-
PDF
- Split View
-
Views
-
Cite
Cite
MICHAEL B. JACKSON, PHOOL C. RAM, Physiological and Molecular Basis of Susceptibility and Tolerance of Rice Plants to Complete Submergence, Annals of Botany, Volume 91, Issue 2, 2 January 2003, Pages 227–241, https://doi.org/10.1093/aob/mcf242
Close - Share Icon Share
Abstract
Rice plants are much damaged by several days of total submergence. The effect can be a serious problem for rice farmers in the rainfed lowlands of Asia, and runs contrary to a widespread belief amongst plant biologists that rice is highly tolerant of submergence. This article assesses the characteristics of the underwater environment that may damage rice plants, examines various physiological mechanisms of injury, and reviews recent progress achieved using linkage mapping to locate quantitative traits loci (QTL) for tolerance inherited from a submergence‐tolerant cultivar FR13A. Progress towards identifying the gene(s) involved through physical mapping of a dominant tolerance locus on chromosome 9 is also summarized. Available physiological evidence points away from responses to oxygen shortage as being inextricably involved in submergence injury. An imbalance between production and consumption of assimilates is seen as being especially harmful, and is exacerbated by strongly accelerated leaf extension and leaf senescence that are ethylene‐mediated and largely absent from FR13A and related cultivars. DNA markers for a major QTL for tolerance are shown to be potentially useful in breeding programmes designed to improve submergence tolerance.
Received: 22 November 2001; Returned for revision: 22 February 2002; Accepted: 3 June 2002
INTRODUCTION
Rice has a reputation for growing well under flooded conditions. This view probably arises because of the widely recognized ability of rice seeds to germinate without O2 (Taylor, 1942) and of deep‐water rice plants to escape aerobically from slowly rising water (2–5 cm d–1) by means of accelerated stem extension that maintains apical parts above water (Catling et al., 1988). However, it is also the case that rice is not well adapted to sudden and total inundation when this is sustained for several days: the effect can be fatal, especially when plants are small. In many lowland areas of Asia subject to monsoon rains, this is a common occurrence. It is a problem for rice farmers and may sometimes happen more than once in a growing season (Catling, 1992). Severe injury arises when the well‐known promotion of coleoptile, leaf or stem elongation that submergence brings about in rice (reviewed by Jackson and Pearce, 1991; Sauter, 2000) is inadequate to renew contact with the atmosphere. This results in sustained total immersion that brings about acute restrictions to normal gas exchange and other damaging features of a total water cover. However, even if underwater elongation succeeds in re‐establishing contact with the air, the subsequent tendency for plants to lodge when water levels fall still poses a problem for farmers. Either way, yields of rice grain are severely decreased (Zeigler and Puckridge, 1995). This review examines the features of the submerged environment that injure plants, and assesses physiological responses to these features that may bring about injury or explain the greater level of tolerance found in a small minority of cultivars. The review centres on the relatively high resilience of the rice cultivar FR13A or its derivatives. Comparison of its behaviour with that of susceptible lines has helped to identify which of many physiological responses are critical for survival or for limiting the extent of injury. The existence of such tolerant lines has also made it possible to employ chromosome linkage mapping, quantitative traits loci (QTL) analysis and physical mapping of QTL. The promise this holds for identifying genes that confer greater tolerance and for breeding crops with improved submergence‐resilience is also reviewed.
There are three other recent reviews of the effects of submergence on rice (Ito et al., 1999; Sauter, 2000; Ram et al., 2002). The present article distinguishes itself by concentrating exclusively on complete submergence, by giving due consideration to pioneering studies and to assessing whether injury and tolerance are necessarily linked to effects of O2 shortage.
BACKGROUND INFORMATION
Complete submergence and its impact on rice farming
Catling (1992) defined submergence tolerance as ‘the ability of a rice plant to survive 10–14 d of complete submergence and renew its growth when the water subsides . . .’. The key word here is ‘complete’; it means that the entire plant, including the tips of the longest leaves, remain underwater for a sustained period. In some experimental studies, leaf tips have been allowed to emerge above the water, or plants have been submerged in stirred and aerated water (e.g. Weerapat and Waranimman, 1974; Suprihatno and Coffman, 1981; Mazaredo and Vergara, 1982). Such work bears little relevance to the effects of complete submergence since some semblance of normal gas exchange is possible. Into this category falls the extensively researched elongation response to submergence by deep‐water rice (Kende et al., 1998). This phenomenon is strongly expressed only when some of the shoot system remains above water.
Complete submergence of plants is particularly serious for rice farmers in the rainfed lowlands of humid and semi‐humid tropics of Asia. They farm approx. 25 % of the world’s rice‐growing area. Here, unexpected and uncontrollable flash floods up to 50 cm deep are common and can be sustained for several days. They are a result of overflowing rivers, accumulated run‐off from higher land, and the practice of making low earth barriers to limit soil erosion and store water (Zeigler and Puckridge, 1995). In a survey of rice breeders’ concerns in south and south‐east Asia (Mackill, 1986), 51 % of respondents rated flash flooding as one of the three most important abiotic constraints on rice yields (the others being drought and salinity). The analysis also revealed that submergence stress occurred regularly in 11 % of the 33 million ha of rainfed lowland covered by the survey and, to a lesser extent, in a further 51 % of this vast area. Maurya et al. (1988) stated that 56 % of rice lands in India are rainfed and 57 % of this area is prone to unscheduled submergence of the rice crop.
Symptoms of injury
Visible symptoms of injury caused by complete and sustained submergence include an initial phase of faster elongation by one or more leaves accompanied by yellowing of older leaves and slow or negative growth in dry mass of roots and shoots. Under some field conditions (especially in overcast weather conditions), decay of the shoot base also occurs underwater. After water levels fall, many leaves, or the whole shoot, may collapse (Fig. 1) and later can die, while surviving plants show poor rates of new leaf emergence and a high incidence of lodging.
The physiological causes of submergence injury and the basis for the greater tolerance shown by some lines or cultivars are increasingly understood and are the major topics of this review. Yamada (1959) pioneered the subject and established the foundations to much of our current understanding. Recent progress has been considerable and has been based on biochemistry, physiology and molecular genetics linked to well‐designed screening work. As a consequence, there is a realistic prospect of submergence tolerance being made a part of breeding programmes that utilize these findings in selecting parents and progeny (Sarkarung et al., 1995). The urgency of such work should be viewed in the context of the current growth rate in the human population of rice‐consuming nations, which is outstripping growth in rice production (Khush, 1995).
The tolerance trait
All rice cultivars are damaged when completely submerged for several days. However, a small number have been found to be relatively more resistant. This characteristic is referred to here as ‘tolerance’, although the term must not be misconstrued as meaning that the plants are unharmed by submergence. This relatively greater ability to tolerate complete submergence for more than a few days is rare. Only 6 % of 3156 rice cultivars tested for tolerance at the Huntra Rice Experiment Research Station in Thailand survived 10 d submergence (Setter et al., 1987). Similarly, phenotypic screening by IRRI revealed that only 2·0 % of 18 115 lines possessed an above‐average level of submergence tolerance (cited by Setter and Laureles, 1996) rivalling that of a tall‐statured indica rice known as FR13A. This cultivar was released in the 1940s by the Central Rice Research Institute, Cuttack, India as a submergence‐tolerant type and is the outcome of selections from a farmer’s variety grown in Orissa and known there as Dhullaputia (Mackill, 1986). It remains the standard against which other rice cultivars are often compared for submergence tolerance (Fig. 1). This review is largely constructed around the physiological and molecular basis of this unusual and agronomically significant trait evident in FR13A and in genetically related cultivars such as Kurkaruppan, Thavalu 15325 and Thavalu 15314 (Mazerado and Vergara, 1982). The genetic and physiological basis of tolerance shown by the unrelated Sri Lankan cultivar Goda Heenati (Senadhira, Mishra and Manigbas, cited by Setter et al., 1997) and by Vaidehi, a tolerant cultivar from Bihar in eastern India (Sarkarung and Prayongsap, pers. comm.) has not yet been explored.
