-
PDF
- Split View
-
Views
-
Cite
Cite
International Committee for Phytolith Taxonomy (ICPT) , International Code for Phytolith Nomenclature (ICPN) 2.0, Annals of Botany, Volume 124, Issue 2, 24 July 2019, Pages 189–199, https://doi.org/10.1093/aob/mcz064
Close - Share Icon Share
Abstract
Opal phytoliths (microscopic silica bodies produced in and between the cells of many plants) are a very resilient, often preserved type of plant microfossil. With the exponentially growing number of phytolith studies, standardization of phytolith morphotype names and description is essential. As a first effort in standardization, the International Code for Phytolith Nomenclature 1.0 was published by the ICPN Working Group in Annals of Botany in 2005. A decade of use of the code has prompted the need to revise, update, expand and improve it.
ICPN 2.0 formulates the principles recommended for naming and describing phytolith morphotypes. According to these principles, it presents the revised names, diagnosis, images and drawings of the morphotypes that were included in ICPN 1.0, plus three others. These 19 morphotypes are those most commonly encountered in phytolith assemblages from modern and fossil soils, sediments and archaeological deposits. An illustrated glossary of common terms for description is also provided.
INTRODUCTION
In the last two decades, the number of studies and publications in all fields of opal phytolith analysis has grown exponentially, mainly due to a diversification of research topics (Hart, 2016; Neumann et al., 2017; Strömberg et al., 2018). Significant progress has been made in the application of phytolith analysis for answering archaeological, palaeoenvironmental, evolutionary, taxonomic and climatological questions, often within the framework of interdisciplinary research. One of the most persistent needs in the discipline of phytolith analysis is a universally accepted nomenclature and classification system. Over the years, numerous synonyms (i.e. different names for the same morphotype) and homonyms (i.e. identical names for different morphotypes) have hampered the communication between researchers and comparison of their data. Historically, there have been several efforts to standardize the naming of opal phytoliths, often connected with the establishment of classification systems (e.g. Bertoldi de Pomar, 1971; Brown, 1984; Ollendorf, 1992; Pearsall and Dinan, 1992; Fredlund and Tieszen, 1994; Runge, 1999; Bowdery et al., 2001; Zucol and Brea, 2005; Pearsall, 2016). However, most of these proposals refer to either geographically restricted areas or single taxonomic groups, and none of them has become universally accepted.
Consistent and universally accepted names of scientific items are essential for successful communication in science. Since Linnaeus’ Species plantarum of 1753, each plant (or animal) species has had a unique binomial name (Knapp, 2000). The species and higher-ranked taxonomic names of algae, fungi and plants are governed by the International Code of Nomenclature for algae, fungi and plants (ICN) (McNeill et al., 2012). Therein, principles are defined for a formal, ‘valid’ publication of a correct name. One of these principles is that the name has to be associated with a precise and detailed description (diagnosis) of the item, which is ‘a statement of that which in the opinion of its author distinguishes the taxon from other taxa’ (Article 38.2), the name must be a unique identifier (i.e. one item, one name) (Article 11.1), and naming must follow transparent and consistent rules (e.g. Article 11.4 and 32), which include its publication in a peer-reviewed journal or similar (Chapter 5, Section 2).
Isolated plant parts in ancient sediments, such as pollen, wood or seeds, can bear the name of the species or taxonomic group from which they come (Cleal and Thomas, 2010). However, there is disagreement about whether taxonomic names are justified for isolated plant organs, which cannot be unambiguously attributed to a species or higher-ranked taxonomic group (Joosten and de Klerk, 2002). Phytoliths are microscopic silica bodies that often take on the shape of the cells in or around which they were deposited; thus they represent selected, variable aspects of preserved plant anatomy. Some phytoliths, especially those from reproductive structures, can be specific for certain taxonomic groups and may be named after them (e.g. fruit and seed phytoliths from Burseraceae, Marantaceae or Commelinaceae; Piperno, 2006; Eichhorn et al., 2010). But the majority of phytoliths show such a degree of redundancy (same shapes produced in multiple taxa) that they typically cannot be attributed to a single taxon. Moreover, often the anatomical origin of a phytolith morphotype is uncertain or unknown, and consequently the name of the morphotype has to be given according to morphological characters, such as shape, size and texture; hence the term ‘morphotype’. A morphotype can be defined as a group of individual specimens that have the same, unique shape (the term ‘shape’ should be understood in a broad sense, and includes different morphological traits). However, using morphological features as part of the name can lead to extremely long and complicated names and constitutes a mixing of naming and describing, reminiscent of the common practice for plant species in pre-Linnaean times (Knapp, 2000). Formalizing and harmonizing the naming of phytolith morphotypes is thus a major challenge for the international phytolith research community.
