-
PDF
- Split View
-
Views
-
Cite
Cite
L. DING, K. J. WANG, G. M. JIANG, D. K. BISWAS, H. XU, L. F. LI, Y. H. LI, Effects of Nitrogen Deficiency on Photosynthetic Traits of Maize Hybrids Released in Different Years, Annals of Botany, Volume 96, Issue 5, October 2005, Pages 925–930, https://doi.org/10.1093/aob/mci244
Close - Share Icon Share
Abstract
• Background and Aims New maize (Zea mays) hybrids outperformed old ones even at reduced N rates. Understanding the mechanisms of the differences in performance between newer and older hybrids under N deficiency could provide avenues for breeding maize cultivars with large yield under N deficiency, and reduce environmental pollution caused by N fertilizers.
• Methods N deficiency effects on grain weight, plant weight, harvest index, leaf area and photosynthetic traits were studied in the field for six maize hybrids released during the past 50 years to compare their tolerance and to explore their physiological mechanisms.
• Key Results N deficiency decreased grain yield and plant weight in all hybrids, especially in the older hybrids. However, there was no significant difference in harvest index, rate of light-saturated photosynthesis (Psat) 20 d before flowering, leaf area or plant weight at flowering between the N-deficient and control plants of all hybrids. Dry matter production after flowering of the N-deficient plants was significantly lower than that of the control plants in all hybrids, especially in the older hybrids, and was mostly due to differences in the rate of decrease in photosynthetic capacity during this stage. The lower Psat of the older hybrids was not due to stomatal limitation, as there was no significant difference in stomatal conductance (gs) and intercellular CO2 concentration (Ci) between the hybrids. N deficiency accelerated senescence, i.e. decreased chlorophyll and soluble protein contents, after anthesis more for the earlier released hybrids than for the later ones. N deficiency decreased phosphoenolpyruvate carboxylase (PEPCase) activity significantly more in older hybrids than newer hybrids, and affected the maximal efficiency of PSII photochemistry (Fv/Fm) only in the old hybrids and at the late stage.
• Conclusions Compared with older (earlier released) hybrids, newer (later released) hybrids maintained greater plant and grain weight under N deficiency because their photosynthetic capacity decreased more slowly after anthesis, associated with smaller non-stomatal limitations due to maintenance of PEPCase activity, and chlorophyll and soluble protein content.
INTRODUCTION
The production system of maize, consisting of high-yielding cultivars and high inputs of fertilizers, agrochemicals and irrigation, contributed to great yield increases in developed and developing countries during the past decades (Conway, 1991; Evans, 1993). However, adverse effects of high input agriculture have been recognized, as fertilizers (nitrate) and agrochemicals discharged from agriculture cause surface water pollution (National Research Council, 1989). Breeding maize cultivars with large production under N deficiency could reduce environmental pollution and increase the economic efficiency of N fertilizer use. Castleberry et al. (1984) and Duvick (1984) reported that new maize hybrids outperformed old ones even at reduced N rates. Indeed, a large proportion of the genetic improvement of maize over the past 50 years is attributable to increased stress tolerance, including N deficiency (Tollenaar and Wu, 1999). Understanding the mechanisms of the different performance of the newer and the older hybrids under N deficiency could aid breeding for future yield increase and increased N use efficiency, and help decrease N pollution.
McCullough et al. (1994) reported that new maize hybrids were more tolerant than earlier hybrids to limited N supply during the early vegetative phase with respect to rate of leaf appearance, photosynthesis, stomatal conductance (gs) and chlorophyll content. However, maize grain weight mostly accumulates during the ‘effective filling period’, beginning 7–14 d post-mid-silk (Johnson and Tanner, 1972), showing that photosynthetic activity during the post-anthesis stage is important for the yield. However, how N deficiency influences photosynthesis of the newer and older hybrids, especially after anthesis, and the influence on dry matter accumulation and grain weight is still unclear. Therefore, the effects of N deficiency on the mass of plant and grain, harvest index, leaf area and photosynthetic traits were studied for six maize hybrids released during the past 50 years to explore the related physiological mechanisms that lead to the different responses to nitrogen deficiency of the newer and older hybrids.
