; ip.) and BEC was stabilized by administration of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg). We have previously demonstrated that these conditions yield relatively stable blood ethanol levels during the ethanol exposure period (Becker, 1994). Mice maintained in the control (air) chamber received injections of saline and pyrazole. The housing conditions in the inhalation chambers were identical to that in the colony room. Immediately after removing mice from the inhalation chambers, blood samples were collected for determination of blood ethanol levels (see below).
Ethanol samples and measurement
Chamber ethanol concentration was determined daily by collecting air samples (2 ml) with a gas-tight syringe through a port in the chamber wall. The samples were then transferred to Venoject™ tubes for later analysis using an enzymatic spectrophotometric assay procedure previously described (Becker and Hale, 1993). Ethanol concentration in the chambers is expressed as mg/l air.
Blood samples were collected from the retro-orbital sinus with heparinized capillary tubes. The samples were centrifuged for phase separation and 5 µl of plasma were injected into an Analox Instrument analyzer (Lunenburg, MA). BEC (in mg/dl) was recorded by measuring oxygen uptake generated by the oxidation of ethanol to acetaldehyde and hydrogen peroxide by ethanol oxidase.
Drug preparation and administration
Pregabalin (generously supplied by Pfizer, Inc.) was prepared by mixing the drug in saline, which served as the vehicle. Pyrazole and ethanol were dissolved in saline. All drugs were administered by intraperitoneal (i.p.) injection in a volume of 20 ml/kg body weight.
Data analysis
BEC upon final removal from inhalation chambers, area under the HIC curves (Experiment 1) and frequency of BSE activity (Experiment 2) were analyzed by analysis of variance (ANOVA), with pregabalin dose as the between-subject variable. HIC activity (area under the curve) in Experiment 3 was analyzed by ANOVA, with chronic ethanol treatment condition and pregabalin dose as between-subject variables and withdrawal cycles as a repeated measure. Post hoc comparisons (Fisher's Protected Least Significant Difference Test) were conducted, as appropriate.
RESULTS
Experiment 1: effect of pregabalin treatment on behavioral convulsions during ethanol withdrawal
Analysis of BEC at the time of final withdrawal revealed no significant differences among pregabalin dosage groups (160.0 ± 5.6, 162 ± 5.0, 141.2 ± 8.1, and 152.5 ± 7.0 mg/dl for vehicle, 50, 100, and 200 mg/kg groups, respectively); [F(3,24) = 2.47, P > 0.08].
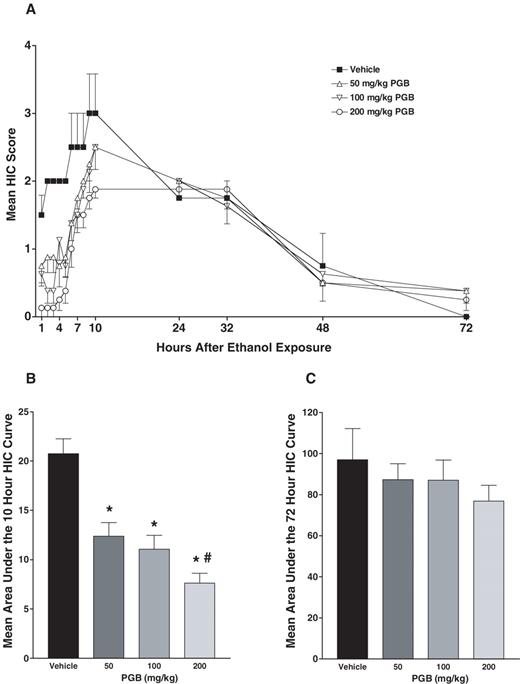
Figure 1A depicts the progressive development of the HIC response for each of the pregabalin treatment groups as a function of time following withdrawal from chronic ethanol exposure. As can be seen, pregabalin was effective in reducing the severity of the HIC response during the acute withdrawal period (first 10 h), and this effect was especially apparent during the first 4–5 h following withdrawal. As illustrated in Figure 1B, this impression is supported by ANOVA of data expressed as area under the 10 h HIC curve [F(3,24) = 12.35, P < 0.0001]. Post hoc analysis revealed that pregabalin reduced HIC activity in a dose-dependent fashion, with all doses significantly differing from the vehicle condition and the 200 mg/kg dose differing from the 50 mg/kg dose condition (Ps < 0.05). As shown in Figure 1C, pregabalin treatment did not significantly alter the overall withdrawal response when data were expressed as area under the 72 h HIC curve [F(3,24) = 0.63, P > 0.60]. This suggests that the drug was most effective during the acute phase of withdrawal when it was administered.