In 2000, the primary governing body for the discipline of phytolith analysis, now the International Phytolith Society (IPS), recognized the need for standardization of nomenclature and terminology in the discipline and subsequently commissioned a committee to draft an International Code for Phytolith Nomenclature. That code, known as ICPN 1.0, was published in the Annals of Botany in 2005 (ICPN Working Group: Madella et al., 2005), and has become a widely cited and utilized standard in phytolith analysis. As anticipated by this first committee, a decade of use of the code revealed the need to revise, update, expand and improve it. Accordingly, in 2014 the IPS commissioned a new committee, the International Committee for Phytolith Taxonomy (ICPT), whose main tasks are: (1) revision and amendment of ICPN 1.0; (2) provision of an extended list of descriptors; and (3) development of the PhytCore database as a platform for identification, exchange of information and discussion of taxonomic issues (Albert et al., 2016; PhytCore DB, 2016).
We publish here ICPN 2.0, with revised names and full descriptions and diagnoses/definitions of the morphotypes that were included in ICPN 1.0, plus three others commonly encountered in phytolith assemblages from modern and fossil soils, sediments and archaeological deposits (Table 1). We also provide an illustrated glossary of common terms for description (Fig. 1A, B and Supplementary Data File 2), based on Stearn’s Botanical Latin (Stearn, 2013). We regard this as a step towards creating a standardized phytolith classification. We do not intend for this to be an exhaustive list because many more morphotypes remain to be described and named in the future.
Morphotypes described in this paper, with codes and ICPN 1.0 synonyms (SD1 = Supplementary Data File 1).
| ICPN 2.0 . | Code . | ICPN 1.0 . | Illustration . |
|---|---|---|---|
| Spheroid psilate | SPH_PSI | – | Fig. 2 |
| Spheroid echinate | SPH_ECH | Globular echinate | Fig. 3 |
| Spheroid ornate | SPH_ORN | Globular granulate | Fig. 4 |
| Acute bulbosus | ACU_BUL | Acicular hair cell/Unciform hair cell | SD1 Fig. 1A–M |
| Blocky | BLO | Parallelepipedal bulliform cell | SD1 Fig. 2 |
| Bulliform flabellate | BUL_FLA | Cuneiform bulliform cell | SD1 Fig. 3 |
| Elongate entire | ELO_ENT | – | SD1 Fig. 4A–M |
| Elongate sinuate | ELO_SIN | – | SD1 Fig. 4O–W |
| Elongate dentate | ELO_DET | Elongate echinate long cell | SD1 Fig. 5A–K |
| Elongate dendritic | ELO_DEN | Dendritic/Dentritic | SD1 Fig. 5L–R |
| Papillate | PAP | Papillae | SD1 Fig. 1N–Q |
| Tracheary | TRA (with subtypes TRA_ANN, TRA_PIT, TRA_BOR) | Cylindric sulcate tracheid | SD1 Figs. 6, 7 |
| Grass silica short-cell phytoliths (GSSCP) | |||
| Saddle | SAD | Saddle | SD1 Fig. 8Bb–Ff |
| Bilobate | BIL | Bilobate short cell | SD1 Fig. 8A–N |
| Polylobate | POL | Cylindrical polylobate | SD1 Fig. 8W–Aa |
| Cross | CRO | Cross | SD1 Fig. 8O–V |
| Crenate | CRE | Trapeziform polylobate/Trapeziform sinuate | SD1 Fig. 9A–F |
| Rondel | RON | Rondel | SD1 Fig. 9G–P |
| Trapezoid | TRZ | Trapeziform short cell | SD1 Fig. 9Q–S |
| ICPN 2.0 . | Code . | ICPN 1.0 . | Illustration . |
|---|---|---|---|
| Spheroid psilate | SPH_PSI | – | Fig. 2 |
| Spheroid echinate | SPH_ECH | Globular echinate | Fig. 3 |
| Spheroid ornate | SPH_ORN | Globular granulate | Fig. 4 |
| Acute bulbosus | ACU_BUL | Acicular hair cell/Unciform hair cell | SD1 Fig. 1A–M |
| Blocky | BLO | Parallelepipedal bulliform cell | SD1 Fig. 2 |
| Bulliform flabellate | BUL_FLA | Cuneiform bulliform cell | SD1 Fig. 3 |
| Elongate entire | ELO_ENT | – | SD1 Fig. 4A–M |
| Elongate sinuate | ELO_SIN | – | SD1 Fig. 4O–W |
| Elongate dentate | ELO_DET | Elongate echinate long cell | SD1 Fig. 5A–K |
| Elongate dendritic | ELO_DEN | Dendritic/Dentritic | SD1 Fig. 5L–R |
| Papillate | PAP | Papillae | SD1 Fig. 1N–Q |
| Tracheary | TRA (with subtypes TRA_ANN, TRA_PIT, TRA_BOR) | Cylindric sulcate tracheid | SD1 Figs. 6, 7 |
| Grass silica short-cell phytoliths (GSSCP) | |||
| Saddle | SAD | Saddle | SD1 Fig. 8Bb–Ff |
| Bilobate | BIL | Bilobate short cell | SD1 Fig. 8A–N |
| Polylobate | POL | Cylindrical polylobate | SD1 Fig. 8W–Aa |
| Cross | CRO | Cross | SD1 Fig. 8O–V |
| Crenate | CRE | Trapeziform polylobate/Trapeziform sinuate | SD1 Fig. 9A–F |
| Rondel | RON | Rondel | SD1 Fig. 9G–P |
| Trapezoid | TRZ | Trapeziform short cell | SD1 Fig. 9Q–S |
Morphotypes described in this paper, with codes and ICPN 1.0 synonyms (SD1 = Supplementary Data File 1).