MATERIALS AND METHODS
Experimental design
Field experiments were conducted at Shandong Agriculture University Research Farm, Taian, Shandong (36°18′N, 117°13′E) on a silt–loam soil. Treatment and control plots were arranged in randomized complete blocks with three replicates. Each plot consisted of four 8 m rows, in East–West orientation, with 0·76 m inter-row spacing. Plants were sampled from the two centre rows. There were four guard rows at each side of the experimental area. Plots were over-seeded with hand planters on June 28, 2003 and seedlings thinned to a final stand of 45 000 plants ha−1. In the control plots, N was applied as urea at 75 kg N ha−1 as basal dose, and 120 kg N ha−1 was top-dressed 25 d after plant emergence. The treatment consisted of growing plants with no N-fertilizer (N-deficient). The total N in the soil at sowing was 1·15 g kg−1. As soil tests indicated high contents of P and K, those fertilizers were not applied. Irrigation was not used either because rainfall was adequate during the year. Plots were kept free from weeds, insects and diseases by appropriate measures.
Six maize (Zea mays) hybrids were used in this experiment. Baimaya and Jinhuanghou (double-cross hybrids) were introduced from the USA in 1931 and 1947, respectively, and were grown widely in China in the 1950s; Zhongdan 2 and Danyu 6 (single-cross hybrids) were released in the late 1960s and were the most widely grown hybrids in north China during the 1970s; Niedan 13 and Nongda 108 (single-cross hybrids) were released in the 1990s and are currently widely grown in China. Seeds of all six hybrids were produced in 2001 or later and stored at the Department of Agronomy at Shandong Agriculture University. All hybrids are characterized by a similar date of mid-silking at 55 d after seeding, but these hybrids differ in physiological maturity. The physiological maturity date of the older hybrids is 40–45 d after silking, approx. 10–15 d earlier than the newer ones (55 d after silking).
Plants were allowed to open-pollinate and all measurements were taken on plants with a fertilized ear. Thelight-saturated photosynthetic rate (Psat) and chlorophyll fluorescence were measured on the topmost ear leaf once per week after flowering. Ear leaves of five plants of each treatment were selected for Psat and chlorophyll fluorescence measurement. Ear leaves in three plants of each treatment were sampled and then the central part of the leaf blades without the midrib were cut into pieces and stored in liquid nitrogen for measurement of chlorophyll and soluble protein content, and enzyme assays. Ten plants per treatment were sampled at flowering to measure leaf area and plant weight per plant. At harvest, another 10 plants per treatment were sampled to measure plant weight and grain weight per plant.
Light-saturated photosynthetic rate and chlorophyll fluorescence measurement
Psat was measured with a portable, open-flow portable photosynthetic system (CIRAS-1, PP Systems, UK) at 1800 µmol m−2 s−1 PPF (photosynthetic photon flux) using the automatic light source of the CIRAS-1 photosynthetic system. Although Psat was measured with an artificial light source, 1000–1100 h of cloudless days were selected for measurements because incident PPF prior to measurement could influence leaf photosynthesis. Five plants of each hybrid were selected for sampling. Measurements were taken on a 2·5 cm2 area in the central part of the leaf blade that did not include the midrib. The flow rate of air through the chamber was set to 200 mL min−1. The CO2 concentration of the intake air was maintained at 350 µmol mol−1, and air vapour pressure deficit (VPD) was approx. 2 kPa during measurements. Leaf temperature was maintained at 30 ± 1 °C by the CIRAS-1 thermoelectric coolers.