Mean ± SEM. (A) HIC score for the different pregabalin (PGB) treatment groups as a function of time following 64 h chronic ethanol exposure (see text for details); (B) area under the 10 h HIC curve; and (C) area under the 72 h HIC curve. *Significantly differs from vehicle condition (P < 0.05); #significantly differs from 50 mg/kg PGB group (P < 0.05).
Experiment 2: effect of pregabalin treatment on electrographic seizure activity during ethanol withdrawal
At the time of withdrawal, BEC for vehicle, 100, and 200 mg/kg pregabalin dosage groups was 148.2 ± 10.0, 148.8 ± 8.9, and 149.2 ± 5.1 mg/dl, respectively. ANOVA revealed no significant difference between groups [F(2,12) < 1.0].
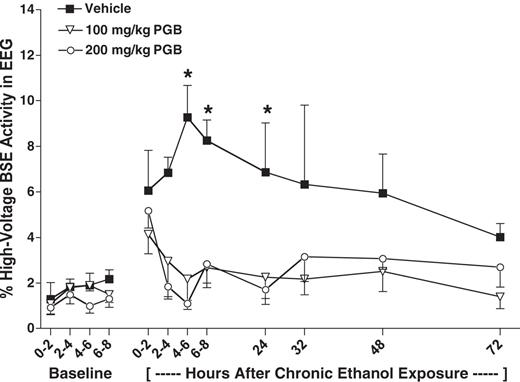
As can be seen in Figure 2, pregabalin treatment reduced the frequency in which EEG activity was interrupted by bursts of high-voltage activity (BSE activity) during ethanol withdrawal. This effect was evident for much of the withdrawal-testing period. ANOVA revealed a significant main effect of pregabalin treatment [F(2,12) = 12.97, P < 0.001] and the pregabalin treatment × time interaction [F(22,132) = 2.40, P < 0.009]. Post hoc analysis indicated that both 100 and 200 mg/kg pregabalin doses significantly reduced BSE activity in comparison to the vehicle condition at 6, 8, and 24 h time points (Ps< 0.05).
Mean ± SEM. Percent of spontaneous EEG activity containing high-voltage BSE for the different PGB treatment groups during baseline and time following 64 h chronic ethanol exposure. *Significantly differs from vehicle condition (P < 0.05).