| ICPN 2.0 . | Code . | ICPN 1.0 . | Illustration . |
|---|---|---|---|
| Spheroid psilate | SPH_PSI | – | Fig. 2 |
| Spheroid echinate | SPH_ECH | Globular echinate | Fig. 3 |
| Spheroid ornate | SPH_ORN | Globular granulate | Fig. 4 |
| Acute bulbosus | ACU_BUL | Acicular hair cell/Unciform hair cell | SD1 Fig. 1A–M |
| Blocky | BLO | Parallelepipedal bulliform cell | SD1 Fig. 2 |
| Bulliform flabellate | BUL_FLA | Cuneiform bulliform cell | SD1 Fig. 3 |
| Elongate entire | ELO_ENT | – | SD1 Fig. 4A–M |
| Elongate sinuate | ELO_SIN | – | SD1 Fig. 4O–W |
| Elongate dentate | ELO_DET | Elongate echinate long cell | SD1 Fig. 5A–K |
| Elongate dendritic | ELO_DEN | Dendritic/Dentritic | SD1 Fig. 5L–R |
| Papillate | PAP | Papillae | SD1 Fig. 1N–Q |
| Tracheary | TRA (with subtypes TRA_ANN, TRA_PIT, TRA_BOR) | Cylindric sulcate tracheid | SD1 Figs. 6, 7 |
| Grass silica short-cell phytoliths (GSSCP) | |||
| Saddle | SAD | Saddle | SD1 Fig. 8Bb–Ff |
| Bilobate | BIL | Bilobate short cell | SD1 Fig. 8A–N |
| Polylobate | POL | Cylindrical polylobate | SD1 Fig. 8W–Aa |
| Cross | CRO | Cross | SD1 Fig. 8O–V |
| Crenate | CRE | Trapeziform polylobate/Trapeziform sinuate | SD1 Fig. 9A–F |
| Rondel | RON | Rondel | SD1 Fig. 9G–P |
| Trapezoid | TRZ | Trapeziform short cell | SD1 Fig. 9Q–S |
| ICPN 2.0 . | Code . | ICPN 1.0 . | Illustration . |
|---|---|---|---|
| Spheroid psilate | SPH_PSI | – | Fig. 2 |
| Spheroid echinate | SPH_ECH | Globular echinate | Fig. 3 |
| Spheroid ornate | SPH_ORN | Globular granulate | Fig. 4 |
| Acute bulbosus | ACU_BUL | Acicular hair cell/Unciform hair cell | SD1 Fig. 1A–M |
| Blocky | BLO | Parallelepipedal bulliform cell | SD1 Fig. 2 |
| Bulliform flabellate | BUL_FLA | Cuneiform bulliform cell | SD1 Fig. 3 |
| Elongate entire | ELO_ENT | – | SD1 Fig. 4A–M |
| Elongate sinuate | ELO_SIN | – | SD1 Fig. 4O–W |
| Elongate dentate | ELO_DET | Elongate echinate long cell | SD1 Fig. 5A–K |
| Elongate dendritic | ELO_DEN | Dendritic/Dentritic | SD1 Fig. 5L–R |
| Papillate | PAP | Papillae | SD1 Fig. 1N–Q |
| Tracheary | TRA (with subtypes TRA_ANN, TRA_PIT, TRA_BOR) | Cylindric sulcate tracheid | SD1 Figs. 6, 7 |
| Grass silica short-cell phytoliths (GSSCP) | |||
| Saddle | SAD | Saddle | SD1 Fig. 8Bb–Ff |
| Bilobate | BIL | Bilobate short cell | SD1 Fig. 8A–N |
| Polylobate | POL | Cylindrical polylobate | SD1 Fig. 8W–Aa |
| Cross | CRO | Cross | SD1 Fig. 8O–V |
| Crenate | CRE | Trapeziform polylobate/Trapeziform sinuate | SD1 Fig. 9A–F |
| Rondel | RON | Rondel | SD1 Fig. 9G–P |
| Trapezoid | TRZ | Trapeziform short cell | SD1 Fig. 9Q–S |
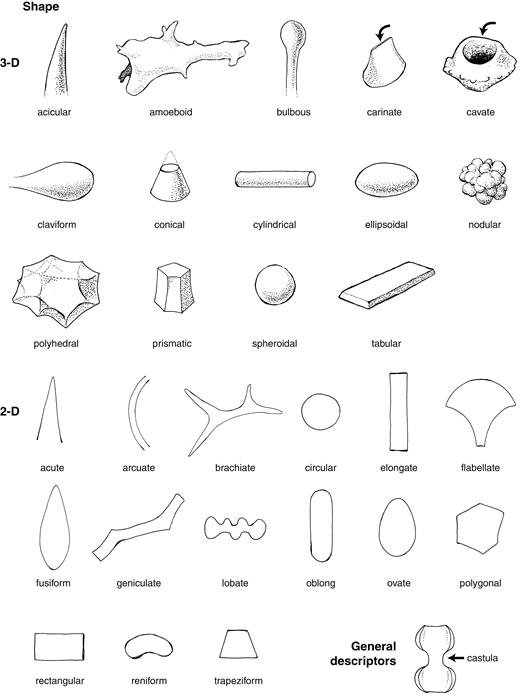
Line drawings illustrating important shape and general descriptors. Drawings by C.A.E. Strömberg.