A theme common to much of the literature is that of comparing the responses of tolerant and susceptible types in attempts to identify processes that contribute to underwater longevity. Such screening work is not as straightforward as it might first seem. Of particular importance is the duration of submergence. If this is too long, all plants show severe symptoms or die, and if too short, most will survive and severe symptoms of damage will then be largely absent (Hille Ris Lambers et al., 1986). The most useful tests derive from skilfully judged intermediate treatments. A further difficulty is deciding how long to wait for signs of recovery before scoring injury or the incidence of plant death. Despite the difficulties, both field‐ and glasshouse‐based studies carried out in different countries—and under very different environmental conditions—often (but not always) agree on which are highly tolerant and which are highly intolerant lines. Varieties such as FR13A and Kurkaruppan consistently score well in tests under contrasting conditions of complete submergence and over different years (e.g. Hille Ris Lambers et al., 1986; Jackson et al., 1987; Adkins et al., 1990; Setter et al., 1998; Mohanty and Ong, 2003). This suggests the existence of inheritable traits connected with tolerance of submergence that apply to a wide range of conditions. However, even tolerant lines grow little, if any dry mass, when underwater, and their subsequent growth is also adversely affected. Thus, identifying the physiological and molecular basis for submergence tolerance in tolerant lines does not identify all the damaging internal lesions induced by submergence since so‐called tolerant lines also are affected adversely, although to a smaller extent.
THE SUBMERGED ENVIRONMENT
Introductory comments
Water itself is chemically harmless to plants. Nevertheless, it inflicts serious injury when present in excess, especially when static or slow moving. The effects are derived from physical properties that damage totally submerged plants as a consequence of one or more of the following:
(1) Imposition of slow rates of gas exchange. This arises from small diffusion coefficients for gases in water compared with those in air (e.g. oxygen, 0·21 cm–2 s–1 in air; 2·38 × 10–5 cm2 s–1 in water) and from the development of substantial unstirred boundary layers around tissues, especially in still water (Armstrong, 1979).
(2) Severe shading. This occurs both in clear water and, more especially, in turbid water.
(3) Mechanical damage. Foliage can suffer physically from strong flow rates or from abrasion by suspended particles (Palada and Vergara, 1972).
(4) Solute‐carrying capacity. The extent to which water dissolves particular solutes can have marked effects on submerged plants. This is especially true for the gases O2 (where solubility is poor), CO2 and possibly the plant hormone ethylene. In the case of CO2, a combination of small concentrations and slow diffusion rates make it impossible for plants to take up sufficient amounts from static water to sustain submerged plant growth (see below). Flowing water may also bring inorganic nutrients or pollutants into contact with flooded plants (Marin et al., 1992).
Some of these points are expanded upon below.
Dissolved oxygen
Even when water is fully equilibrated with air, the inward diffusion gradient that drives O2 entry into respiring cells is much reduced. This arises because of the modest solubility of O2 in water which decreases the concentration outside the plant from approx. 266 × 10–6 g cm–3 (8·31 mol m–3) in air to only 8·26 × 10–6 g cm–3 (0·25 mol m–3) in fully aerated water at 25 °C, a 33‐fold decrease (based on tabular data in Armstrong, 1979). However, this is still enough to support normal respiration rates of small or thin organs, such as fine roots, if the water is flowing fast enough to narrow the boundary layer of unstirred water. In this situation, O2 respiratory demand is satisfied by mass flow of dissolved O2 to the tissue surface rather than by diffusion. This may not happen often in the field but is commonly achieved in laboratory‐based nutrient solution cultures or commercial nutrient flow systems such as nutrient film technique (Jackson et al., 1984). By contrast, in the standing water of flooded fields, respiration by the plant will quickly deplete the small reserve of dissolved O2. The resulting concentrations are too small to sustain mitochondrial electron transport by means of inward diffusion through highly resistant water. Roots are especially vulnerable to this effect since they lack the capacity to generate photosynthetic oxygen in compensation.
Dissolved carbon dioxide
The influx of CO2 required by photosynthesis is similar in magnitude to the mole influx of O2 needed for aerobic respiration. Compared with O2, access to CO2 underwater is favoured by the gas being much the more soluble in water. At 25 °C, the ratio of dissolved CO2/gas‐phase CO2 is approx. 0·76, depending on the pH (Sisler and Wood, 1988), while the ratio for oxygen is only 0·03. This high solubility results in a close similarity between concentrations of CO2 in air (approx. 0·0146 mol m–3, at 25 °C) and in water in equilibrium with air (0·0114 mol m–3). Nevertheless, photosynthesis by submerged leaves remains severely CO2 limited because of the small absolute concentrations involved compared with O2 (air‐saturated water contains 0·268 mol m–3 O2 but only 0·0114 mol m–3 CO2 at 25 °C) and slow diffusion rates through the inevitable boundary layer. For these reasons, even a flow of water in equilibrium with air cannot support photosynthesis at normal rates by mass carriage of CO2 (Setter et al., 1989), even though this same water flow could probably supply enough oxygen to sustain normal rates of respiration. Furthermore, the affinity of the Rubisco enzyme that adds CO2 to ribulose‐1,5‐P2 (Km about 0·01 mol–3) is very much less than the affinity of cytochrome oxidase for O2 (Km about 0·14 mmol m–3). In addition, stomata of submerged rice plants are closed (pers. obs.) adding a further resistance to inward CO2 flow. These effects curtail drastically the rate of photosynthetic accretion of externally derived carbon by submerged plants.
Experimental tests with aquatic species have backed up the theoretical predictions. Concentrations of dissolved CO2 need to be raised artificially to more than 0·35–0·65 mol m–3 in agitated water if aerial rates of photosynthesis are to be achieved underwater (Madsen, 1993). In rice, dissolved CO2 in stirred water must be raised to 0·9–6·3 mol m–3, using 3–20 % (v/v) in a gas phase, before growth in dry mass approaches that of air‐grown plants over 6 d (Setter et al., 1987, 1989). However, interpretation of these tests is difficult because the gas mixtures used also contained O2. Furthermore, CO2 assimilation in the light also produces O2 by photolysis. These features may help to offset any tendency to hypoxia or anoxia that submerged tissues would otherwise experience. The sparging action may also deplete the plant of entrapped gases such as ethylene. These problems of interpretation have been largely overcome by abandoning sparging and, instead, supplying inorganic carbon in the form of 0·1–5 mol m–3 KHCO3 at a neutral or slightly acidic pH (note that acidity influences the dissociation of HCO3– in favour of CO2; according to the Henderson–Hasselbalch equation, pH = pKa + log [HCO3–]/[CO2]). This method of CO2 fertilization strongly promoted growth in dry mass, and improved survival rates of intolerant cultivars after 10 d underwater (Setter et al., 1989; Ramakrishnayya et al., 1999; Krishnan and Ramakrishnayya, 1999). The extent of the effect was sufficient to sustain underwater growth at rates similar to those of submerged plants gassed with CO2 in air (Setter et al., 1989; Krishnan and Ramakrishnayya, 1999) demonstrating a strong CO2 limitation to underwater growth under most circumstances.
In addition to being a co‐substrate for photosynthesis, CO2 can have other physiological effects. These include an inhibition of leaf senescence (Jackson et al., 1987) and a slowing of leaf extension (Summers and Jackson, unpubl. res., but see Raskin and Kende, 1984). The gas phase concentrations needed for these effects are in the order of 1 % v/v CO2 (0·317 mol m–3 in the water). However, since similar concentrations have been reported to occur naturally in floodwater in the field (see below), this raises the possibility that natural accumulations of the gas may, on occasions, slow the rate of underwater leaf elongation and senescence in the field. The effects may amount to an antagonism of the action of ethylene. Such effects have been reported for other ethylene responses such as petiole epinasty, and root growth, although the mode of action is unlikely to be direct competition for ethylene receptor sites (Abeles et al., 1992).
Entrapped gases
Slow gas diffusion imposed by submergence not only retards the influx of aerial O2 and CO2, it also favours the accumulation, within the plant, of physiologically active gases produced in situ. These include the hormone ethylene (Konings and Jackson, 1979), O2, and respiratory CO2 where and when this is not used in photosynthesis (Stünzi and Kende, 1989). In rice seedlings, up to 12 p.p.m. (v/v) ethylene (0·49 mmol m–3) was extracted from the gas phase of shoots of 12‐d‐old seedlings of cultivar IR42 after submergence for up to 55 h (Jackson et al., 1987). This exceeds the minimum concentrations of ethylene needed to stimulate shoot extension in other species (e.g. approx. 0·01 p.p.m. in Callitriche platycarpa; Musgrave et al., 1972). In addition to promoting leaf elongation (see ‘Fast underwater extension’ below), the gas may also promote senescence of older leaves (Jackson et al., 1987). This can be overcome to some extent with additional CO2 and more effectively by applying the ethylene action inhibitor 1‐methylcyclopropene (1‐MCP) to the floodwater or as a pre‐treatment (Jackson and Saker, unpubl. res.).
