-
PDF
- Split View
-
Views
-
Cite
Cite
Juan M. Losada, María Herrero, Arabinogalactan-protein secretion is associated with the acquisition of stigmatic receptivity in the apple flower, Annals of Botany, Volume 110, Issue 3, 1 August 2012, Pages 573–584, https://doi.org/10.1093/aob/mcs116
Close - Share Icon Share
Stigmatic receptivity plays a clear role in pollination dynamics; however, little is known about the factors that confer to a stigma the competence to be receptive for the germination of pollen grains. In this work, a developmental approach is used to evaluate the acquisition of stigmatic receptivity and its relationship with a possible change in arabinogalactan-proteins (AGPs).
Flowers of the domestic apple, Malus × domestica, were assessed for their capacity to support pollen germination at different developmental stages. Stigmas from these same stages were characterized morphologically and different AGP epitopes detected by immunocytochemistry.
Acquisition of stigmatic receptivity and the secretion of classical AGPs from stigmatic cells occurred concurrently and following the same spatial distribution. While in unpollinated stigmas AGPs appeared unaltered, in cross-pollinated stigmas AGPs epitopes vanished as pollen tubes passed by.
The concurrent secretion of AGPs with the acquisition of stigmatic receptivity, together with the differential response in unpollinated and cross-pollinated pistils point out a role of AGPs in supporting pollen tube germination and strongly suggest that secretion of AGPs is associated with the acquisition of stigma receptivity.
INTRODUCTION
Flower receptivity plays a crucial part in pollination dynamics, reproductive success and plant productivity. The stigma is the first surface of the pistil to make contact with the pollen grains, and pollen grain adhesion on the stigma is followed by pollen hydration and germination (Lord and Russell, 2002; Hiscock and Allen, 2008). Stigmatic receptivity is the temporal interval in which the stigma provides an adequate environment for pollen germination (González et al. 1995a; Sanzol et al., 2003), and this parameter has significant biological implications. In dichogamous species it has a clear role separating the female and male phase and encouraging out-breeding (Lora et al., 2011). Also, in hermaphrodite flowers, a late acquisition of stigmatic receptivity favours pollen gathering in the stigma prior to pollen germination, and encourages pollen competition (Hormaza and Herrero, 1992). Finally, stigmatic receptivity is susceptible to temperature fluctuations (Hedhly et al., 2003, 2005; Lora et al., 2011), and is a clear candidate vulnerable to climate change (Hedhly et al., 2009; Hedhly, 2011), since a short period of stigmatic receptivity may hinder crop production (Sanzol et al., 2003). But in spite of the widespread biological implications of stigmatic receptivity, very little is known about what confers on a stigma the capability to be receptive for pollen grain germination.
Stigmatic receptivity is evaluated in an indirect way by pollen-germination ability, the capacity to promote fertilization, or some enzymatic activities (Edlund et al., 2004). Attention has been focused on the nature of stigmatic secretions and their genetic control (Verhoeven et al., 2005), and an interaction between stigmatic secretions and pollen tubes has been shown (Wolters-Arts et al., 1998; Inamura et al., 2007), opening a search for the components of the stigmatic secretion that may play a role in stigmatic receptivity. At maturity stigmas have high levels of esterase (Hiscock et al., 1994, 2002) and also peroxidase activity (Dupuis and Dumas, 1990; McInnis et al., 2006b).
The association of peroxidase activity with stigmatic receptivity prompted an investigation of the activity of reactive oxygen species and reactive nitrogen species in stigmas and pollen, which revealed that receptive stigmas accumulate high levels of reactive oxygen species, particularly hydrogen peroxide, suggesting a possible role in stigma receptivity (McInnis et al., 2006 a, b; Hiscock and Allen, 2008). Some glycoproteins such as arabinogalactan-proteins (AGPs) (Quiapim et al., 2009) are also potential candidates for markers of stigma receptivity, pointing to a co-ordinated cell response on the stigma; however, evidence of their biological roles remains elusive.