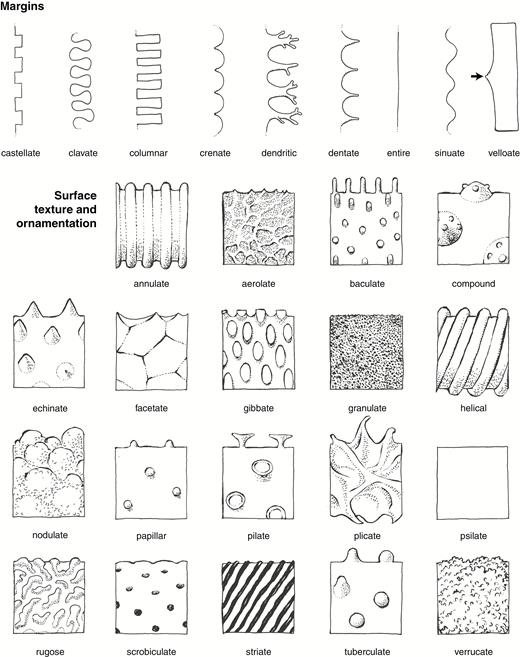
Line drawings illustrating important phytolith descriptors for margins, surface, texture and ornamentation. Drawings by C.A.E. Strömberg.
PRINCIPLES FOR NAMING PHYTOLITH MORPHOTYPES: ICPN 2.0
(1) Each name shall be a unique identifier, concise (three words maximum) and clearly related to the morphotype.
(2) The naming of phytoliths shall follow a hierarchical order, from taxonomic to anatomical to morphological. Those morphotypes that can be unambiguously attributed to a taxon shall begin with the taxonomic name; those that have an unambiguous origin in a plant organ or anatomical tissue, but that are not restricted taxonomically, shall begin with the anatomical name, following Esau (1965). Those morphotypes that are not unique to a taxon or plant organ shall begin with a morphological name.
The grass silica short-cell phytoliths (GSSCP) are an exception to this rule. For GSSCP it is implicit that they are produced in certain short cells of the Poaceae epidermis. GSSCP can be clearly distinguished based on morphological features, and their names (such as rondel or bilobate) are well established (nomina conservanda). Specifically, adding ‘Poaceae epidermis’ to an existing GSSCP name does not clarify the identification; it would merely lengthen the name.
(3) In cases where morphological names are given, descriptors from the glossary shall be used when possible. The first word for a morphological name shall normally refer to the overall shape, the second one either a specification of the shape, or texture/ornamentation. Specification of the shape or texture/ornamentation can be used as a first descriptor if it is the most conspicuous and consistent feature of a morphotype with variable shape.
In rare cases a name not consistent with principle 3 but consistent with principle 1 may be given to a morphotype. Such exceptions include names that are either (a) commonly used (nomina conservanda) or (b) capture unambiguous and fundamental aspects of the morphotype. An example that incorporates both aspects, (a) and (b), is the name Blocky.
(4) Phytoliths that exhibit features of two closely related morphotypes may bear combined names with descriptors in alphabetical order, separated by a slash. Examples are Elongate dendritic/dentate and Elongate entire/sinuate.
(5) Names shall be written in small capitals according to the suggestion of Joosten and de Klerk (2002). The first descriptor is capitalized, the second and third are not.
(6) To facilitate data management, each name shall be assigned a unique code. The code shall consist of the first three letters (in capitals) of each of the first two descriptors in the morphotype name, separated by an underscore. Examples are SPH_PSI for Spheroid psilate or CRO for Cross (name consisting of only one descriptor). In cases where a code has already been given to another morphotype, other letters from the descriptors shall be used. Example: Elongate dendritic (ELO_DEN) versus Elongate dentate (ELO_DET). Additional subdivision of the code shall include numbers, separated by underscores when more than one number is required (e.g. ELO_DET, ELO_DET_1, ELO_DET_1_3, with each code after the first being a subtype of the preceding code).
(7) A rationale for naming shall be provided, including information about why the morphotype deserves a taxonomical, anatomical or morphological name, why a new name is given or an established name is retained (nomen conservandum).
PRINCIPLES FOR DESCRIBING PHYTOLITH MORPHOTYPES (MODIFIED FROM ICPN 1.0)
(1) To be validly published, a morphotype name plus code must be accompanied by the rationale for naming, description, size, anatomical origin and taxonomic occurrence if known, discussion and interpretation, illustrations and synonyms. Terminology from the provided glossary (Supplementary Data File 2) and/or from Stearn’s Botanical Latin (Stearn, 2013) and Esau (1965) shall be used.