Chlorophyll fluorescence was measured with a portable fluorimeter (FMS2, Hansatech, UK) on the same leaves and at the positions used to measure photosynthesis. The incident PPF was approx. 1800 µmol m−2 s−1 at 1000–1100 h on cloudless days when chlorophyll fluorescence was measured. The initial fluorescence (F0) was recorded on leaves adapted to dark for 30 min. A single saturating radiation pulse was applied to obtain the maximum fluorescence (Fm). The maximum efficiency of PSII photochemistry in the dark-adapted state (Fv/Fm) was calculated according to Demmig-Adams et al. (1996).
Chlorophyll and soluble protein content and enzyme determination
Chlorophyll content was determined according to Arnon (1949). Soluble protein content was determined as described by Bradford (1976): each 0·1 mL protein sample was mixed with 5 mL of protein reagent [0·01 % (w/v) Coomassie brilliant blue G-250, 4·7 % (w/v) ethanol and 8·5 % (w/v) phosphoric acid]. The absorbance at 595 nm was measured after 2 min. The content of protein was calculated by comparison with the standard curve prepared from a bovine serum albumin (BSA) solution.
Ribulose-1,5-bisphosphate carboxylase (RuBPCase) was measured according to Lilley and Walker (1974). Fresh leaf segments (0·5 g) were homogenized in a pre-cooled pestle and mortar with acid-washed quartz sand and 2·5 mL of extraction medium [0·1 m Tricine-HCl (pH 8·4), containing 10 mM MgCl2, 1 mm EDTA, 7 mm β-mercaptoethanol, 5 % glycerol (v/v) and 1 % polyvinylpyrrolidone (PVP)] followed by centrifuging at 10 000 g for 10 min below 4 °C. The clear supernatant was decanted slowly and used as the crude RuBPCase source. Before quantification, the crude extract was activated with 200 m NaHCO3 and 1 m MgCl2, at 25 °C for 10 min. The reaction mixture contains 50 mm Tricine-HCl (pH 7·8), 10 mm KCl, 1 mm EDTA, 15 mm MgCl2, 0·21 mm NADH, 5 mm dithiothreitol (DTT), 5 mm phosphocreatine, 5 mm ATP, 2 U of creatine phosphokinase, 15 U of phosphoglyceric phosphokinase, 5 U of glyceraldehyde-phosphate dehydrogenase and 10 mm NaHCO3. Reactions were initiated by the addition of 0·5 µmol of RuBP. The activity of RuBPCase was determined as the oxidation rate of NADH at 340 nm.
PEPCase activity was measured according to Arnozis et al. (1988). Leaf samples were homogenized in 0·1 m Tricine-HCl (pH 8·4) buffer, containing 10 mm MgCl2, 1 mm EDTA, 7 mm β-mercaptoethanol, 5 % glycerol (v/v) and 1 % PVP, followed by centrifuging at 10 000 g for 10 min. All the procedures were done at a temperature below 4 °C. The clear supernatant was used for the enzyme assay. Enzyme extract was added to the reaction mixture which contained 0·5 µmol phosphoenolpyruvate (PEP), 3·68 µmol NaHCO3, 0·16 µmol NADH, 11·2 µmol MgCl2 and 112 µmol Tris-NaOH (pH 9·2). PEPCase activity was measured spectrophotometrically by coupling the reaction to the NADH oxidation at 340 nm mediated by 15 U of malate dehydrogenase.