AGPs are a superfamily of highly glycosylated hydroxyproline-rich glycoproteins which are constituents of cell walls of many flowering plants in different tissues of every taxonomic group studied (Clarke et al., 1979; Seifert and Roberts, 2007), from some early angiosperms (Prychid et al., 2011) to eudicots (Vaughn et al., 2007). They are classified into two main groups (classical or non-classical AGPs), depending on the presence or absence of a hydrophobic glycosylphosphatidilinositol-lipid membrane anchor at the C terminus. This glycosylphosphatidilinositol anchor makes AGPs capable of being secretory molecules involved in signalling (Ellis et al., 2010). While the Hyp-rich protein backbone is fairly well conserved, the complex carbohydrate moiety that occupies 90 % of the molecule varies between AGPs types and determines their biological function (Ellis et al., 2010). AGPs participate in several physiological processes in plant growth and development of both monocots (Ma and Zhao, 2010) and dicots (Majewska-Sawka and Nothnagel, 2000; Showalter, 2001; Seifert and Roberts, 2007), as cell expansion and proliferation (Yang et al., 2007), programmed cell death and xylem differentiation (Zhang et al., 2003; Motose et al., 2004), or somatic embryogenesis (van Hengel et al., 2002).
In reproductive tissues, the expression of AGPs is associated with the sporophyte–gametophyte transition (Acosta-García and Vielle-Calzada, 2004), or components of pollen-tube wall elongation (Wu et al., 2001; Pereira et al., 2006; Coimbra et al., 2010). Several AGP-like molecules have also been reported along different pistil structures of lily (Kim et al., 2003), tobacco (Wu et al., 2000; Hancock et al., 2005), and arabidopsis (Lee et al., 2009; Coimbra et al., 2010). Their abundance and generalized presence along the pistil makes AGPs clear candidates for pollen–pistil interactions, but their possible function remains to be deciphered. The difficulty in unveiling the role of AGPs is partly due to the fact that it is difficult to find a system where an on/off function can be found. In this sense, the stigma that becomes receptive at a particular stage of development offers the opportunity to look for the possible implications of AGPs on pollen germination.
This work investigates the possible role of AGPs on the acquisition of stigmatic receptivity in the flowers of apple (Malus × domestica). There has been extensive research on agronomic properties of apple, but there is relatively little information on the reproductive process. The anatomy of the pistil (Cresti et al., 1980) and gynoecial structure (Sheffield et al., 2005) have been examined. Some aspects referring to flower receptivity and fruit set have further been considered (Williams et al., 1984; Buszard and Schwabe, 1995; Sheffield et al., 2005). Apple flowers have a wet stigma (Heslop-Harrison and Shivanna, 1977), but the nature of secretions contributing to the acquisition of stigmatic receptivity is lacking. To gain insight into the key steps of stigmatic receptivity competency, in this work we combined a developmental approach to evaluate the functional capacity of the stigmas, with detailed anatomical observations and the cytoimmunodetection of AGP epitopes.
MATERIALS AND METHODS
Plant materials
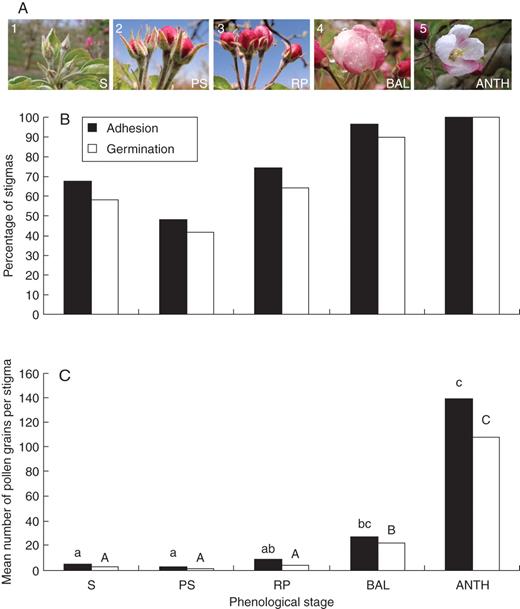
Evaluation of stigmatic receptivity in different apple-flower phenological stages. (A) Flower phenology: (1) petals enclosed by sepals (S); (2) petals show between sepals (PS); (3) petals protruding and showing red colour – red-petal stage (RP); (4) balloon stage (BAL) where petals are pink; and (5) anthesis (ANTH). (B) Percentage of stigmas supporting at least one pollen grain adhered and germinated (as indicated) in each phenological stage. (C) Mean number of adhered and germinated pollen grains per stigma on each phenological stage, showing that only stages close to flower opening reached a high number of adhered and germinated pollen grains. Letters over the columns show differences at a P ≤ 0·05 on either adhesion (lower-case letters) or germination (upper-case letters).
Since apples are self-incompatible, anthers from the compatible ‘Royal Gala’ were collected from flowers at an advanced balloon stage and left to dry on a paper at room temperature around 20 °C for 24–48 h until dehiscence. Pollen was sieved with a mesh with a diameter pore of 0·26 mm and then stored at –20 °C until required.
