-
PDF
- Split View
-
Views
-
Cite
Cite
R. ALONI, E. ALONI, M. LANGHANS, C. I. ULLRICH, Role of Cytokinin and Auxin in Shaping Root Architecture: Regulating Vascular Differentiation, Lateral Root Initiation, Root Apical Dominance and Root Gravitropism, Annals of Botany, Volume 97, Issue 5, May 2006, Pages 883–893, https://doi.org/10.1093/aob/mcl027
Close - Share Icon Share
Abstract
• Background and Aims Development and architecture of plant roots are regulated by phytohormones. Cytokinin (CK), synthesized in the root cap, promotes cytokinesis, vascular cambium sensitivity, vascular differentiation and root apical dominance. Auxin (indole-3-acetic acid, IAA), produced in young shoot organs, promotes root development and induces vascular differentiation. Both IAA and CK regulate root gravitropism. The aims of this study were to analyse the hormonal mechanisms that induce the root's primary vascular system, explain how differentiating-protoxylem vessels promote lateral root initiation, propose the concept of CK-dependent root apical dominance, and visualize the CK and IAA regulation of root gravitropiosm.
• Key Issues The hormonal analysis and proposed mechanisms yield new insights and extend previous concepts: how the radial pattern of the root protoxylem vs. protophloem strands is induced by alternating polar streams of high IAA vs. low IAA concentrations, respectively; how differentiating-protoxylem vessel elements stimulate lateral root initiation by auxin–ethylene–auxin signalling; and how root apical dominance is regulated by the root-cap-synthesized CK, which gives priority to the primary root in competition with its own lateral roots.
• Conclusions CK and IAA are key hormones that regulate root development, its vascular differentiation and root gravitropism; these two hormones, together with ethylene, regulate lateral root initiation.
INTRODUCTION
A major challenge for plant developmental biologists is to understand the mechanisms that control patterned development of complex systems. The focus of the present study is the hormonal mechanisms that shape growth, differentiation and architecture of root systems. The role of the main root signal, namely cytokinin (CK), is analysed, together with the role of the shoot signal, namely auxin (indole-3-acetic acid, IAA), and their interactions (Coenen and Lomax, 1997; Bangerth et al., 2000; Nordström et al., 2004; Woodward and Bartel, 2005).
Cytokinins are signalling hormonal molecules that may play an essential role in regulating cytokinesis, growth and development in plants. In an intact plant, the living cells in both the root and the shoot are capable of producing CKs (Miyawaki et al., 2004; Nordström et al., 2004; Aloni et al., 2005; Tanaka et al., 2005) as well as the auxin signalling hormone (Aloni et al., 2003; Ljung et al., 2005). However, although both CK and IAA can be produced in roots and shoots (Nordström et al., 2004; Ljung et al., 2005), the production of these major hormonal signals does not occur randomly but is regulated by the location of the synthesizing cells in the plant body and their developmental stage, and is influenced by environmental conditions. Young shoot organs are the major sites of IAA production (Aloni et al., 2003, 2006), while root tips are major sites of CK synthesis (Aloni et al., 2004, 2005). From the sites of hormone production, the signals move in specific structural pathways and by different mechanisms (Aloni, 2004; Aloni et al., 2005) to regulate plant development and differentiation.
The absence of isopentenyltransferase (IPT) expression in shoot apical meristems (Miyawaki et al., 2004) is consistent with the absence of free CK in shoot apical meristems of 3-day-old Arabidopsis thaliana seedlings (Aloni et al., 2004), and its absence in lateral buds of older plants grown under conditions of almost no transpiration (Aloni et al., 2005) indicates that the shoot apical buds are apparently not the primary sites of CK synthesis (Fig. 1). The suggestion of Letham (1994) that the main site of CK synthesis is the root tip, specifically the root cap cells, was confirmed by Aloni et al. (2004, 2005), showing that in seedlings as well as in plants grown under conditions of almost no transpiration (Fig. 1B) the highest concentration of free CK occurs in the root cap statocytes (Fig. 1A). From the root cap, the CK is transported upward through plasmodesmata, which provide symplastic continuity (Tirlapur and König, 1999; Kim and Zambryski, 2005) in the meristematic and elongation zones (Scheres et al., 2002), and from the differentiation zone through vessels of the xylem by the transpiration stream, mainly to developing shoot organs with high transpiration rates (Lejeune et al., 1998; Emery and Atkins, 2002; Aloni et al., 2005). CKs have opposing roles in shoots and roots; in young shoot organs the CKs positively regulate development and promote shoot growth (Howell et al., 2003; Rahayu et al., 2005), but in roots they are negative regulators of growth and development (Werner et al., 2001, 2003).