Experiment 3: effect of pregabalin treatment on development of sensitized behavioral convulsions during repeated cycles of ethanol withdrawal
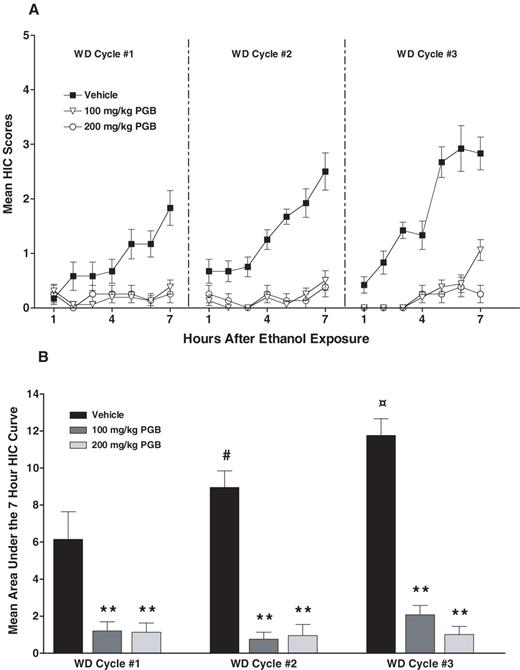
Figure 3A depicts HIC activity exhibited by chronic ethanol and control mice across the three withdrawal cycles as a function of pregabalin treatment. Since HIC activity in the control groups was negligible, these data are not presented in the figure. As illustrated when data are expressed as HIC scores (Fig. 3A) or area under the 7 h HIC curve (Fig. 3B), HIC responses in vehicle-treated mice progressively intensified over successive withdrawal cycles. In addition, pregabalin treatment reduced HIC activity during each of the withdrawal cycles. This impression was supported by ANOVA of the area under the HIC curve data, which revealed a significant main effect of ethanol group [F(1,40) = 4.80, P < 0.04], pregabalin treatment [F(2,40) = 37.0, P < 0.0001] and withdrawal cycle [F(2,80) = 4.92, P < 0.01], as well as the ethanol group × pregabalin treatment × withdrawal cycle interaction [F(4,80) = 3.74, P < 0.01]. Separate analyses of ethanol-exposed mice indicated a significant pregabalin treatment × withdrawal cycle interaction term [F(4,42) = 4.12, P < 0.02]. Post hoc analyses indicated that the severity of HIC activity significantly increased from one cycle to the next in vehicle-treated mice (Ps< 0.02). Both the 100 and 200 mg/kg doses of pregabalin were equally effective in decreasing HIC activity to negligible levels during each of the withdrawal cycles (Ps< 0.001).
Mean ± SEM. (A) HIC score for the different PGB treatment groups as a function of time following 16 h ethanol exposure during each of three successive exposure/withdrawal cycles; (B) area under the 7 h HIC curve. #Significantly differs from vehicle condition during WD cycle 1 (P < 0.02);  significantly differs from vehicle condition during WD cycles 1 and 2 (P < 0.01); **significantly differs from respective vehicle condition (P < 0.001).
significantly differs from vehicle condition during WD cycles 1 and 2 (P < 0.01); **significantly differs from respective vehicle condition (P < 0.001).
DISCUSSION
Results from these experiments indicate that pregabalin may be effective in the treatment of alcohol withdrawal and, in particular, against withdrawal-related seizure activity. Pregabalin was found to reduce HIC activity in a dose-related manner during the acute phase of withdrawal (first 10 h), with no evidence of a rebound-like effect during the later (protracted) period of withdrawal (24–72 h). Both the 100 and 200 mg/kg doses of pregabalin were equally effective in reducing electrographic measures of seizure activity (Experiment 2) and blocking sensitization (kindling) of behavioral convulsions that typically develop over repeated cycles of withdrawal (Experiment 3). These results are similar to those obtained with the related anticonvulsant gabapentin (Veatch and Becker, 2000; Veatch et al., 2001), as well as the benzodiazepine lorazepam (Becker and Veatch, 2002). Pilot (HCB and LMV, unpublished data) work by our group has suggested that pregabalin is more potent and exerts its anticonvulsant effects against ethanol withdrawal seizures for a longer period of time in comparison to gabapentin. This is in agreement with pre-clinical studies comparing the two compounds in a variety of seizure models (Ben-Menachem, 2004). The doses of pregabalin shown to effectively reduce behavioral seizures during alcohol withdrawal in the present study (50–200 mg/kg) are higher than those reported to antagonize maximal electroshock convulsions, but within the same range of doses that effectively antagonize pentylenetetrazole-induced seizures in mice (Vartanian et al., 2006). These doses are also below the range of parenteral doses shown to produce ataxia in mice (Vartanian et al., 2006). Clearly, additional studies are needed to more fully characterize dose–effect functions for pregabalin against alcohol withdrawal seizures, as well as other withdrawal-related behaviors (e.g. motor effects, anxiety).