Pollination experiments
Stigmatic receptivity was evaluated through the capacity of stigmas to offer support for pollen germination and pollen tube growth (González et al., 1995b). Following hand pollinations with a paintbrush, six flowers – 30 gynoecia – from each phenological stage were sampled 1 d after pollination and fixed in FAA (formalin : acetic acid : 70 % ethanol) (1 : 1 : 18) (Johansen, 1940). Once fixed, the pistils were washed three times in distilled water for 1 h each, and left in 5 % sodium sulfite for 24 h. Samples were autoclaved for 10 min at 1 kg cm−2 in 5 % sodium sulfite (Jefferies and Belcher, 1974), and then the individual styles were squashed onto glass slides with 0·1 % aniline blue in 0·1 n K3PO4 to visualize callose (Currier, 1957) and pollen tubes (Linskens and Esser, 1957) with a LEICA DM2500 fluorescence microscope with 340/400-nm filter. The percentage of stigmas bearing either adhered or germinated pollen grains was subjected to a chi-square homogeneity test at P ≤ 0·05. Then the mean number of adhered and germinated pollen grain/tubes on the stigma was compared by one-way ANOVA, and groups separated by the Duncan multiple-range test at P ≤ 0·05. Statistical analysis was performed with the SPSS software (SPSS Inc., Chicago, IL, USA).
Histochemical preparations
Flowers for histochemical examination were selected according to the stigmatic receptivity results. Pistils from three phenological stages (3, 4 and 5) were fixed in 2·5 % glutaraldehyde in 0·03 m saline phosphate buffer pH 7·3 for 4 h (Sabatini et al., 1963). Then the gynoecia were washed in 0·03 m saline phosphate buffer and sequentially dehydrated in an ethanol series (30 %, 50 %, 70 %, and 96 %) leaving them for 1 h in each ethanol concentration. The pistils were left for 5 d in the embedding solution at 4 °C, and then embedded in JB4 plastic resin (0226A; Polysciences Inc.). Sections, 2 µm thick, were cut on a LEICA EM UC6 ultramicrotome with a glass knife and then placed onto distilled water on a glass slide previously coated with 1 % gelatine.
For general structure and presence of polyphenols, 0·02 % toluidine blue in water was used (Feder and O'Brien, 1968). Insoluble polysaccharides were stained with periodic acid Shiff reagent (Feder and O'Brien, 1968). Cellulose was stained with 0·07 % calcofluor white (Hughes and McCully, 1975) and cutin and lipids with 0·01 % auramine in 0·05 m phosphate buffer, pH 7·8 (Heslop-Harrison, 1977). General structure and secretions were observed following staining with 0·01 % acridine orange in 0·03 % phosphate buffer, pH 7·4 (Nicholas et al., 1987). Slides were observed under bright field LEICA DM2500 microscope carrying a 100-W light source, and photographs were obtained with a Leica DFC320 camera linked to the software Leica Application Suite. Fluorescence microscopy was developed with the same device provided with an epifluorescence source and connected to a CANON Power Shot S50 camera linked to the CANON Remote Capture software. Filters used were 355/455 nm for calcofluor white, and 450/510 nm for auramine- or acridine orange-stained sections.
In situ detection of AGPs
To detect the presence of AGPs in fresh tissue, Yariv reagents were used, both β-d-glucosyl Yariv reagent (β-GlcYR), which specifically binds to and precipitates AGPs, giving a red to brown colour, and α-d-galactosyl Yariv reagent (α-GalYR) as a negative control (Yariv et al., 1967; Anderson et al., 1977; Capataz-Tafur et al., 2010). Following the manufacturer's instructions, 2 mg of Yariv powder reactive (Biosupplies, Australia) were dissolved in 1 mL of 0·15 m NaCl. After that, aliquots of 20 µL of the stock solution were dissolved in 500 µL of distilled water. While this reaction has been previously used in a biochemical context, here the presence of AGPs on the stigmatic surface was evaluated in fresh tissue. For this purpose, batches of five flowers – 25 stigmas – per phenological stage were emasculated, and the stigmas were placed in contact with the solutions for 5 h at room temperature. The presence of AGPs was detected through the presence of a red to brown colouration on the stigma, which was observed under a binocular Leica MZ16 with a light source Leica KL1500LCD. Photographs were taken with the bright-field system mentioned above. Reaction for both stains was quantified, scoring the percentage of stigmas showing colouration on the stigma surface.