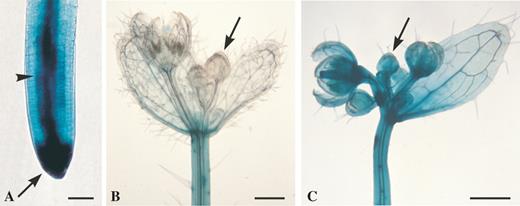
Apparent free-CK distribution in a root (A), in young flowers protected from the wind (B), or exposed to gentle wind (C), as detected by free bioactive-CK-dependent GUS expression in the CK-responsive ARR5::GUS transformant of 5-week-old Arabidopsis grown under long-day conditions (described in Aloni et al., 2005). (A) Root of wind-protected plant with strong ARR5::GUS expression reflecting high bioactive CK concentration in the cap (arrow), considerable concentration in the vascular cylinder (arrowhead) and lower concentration in the cortex. (B) Wind-protected inflorescence (by tight translucent plastic bags, under 95–100 % humidity) showing young apical flower buds (arrow) without GUS expression. (C) Plant from the same experiment as in A and B, but exposed to gentle wind (of 0.2–0.7 m s−1 under lower humidity of 60–70 %) only for the last 3 h before harvesting for the GUS assay, resulting in very strong ARR5::GUS expression in the young apical flower buds (arrow). Scale bars = 40 μm (A), 500 μm (B, C).
Nitrate (not NH4+) supply to nitrogen-depleted roots causes a rapid up-regulation of IPT genes and an increase in CK content in the root, which is transported via the xylem upward into the shoot (Yong et al., 2000; Gessler et al., 2004; Miyawaki et al., 2004; Rahayu et al., 2005) and could possibly also be transported downward from shoot to root through the phloem (Gessler et al., 2004). CKs were reported to down-regulate the expression of IPT1,3,5,7 rapidly (Miyawaki et al., 2004), which may emphasize the regulatory role of root-to-shoot CK mass transport on shoot CK synthesis. This suggests that synthesis of CK in the shoot could guarantee CK availability under conditions of insufficient CK supply from the root, e.g. under nitrogen deficiency (Miyawaki et al., 2004; Takei et al., 2004) and in young shoot organs of trees (Gessler et al., 2004) which are further away from the roots. Shoot-synthesized CKs (Nordström et al., 2004) as well as CKs released from xylem sap at the hydathodes by guttation (Aloni et al., 2003; fig. 2i) and re-absorbed into the phloem (Bürkle et al., 2003) can be transported from the leaves downward by phloem transport.
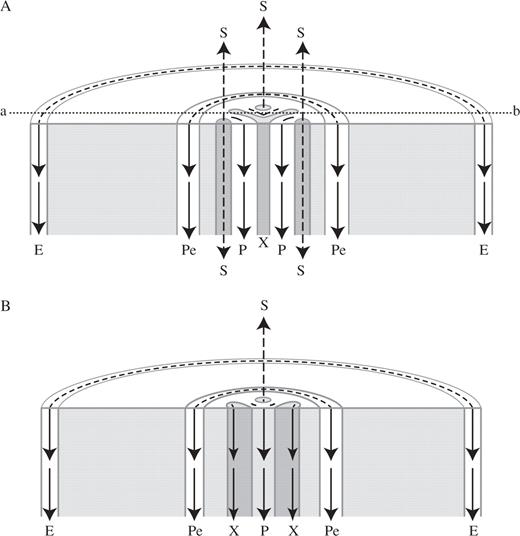
Schematic diagrams at the root's differentiation zone, showing free-IAA transport pathways in a triarch root at a phloem plane (A), and in the same root at a xylem plane (B); the xylem plane location is marked by a dotted line (a–b) in A. The long arrows in the diagrams illustrate IAA movement in a polar manner preferably in the vascular cylinder (the internal route): through the procambium (P), the differentiating xylem (X) and the pericycle (Pe). In the peripheral route (from the shoot to the root's differentiation zone), the IAA arriving from the young leaves moves downward in a polar manner (see Terasaka et al. 2005, fig. 3D, E) through the epidermis (E) (this downward peripheral IAA movement probably stops the upward peripheral IAA movement arriving from the root tip; they possibly merge and together move into the cortex. This probably induces the meristematic cortical downward IAA movement detected by the polar pattern of PIN1 in the acropetal membranes of the differentiating cortex near the root tip). In the non-polar route, IAA moves up and down (illustrated by broken-line arrows) in mature protophloem sieve tubes (S).