It is also likely that the water covering, especially if static, would entrap photosynthetic O2 in leaves. The release of gas bubbles in daytime is visible evidence that the O2 generated is not all consumed by respiration and internal transport to the roots. Setter et al. (1988a) also deduced that floodwater was enriched considerably with O2 generated photosynthetically by the leaves. Entrapped photosynthetic O2 will help offset any O2 shortage, including in the roots (Setter et al., 1989), but the downside is a possible promotion of photorespiration that will depress the efficiency of underwater photosynthesis (Setter et al., 1989)
Measurement in the field
Dissolved gases.
Measurements made of gases dissolved in the submerging floodwaters in the field are highly variable (Setter et al., 1987, 1988a, 1995; Ram et al., 1996, 1999). Dissolved concentrations exhibit diurnal patterns and are affected by geographical location, flow rate, duration of flooding, temperature, acidity, total depth of the floodwater and depth within the floodwater (Setter et al., 1987). In slowly moving or stagnant floodwater in eastern India, concentrations of dissolved O2 were often smaller at depth than at the surface, especially at the end of the night, and could approach zero. Ram et al. (1999) found the largest O2 concentrations at or shortly after midday and near to the water surface. At some locations, O2 concentrations exceeded those of water in equilibrium with air (presumably the outcome of plant and algal photosynthesis). However, despite this photosynthetic activity, concentrations of dissolved CO2 invariably exceeded by 31–217 times those of water equilibrated with air, especially in the mornings and close to the water surface. An increase in O2 in association with increases in CO2 or in the absence of concomitant decreases in dissolved CO2 can only be the result of extra inputs of external CO2 into the floodwater to compensate for that consumed in photosynthesis. A likely source is microbial respiration in the soil. These augmented concentrations of CO2 can be expected to support some photosynthesis but are not sufficiently large to compensate for the suppressing effects of slow diffusion and unstirred boundary layers. Measurements in Thailand (Setter et al., 1987) also showed CO2 concentrations in floodwater in most locations to be in the order of 0·7–1·6 mol m–3 and thus somewhat above those expected from straight equilibration with air.
On occasions, there will undoubtedly be ethylene in natural floodwater, as there is in waterlogged soil (Arshad and Frankenberger, 1990) since the gas is soluble in water [water in equilibrium with 1 p.p.m. (v/v) in air contains 4·5 µmol m–3]. In the field, this would be gas generated by plants, algae and soil micro‐organisms. Setter et al. (1988b) found equilibrium partial pressures of up to 3 Pa (equivalent to 30 p.p.m. or 135 µmol m–3) in the floodwater of a Thai rice field. In contrast, in laboratory conditions where submergence had strongly damaging effects on rice plants, dissolved concentrations were <1·03 µmol m–3 and too small to have affected the plants significantly (Jackson et al., 1987). Thus, environmental ethylene is not an essential component of the injurious impact of submergence on rice, although when present in appreciable amounts it can be expected to affect the plant.
Shading.
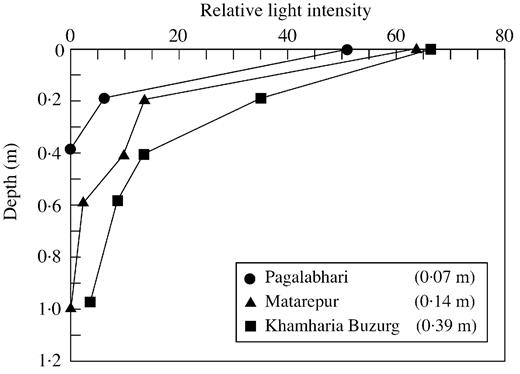
In addition to its effects on gas exchange, water shades submerged plants logarithmically with increasing depth. The attenuation can be described by the relationship Iz = Ioe–kz (Madsen, 1993). For light with an initial intensity Io, this relationship gives the light intensity, Iz, that remains after it has passed through z metres of water of attenuation constant k. The attenuation constant can vary from below 0·3 in clear water to 0·9 in turbid or algae‐rich water. This shading effect lowers the light intensity or extinguishes it. Not surprisingly, increasing the depth of water and thus the amount of shading has been shown to promote submergence injury in rice (Palada and Vergara, 1972; Adkins et al., 1990) and other species (e.g. Rumex palustris; Nabben et al., 1999). Figure 2 shows some light intensity profiles in floodwater from various field sites in India with differing turbidities. Low light may be injurious (Yamada, 1959; Adkins et al., 1990) because the resulting slower photosynthesis reduces production of respirable assimilates and depresses O2 production (Waters et al., 1989).
FAST UNDERWATER EXTENSION
Evidence for the view that fast leaf extension rates may increase leaf damage and prejudice survival is now examined, together with the related hypothesis that the difference in tolerance between cultivars is, in some measure, an outcome of inherent differences in the vigour of underwater leaf extension. The underpinning theory is that the metabolic costs of underwater elongation shorten underwater survival times by competing with cell maintenance processes for limited energy and hydrocarbon skeletons (Jackson et al., 1987; Greenway and Setter, 1996; Sarkar et al., 1996; Setter et al., 1997). The corollary of this is that cultivars with unusually slow rates of underwater elongation should be more resistant to total and sustained submergence.
Experimental evidence linking extension to injury
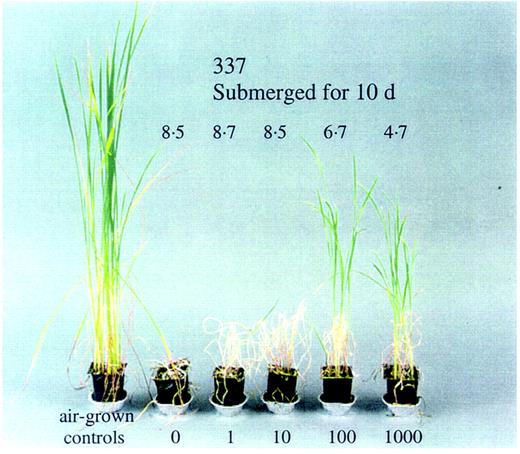
In early field tests, Yamada (1959) found no positive connection between the amount by which plants increased their height underwater and loss of resistance to 12 d of submergence. However, more recent findings have indicated that a strong link is often present, especially when tolerance is inherited from cultivar FR13A. For example, Jackson et al. (1987) found that two 10‐d‐old submergence‐tolerant types (FR13A and Kurkaruppan) extended the most rapidly growing leaf more slowly than did intolerant IR42 when inundated. In tests using large outdoor ponds, Singh et al. (2001) also found a strong negative correlation between elongation and survival amongst four different cultivars. A computer survey of the agronomic characteristics of 903 entries in the IRRI Genebank database by Setter and Laureles (1996) indicated that fast underwater extension was always linked with poor tolerance, although slow elongation was not always linked with high tolerance. Setter and Laureles (1996) also performed three types of experiment to probe the association between underwater elongation and submergence tolerance. They assessed elongation and submergence tolerance in five cultivars of contrasting tolerance, manipulated elongation underwater in two cultivars using a growth‐promoting hormone treatment (gibberellin, GA) or the GA‐biosynthesis inhibitor paclobutrazol, and examined the submergence tolerance of the naturally short‐statured and GA‐deficient mutant Tanginbozou. The results from each approach indicated that more underwater elongation equates with more injury and less tolerance. Support for this conclusion has also been obtained using plants pre‐treated with the growth‐retarding hormone abscisic acid (ABA) (Summers and Jackson, unpubl. res.; Fig. 3) which show some promotion of survival being linked to a slowing of leaf extension. However, there are some problems with the quantitative detail of some aspects of these studies. For example, the link between slow elongation and greater survival was not sustained in studies with the gibberellin‐deficient cultivar Tanginbozou. This survived submergence well, but despite its dwarf non‐submerged phenotype, elongated more strongly in response to submergence than did intolerant IR42. Similarly, paclobutrazol increased survival by almost 100 % but slowed shoot elongation by only 36–51 %. This indicates that other characteristics of the plants can interfere with a simple arithmetic relationship between extension and tolerance level.