RESULTS
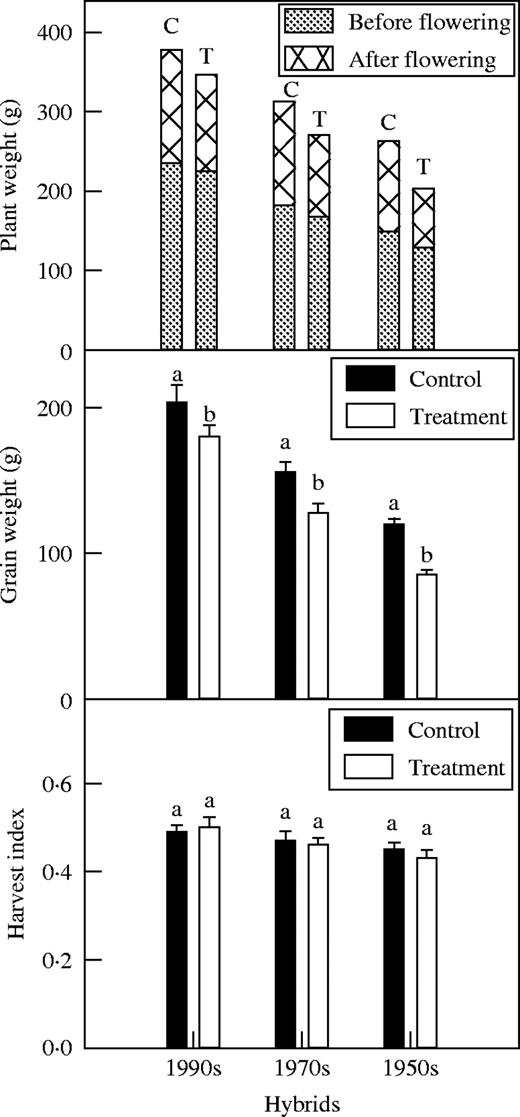
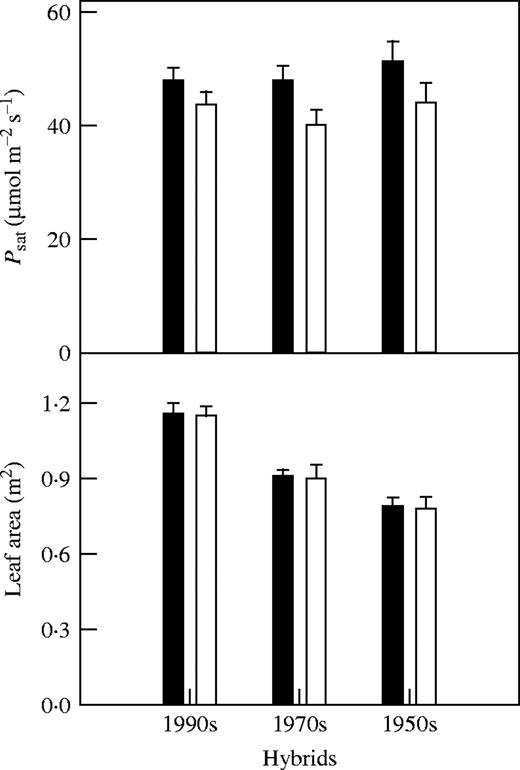
N deficiency decreased grain yield and plant weight significantly in all hybrids, with the older, earlier released hybrids more affected than those released more recently (P < 0·05) (Fig. 1). However, there was no significant difference in harvest index and plant weight at flowering between the N-deficient and control plants of all hybrids (P > 0·05). Plant weight produced after flowering decreased significantly in all hybrids, especially the older ones (P < 0·05). There was also no significant difference in Psat at 20 d before flowering and in leaf area between the N-deficient plants and the control plants of all hybrids (P > 0·05) (Fig. 2).
Effect of N deficiency on plant weight produced before and after flowering (C = control, T = treatment), grain weight and harvest index at harvest. Data represent the mean ± s.e. (n = 10). Different letters indicate significant differences at P < 0·05.
Effect of N deficiency on Psat 20 d before flowering and leaf area at flowering. Data represent the mean ± s.e. (n = 5). There were no significant differences between means.