Immunolocalization of AGPs
Two monoclonal antibodies (mAbs), Jim13 (Knox et al., 1991) and Jim8 (Pennell et al., 1991), against AGPs glycosyl moeties were obtained from Carbosource Services (University of Georgia, USA). As a secondary antibody, Alexa 488 fluorescein isothiocyanate (FITC)-conjugated anti-rat IgG was used (F-1763; Sigma).
Gynoecia from flowers of developmental stages balloon (stage 4), anthesis (stage 5), 1 d after anthesis (1DAA), 1 d after pollination (1DAP) and 3 d after pollination (3DAP) were fixed in 4 % paraformaldehyde in phosphate-buffered saline (PBS), pH 7·3, and left overnight at 4 °C. Samples were dehydrated in an acetone series (30 %, 50 %, 70 %, 90 %, 100 %) and embedded in Technovit 8100 (Kulzerand Co., Germany) for 2 d. The resin was polymerized at 4 °C, and slices sectioned at 4 µm width. Sections were placed in a drop of water on a slide covered with 2 % (3-aminopropyl) triethoxysilane (Sigma-Aldrich) and dried at room temperature.
Sectioned samples were soaked in PBS, pH 7·3, for 5 min followed by 5 % bovine serum albumin in PBS for 5 min. Then, they were incubated at room temperature for 1 h with both primary mAbs, Jim8 and Jim13, followed by three washes in PBS. After that, samples were incubated with a 1/25 dilution of the secondary anti-rat antibody conjugated with FITC in 1 % bovine serum albumin in PBS for 45 min in the dark, followed by three washes in PBS (Solís et al., 2008). Controls were performed for each sample, by incubating with the blocking solution instead of the primary antibody. For better localization of the epitopes, some sections were counterstained with calcofluor white for cellulose (Coimbra et al., 2007), mounted in PBS or Mowiol and examined under a LEICA DM2500 epifluorescence microscope connected to a LEICA DFC320 camera. Filters were 355/455 nm for calcofluor white and 470/525 nm for the Alexa488 fluorescein label of the antibodies. Overlapping photographs were obtained with the Leica Application Suite software.
RESULTS
Acquisition of stigmatic receptivity
Five phenological stages where chosen from the sepals stage through to anthesis (Fig. 1A): (1) petals enclosed by sepals (S); (2) petals show between sepals (PS); (3) petals protruding and showing red colour – red-petal stage (RP); (4) balloon stage (BAL) where petals are pink; and (5) anthesis (ANTH). The style was fully formed at stage 1; however, the stylar length varied from 6 mm at this stage to 11 mm at stage 5. The different phenological stages were hand pollinated and evaluated for their ability to adhere and germinate pollen grains. Whereas in early stages (1 and 2) a proportion of the stigmas were able to support adhesion and germination of at least one pollen grain (Fig. 1B), no significant differences were recorded between them, and only advanced stages (4 and 5) showed significant differences with earlier stages 1 and 2 (P ≤ 0·05). But the number of pollen grains adhering and germinating gave a more precise picture and a better estimate of stigmatic receptivity. The number of pollen grains adhering and germinating per stigma increased at later developmental stages (Fig. 1C). Few pollen grains (under ten) were able to germinate in the early developmental stages (1–3) compared with 40 pollen grains per stigma at stage 4, and 100 at stage 5. Significant differences were recorded in the number of adhered and germinated pollen grains between early (1–3) and later (4 and 5) stages (P ≤ 0·05). At stage 4, germinating pollen grains were localized mainly to the outermost edges, marking the first receptive area in the stigma. By stage 5, they included the whole stigma. Therefore, the acquisition of stigmatic receptivity evaluated by pollen behaviour began at balloon stage (4), but a spatial distribution was observed and the stigmas started to be receptive at the stigmatic edges, progressing then centripetally to the inner stigma.