The experiments discussed so far suffer from comparing rather few lines that have very different genetic backgrounds. A preferable approach is to screen larger numbers of lines that are more closely linked genetically. Lines of a homozygous mapping population fit the requirement well. Not only will their use allow a more statistically certain correlation to be established between tolerance and underwater elongation, but it will also help locate one or more loci on a linkage map that are responsible for conferring the traits (Jones et al., 1997). Such a study has now been carried out using several hundred lines from various crosses and mapping populations (Toojinda et al., 2003). The work demonstrates a clear link between elongation by submerged plants and various symptoms of injury or rates of survival. Introgressing the tolerance trait from FR13A or its progeny into Thai fragrant rice also introduced a suppressed elongation response to submergence (Siangliw et al., 2003). Overall, the balance of evidence points to greater underwater leaf extension being associated with poorer rates of survival. The likely explanation is that the extra growth exhausts limited substrate supplies or redirects them away from maintenance processes needed to prolong cell survival. An indication of the likely energy saving developments inherent in FR13A is the dampening of adenylate kinase activity seen after more than 48 h underwater (Kawai et al., 1998)
Mechanism of submergence‐promoted leaf extension
Promotion of shoot elongation by submergence is known to occur in wetland and amphibious species over a wide taxonomic range (reviewed in Jackson, 1990). It constitutes an escape mechanism from asphyxiation by submergence that appears to be futile and debilitating in small rice plants where the water is too deep for prompt renewal of contact with air. In rice, the coleoptiles, leaves and stems all respond to submergence by elongating more quickly (reviewed in Jackson and Pearce, 1991). There are differences between species and plant organs in terms of interaction with CO2 enrichment or O2 depletion. However, common to almost all species that elongate faster underwater is the adoption of entrapped ethylene as the signal. A notable exception is Potamogeton pectinatus, an aquatic monocot that makes no ethylene (Summers and Jackson, 1998). Across the various plant taxa, promotion of elongation growth by ethylene is unusual since in the majority of species tested (mostly land plants), the gas slows elongation rather than promotes it. In contrast, for amphibious and aquatic species, entrapped ethylene acts as a fast and effective instigator of the elongation‐based escape from submergence.
The growth response of rice leaves to submergence and ethylene has not received intense physiological study beyond demonstrations that submerged plants contain physiologically active amounts of ethylene and that ethylene treatment mimics submergence by promoting leaf elongation (discussed above). But it has recently been shown that the non‐toxic and highly specific ethylene action inhibitor 1‐MCP can suppress strongly the growth‐promoting action of submergence (Jackson and Saker, unpubl. res.), thus supporting the view that underwater leaf extension is indeed ethylene‐driven. As with dicots such as Callitriche platycarpa (Musgrave et al., 1972), gibberellins are probably required for ethylene action in rice leaves since the gibberellin biosynthesis inhibitor paclobutrazol slows underwater elongation considerably (Setter and Laureles, 1996). A submergence‐induced increase in GA (Yamaguchi, 1974; Kende et al., 1998) and an associated decline in the hormone abscisic acid (Hoffmann‐Benning and Kende, 1992; Van der Straeten et al., 2001) may also play a role in promoting underwater extension. This appears to be an outcome of releasing ethylene/GA‐promoted elongation from the inhibition ABA may otherwise impose. Work with rice and Rumex palustris has shown that the decline in ABA is ethylene‐mediated (Hoffmann‐Benning and Kende, 1992; Benschop et al., 2001). Extensive research on rice stems and the petiole of the dicot R. palustris gives further clues to the underpinning cell biology. It is not appropriate here to review these findings comprehensively. In summary, there is evidence that changes in cell wall properties underpin the faster expansion. In addition, enhanced expression of GA‐inducible genes and genes coding for cell wall softening enzymes, such as expansins, and putative ethylene receptor proteins have been described in the literature (reviewed in Kende et al., 1998; Voesenek et al., 2003). Of particular interest is the extensive mobilization and transport of respirable reserves needed to sustain faster growth in ethylene‐treated rice stems. This is based largely on the enhanced synthesis of an α‐amylase isozyme, transport of sucrose to sites of faster growth, and an underlying availability of starch reserves (Smith et al., 1987). This gives an indication of the large metabolic demands generated by enhanced elongation, thus pointing to the likely reason for its prejudicial effects when complete submergence is prolonged. The importance of photosynthate in influencing the severity of submergence injury is considered in the section ‘Carbohydrate reserves’.
The question of whether or not ethylene biosynthesis is promoted by submergence is uncertain. On the one hand, expression of a gene (OS‐ACS5) coding for 1‐aminocyclopropane‐1‐carboxylic acid synthase (ACC synthase), a rate‐limiting step in ethylene biosynthesis, is up‐regulated by submergence within 1 h (Zhou et al., 2001), as is the capacity to convert ACC to ethylene itself (Van der Straeten et al., 2001). On the other hand, ethylene production underwater is probably slower than in air as indicated by work with Rumex palustris (Voesenek et al., 1993). Any extra ACC produced during submergence may have a role in its own right by retarding leaf senescence (Van der Straeten et al., 2001).
CARBOHYDRATE RESERVES
This section discusses the impact of respirable reserves on the extent of submergence tolerance and evidence linking inter‐cultivar differences in submergence tolerance to variation in carbohydrate levels. Yamada (1959) provided extensive data showing the rapid loss of starch and total carbohydrates during submergence in leaves, leaf sheaths and roots. Inter‐varietal comparisons showed that high starch levels prior to submergence favoured tolerance, and that treatments such as shading or nitrogen fertilization which depressed carbohydrate reserves also decreased survival. Yamada also found that a stronger ability to survive darkness was related to greater submergence tolerance. Accordingly, survival of IR42 submerged for 10 d either with natural diurnal irradiance or in complete darkness was 65 % and 0 %, respectively (Setter et al., 1997). Palada and Vergara (1972) showed that starch and more especially total sugars (anthrone assay) in the seedlings were decreased by up to 10 d of submergence, especially if the water was turbid (see also Ram et al., 2002). In general, greater injury and mortality are associated with a smaller dry weight at the end of submergence (Yamada, 1959; Palada and Vergara, 1972; Adkins et al., 1990). Thus, the amount of carbohydrate in plant parts is often positively correlated with the level of submergence tolerance (Palada and Vergara, 1972; Emes et al., 1988; Chaturvedi et al., 1995; Mallik et al., 1995). Older seedlings with higher levels of carbohydrates were more tolerant of submergence (Vergara, 1985; Chaturvedi et al., 1995) than younger plants. Convincing evidence for the involvement of carbohydrates in submergence tolerance of rice has come from experiments in which 14‐d‐old plants were submerged at 0600 h or at 1800 h for 24–48 h in the dark. Plants submerged in the morning (0600 h) before the onset of dawn showed poor survival (0–25 %) compared with those submerged in the evening (100 % survival) (Ram et al., 2002). As expected, carbohydrate (soluble sugars and starch) concentrations of shoots were higher in plants submerged in the evening as compared with those submerged in the morning (Ram et al., 2002). There is no experimental evidence that tolerant types undergo more vigorous underwater photosynthesis than intolerant types (Mazaredo and Vergara, 1982).
It is concluded that high amounts of total water‐soluble carbohydrate and starch prior to submergence and slower rates of their depletion during submergence are adaptive traits that prolong survival underwater (Mallik et al. 1995; Chaturvedi et al. 1996), the carbohydrate presumably being utilized in maintenance processes. Carbohydrate status is susceptible to genotype, plant age, nature of submergence (partial or complete), and floodwater environment (turbidity, O2 and CO2 concentrations, temperature and pH) (Setter et al., 1989; Ram et al., 2002). Carbohydrate remaining after submergence will presumably be especially important in recovery growth after desubmergence (Chaturvedi et al., 1996; Singh et al., 2001; Ram et al., 2002).