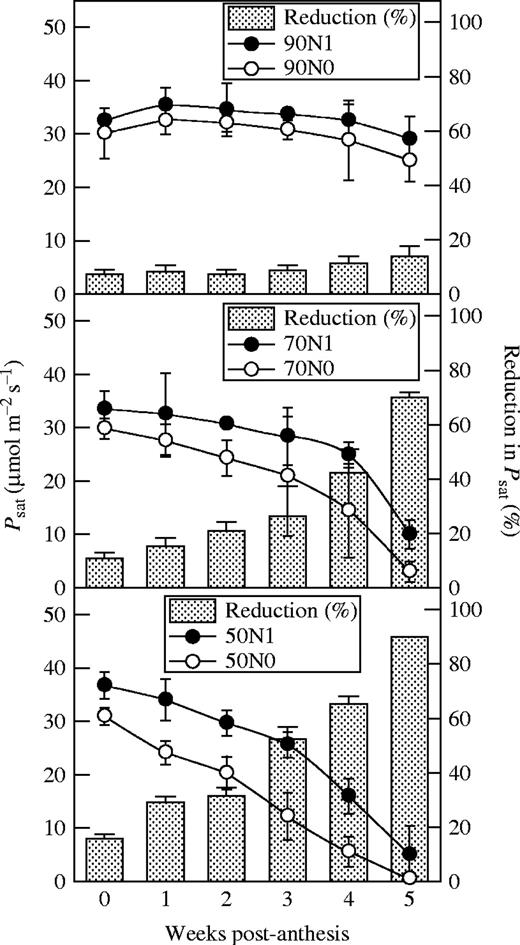
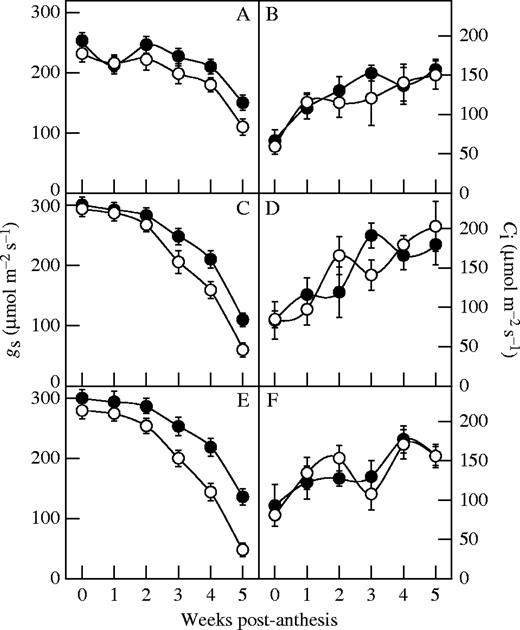
N-deficient plants of all hybrids had lower Psat than their controls after flowering, and the effect became more significant later (P < 0·05) (Fig. 3). Moreover, Psat of the older hybrids declined more than that of the newer ones under N deficiency (P < 0·05). During the first 4 weeks after flowering, the gs of all hybrids decreased little with N deficiency and there was no significant difference between the older and newer hybrids (P > 0·05) (Fig. 4) but, during the fifth week, the gs of older hybrids decreased significantly (P < 0·05) more than that of newer ones under N deficiency. At the flowering stage, the intercellular CO2 concentration (Ci) was lower in the N-deficient plants of all hybrids but it increased gradually after anthesis and there was no significant difference between hybrids (P > 0·05).
Psat and its reduction in N-deficient plants of maize hybrids released in different years. Data represent the mean ± s.e. (n = 5).
Effect of N deficiency on stomatal conductance (gs) (A, C and E) and intercellular CO2 concentration (Ci) (B, D and F) of the hybrids from the 1990s (A and B), 1970s (C and D) and 1950s (E and F). Filled circles represent the treatment plants; open circles represent the control plants. Data represent the mean ± s.e. (n = 5).