Developmental changes in the stigma
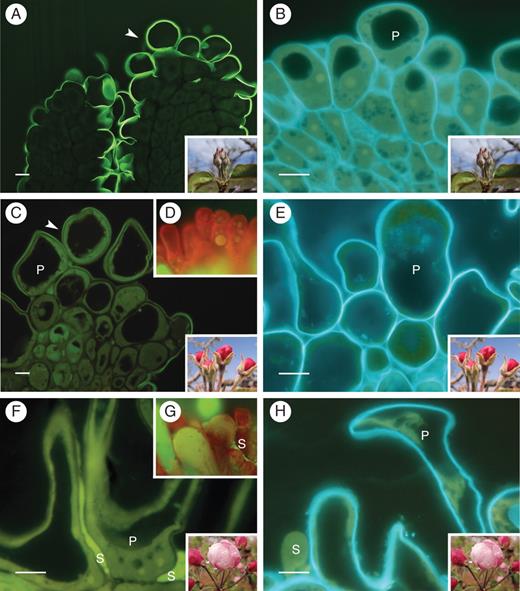
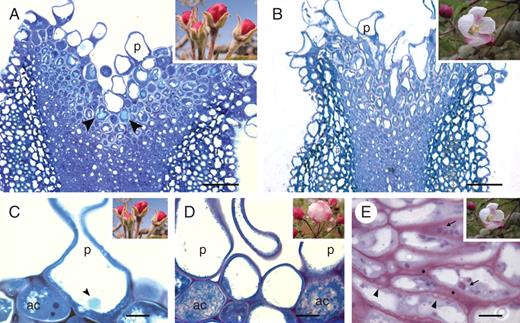
Papillae development in the apple flower stigma: (A) thick cuticle layer (arrowhead) covering the stigma and suture line along the short style at stage 1; (B) undifferentiated stigma at this stage showing papillae with small vacuoles; (C) stigma with a continuous cuticle layer covering papillae at stage 3; and (D) whole mounts without secretion; (E) turgid papillae after the vacuoles enlarged at stage 3; (F) lipidic secretions from stigmatoid tissue cells merged between papillae at stage 4; (G) stigma whole mount with secretions at this stage; (H) papillae loose turgidity at stage 4. (A, C, F) Auramine O staining. (B, E, H) calcofluor white for cellulose; 2-μm sections embedded in JB4 resin; (D, G) acridine orange of squashed preparations. Abbreviations: p, papilla; s, secretions. Scale bars = 10 µm.
Changes in the stigmatoid tissue with development in the apple flower stigma: (A) general view of the stigma at stage 3 showing a vacuolar reaction for polyphenolic compounds (arrowheads) in the cells underneath papillae; (B) at stage 5, stigmatoid cells without phenolic compounds, as the intercellular substance appeared in the stigmatoid tissue; (C) phenolic bodies were conspicuous within papillar vacuoles (arrowhead) at stage 3 (autolytic cell at the interpapillar valley with a dense cytoplasm); (D) autolytic cell degeneration at stage 4; (E) at stage 5, stigmatoid cells contained starch (arrows) and wall ingrowths (arrowheads), as the intercellular substance (*) stained for carbohydrates. (A–D) Toluidine blue staining for general structure showing metachromasia. (E) periodic acid Shiff reagent staining for insoluble carbohydrates. 2-μm sections embedded in JB4 resin. Abbreviations: ac, autolythic cells; p, papilla. Scale bars: (A, B) = 50 µm; (C–E) = 10 µm.
AGPs in the stigma
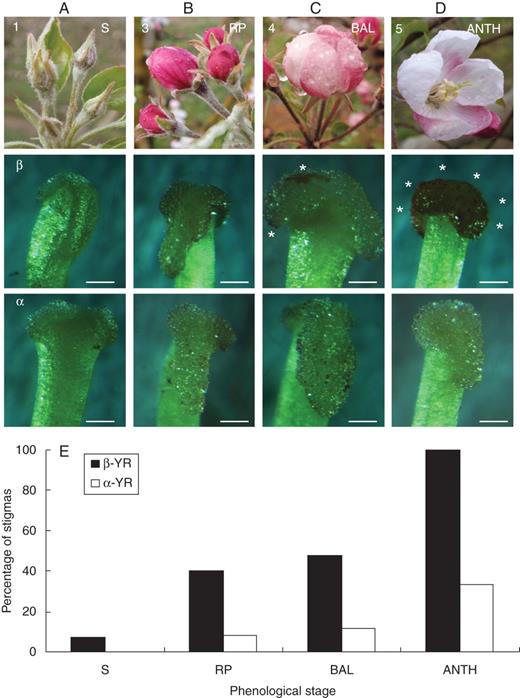
In situ detection of AGPs on whole mounts of the apple flower stigma. Each column corresponds to a flower phenological stage. The first row shows the phenological stages, the second row shows reactions to positive β-glucosyl-Yariv reagent (β-GlcYR) indicating the presence of AGPs, and the third row shows the negative control using α-d-galactosyl-Yariv reagent (α-GalYR). While no reaction appeared on the stigmatic surfaces at the early stage 1 (A), some scattered points stained at stage 3 (B). The colour spreads first to the stigmatic edges at stage 4 (C), but a maximum intensity was clear all over the stigma at stage 5 (D, asterisks). (E) Percentage of stigmas with reaction to β-GlcYR, with almost 100 % of the mature stigmas reacting at anthesis, and the negative control α-GalYR. Scale bars = 0·25 mm.