The auxin hormone and its polar movement, originating in young shoot organs (Jacobs, 1952; Aloni et al., 2003, 2006), play a crucial role in many aspects of root growth, development and differentiation. IAA regulates the development of the primary and lateral roots (Sabatini et al., 1999; Casimiro et al., 2001; Taiz and Zeiger, 2002; Benkova et al., 2003; Blilou et al., 2005; Raven et al., 2005; Teale et al., 2005), the quiescent centre (Kerk et al., 2000; Jiang and Feldman, 2005), root apical meristem (Jiang and Feldman, 2005), root cap (Ponce et al., 2005) and root vascular differentiation (Sachs, 1981; Aloni, 2004).
The PINFORMED1 (PIN1), an essential protein component involved in auxin efflux, cycles between the plasma membrane and intracellular vesicular compartments, facilitating rapid transfer and accumulation of the protein in various sites of the plasma membrane (Geldner et al., 2001; Balušká et al., 2003; Paponov et al., 2005). Polar accumulation of PIN proteins at specific sites of the plasma membrane enables detection of the polar IAA transport direction (Friml et al., 2002, 2004; Benková et al., 2003; Blilou et al., 2005). In the root and shoot apices, probably due to high local IAA concentrations at and near the IAA maxima, polar patterns of PIN proteins in the cell membranes are easily detected (Benková et al., 2003; Blilou et al., 2005; Heisler et al., 2005). However, further away from the apices, perhaps owing to decreased local IAA concentrations, the polar IAA movement might not be enough to induce detectable polar patterns of PINs, and therefore further away from the tips the IAA routes remain obscure. Until more sensitive methods become available for detecting low IAA concentrations in individual cells, other possible clues are needed to detect possible preferable IAA pathways along the plant body, as will be discussed below.
IAA moves in a polar manner in preferable and defined pathways from the hydathodes of young leaves (Aloni, 2001, 2004; Aloni et al., 2003) downward to the root tips (Friml and Palme, 2002; Friml et al., 2002; Booker et al., 2003; Aloni, 2004; Blilou et al., 2005). Recently, a new IAA flow model was suggested in which the IAA arriving from the shoot to the root's auxin-maximum site (located at the quiescent centre, Jiang and Feldman, 2005) is channelled back up the root through the epidermis and can be re-cycled from the zone of cell elongation into the vascular cylinder downward to the auxin maximum (Blilou et al., 2005; Leyser, 2005). Yet, there is no physiological evidence that fluxes of IAA can penetrate from the cortex into the vascular cylinder, which is already overloaded with the IAA arriving from the shoot. Conversely, evidence from the meristematic root tip and the zones above it shows that the differentiating endodermis is a physiological barrier that prevents CK outflow from the vascular cylinder into the cortex during gravitropic bending (see Aloni et al., 2004, fig. 1d, e); therefore, it is possible that the differentiating endodermis might also block cortical IAA transport into the vascular cylinder. These results emphasize the urgent need further to explore and understand the role of specific IAA routes in cell differentiation and organ formation. This article presents an overview on the hormonal regulation of root vascular differentiation, growth and architecture, with specific emphasis on the role of auxin in roots, analysing specific IAA pathways in the vascular cylinder. Here the focus will be on the zone of cell differentiation where the PIN patterns are unclear, and where the primary vascular tissues start to differentiate and the lateral roots are initiated. We analyse fundamental questions in patterned root differentiation and propose how neighbouring IAA transport routes can simultaneously affect each other and concurrently induce the radial gradient in primary vessel width and the initiation of lateral roots. Likewise, cytokinin's role in regulating vascular tissues, root apical dominance and gravitropism are discussed. Finally, as ethylene is involved in regulating lateral and adventitious root formation (Yamamoto et al., 1995; Visser et al., 1996; Mergemann and Sauter, 2000), its possible role will be discussed regarding our suggested new model for lateral root initiation.