The mechanism of starch mobilization is apparently linked to rapid starch breakdown in amyloplasts in the submerged leaves (Emes et al., 1988). Parallels with studies on partially submerged rice (Smith et al., 1987) suggest that hydrolysis by α‐amylase is the likely means of achieving amyloplast breakdown (Dunn, 1974) and is susceptible to the action of ethylene and GA, two hormones that also stimulate leaf extension.
While low carbohydrate reserves prejudice survival of submergence, it is less certain that differences in tolerance between cultivars are linked to differences in carbohydrate status. Ram et al. (2002) reported that the amount contained within the dry seed or in the shoots of 10‐d‐old seedlings prior to submergence was not especially high in submergence‐tolerant types. However, the latter tended to lose less carbohydrate when underwater, and recovered more quickly after submergence (Mazaredo and Vergara, 1982). This difference applied to FR13A and Kurkaruppan (resistant types) and IR42 (intolerant), but not to two Thavalu strains that were tolerant despite low carbohydrate levels. This suggests a faster utilization rate in intolerant types when underwater or a smaller proportional contribution to respiration pathways by current photosynthate (Emes et al., 1988). Vegetatively propagated plants may also survive better than seedlings up to 6 weeks old (Richharia and Parasuram, 1963) because of their larger photosynthate content.
NITROGEN AND PHOSPHORUS SUPPLY
Submergence strongly affects protein content, while nitrogen and phosphorus availability and assimilation can influence submergence responses and have been implicated in differences in tolerance between cultivars. Submergence rapidly depletes protein reserves through hydrolysis to amino acids and other soluble nitrogen‐containing compounds (Yamada, 1959). Palada and Vergara (1972) found the increase in the percentage nitrogen content that normally occurs between 10 and 20 d after germination (from 3·1 to 4·3 %) to be almost abolished by submergence or even reversed if the water is turbid. However, attempts to raise nitrogen levels by feeding ammonium sulfate were not beneficial but instead were prejudicial to survival (79 % vs. 15 % survival), an effect associated with a 61 and 34 % decrease, respectively, in initial pre‐submergence starch and total sugar concentrations (see also Yamada, 1959). Furthermore, compared with intolerant lines, submergence‐susceptible cultivars were not noticeably richer in nitrogen after damaging lengths of submergence (Mazaredo and Vergara, 1982). Yet, when plants were analysed before submergence, the shoots of tolerant lines such as FR13A were found to be richer in nitrate than those of susceptible types: the difference was large, with the most tolerant lines containing over 70 µg per plant shoot whereas the most susceptible line contained less than 20 µg per plant. Similarly, Chaturvedi et al. (1995) observed a higher total nitrogen content in leaf sheaths, culms and leaves of submergence‐tolerant than susceptible rice genotypes. Thus, leaf sheath nitrogen could have a major accumulative and supportive role during submergence, perhaps by proteins acting as respirable reserves. However, lack of information does not allow firm conclusions to be drawn.
As expected, addition of phosphorus to soil prior to seed sowing can stimulate overall growth in height, dry mass and carbohydrate content. Surprisingly, cultivars normally intolerant of submergence then elongate more slowly underwater and recover more strongly after desubmergence (Singh and Ram, unpubl. res.). However, applying phosphate to plants at the time of submergence does not improve tolerance. On the contrary, Ramakrishnayya et al. (1999) reported that it reduced rice plant survival by 35 %. The adverse effects of a high phosphorus concentration in floodwater were mainly attributed to a promotion of algal growth and the resulting competition between the algae and submerged plants for CO2 and light. Overall, it seems likely that adequate N and P supply prior to submergence reduces injury, but that fertilizing the floodwater may increase injury. The possibility that tolerant cultivars are naturally rich in nitrogen warrants closer examination.
INVOLVEMENT OF TISSUE ANOXIA IN SUBMERGENCE INJURY
Study of the damaging effects of excess water on plants has generally been dominated by concerns over oxygen supply (e.g. Vartapetian and Jackson, 1997; Sauter, 2000). As a consequence, the terms flooding, submergence and anaerobiosis have become almost synonymous. On this basis it is to be expected that a lack of oxygen (environmental or photosynthetic) is at the centre of the injury inflicted by sustained submergence, and that the greater tolerance shown by cultivars such as FR13A is largely a question of greater tolerance of anoxia. This assumption was considered in depth by Greenway and Setter (1996) and is now re‐examined in the light of additional work.
There is no doubt that submerged plants can experience anaerobiosis. This is clear from measurements of very low concentrations of O2 in the field (see above), and direct tests show that the plants will suffer severely as a result (Ellis and Setter, 1999). However, it is surprising how little O2 is needed to avoid fatality and severe foliar damage. Even in continuous darkness, 1·5 mmol m–3 O2 dissolved in the water is enough to secure survival for several days (Ellis and Setter, 1999) (water in equilibrium with air contains approx. 0·25 mol m–3 at 25 °C). Furthermore, floodwater does not need to be anaerobic for submergence to damage rice severely, nor to distinguish between tolerant and intolerant cultivars (Boamfa et al., 2003; Mohanty and Ong, 2003). Under these less harsh conditions, tissue anoxia is only likely to develop at night or when water turbidity and/or overcast skies prevent photosynthetic O2 generation during the day. Under such circumstances, roots will be especially vulnerable to O2 shortage since the soil will be anaerobic, rendering them entirely dependent upon internal O2 transport from the shoot, day and night. Thus, one possibility is that visible injury to the shoots inflicted by submergence (Fig. 1) could be an indirect result of anoxic damage to the roots. [This is the situation for shoots of conventional land plants (e.g. Pisum sativum) which are quickly and severely damaged indirectly by waterlogging of the soil.] In support of this view, submergence in water, even when rich in dissolved O2 and CO2, is known to lower O2 in roots sufficiently at night to promote fermentation and slow their elongation (Waters et al., 1989). It can also affect hydraulic properties and ion transport ability (Singh et al., 2001). But it has been found that roots recover quickly each morning and promptly recommence normal elongation (Waters et al., 1989). It is also known from excision and illumination studies that the state of the shoot determines the extent of root injury rather than the reverse (Singh et al., 2001). Thus, although a lack of internal O2 resulting from submergence can occur routinely in root tips at night even when floodwater around the shoots contains O2, this deprivation is neither sufficiently severe nor prolonged to be highly damaging. These results point away from anoxically damaged roots as the prime cause of submergence injury to the shoots, and thus away from O2 shortage as a prime cause of injury. This view has been supported experimentally by photoacoustic laser analyses of ethanol and acetaldehyde outputs from whole rice plants submerged in water that was initially in equilibrium with air (Boamfa et al., 2003). These conditions were demonstrably damaging to rice seedlings under a 12‐h day/night regime, and discriminated between tolerant FR13A and susceptible CT6241. However, they have been found to increase rates of ethanol and acetaldehyde evolution in the shoot by very little, even after extending the dark period to 16 h. This finding indicates that up‐regulation of fermentation (a marker for tissue anoxia) is not an obligatory component of submergence injury nor of the mechanism underlying the tolerance of FR13A (Boamfa et al., 2003).
Gene expression and biochemical studies also point away from anaerobic effects dominating the submergence response. There are reports of increases in the accumulation of mRNA coding for alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC) enzymes in plants submerged in unstirred water and given a 12‐h day/night cycle (Umeda and Uchimiya, 1994). ADH and PDC have been termed anaerobic proteins and were once thought to be good markers for tissue anoxia; it is now known that this is not the case. Hypoxia rather than anoxia (Saglio et al., 1999; Chang et al., 2000) induces genes coding for these enzymes. Paul and Furl (1991) showed that a small amount of oxygen (e.g. 3 % v/v or 1·19 mol m–3) is actually required for maximal accumulation of mRNA for ADH genes. Increases in ADH and PDC mRNA reported by Umeda and Uchimiya (1994) were probably associated with the onset of night‐time and the arrest of photosynthetic O2 formation. Although whole seedlings were sampled, these increases in ADH and PDC messages may well have been confined to the root tips suggesting highly localized occurrence of hypoxia rather than anoxia. In support of this, Mohanty and Ong (2002) found submergence in O2‐rich water increased the activity of PDC in root tips, but the effect was restricted to the 12‐h‐long night, and illumination promptly reversed the effect, as already shown for root extension by Waters et al. (1989).