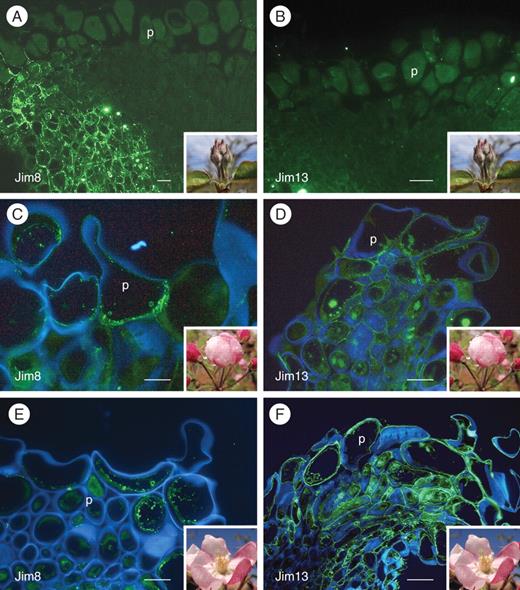
Changes in AGPs on the apple flower stigma in different phenological stages: stage 1 (A, B), stage 4 (C, D) and stage 5 (E, F). The left column of photographs shows epitopes labelled with Jim8 mAb FITC-conjugated mAb signal in fluorescent green. The right column shows the same staining but labelled with Jim13 mAb. At stage 1, neither Jim8 (A) nor Jim13 mAbs (B) showed labelling on the papillae, only in the stylar cortical tissue. (C) At stage 4, Jim8 epitopes appeared in the papillae cytoplasm. (D) In contrast, Jim13 labelled the stigmatic edges in the stigmatoid tissue, and the intercellular substance. (E) At stage 5, Jim8 epitopes still labelled inside the papillae. (F) Conspicuous signal of Jim13 epitopes in the intercellular substance mainly at the stigmatic edges. (A, B) FITC signal; (C–F) FITC signal counterstained with calcofluor white for cellulose (blue); 4-μm sections embedded in Technovit8100. Abbreviation: p, papilla. Scale bars: (A, B) = 10 µm; (C–F) = 50 µm).
Changes in AGPs with pollination
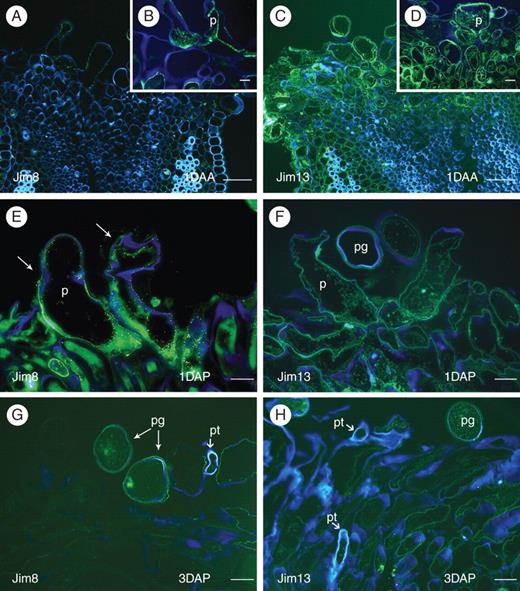
Changes in apple flower stigmatic AGPs with pollination. Unpollinated stigmas (A–D) 1 d after anthesis (1DAA), and pollinated stigmas (E, F) 1 d after pollination (1DAP) and (G, H) 3 d after pollination (3DAP). (A) Unpollinated stigmas 1DAA showing Jim8 epitopes in the stigmatic papillae. (B) Detail of epitopes inside of a papilla. (C) Jim13 epitopes secreted to the external cell walls in the stigmatoid tissue of unpollinated stigmas, and also (D) on the papillae. (E) Jim8 epitopes 1DAP showed now a reaction outside the papillae cell walls (arrows). (F) Jim13 labelled epitopes decreased 1DAP. (G) Jim8 epitopes recognized only in pollen grains and papillae walls contacting them 3 DAP. (H) Jim13 labelling decreased 3DAP, especially at places of pollen tube passage. FITC fluorescent green counterstained with calcofluor white – in blue; 4-μm sections embedded in Technovit 8100. Abbreviations: p, papilla; pg, pollen grain; pt, pollen tube. Scale bars: A, C = 50 µm; B, D–H = 10 µm.