As previously reported (Veatch and Becker, 2002), spontaneous EEG activity recorded in freely moving mice was increasingly interrupted by repetitive, high-voltage spike-and-wave activity during the course of ethanol withdrawal. This electrographic activity was most prominent in cortex, to a lesser extent in amygdala, and minimal in hippocampus. It is noteworthy that pregabalin's anticonvulsant effects were apparently longer lasting against electrographic in comparison to behavioral indices of ethanol withdrawal (compare pregabalin effects in Figs 1A and 2). Further, the withdrawal-associated BSE activity was accompanied by a concomitant cessation of on-going behavior. While this paroxysmal EEG activity and associated behavioral profile resembles generalized absence seizures (Snead, 1995), the protective effect of pregabalin against abnormal EEG (BSE) activity during withdrawal in the present study is somewhat surprising in that the drug has been reported to be ineffective against absence seizures (Taylor and Vartanian, 1997). However, non-convulsive seizures may occur under a variety of conditions including focal ischemia, where similar spike-and-wave activity occurs in the absence of overt clinical manifestations (Hartings et al., 2003). In this vein, it is interesting that the related anticonvulsant gabapentin was found to be effective in reducing non-convulsive seizure activity induced by focal brain ischemia (Bryans and Wustrow, 1999; Williams et al., 2004). Whether the abnormal withdrawal-related EEG (BSE) activity in the present study reflects such an effect remains to be determined.
The mechanism by which pregabalin exerts its anticonvulsant effects against ethanol withdrawal-related seizure activity is presumably through its interaction with the alpha-2-delta subunit on VGCCs (Ben-Menachem, 2004; Bialer et al., 2004). CNS hyperexcitability associated with alcohol withdrawal is thought to reflect the manifestations of an imbalance in excitatory and inhibitory activity that results from adaptive changes following chronic exposure to the drug. This includes blunted GABA-mediated inhibitory transmission along with an upregulation in glutamate-mediated excitatory transmission (Becker, 2000). An increase in L-type VGCC activity has been noted as a contributory factor as well (Littleton et al., 1990; Guppy et al., 1995; Veatch and Gonzalez, 1997; Watson and Little, 1997). The effects of chronic ethanol exposure and withdrawal on other VGCCs (N and P/Q-type) have not been fully explored. Results from the present study suggest that the alpha-2-delta subunit of these VGCCs may represent a novel target for pharmacotherapeutics in treatment of alcohol withdrawal.
Pregabalin has several novel characteristics suggesting that it may be a compound worth screening in humans as a treatment for alcohol withdrawal. The fact that pregabalin does not bind to plasma proteins and is excreted unchanged in the urine is of major clinical importance. This lack of hepatic metabolism is important given the high prevalence of hepatic disease associated with chronic alcohol use. The lack of hepatic metabolism would also make it a preferential agent to use in the detoxification of patients receiving medications for other indications in order to avoid drug-to-drug interactions.
In addition to pregabalin's potential for use in the treatment of alcohol withdrawal, the agent may have other utility in alcoholism treatment. While alcohol withdrawal is typically thought to encompass the first 2–5 days after alcohol cessation, a more protracted phase may last for weeks to months after the acute symptoms of withdrawal have abated (Anton and Becker, 1995). During this protracted phase, persistent signs of sleep dysregulation, irritability, and mood instability can be experienced (Gillin et al., 1990; Brower et al., 1998). Evidence from both pre-clinical and clinical trials suggesting anxiolytic effects of pregabalin is important given the anxiety-related symptoms associated with not only acute alcohol withdrawal, but also the protracted phase. Pregabalin has been reported to be a novel sleep modulator with the ability to induce restorative sleep (Kubota et al., 2001), which suggests it may also be able to aid with the sleep dysfunction experienced during and after acute alcohol withdrawal. Further, improvement in anxiety and sleep as a consequence of anticonvulsant treatment for alcohol withdrawal has been found to impact relapse to alcohol consumption (Malcolm et al., 2001). As such, clinical trials utilizing pregabalin in the treatment of acute withdrawal as well as protracted withdrawal appear warranted.