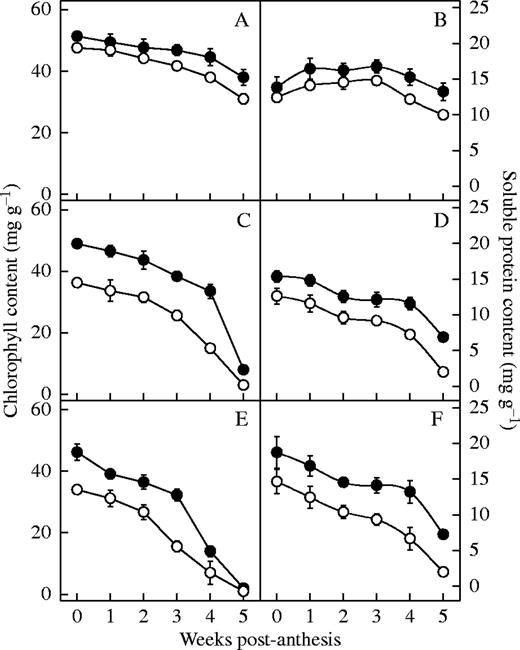
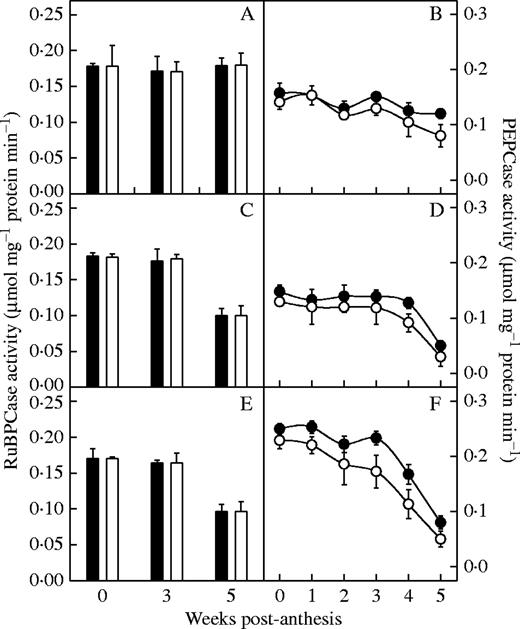
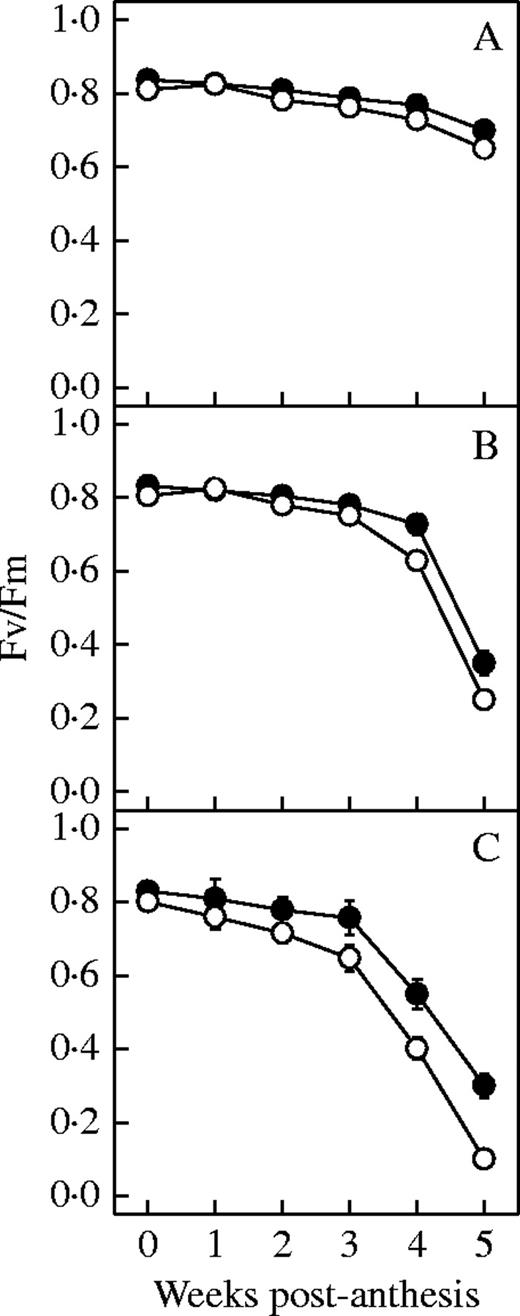
Chlorophyll and soluble protein content in N-deficient plants was significantly lower than in control plants (P < 0·05) (Fig. 5), particularly for the older hybrids compared with newer ones (P < 0·05). Figure 6 show the variation in activities of the PEPCase and RuBPCase after anthesis of six hybrids released in different years. PEPCase activity decreased with the diminution of N, and the effect was more significant in the older than newer hybrids (P < 0·05) (Fig. 6). In contrast, there were no significant changes in RuBPCase activities under N deficiency conditions (P > 0·05) for the hybrids. During the first 4 weeks after anthesis, the Fv/Fm of all hybrids did not change significantly with N deficiency (P > 0·05) (Fig. 7). At the fifth week, however, the Fv/Fm ratio of the older hybrids declined much more than their controls under nitrogen deficiency (P < 0·05).
Effect of N deficiency on chlorophyll content (A, C and E) and soluble protein content (B, D and F) of the hybrids from the 1990s (A and B), 1970s (C and D) and 1950s (E and F). Filled circles represent the treatment plants; open circles represent the control plants. Data represent the mean ± s.e. (n = 3).
Effect of N deficiency on ribulose-1,5-bisphosphate carboxylase (RuBPCase) activity (A, C and E) and phosphoenolpyruvate carboxylase (PEPCase) activity (B, D and F) of the hybrids from the 1990s (A and B), 1970s (C and D) and 1950s (E and F). Filled squares and circles represent the treatment plants; open squares and circles represent the control plants. Data represent the mean ± s.e. (n = 3).
Effect of N deficiency on maximum efficiency of PSII photochemistry (Fv/Fm) of the hybrids from the 1990s (A and B), 1970s (C and D) and 1950s (E and F). Filled circles represent the treatment plants; open circles represent the control plants. Data represent the mean ± s.e. (n = 5).
DISCUSSION