Conspicuous changes occurred 1 d following pollination (1DAP). The epitope detected with Jim8 changed and appeared now outside of the papillae (Fig. 6E). In contrast, the epitope detected with Jim13 at this stage (Fig. 6F) showed a weaker reaction at the intercellular spaces and labelling decreased compared with that observed in unpollinated stigmas at the same stage (Fig. 6C, D).
After the passage of the pollen tube, when examining stigmas 3 d after pollination (3DAP), a reaction could be seen on the pollen grain wall and cytoplasm for the Jim8 mAb. But on the stigma, the staining just remained on cell surfaces contacting pollen grains and tubes (Fig. 6G). At this stage the Jim13 label was also much weaker in the intercellular substance of the stigmatoid tissue (Fig. 6H), and appeared to be absent close to the passage of the pollen tube.
DISCUSSION
The results described show that the acquisition of stigmatic receptivity in apple flowers was concomitant with the secretion of AGPs, which further vanished with pollination as pollen tubes grew through the stigmatoid tissue.
The onset of stigma receptivity: cell differentiation and secretions
Apple stigmas are of the wet type (Heslop-Harrison and Shivanna, 1977), and acquired the capability to support pollen germination close to flower opening, being fully receptive at anthesis. Stigmatic receptivity was acquired in a developmentally controlled way, maturing in a basipetal fashion as in other species (Herrero and Arbeloa, 1989), but our results also show a centripetal developmental sequence from the stigmatic edges to the groove of the suture line. Previous reports in species with wet stigmas have shown a fairly well-conserved structure at anthesis with papillae covered with secretion and a stigmatoid tissue beneath (Konar and Linskens, 1966; Ciampolini et al., 1990; Heslop-Harrison, 2000). This work shows that three types of cells developed and matured in an orchestrated manner: the stigmatic papillae, cells of the stigmatoid tissue, and some autolythic cells, not previously reported, located at the stigmatoid tissue underneath the interpapillar valleys. These three cell types all contributed to the production of stigmatic secretion. At maturity, as papillae loose turgidity, autolysis of interpapillar stigmatoid cells and secretion by stigmatoid cells resulted in a copious secretion located both on the stigma surface and also in the stigmatoid intercellular spaces. The contribution of different types of cells to a common stigmatic secretion may be in line with the different functions of a stigma, from pollen recognition (Dickinson, 2000) and support for pollen germination (Herrero and Dickinson, 1979), to xenobiotic resistance (Heslop-Harrison, 2000; McInnis et al., 2006a).
Also in time with these concurrent functions that occur in the small stigma is the varied and rich composition of the stigmatic secretion. Thus stigmatic secretion was rich in polysaccharides and lipids, similar to other wet stigmas (Cresti et al., 1980, 1986). In other species, lipids appear to play a role in pollen-grain hydration and pollen-tube orientation on the stigma (Wolters-Arts et al., 1998). Sugars have been reported to provide an adequate pollen germination medium (Herrero and Dickinson, 1979). Apple stigmatoid tissue cells also secreted phenolic compounds into the intercellular space, and lipids onto the surface. In some species, secretions of phenolics have been considered as peroxidase substrates (Šukalović et al., 2010), which may act as indicators of stigma receptivity (Shivanna and Sastri, 1981; McInnis et al., 2006 a, b; Hiscock and Allen, 2008). Phenolic accumulation has also been associated with senescence (Beckam, 2000), and also reported during maturation of floral organs (Wu and Cheung, 2000; Rogers, 2006). In the apple stigma, lipophenolic cell release occurred at a time close to the end of the secretory process, suggesting that they could also be defensive molecules (González et al., 1995a), just as the presence of reactive oxygen species and reactive nitrogen species (McInnis et al., 2006a, Hiscock and Allen, 2008), which have been shown to clearly contribute to stigmatic receptivity, could be involved in a prophylactic role (Heslop-Harrison, 2000). Clearly, the rich display of different activities that occur in a receptive stigma support the fact that stigmatic receptivity depends on multiple factors. However, from an interactive perspective, the specific and tuned secretion of AGPs point to them as good candidates playing also a part in the acquisition of stigmatic receptivity.