Supported by the National Institute on Alcohol Abuse and Alcoholism (Grant P50-AA10761) and Office of Research and Development, Department of Veterans Affairs. The authors wish to acknowledge Laura Ralston for her technical assistance.
REFERENCES
Andre, V., Rigoulot, M. A., Koning, E. et al. (
Anton, R. F. and Becker, H. C. (
Arroyo, S., Anhut, H., Kugler, A. R. et al. (
Ballenger, J. C. and Post, R. M. (
Becker, H. C. (
Becker, H. C. (
Becker, H. C. and Hale, R. L. (
Becker, H. C. and Littleton, J. M. (
Becker, H. C. and Veatch, L. M. (
Becker, H. C., Diaz-Granados, J. L. and Hale, R. L. (
Becker, H. C., Diaz-Granados, J. L. and Weathersby, R. T. (
Ben-Menachem, E. (
Bialer, M., Johannessen, S. I., Kupferberg, H. J. et al. (
Brodie, M. J. (
Brower, K. J., Aldrich, M. S. and Hall, J. M. (
Bryans, J. S. and Wustrow, D. J. (
Chesler, E. J., Ritchie, J., Kokayeff, A. et al. (
Crabbe, J. and Kosobud, A. (
Cunningham, M. O., Woodhall, G. L., Thompson, S. E. et al. (
Dooley, D. J., Donovan, C. M., Meder, W. P. et al. (
Dworkin, R. H., Corbin, A. E., Young, J. P. et al. (
Ellis, R. and Gulick, D. (
Fehrenbacher, J. C., Taylor, C. P. and Vasko, M. R. (
Feltner, D. E., Crockatt, J. G., Dubovsky, S. J. et al. (
Field, M. J., Oles, R. J. and Singh, L. (
Fink, K., Dooley, D. J., Meder, W. P. et al. (
Franklin, K. B. J. and Paxinos, G. (
French, J. A., Kugler, A. R., Robbins, J. L. et al. (
Gillin, J. C., Smith, T. L., Irwin, M. et al. (
Griffiths, R. R. (
Griffiths, R. R. and Sannerud, C. A. (
Groseclose, C. H., Draughn, R. A., Tyor, W. R. et al. (
Guppy, L. J., Crabbe, J. C. and Littleton, J. M. (
Hartings, J. A., Williams, A. J. and Tortella, F. C. (
Kubota, T., Fang, J., Meltzer, L. T. et al. (
Littleton, J. M., Little, H. J. and Whittington, M. A. (
Malcolm, R., Roberts, J. S., Wang, W. et al. (
Malcolm, R., Myrick, H., Brady, K. T. et al. (
Myrick, H. and Anton, R. F. (
Myrick, H., Brady, K. T. and Malcolm, R. (
Myrick, H., Malcolm, R. and Anton, R. F. (
Pande, A. C., Crockatt, J. G., Feltner, D. E. et al. (
Pande, A. C., Feltner, D. E., Jefferson, J. W. et al. (
Rosenstock, J., Tuchman, M., LaMoreaux, L. et al. (
Sabatowski, R., Galvez, R., Cherry, D. A. et al. (
Snead, O. C. (
Stahl, S. M. (
Taylor, C. P. and Vartanian, M. G. (
Taylor, C. P., Vartanian, M. G., Andruszkiewicz, R. et al. (
Vartanian, M. G., Radulovic, L. L., Kinsora, J. J. et al. (
Veatch, L. M. and Becker, H. C. (
Veatch, L. M. and Becker, H. C. (
Veatch, L. M. and Gonzalez, L. P. (
Veatch, L. M., Myrick, D. L. and Becker, H. C. (
Watson, W. P. and Little, J. J. (
Author notes
1Charleston Alcohol Research Center, Center for Drug and Alcohol Programs, Institute of Psychiatry, Charleston, SC, USA
2Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA
3Department of Veterans Affairs, Ralph H. Johnson VA Medical Center, Charleston, South Carolina, USA