(2) The Description shall include the typical features of the morphotype and also cover the full range of its variations. The description shall provide information about the overall 3-D and/or 2-D shape. Rotation in a liquid medium may be necessary to ensure that all sides of the morphotype are observed and described. Degrees, planes or axes of symmetry shall be included if applicable. In addition, more detailed morphometric shape data can be provided (for current morphometric standards see Ball et al., 2016).
Surface texture and ornamentation shall be described. Weathering features shall not be described as ornamentations or texture. Material texture (e.g. granular, laminar, homogenous translucent) and presence of inclusions (e.g. organic inclusions, crystals) may be described if regarded as being of diagnostic importance.
(3) Size description shall include information about the general size range of the morphotype (e.g. length, width and diameter). We encourage authors to consult ICPN 1.0 for a primer on how to supply statistically robust size measurements. For more detailed measurements in the context of morphometric studies, see Ball et al. (2016).
(4) Anatomical origin shall describe in which organ, tissue and/or cell types a morphotype is formed. If the anatomical origin is not known, this shall also be stated. If known, the locus of silicification shall be specified (e.g. cell wall, lumen and intercellular spaces). For GSSCP, Anatomical origin is replaced by Orientation in epidermis, which shall describe the orientation of the GSSCP morphotype in the grass epidermis relative to the long axis of the leaf or stem, if known. Taxonomic occurrence shall describe in which taxa the morphotype is present. If the taxonomic origin is unknown, this shall also be stated.
(5) Discussion and interpretation shall describe how the morphotype compares to similar silica bodies by specifying the features that set it apart from others [see also point (2) above]. It shall also provide information about how the morphotype is interpreted (e.g. taxonomically, ecologically) in the literature (although such information need not be exhaustive).
(6) Illustrations shall include at least one but preferably several light microscope photographs showing typical examples and the broader range and variations of morphology and/or texture. Scanning electron microscope (SEM) images can also be provided; however, because the routine work in phytolith analysis is done with the light microscope, SEM images should not be substituted for optical micrographs. Illustrations must show a labelled scale bar, indicate orientation when relevant (e.g. for GSSCP) and indicate authorship. Line drawings and/or computer-generated graphics depicting all relevant orientations of the morphotype are recommended.
(7) Synonyms shall contain names that have previously been given, as well as specific references to the publication and illustration (if applicable), in chronological order. It is recommended that the author specifies the degree of synonymy, i.e. whether the morphotype corresponds directly, is a subset of the previous type, or incorporates a broader set of morphologies. References to previous figures of phytoliths that were not explicitly named may also be listed.
CURRENT STANDARD MORPHOTYPES
This section publishes the morphological names and codes of 19 morphotypes according to the principles for naming and description of ICPN 2.0. Three of them (Spheroid psilate, Spheroid echinate and Spheroid ornate) are presented here. The 16 remaining are published as Supplementary Data File 1. These 19 morphotypes are those most commonly encountered in phytolith assemblages from modern and fossil soils, sediments and archaeological deposits.
Taxonomical attribution of phytolith morphotypes was obtained from the literature and through the study of phytolith reference collections, most of them at the University of Barcelona (Spain) (UB), the University of California Museum of Paleontology, Berkeley (UCMP), the University of Washington Burke Museum of Natural History and Culture, Seattle (UWBM) and Goethe University Frankfurt (Germany) [Phytolith-Vergleichs-Sammlung (PHV)].
Spheroid psilate
Code: SPH_PSI
Rationale for naming: The term ‘spheroid’ designates a sphere-like body. The name encompasses a broad range of variation from perfectly spherical to slightly ellipsoidal or somewhat irregular, but with the basic shape resembling a sphere.
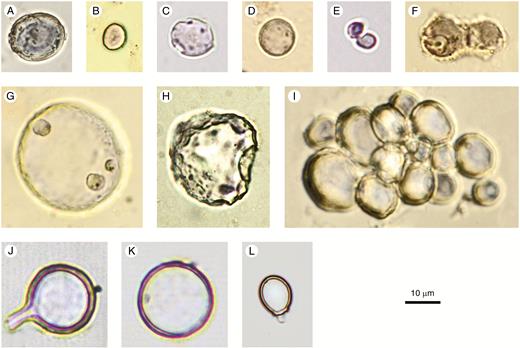
Description: Solid or hollow phytoliths, spheroidal to slightly ellipsoidal or somewhat irregular, but with the basic shape resembling a sphere. Surface smooth, without surface ornamentation or projections. Internal texture homogeneous translucent silica, or granular (best discernible in SEM), or with concentric laminae. Occurring singly or in articulated groups.
Size: 3–30 µm.