REGULATION OF VASCULAR DIFFERENTIATION IN ROOTS
To understand the control of root architecture and the sites of lateral root initiation, there is a need to understand the hormonal mechanism that regulates the formation of the radial pattern of the primary vascular system in roots. Most of the studies on vascular differentiation and vascular patterning were made on shoots of soil-grown plants (Jacobs, 1952; Sachs, 1981; Nelson and Dengler, 1997; Berleth et al., 2000; Aloni, 2001; Turner and Sieburth, 2002). It is relatively difficult to study the role of CK in vascular differentiation because of considerable concentrations of CK in plant tissues (Torrey et al., 1971; Baum et al., 1991). This difficulty may be overcome by using transgenic plants with lowered CK concentrations (Werner et al., 2001, 2003), by reducing the endogenous CK concentration via continuous removal of initiating adventitious roots from excised internodes (Aloni et al., 1990; Baum et al., 1991), and by minimizing the rate of CK movement from root tips to shoot by growing plants under conditions of almost no transpiration (Saks et al., 1984; Aloni et al., 2005). Interestingly, the hormonal control of vascular differentiation and regeneration in both stem and root follows similar general principles (Robbertse and McCully, 1979; Aloni and Plotkin, 1985; Sachs, 1981; Aloni, 1987), which provide an explanation of how the root vascular system is induced. Three types of vascular differentiation can be distinguished: (1) primary differentiation, which occurs in cells originating from the primary vascular meristem, the procambium; (2) secondary differentiation, where the derivates originate from the vascular cambium; and (3) regenerative differentiation, in which the vascular elements re-differentiate from parenchyma cells at lateral root junctions (Aloni and Plotkin, 1985) and around wounds in parenchyma or cambium (Sachs, 1981; Aloni, 2004).
Vascular development in a plant continues as long as the plant grows from apical and lateral meristems. The continuous development of new vascular tissues enables regeneration of the plant and its acclimation to changes in the environment. The vascular tissues are induced and controlled by polar IAA movement along the plant body, from the hydathodes of young leaves downward to the root tips (Aloni, 2004). CK by itself does not induce vascular tissues, but in the presence of IAA, CK promotes vascular differentiation and regeneration (Aloni, 1995). CK, which promotes cell divisions in the vascular tissues (Roberts et al., 1988), is a limiting and controlling factor that increases the number of xylem fibres in tissue culture (Aloni, 1982) and along the plant axis (Saks et al., 1984; Roberts et al., 1988, fig. 3.3). CKs stimulate vascular cambium activity in mature internodes of Coleus blumei (Aloni et al., 1990; Baum et al., 1991), and they promote vascular regeneration by increasing the number of phloem and xylem strands around the wound (Aloni et al., 1990; Baum et al., 1991; Aloni, 1995). During evolution, in plants grown under continuous selective pressures in limiting environments that reduced vegetative shoot growth, CK has mediated environmental pressure by promoting an increase in vascular cambium sensitivity, making it more responsive to the decreasing IAA concentrations from the reduced vegetative shoot (Aloni, 1991, 2001). The extreme example is the very sensitive cambium in ring-porous trees, which is reactivated at the beginning of the growth season by CK originating in their roots. These trees then respond to the initial IAA concentration originating from dormant looking buds by inducing the differentiation of their wide earlywood vessels a few weeks before bud break (Aloni, 1991, 2001).
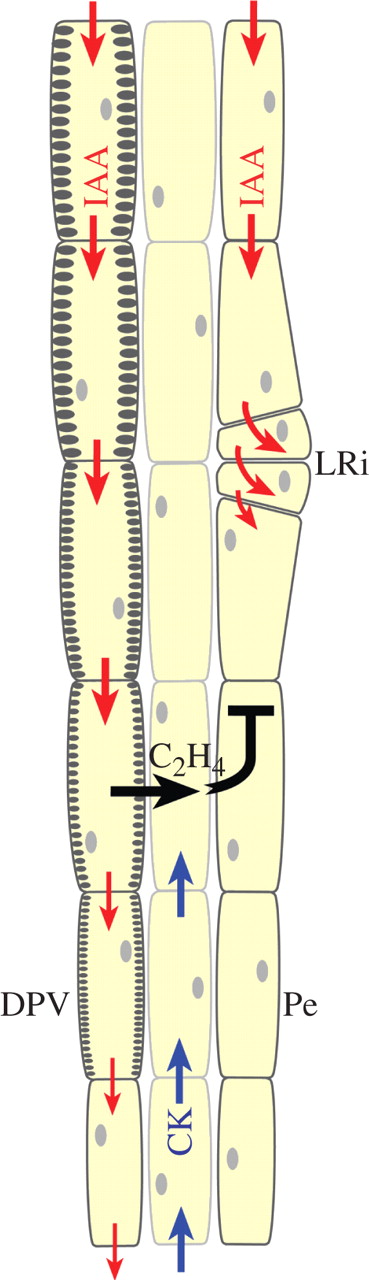
Model of IAA-, ethylene- and CK-regulated lateral root initiation (LRi) in the cell differentiation zone of a growing root. The schematic diagram shows the three outermost cell columns of the vascular cylinder in a young dicotyledonous root in the xylem plane, with a differentiating protoxylem vessel (DPV) and the pericycle (Pe). Two pathways of parallel streams of polar IAA transport (marked by red arrows) are shown: the left auxin stream induces the vessel (marked by the gradual development of secondary wall thickenings) and the right stream maintains the meristematic identity of the pericycle (which is a major preferable pathway of polar auxin transport). During vessel differentiation, a local increase of IAA concentration in a differentiating-protoxylem vessel element induces ethylene production. This C2H4 is released, and in the centrifugal direction (black arrow) it locally blocks the IAA transport in the adjacent pericycle cell, causing a local increase of IAA concentration immediately above the blockage, which induces cell division and lateral root initiation (LRi). Cytokinin (blue arrows) arriving from the root cap may inhibit lateral root initiation near the root tip.