A range of other observations also downplays the essential involvement of oxygen shortage in submergence injury. For example, Ramakrishnayya et al. (1999) enriched floodwater with O2 indirectly using inorganic phosphorous to boost the growth of O2‐generating algae. Despite raising dissolved O2 concentrations to twice those of air‐saturated water, plants were more rather than less damaged by this treatment. Similarly, Krishnan and Ramakrishnayya (1999) used 3 p.p.m. copper sulfate to suppress algal growth and thus indirectly to depress O2 concentrations. This did not increase submergence damage. On the contrary, small improvements to survival of submergence‐intolerant IR42 were recorded. Submergence usually promotes leaf yellowing, but this requires oxygen to support an Fe‐containing oxygenase that cleaves the porphyrin moiety of chlorophyll (Rodoni et al., 1996). Thus, oxygen must be available to this tissue for at least some of the time to permit the yellowing commonly seen in submerged rice. If steps are taken to remove all oxygen experimentally, plants can die whilst green (Yamada, 1959); this rarely happens to submerged plants.
There is no doubt that on occasions, submerged rice plants can experience anaerobiosis. When this happens, submergence‐tolerant types are also remarkably tolerant of anaerobiosis provided that they have first experienced some hours of partial O2 shortage as whole plants (Ellis and Setter, 1999). Such hypoxic pre‐treatment (water in equilibrium with approx. 0·023 mol m–3 O2) for several hours raised the activity of ADH, and especially PDC, in the leaves (Ellis and Setter, 1999). This finding is in accord with earlier work indicating that transcription of genes that code for steps in fermentation, glycolysis and translation into protein is promoted by a low but not extinguished O2 supply (Drew et al., 1994). Without this hypoxic training, even the submergence‐tolerant variety FR13A dies within 1 d when subjected to oxygen‐free conditions, and is no more resistant to anoxia than submergence‐intolerant types such as IR42 (Ellis and Setter, 1999). Quimio et al. (2000) tested this link between submergence tolerance and faster ethanolic fermentation by over‐expressing a rice PDC gene (PDC1) in the lowland variety Taipei 309. The transformation increased PDC activity and ethanol production rates in leaves given 24 h of sudden anoxia. This production rate was linked positively to the ability of progeny of these lines to survive 14 d total submergence. However, the floodwater used was rich in O2 (0·024–0·173 mol m–3) and thus close to ambient for much of the submergence test (Quimio et al., 2000). Clearly, there was little likelihood of severe anaerobiosis developing in the shoots, and the submergence treatment was not a test of anoxia tolerance. A more thorough examination of these interesting lines is needed before firm conclusions can be drawn.
On balance, the available evidence suggests that anaerobiosis is neither the principal cause of submergence injury nor the basis of submergence tolerance of cultivars such as FR13A. However, tolerance of anaerobiosis will be important in circumstances when O2 is completely absent from floodwater since 24 h of whole‐plant anoxia can be fatal.
POST‐SUBMERGENCE INJURY
It is widely believed that when plants suffering from anoxia are returned to air they can be damaged by oxidative stress initiated by the re‐entry of atmospheric O2 (Wollenweber‐Ratzer and Crawford, 1994). This possibility arises because of the related notion that an absence of oxygen can prejudice the ability of cells to protect themselves against uncontrolled oxidations when re‐exposed to O2. The mechanisms behind post‐anoxic formation of active oxygen species (e.g. superoxide radicals) and possible loss of protection against their effects are complex. Formation of active oxygen species is linked to a build‐up of chemical reducing power, cytoplasmic acidosis, low energy charge and a saturated electron transport chain. This swing to highly reducing conditions favours the transfer of electrons, one at a time, to O2 molecules re‐entering previously anoxic cells. This generates superoxide radicals (O2–) (Hendry and Crawford, 1994). The basic assumption is that the extent to which the post‐anoxic cells can detoxify by ‘scavenging’ free radicals using enzymic and chemical means will determine the level of injury. This injury is a result of auto‐oxidative chain reactions promoted by free radicals which damage lipid membranes through peroxidation (Monk et al., 1989). There are numerous scavenging enzymes and anti‐oxidants present in plant cells. These include the superoxide dismutase (SOD) enzyme complex (generates H2O2 from O2–), catalase (converts H2O2 to H2O and O2 in microbodies), ascorbate peroxidase (concomitantly converts H2O2 to H2O, and ascorbate to monodehydroascorbate which can dismutate back to ascorbate and dehydroascorbate), dehydroascorbate reductase (regenerates ascorbate from dehydroascorbate by reacting it with ‘reduced glutathione’), and monodehydroascorbate reductase and glutathione reductase enzymes that recycle ascorbate or use NADPH to chemically reduce ‘oxidized glutathione’ back to ‘reduced glutathione’ (Asada and Takahashi, 1987). Hydrogen peroxide can also be generated by β‐oxidation in peroxisomes and by photorespiration. In addition to ascorbate and glutathione just mentioned, other non‐enzymic substances such as α‐tocopherol and carotenoids can also act as antioxidants. Clearly, plants normally have an abundance of systems for free‐radical scavenging enzymes. The question arises as to whether they are adequate to deal with the post‐submergence influx of O2. The question also presumes that submergence necessarily generates anoxic interiors in the plants. As discussed above, this is by no means an essential component of submergence injury.
Direct experimental evidence implicating active oxygen species in post‐submergence injury is still incomplete and contradictory. Unlike roots of soybean, those of rice were shown by Van Toai and Bolles (1991) not to suffer from oxidative stress after being returned to air after 6 h without oxygen. This was possibly because concentrations of ascorbate, and superoxide dismutase activity and transcript accumulation were higher in rice than in soybean. However, the possibility cannot be excluded that the shoots of submerged plants are damaged by oxidative stress. Support for the notion has been provided by assays of ethane evolution. Ethane, a product of lipid peroxidation, was found to be produced in greater amounts by intolerant rather than tolerant lines, and from plants submerged in the mornings rather than the evenings; the latter being much the less damaged (Santosa et al., 2001). Similarly, submergence of rice plants has been reported to increase the generation of free radicals as detected in leaves by electron paramagnetic resonance (Thongbai and Goodman, 2000). Furthermore, supplying plants with ascorbate 24 h before desubmerging to scavenge these free radicals improved plant survival rates, especially in a submergence‐sensitive cultivar (Thongbai and Goodman, 2000). This raises the clear possibility that in addition to suffering stress from submergence per se, further damage is inflicted by the re‐entry of oxygen on return to air. Ushimaru et al. (1999) showed that submergence for 6 d in the dark strongly depressed the six main free radical‐scavenging enzymes in the shoots, and even these low levels were dependent on new protein synthesis. The decreased SOD activity was entirely plastid SOD rather than mitochondrial or cytoplasmic SOD. This indicates that submergence can damage the enzymic basis for disposing of free radicals. However, contrary results reported by Singh et al. (2001) indicate that submergence for 4–7 d increased SOD, catalase and peroxidase activity, and that 24 h after desubmergence, SOD activity in tolerant lines was twice that of susceptible plants. More detailed time course studies of free radicals, H2O2, antioxidative enzymes and scavenger levels are clearly required for plants submerged under normal daily light/dark regimes and known external O2 concentrations.
The possibility that oxidative damage may take place even during submergence has not been explored experimentally. This is entirely possible if parts of the plants develop anoxic tissues at night but become oxygenated by day from photosynthetically derived O2. Overall, it seems possible that post‐anoxic stress contributes to the damage that follows desubmergence and may be a part of the physiological package that differentiates tolerant and intolerant cultivars. Further work on this subject appears justified.