AGPs were secreted concurrently with the acquisition of stigmatic receptivity
Stigmatic receptivity was acquired concomitantly with the secretion of AGPs. In addition to displaying the same developmental timing, AGP secretion followed the same centripetal and basipetal spatial development pattern. While the use of Yariv reagent to precipitate and quantify AGPs in other systems has been put forward (Baron-Maurer et al., 2010), results in this work show the appearance of AGPs on the stigmatic surface in fresh tissues by a colour reaction. These results are consistent with a previous biochemical approach using Yariv reagent in Nicotiana stigmatic extracts (Gell et al., 1986). Our results in apple suggest that this simple procedure could be used to evaluate the acquisition of stigmatic receptivity in additional species, but it requires further evaluation.
The immunocytochemical approach with Jim8 and Jim13 mAbs was consistent with the Yariv biochemical reaction, and confirmed the presence of AGPs on the apple stigma. AGPs have been previously reported in reproductive tissues (Juah and Lord, 1995; Coimbra et al., 2007), but they are also ubiquitous in plant tissues derived from the epidermis (Vaughn et al., 2007; Qin and Zhao, 2007). While the possible function of AGPs has not been previously considered in a developmental context, their immunolocalization followed the same developmental pattern as the maturing stigma. Furthermore, secretion of AGPs was co-ordinated with the initiation of stigmatic receptivity. A differential expression pattern of proteins recognized by Jim8 and Jim13 mAbs was observed during stigma development. While AGPs recognized by Jim8 appeared to be constitutive of the papillae cell wall at vacuolation developmental stages, those recognized by Jim13 were secreted at the stigmatoid tissue concomitantly with tissue maturation, suggesting an additive role on the acquisition of stigmatic receptivity. The epitopes recognized by both mAbs were located in the glycan side of the glycoproteins, and they represent post-translational modifications of the glycoproteins within the cells (Estévez et al., 2006). This work shows, for the first time, a different location of developmental tissue for both mAbs that recognize different AGPs. The ubiquitous nature of AGPs combined with their glycan heterogeneity questions their rapid functional adaptability in plant tissues and/or cell types (Ellis et al., 2010), but current results suggest that small changes may have been recruited to fulfil different functions leading to the functional plasticity of these glycoproteins.
Changes in AGPs following pollination
The different nature and function of the different epitopes recognized by Jim8 and Jim13 mAbs was further apparent following pollination. Pollination elicited the secretion of Jim8 epitopes to the papillar cell-wall surface, and their presence was stronger at contact points with pollen tubes, suggesting a possible adhesion cue. The involvement of proteins influencing adhesion in wet stigmas has been previously documented in lily, with a hollow style (Park et al., 2000), as well as in the arabidopsis dry stigma (Dong et al., 2005); of special interest are small cystein-rich proteins that influence pollen-tube behaviour (Kim et al., 2003). Our observations in this work showed an induced expression of Jim8 epitopes by pollen arrival on the papillae, suggesting a potential involvement in an early male–female physical contact on the papillar surface.
A different pattern was followed by Jim13-recognized epitopes, which were abundant in the intercellular substance of unpollinated stigmas, and disappeared as pollen tubes passed by, suggesting a pollen tube–AGP interaction within the stigmatoid tissue in apple. The possible relationship between small, secreted proteins and pollen-tube performance in the pistil has been suggested (Lee et al., 2009; Chae et al., 2010; Higashiyama, 2010; Chae and Lord, 2011). AGPs in the stylar transmitting tissue follow a deglycosylation gradient in the style (de Graaf et al., 2003; Hancock et al., 2005; Kim et al., 2006). Since AGPs exert a crucial control in rapidly expanding tissues such as the stigma, the intrinsic developmental regulation of Jim13 AGP expression and their vanishing after pollen-tube passage further supports a possible contribution to pollen-tube wall-building and orientation towards the style.
This work shows that the developmental regulation of the secretion of AGPs is associated with the onset of stigmatic receptivity in the apple flower. Changes in their labelling following pollination suggest a role in pollen–stigma interaction. The presence of AGPs in the pistil has been shown in divergent species, from monocots (Ma and Zhao, 2010) and eudicots (Coimbra et al., 2007) to early divergent angiosperms (Prychid et al., 2011). This together with their functional plasticity opens a way for an understanding of glycoprotein evolution modulating pollen–pistil interactions in angiosperms.
ACKNOWLEDGEMENTS
We thank Iñaki Hormaza and Faye Rosin for valuable comments on the manuscript. This work was supported by Ministerio de Ciencia e Innovación (MICINN)-FEDER [AGL2006-13529-C02-01, AGL 12621-C02-01], and Gobierno de Aragón [group A43]. Antibodies distribution was partly supported by NSF grants [DBI-0421683, RCN 009281]. J.M.L. was supported by a FPI fellowship [BES-2007-16059] from the MICINN.
LITERATURE CITED