N supply has substantial effects on plant growth, development and grain yield, as it is a fundamental constituent of many leaf cell components, particularly those associated with the photosynthetic apparatus, including carboxylating enzymes and proteins of membranes (Greenwood, 1976; Pandey et al., 2000). In our study, N deficiency decreased grain yield and plant weight in all hybrids, especially in the older ones, but there was no significant difference in harvest index between the N-deficient plants and the control plants of any hybrids. So the response of grain yield to N deficiency was associated with much larger effects on biomass production than on harvest index, consistent with the report of Muchow (1994). The different responses of grain yield to N deficiency between the older and the newer hybrids were mostly due to their different rate of accumulation of dry matter after anthesis.
Much of the effect of nitrogen deficiency on crops can be explained through radiation interception and radiation use efficiency that relate directly to plant growth and carbon assimilation, respectively (Boyer, 1970; Gifford et al., 1984; Uhart and Andrade, 1995). Leaf area may be decreased by N deficiency, depending on the severity. Photosynthesis depends on many physiological and biochemical processes, such as gs, photochemical capacity of PSII, and amounts and activities of carbon fixation enzymes. Chloroplasts contain 70–80 % of the cell N (Makino and Osmond, 1991), and photosynthetic performance requires proteins for all steps of the process including formation of the light-harvesting chlororphyll–protein complexes of the antenna (Bungard et al., 1997), so N deficiency affects these processes, depending on the severity. Although leaf area at flowering, and Psat 20 d before flowering, decreased a little under N deficiency, the decreases were not significant; this could explain the lack of a significant difference in plant weight at flowering between the N-deficient plants and the control plants of all hybrids. However, dry matter production after flowering of the N-deficient plants was significantly lower than that of the control plants in all (especially the older) hybrids, mostly due to their faster rate of loss of photosynthetic capacity.