GENETICS OF SUBMERGENCE TOLERANCE
Segregation analysis of progeny from crosses between tolerant and intolerant types (Suprihatno and Coffman, 1981) gave an early indication that submergence tolerance is controlled by a small number of genes that are partially or completely dominant. Subsequently, Mackill et al. (1993) introduced the tolerance trait into an agronomically useful cultivar by conventional plant breeding, an achievement made possible by the existence of a single, dominant locus (Mishra et al., 1996). The strong effect of one locus was subsequently confirmed in a QTL mapping exercise (Xu and Mackill, 1996) that located a region on chromosome 9 (designated SUB1) that accounted for 69 % of the variation in tolerance. The work utilized an F3 generation derived from a cross between an intolerant japonica rice and an indica rice receiving its tolerance genes from FR13A. This finding has since been confirmed and extended by the same laboratory (Xu et al., 2000) and by two other groups using different DNA markers, restriction enzymes, probes and mapping populations, but retaining the FR13A parentage (Nandi et al., 1997; Toojinda et al., 2003). The most recent maps are highly refined (Vanavichit et al., 1996; Xu et al., 2000). They have located molecular markers (i.e. short lengths of genomic DNA of known location) very close to the SUB1 locus. The best markers have proved inseparable from the tolerance trait (Xu et al., 2000) during recombination processes that potentially can split such associations during crossing‐over of chromatids at the diplotene stage of meiosis. In addition, minor QTL affecting tolerance have been mapped to chromosomes 1, 2, 5, 7, 10 and 11 (Siangliw et al., 2003).
Creating these chromosome linkage maps and locating the submergence tolerance trait on the map have several important implications. First, the markers that map close to SUB1 should be useful in breeding programmes to select submergence‐tolerant offspring and develop new submergence‐tolerant lines without the need for as much time‐consuming and costly outdoor screening (Mackill et al., 1999). New submergence‐tolerant cultivars such as a fragrant, high‐value culinary rice derived from KDML 105 (Siangliw et al., 2003) carry these markers, thus heightening the promise of using marker‐aided selection in future breeding programmes. Secondly, markers for SUB1 located with fine mapping are now sufficiently close to the locus for physical mapping (map‐based positional cloning) of the locus to be carried out. Chromosome walking using large insert libraries based on bacterial artificial chromosomes (BACs) and yeast artificial chromosomes (YACs) is being used (Kamolsukyunyong et al., 2001). Ultimately, this approach may enable the base sequences of the locus to be determined and may allow putative functions of the genes in the region to be assigned on the basis of sequence homology and on complementation studies using transformed plants. Thirdly, fine mapping opens up the possibility of checking whether physiological traits thought to be involved in explaining the tolerance trait also map closely to this same locus. Traits that map to SUB1 are likely to be inextricably involved in the tolerance mechanism. Two traits that have already been mapped to SUB1 are a resistance to visible leaf senescence (yellowing) and slow underwater elongation (Siangliw et al., 2003). The reduction in senescence would conceivably help maintain photosynthesis (mostly utilizing respiratory CO2 when underwater) and thus O2 and sugar production. The slower leaf extension rate can be expected to conserve energy and assimilates.
Both leaf senescence and underwater leaf elongation have a common basis in that they are linked to ethylene action. This opens up the possibility that SUB1 incorporates one or more genes coding for signal transduction steps for this hormone or for gibberellin (GA) with which it interacts. GA is probably essential for ethylene action in leaf elongation (Setter and Laureles, 1996), its tissue concentrations being maintained at near‐normal levels during the first few days of submergence (Van der Straeten et al., 2001). Ethylene‐promoted senescence may also involve antagonizing the action of GAs which are known to retard senescence in some plants (Fletcher and Osborne, 1966). Alternatively, the link may be less direct and may involve one or more proteins that interact with signal transduction pathways of both hormones. These complexities are beyond the scope of this review but may be glimpsed by reference to the interactions of groups of mitogen‐activated protein (MAP) kinases operating in signal transduction pathways in yeast and human cells (Widemann et al., 1999). It can be concluded that physical mapping and the associated trait correlation are a promising basis for identifying the functional genes residing in the SUB1 locus. Genes coding for alcohol dehydrogenase do not map to the SUB1 locus (Nandi et al., 1997), lending further support to the notion that tolerance inherited from FR13A is unlikely to be mediated through regulation of fermentation.
A parallel strategy for identifying genes responsible for conferring submergence tolerance may be through identification of genes that are up‐ or down‐regulated by submergence and comparing the patterns in tolerant and intolerant lines that have a similar genetic background. The recently developed KDML 105 lines with or without an introgressed locus for submergence tolerance (Siangliw et al., 2003) hold particular promise for such work. Such lines could be examined with probes for mRNA using micro‐array hybridization techniques (Dolferus et al., 2003). The use of such panoramic gene expression methodology will be accelerated by the release of sequence data from the japanese‐led International Rice Genome Sequencing Project, and draft sequences published recently by privately and publicly financed laboratories (Goff et al., 2002; Yu et al., 2002).
CONCLUSIONS AND OUTLOOK
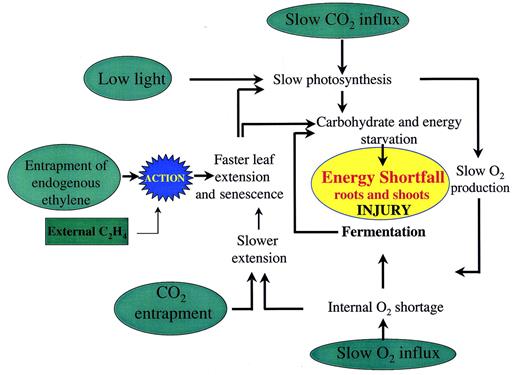
Small rice plants are severely injured when totally submerged for several days. The physiology of the response has been extensively studied since the pioneering work of Yamada (1959). The damage may have several causes linked to the interference by water in normal gas exchange and illumination. Reactions to impeded gas exchange include faltering of photosynthesis, an acceleration of leaf elongation, and faster leaf senescence. These effects may prejudice survival by creating a shortfall in the energy needs for maintaining cell integrity in key tissues such as root tips and meristematic regions of leaf bases and stem. An integration of these and other influences on submergence injury is shown diagrammatically in Fig. 4.
The mechanism by which the debilitating effects of submergence are suppressed in tolerant cultivars such as FR13A is becoming clearer. Large differences in the rates of anaerobic metabolism do not seem to offer the explanation, although it is abundantly clear that the level of available substrates for respiration strongly influences resilience. It is proposed that tolerance is favoured by a minimizing of the imbalance between energy generation and usage. This is favoured by a suppression of the invigorated rates of leaf elongation that submergence usually brings about. In addition, a lessening of leaf senescence during submergence has been implicated. Both underwater leaf elongation and senescence can be promoted by ethylene, a hormone that is trapped within submerged tissues because its diffusive escape is strongly inhibited by water. In tolerant lines, these ethylene‐like effects are suppressed.
Linkage mapping by several laboratories has identified several chromosomal loci that influence submergence tolerance. Prominent amongst these is the SUB1 locus on chromosome 9. One or more genes in this short region have effects on injury, survival, senescence and leaf elongation. The effect is dominant (i.e. effective in the heterozygote carrying only one FR13A‐derived segment; Siangliw et al., 2003) and may affect ethylene responsiveness.
Harnessing molecular biology to ameliorate the effects of environmental stress is a major challenge for biotechnologists (Grover et al., 1999; Rathinasabapathi, 2000), and one that is being met with respect to submergence tolerance. The identification of tightly linked DNA markers holds particular promise for transferring the submergence‐tolerance trait from FR13A or related lines into commercially important cultivars. Such markers are already known to be carried over when tolerance is introduced into an agronomically desirable background by conventional crossing and progeny phenotyping (Siangliw et al., 2003). The potential for identifying other mechanisms of tolerance derived from resilient cultivars unrelated to FR13A (e.g. Goda Heenati and Vaidehi) remains unexplored and represent a promising additional resource for plant breeders.
ACKNOWLEDGEMENTS
We thank the European Commission for support through its INCO‐DC programme, International Co‐operation with Developing Countries (project IC18‐CT96‐00078: ‘Rice for Life’).
Fig. 1. Effect of complete submergence for 10 d on the appearance of initially 3‐week‐old rice plants. Different rice lines are in double rows running from top to bottom of the picture. Rice lines on the right are intolerant to submergence and show evidence of leaf degeneration and collapse. The left‐hand rice line (cultivar FR13A) is relatively undamaged by 10 d of submergence. Picture taken 1 d after desub mergence by Dr Panatda Bhekasut (Prachinburi Rice Research Center, Department of Agriculture, Thailand).
Fig. 2. Influence of depth in the water on the percentage loss of incident light intensity at three field sites in eastern India. The inset values show the depth in metres at which light intensity would have been halved (taken from Ram et al., 1999).