Anatomical origin and taxonomic occurrence: Many Spheroid psilate arise as vesicular infillings of epidermal and parenchyma cells of foliage and reproductive organs in a wide range of dicots, monocots (e.g. Arecaceae, Poaceae and Cyperaceae) and some gymnosperms (Geis, 1973; Strömberg, 2003; Piperno, 2006: 38f). They sometimes occur in ray and parenchyma cells of silica-accumulating wood and have also been observed in bark (Kondo et al., 1994; Albert and Weiner, 2001; Collura and Neumann, 2017).
In dicots, some articulated groups of Spheroid psilate seem to be parenchyma cells filled with silica, e.g., in the fruits of Chrysobalanaceae (Fig. 2I).
Discussion and interpretation: Articulated groups of Spheroid psilate can be confused with the ‘Shallow honeycomb assemblages’ described by Bozarth (1992, Fig. 10.4) which are clusters of palisade parenchyma cells with silicified end walls.
Non-phytolith confusers for Spheroid psilate are silicified Chrysophyceae stomatocysts with a psilate surface (Fig. 2J–L). Stomatocysts, however, have a distinct pore, sometimes with a collar. In case of doubt, it is recommended to check images in the relevant Chrysophyceae literature (e.g. Wilkinson et al., 1995, 2001).
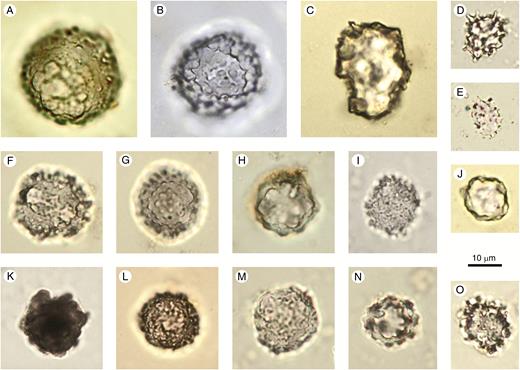
Spheroid psilate (A–I) and confusers (J–L). (A–D, F–H) From surface samples and archaeological sites in West and Central Africa. (C, F–H) With corrosion features. (E) Elodea canadensis (Hydrocharitaceae), stem. (I) Parinari curatellifolia (Chrysobalanaceae), fruit. (J, K) Chrysophyte cyst, agricultural feature, Hawaii, Late Holocene. (L) Chrysophyte cyst, Palaeolake sediments, Northern Awash, Ethiopia, Late Pliocene. Authors: K. Neumann (A–D, F–I); C. Yost (E, J–L).
Due to their wide distribution in different tissues, organs and taxonomic groups, the diagnostic value of the Spheroid psilate morphotype is low. However, because of their abundance in some woody dicots, they have been used as evidence of non-grass plants, for example as part of a ‘forest indicator’ class (e.g. Strömberg, 2004, 2005; Strömberg et al., 2018).
Synonyms: Grain (ter Welle, 1976a, b). Spherical smooth (Kondo et al., 1994, Plate 10). Spherical phytolith with a smooth surface (Runge, 1999, Plate I4: Type B1/B7). Smooth VI spheres and subspheres (Cl-1), VI spheres (VI-1) (in part) (Strömberg, 2003, Fig. 4.11e). Globulolithum sphaeropsilathum (Zucol and Brea, 2005, Fig. 2D). Smooth sphere (Piperno, 2006, Fig. 2.10). Globular smooth (Barboni et al., 2007, Fig. 2.13; Iriarte and Paz, 2009, Fig. 2c). Globular psilate (Bremond et al., 2008, Fig. 2.13). Small globular (Bremond et al., 2017, Fig. 2b). Spheroid psilate (PhytCore DB).
Illustrations:Fig. 2A–I.
Spheroid echinate
Code: SPH_ECH
ICPN 1.0: Globular echinate
Rationale for naming: For the overall shape, see rationale for Spheroid psilate.
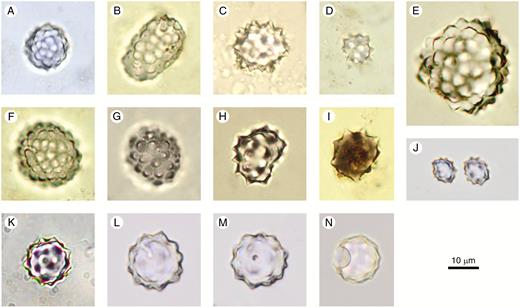
Description: Solid spheroidal phytoliths with conical projections distributed over the entire surface. The shape ranges from spherical to slightly ellipsoidal or somewhat irregular, but with the basic shape resembling a sphere. The conical projections vary in size, are more or less closely spaced, and can be distinctly pointed to rounded. Individual conical projections are either simple or compound, with apices having one or two smaller satellite projections or being split in two (two-headed). Occurring singly or in articulated groups/rows.
Size: 2–25 (30) µm.