In our study, there was no significant difference in gs and Ci between the hybrids. So the faster decrease in Psat of the older hybrids under N deficiency was not due to stomatal limitation. Loss of chlorophyll is the most obvious symptom of senescence (Matile, 1992). N deficiency accelerated loss of chlorophyll (senescence) of the older hybrids more than of the newer ones after anthesis. In our study, the older hybrids showed greater reduction in soluble protein content than the newer hybrids at the late stage, which indicated either greater inhibition of amino acid or protein synthesis, or greater degradation of existing proteins under N stress during leaf senescence. Sugiharto et al. (1990) showed that N deficiency inhibits photosynthetic capacity through decreased stromal and thylakoid proteins, including several key enzymes of the Calvin cycle. PEPCase and RuBPCase are major carbon-assimilating enzymes in C4 plants. We found that the decrease in PEPCase under N deficiency was more significant in the older hybrids. There was no significant change in the RuBPCase activities under N deficiency in hybrids, perhaps because degradation of RuBPcase parallels that of the total soluble protein during N starvation (Maria et al., 2000) and/or PEPCase was lost rather than RuBPCase during N availability (Sugiharto et al., 1990).
Chlorophyll fluorescence in our study showed negative effects of N deficiency on the photochemistry reactions in the thylakoid membranes, only affecting Fv/Fm of the old hybrids and at the late stage. McCullough et al. (1994) showed that Fv/Fm is associated with loss of chlorophyll only when chlorophyll content is low, and PSII is damaged, and may be related to maintenance of oxidized PSII when the capacity for carbon assimilation is impaired by N deficiency (Khamis et al., 1990).
In conclusion, compared with the more recently released hybrids, the earlier released hybrids had smaller plant and grain weight under N deficiency, due mainly to a more rapid decrease in photosynthetic capacity after anthesis, associated with the greater non-stomatal limitations caused by decreased PEPCase activity, chlorophyll content and soluble protein concentration.
Financial support from the National Natural Science Foundation of China (NO. 30100108) is gratefully acknowledged. We are grateful for technical assistance from the Agronomy Department, Shandong Agricultural University. Thanks are due to Dr Zhang Xiaoyan, Wu Zhengfeng, MS, Wang Yongjun, MS, Zhuang Kezhang, MS, and Li Congfeng, MS, for their help in this study.
LITERATURE CITED
Arnon DI.
Arnozis PA, Nelemans JA, Findenegg GR.
Boyer JS.
Bradford MM.
Bungard RA, McNeil D, Morton JD.
Castleberry RM, Crum CW, Krull F.
Conway G.
Demmig-Adams B, Adams WW, Baker DH, Logan BA, Bowling DR, Verhoeven AS.
Duvick DN.
Gifford RM, Thorne JH, Hitz WD, Giaquinta RT.
Johnson DR, Tanner JW.
Khamis S, Lamaze T, Lemoine Y, Foyer C.
Lilley RM, Walker DA.
McCullough DE, Giradin P, Mihajlovic M, Guilera AA, Tollenaar M.
Makino A, Osmond B.
Maria GE, Ricardo BF, Artur RT.
Matile P.
Muchow RC.
Pandey RK, Maranville JW, Admou A.
Sugiharto B, Miyata K, Nakamoto H, Sasakawa H, Sugiyama T.
Tollenaar M, Wu J.
Author notes
1Laboratory of Quantitative Vegetation Ecology, Institute of Botany, The Chinese Academy of Sciences, 20 Nanxincun, Xiangshan, 100093 Beijing, PR China and 2Agronomy Department, Shandong Agriculture University, Tai'an, Shandong, 271018, PR China