Fig. 3. Effect of a 1‐d pre‐treatment with the growth‐inhibiting hormone abscisic acid (0, 10, 100 and 1000 mmol m–3) applied through the roots of the submergence‐susceptible doubled haploid line 337. The number above each plant is a visual submergence tolerance score on a scale where 9 is dead and 1 is undamaged. Plants were submerged for 10 d at the three‐leaf stage at the time submergence began and photographed 7 d after desubmergence. Photograph by Dr J. E. Summers.
Fig. 4. Diagrammatic summary of several environmental components (in green) that may affect rice plants during complete submergence, and their likely physiological impact. The central assumption is that submerged plants are damaged by a shortage of energy. The relatively high tolerance shown by cultivars such as FR13A can be linked to: (1) a suppression of energy‐demanding processes such as underwater leaf extension, leaving a larger surplus for cell maintenance; and (2) retention of older leaves in a non‐senescent state. Note that: leaf elongation is promoted by ethylene but decreased by slow O2 supply or accumulations of CO2; leaf senescence is promoted by ethylene; evidence that submerged plants necessarily suffer severely from O2 shortage is limited; and O2 from daytime photosynthesis underwater or from the air at the time of desubmergence may cause oxidative damage.
References
AbelesFB, Morgan PW, Saltveit ME Jr.
AdkinsSW, Shiraishi T, McComb JA.
ArshadM, Frankenberger WT.
AsadaK, Takahashi M.
BenschopJJ, Jackson MB, Peeters AJM, Voesenek LACJ.
BoamfaEI, Ram PC, Jackson MB, Reuss J, Harren FJM.
CatlingHD, Puckridge DW, Hille Ris Lambers D.
ChangWPP, Huang L, Shen M, Webster C, Burlingame AL, Roberts JKM.
ChaturvediGS, Ram PC, Singh AK, Ram P, Ingram KT, Singh BB, Singh RK, Singh VP.
ChaturvediGS, Mishra CH, Singh ON, Pandey CB, Yadav VP, Singh AK, Dwivedi JL, Singh BB, Singh RK.
DolferusR, Klok EJ, Delessert C, Wilson S, Ismond KP, Good AG, Peacock WJ, Dennis ES.
DrewMC, Cobb BG, Johnson JR, Andrews D, Morgan PW, Jordan W, He CJ.
EllisMH, Setter TL.
EmesMJ, Wilkins CP, Smith PA, Kupkanchanakul K, Hawker K, Charlton WA, Cutter EG.
FletcherRA, Osborne DJ.
GoffSA, Ricke D, Lan T‐H, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma Het al.
GreenwayH, Setter TL.
GroverA, Sahi C, Sanan N, Grover A.
HendryGAF, Crawford RMM.
Hille Ris LambersD, Gomosta AR, Kupkanchanakul T.
Hoffmann‐BenningS, Kende H.
ItoO, Ella E, Kawano N.
JacksonMB.
JacksonMB, Pearce DME.
JacksonMB, Blackwell PS, Chrimes JR, Sims TV.
JacksonMB, Waters I, Setter T, Greenway H.
JonesN, Ougham H, Thomas T.
KamolsukyunyongW, Ruanjaichon V, Siangliw M, Kawasaki S, Sasaki T, Vanavachit A, Tragoonrung S.
KawaiM, Umeda M, Uchimiya H.
KeithKA, Raskin I, Kende H.
KendeH, van der Knaap E, Cho HT.
KoningsH, Jackson MB.
KhushGS.
KrishnanP, Ramakrishnayya G.
MackillDJ.
MackillDJ, Amante MM, Vergara BS, Sarkarung S.
MackillDJ, Nguyen HT, Zhang JX.
MadsenTV.
MallikS, Kundu C, Banerji C, Nayak DK, Chatterji SD, Nanda PK, Ingram KT, Setter TL.
MarinAR, Masscheleyn PH, Patrick WH.
MauryaDM, Bottrall A, Farrington J.
MazaredoAM, Vergara BS
MishraSB, Senadhira D, Manigbas NL.
MohantyBL, Ong B‐L.
MonkLS, Fagerstedt KV, Crawford RMM.
MusgraveA, Jackson MB, Ling E.
NabbenRHM, Blom CWPM, Voesenek LACJ.
NandiSP, Subudhi K, Senadhira D, Manigbas NL, Sen‐Mand S, Huang N.
PaladaMC, Vergara BS.
QuimioCA, Torrizo LB, Setter TL, Ellis M, Grover A, Abrigo EM, Oliva NP, Ella ES, Carpena AL, Ito O, Peacock WJ, Dennis E, Datta SK.
RamPC, Singh BB, Singh AK, Singh VK, Singh ON, Setter TL, Singh RK, Singh VP.
RamPC, Singh K, Singh BB, Singh VK, Singh HP, Setter TL, Singh VP, Singh RK.
RamPC, Singh BB, Singh AK, Ram P, Singh PN, Singh HP, Boamfa EI, Harren FJM, Santosa E, Jackson MB et al.
RamakrishnayyaG, Setter TL, Sarkar RK, Krishnan P, Ravi I.
RathinasabapathiB.
RodoniS, Mühllecker W, Anderl M, Kräutler B, Moser D, Thomas H, Matile P, Hörtensteiner S.
SaglioP, Germain V, Ricard B.
SantosaE, Boamfa I, Ram PC, Jackson MB, Harren FJM.
SarkarRK, De RN, Reddy JN, Ramakrishnayya G.
SarkarungS, Singh ON, Roy JK, Vanavichit A, Bhekasut P.
SauterM.
SetterTL, Laureles EV.
SetterTL, Kupkanchanakul T, Waters I, Greenway H.
SetterTL, Ramakrishnayya G, Ram Maurya PC, Singh BB.
SetterTL, Waters I, Wallace I, Bhekasut P, Greenway H.
SetterTL, Kupkanchanakul T, Kupkanchanakul K, Bhekasut P, Wiengweera A, Greenway H.
SetterTL, Kupkanchanakul T, Kupkanchanakul K, Bhekasut P, Wiengweera A, Greenway H.
SetterTL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB, Sarkarung S, Datta S.
SetterTL, Ramakrishnayya G, Ram PC, Singh BB, Mallik S, Roy JK, Kundu C, Laureles EV, Sarkarung S, Nayak SK.
SiangliwM, Toojinda T, Tragoonrung S, Vanavichit A.
SinghHP, Singh BB, Ram PC.
SmithMA, Jacobsen JV, Kende H.
StünziJT, Kende H.
SummersJE, Jackson MB.
SuprihatnoB, Coffman WR.
TaylorDL.
ThongbaiP, Goodman BA.
ToojindaT, Siangliw M, Tragoonrung S, Vanavichit A.
UshimaruT, Kanematsu S, Shibasaka M, Tsuji H.
UmedaM, Uchimiya H.
VanavichitA, Siangliw M, Sarkarung S, Tragoonrung S.
Van der StraetenD, Zhou ZY, Prinsen E, Van Onckelen HA, Van Montagu MC.
Van Toai, TT, Bolles CS.
VartapetianBB. Jackson MB.
VergaraBS.
VoesenekLACJ, Banga M, Thier RH, Mudde CM, Harren FJM, Barendse GWM, Blom CWPM.
VoesenekLACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM.
WatersI, Armstrong W, Thompson CJ, Setter TL, Atkins S, Gibbs J, Greenway H.
WeerapatP, Waranimman P.
WidemannC, Gibson S, Jarpe MB, Johnson GL.
Wollenweber‐RatzerB, Crawford RMM.
XuK, Xu X, Ronald PC, Mackill DJ.
XuKN, Mackill DJ.
YamadaN.
YamaguchiT.
YuJ, Hu S, Wang J, Wong GK‐S, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X.
ZeiglerRS, Puckridge DW.
ZhangQS, Greenway H.
Author notes
1School of Biological Sciences, University of Bristol, Woodland Road, Bristol BS8 1UG, UK, 2Department of Plant Ecophysiology, Faculty of Biology, University of Utrecht, Sorbonnelaan 16, 3584 CA Utrecht, The Netherlands and 3Department of Crop Physiology, Narendra Deva University of Agriculture and Technology, Kumarganj, 224‐229, Faizabad, Uttar Pradesh, India