Anatomical origin and taxonomic occurrence:Spheroid echinate are common in vegetative and reproductive organs of Arecaceae, where they are produced in stegmata, i.e., longitudinal files of cells adjacent to vascular or non-vascular fibres (Tomlinson, 1961). They also occur in the leaves of Bromeliaceae (Tomlinson, 1969). Piperno (1988, 2006) and Benvenuto et al. (2015) distinguish the two families by the size of the spheroids, which range from <2 to about 10 µm in Bromeliaceae, while those of the Arecaceae vary from 6 to 25 µm. In the Arecaceae, the conical projections are usually larger and more closely distributed on the surface. Among the Arecaceae, differentiation at lower taxonomic levels seems to be possible based on a combination of qualitative and quantitative variables (Albert et al., 2009; Fenwick et al., 2011; Bowdery, 2014; Benvenuto et al., 2015), but there is still no general agreement on the diagnostic features. Recent research on the phytoliths of Amazonian and Andean forest palms has shown that several taxonomically relevant subtypes of Spheroid echinate can be distinguished (Morcote-Rios et al., 2016; Huisman et al., 2018). Many more studies on modern reference material from other tropical areas, including morphometric data, are necessary to reach a higher level of taxonomic resolution.
Discussion and interpretation: In unknown assemblages of tropical soils and sediments, special attention should be devoted to the distinguishing features of Spheroid echinate and spheroids with other ornamentations, such as those with an irregularly plicate, ‘crushed’, or nodulose surface occurring in the Zingiberales (Piperno, 1988; Chen and Smith, 2013).
Silicified asterone microscleres of marine sponges (Uriz et al., 2003; Łukowiak, 2016) may appear similar to Spheroid echinate at first sight, but they are perfectly spherical and exhibit one pore on the surface (Fig. 3K–N). The pore may be difficult to see.
Spheroid echinate (A–J) and potential confusers (K–N). (A–I) From soil surface samples and Holocene archaeological sites in West and Central Africa. (J) Cocos nucifera (Arecaceae), leaf. (K–N) Silica bodies with similarity to both spheroid echinate and asterone microscleres. Palaeolake sediments, Northern Awash, Ethiopia, Late Pliocene. Authors: K. Neumann (A–I); C. Yost (J–N).
In the phytolith literature, the morphotype is most often associated with palms (Arecaceae) (e.g. Albert et al., 2009; Strömberg et al., 2013). However, if not further subdivided, the Spheroid echinate morphotype should not uncritically be assigned to a taxon or taxonomic group because spheroidal phytoliths with an echinate ornamentation also occur in Bromeliaceae (Piperno, 1988). The latter is not distributed in the Old World and can therefore be excluded in unknown assemblages from these regions. Some morphotypes from Commelinaceae and Orchidaceae are also similar to Spheroid echinate (see discussion in Strömberg, 2003, p. 672f; Chen and Smith, 2013).
Synonyms: Globulolitha esferoequinolata (Bertoldi de Pomar, 1971, p. 319, 323). Spherical spinulose (Piperno, 1888, Plate 1, 2; Kondo et al., 1994, Plate 11a–d). Circular crenate, Palmae type (Barboni et al., 1999, Plate I1–3). Spherical Palmae phytolith with spinulose surface (Type B3) (Runge, 1999, Plate III7). Mamillated spheroid (Vrydaghs et al., 2001). Echinate sphere (Clm-2) (Strömberg, 2003, p. 672, Fig. 4.11a, b; Wallis, 2003, Fig. 2). Crenate spherical (Bremond et al., 2005, Fig. 2.9). Globulolithum sphaeroechinulathum (Zucol and Brea, 2005, Fig. 2A–C). Spheroid echinate (Bamford et al., 2006; PhytCore DB).
Illustrations:Fig. 3A–J.
Spheroid ornate
Code: SPH_ORN
ICPN 1.0: Globular granulate
Rationale for naming: For the overall shape, see rationale for Spheroid psilate. The name encompasses spheroidal phytoliths with a wide range of surface textures and processes, including the ICPN 1.0 ‘Globular granulate’.
Description: Spheroidal solid phytoliths with complex surface ornamentation. The shape can range from spheroidal to slightly ellipsoid or largely irregular, but with the basic shape resembling a sphere. Occurring singly or in articulated groups. The morphotype includes a large range of surface ornamentations, such as areolate, granulate, rugose, verrucate, columellate and plicate, and various combinations and/or transitional forms of all of these.
Size: 3–30 (50) µm, very variable.
Anatomical origin and taxonomic occurrence:Spheroid ornate occur in leaves, branches, trunks, and fruits of woody dicots (in the sense of non-monocot angiosperms), and in vegetative structures (less often in seeds) of some herbaceous monocots (Piperno, 2006, p. 39). In Neotropical Marantaceae leaves, the spheroids are randomly distributed in the mesophyll (Albuquerque et al., 2013). Spheroid ornate have been found in rays and axial parenchyma of wood and in the bark of some tropical trees (Amos, 1952; Scurfield et al., 1974; Collura and Neumann, 2017). They are very common in soils underneath tropical forests. As only 10 % of all woody species worldwide have silica in their wood, it is reasonable to assume that a larger proportion of these phytoliths in tropical forest soils originate either from dicot leaves and fruits or from monocots, notably of the order Zingiberales. Iriarte and Paz (2009) distinguish the ornamented spheroids of tropical woody dicots with size ranges of mainly 3–10 µm from the larger ‘spherical rugose’ phytoliths (10–30 µm) produced in Marantaceae and Cannaceae. After Piperno (2006, p. 39), ‘verrucate phytoliths’ from monocots usually range from 9 to 25 µm, being much smaller in eudicots (3–9 µm). A recent study has shown that ornamented spheroids, occurring in vegetative as well as reproductive structures of several families of Zingiberales, have a larger range of size variation (Chen and Smith, 2013). When more studies on modern plants are available, it is likely that more diagnostic morphotypes among the Spheroid ornate will be defined.
Discussion and interpretation: The morphotype is quite broadly defined in shape, size and surface ornamentation. When a distinct surface ornamentation can be effectively recognized, the spheroid may be named after it using terms in the glossary (e.g. Spheroid verrucate). For further differentiation, refer to the descriptors of Bowdery et al. (2001) in their definition of the Class Spherical/Spheroidal, using the following features: composition (single/compound), shape, size, attachments, and ornamentation.
In assemblages of tropical soils and sediments it may be useful to distinguish the Spheroid ornate from the Spheroid echinate, and from the spheroids with a plicate surface (Maranta-type and Costus-type, Strömberg, 2003, 2005; ‘nodulose/folded phytoliths’, Piperno, 2006; ‘druses,’ Chen and Smith, 2013; ‘globular with ridges,’ Albuquerque et al., 2013), which are typical in the families Marantaceae, Cannaceae, Costaceae and Strelitziaceae in the order Zingiberales.
Spheroidal ornate phytoliths with a stalk or attachment, which are cystoliths, are a different morphotype (Bozarth, 1992; Strömberg, 2003) and are not included here.
In wood, a special morphotype is produced with a texture consisting of tiny granules or nodules (cauliflower-like structure). This feature is best visible in the SEM (Kondo et al., 1994; Collura and Neumann, 2017). These granular and nodular types, which sometimes show spheroidal but often ellipsoid or irregular outlines, are specific for wood (Amos, 1952; Scurfield et al., 1974; Collura and Neumann, 2017), where they form a continuum with the Spheroid ornate morphotype, and it is often not possible to differentiate between them. When Spheroid ornate are used as indicators of woody vegetation in the calculation of the D:P (dicotyledon vs. Poaceae morphotypes) index (Alexandre et al., 1997; Barboni et al., 2007; Bremond et al., 2008), the granular and nodular morphotypes are usually included in the D (= dicotyledon) class.
Synonyms: Irregular sphere- or oval-shaped opals (Kondo and Peason, 1981, Fig. 7a–d). Spherical rugulose (Piperno, 1988, Plate 5). Spherical verrucose (Kondo et al., 1994, Plates 12, 13). Circular rugose (Alexandre et al., 1997, Fig. 2h). Spherical with a rough surface (B2) (Runge, 1999). Spherical class, spheroid with various surface ornamentations (Bowdery et al., 2001). Small rugose/rugulose sphere (Cl–7) (Strömberg, 2003, Fig. 4.11k). Verrucate sphere (class D1) (Strömberg, 2004, Fig. 5d). Rough spherical (Bremond et al., 2005, Fig. 2.10). Decorated sphere (Piperno, 2006, Fig. 2.13). Globular decorated (class A3) (Neumann et al., 2009, Fig. 2c–f). Globular, with various patterns of surface sculpturing (Chen and Smith, 2013, Figs. 1D, 3M, 4O, W, Y, Z, AA). Spheroid ornate (PhytCore DB).
Illustrations:Fig. 4A–O.
Spheroid ornate. (A–O) From soil surface samples and Holocene archaeological sites in West and Central Africa. Author: K. Neumann.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. File 1: Morphotype descriptions. File 2: Glossary of descriptive terms.
FUNDING
The work of K.N. is supported by Goethe University Frankfurt. Financial support was given to R.M.A. by the Spanish Ministry of Economy and Competitiveness (HAR2016-75216-P). The work of C.A.E.S. was in part funded by US National Science Foundation grant EAR-1253713 as well as the Burke Museum of Natural History and Culture, University of Washington. The work of L.V. is supported by the Brussels Capital Region, supplied by travel funds from the Fond National de la Recherche Scientifique (FNRS). Workshops related to this work were funded through the Quaternary Research Center, University of Washington, Seattle, and the University of Barcelona, Barcelona.
ACKNOWLEDGEMENTS
We thank C. Yost for useful comments and for providing images, and M. Heckner and J. Markwirth for artwork. Images for the glossary and the morphotype descriptions were provided by D. Bowdery, T. E. Buchanan, V. Collura, C. Crifò, I. Esteban, A. Garnier, B. Eichhorn, A. Emery-Barbier, M. J. Hodson, Z. Lisztes-Szabó, M. Pearce, A. Rodriguez-Cintas and S. A. Shakoor. We are very much indebted to Dolores Piperno, Doris Barboni and one anonymous reviewer whose valuable comments helped to improve the paper substantially.