Vessels in the secondary xylem are induced by polar IAA streams moving in the vascular cambium between the secondary vessels and sieve tubes, but not in vascular rays (Aloni, 2004, fig. 5B). The secondary xylem along the plant axis, in both the stem (Aloni and Zimmermann, 1983; Aloni, 2001; Leitch, 2001) and the root (Fahn, 1964), shows a gradual and continuous increase in vessel width and decrease in vessel density with increasing distance from the leaves. These gradual vascular patterns are probably induced by a gradient of decreasing IAA concentrations from young leaves to root tips (Aloni and Zimmermann, 1983).
In tissue cultures, low IAA concentrations induced sieve elements but not tracheary elements, whereas high IAA concentrations resulted in the differentiation of both phloem and xylem (Aloni, 1980, 1987). However, even in cultures grown at a high IAA concentration, only phloem developed at the surface further away from the high auxin-containing medium (Aloni, 1980). The same basic rules on phloem and xylem relationships occur in both the stem and the root. Low-IAA streams produce phloem strands (with no xylem) and phloem anastomoses in stems of many species (Aloni, 2001). It has been suggested that the differentiation of phloem strands and phloem anastomoses between the vascular strands are induced by streams of low IAA concentrations (Aloni, 1987, 1995). When a high auxin concentration was applied to decapitated Luffa cylindrica stems, it induced xylem differentiation in its phloem anastomoses (Aloni, 1995, fig. 1B), indicating the need for high auxin stimulation to induce xylem differentiation. In leaves, the proximity between the sites of IAA production and the site of differentiating vascular cells probably results in relatively high local IAA concentrations at the differentiating sites (Aloni, 2001, 2004), which may explain why in leaves xylem can differentiate in the absence of phloem at the freely ending veinlets (Horner et al., 1994) and hydathodes (Aloni et al., 2003).
Primary vascular differentiation in the root vascular cylinder is characterized by a radial pattern of alternating strands of xylem and phloem. The primary vascular tissues and the pericycle (both originate from the procambium) are the main pathways of polar IAA transport (Fig. 2A). In flowering plants there are two common primary vascular morphologies, as exemplified by monocot roots and dicot roots. Xylem archs (strands) made up of vessels are either distributed around the periphery of the vascular cylinder and the centre of the cylinder is parenchymatous (monocots), or the centre of the vascular cylinder is occupied mainly by vessels with xylem archs radiating out to the periphery of the cylinder (dicots). In both cases the phloem strands are always separated and located at the periphery of the vascular cylinder (Raven et al., 2005).
To explain the mechanism that controls the radial pattern of the primary vascular tissues in a root, we propose that the primary xylem and phloem strands are induced by alternating streams of high vs. low IAA concentrations that flow in a polar manner toward the root tip. These alternating high- vs. low-concentration IAA streams flow near the surface of the vascular cylinder and induce strands of xylem and phloem. The high-concentration IAA streams induce the protoxylem vessels. The relatively high-concentration IAA flow may also occupy the centre of the vascular cylinder, resulting in differentiation of metaxylem vessels. By contrast, the low-concentration IAA streams induce the protophloem strands, which are small and always separated and limited to the periphery of the vascular cylinder (Fig. 2A). Because low-concentration IAA streams are enough to induce protophloem sieve tubes, they are induced and mature first and closer to the root tip than the protoxylem vessels (which require greater IAA stimulation for vessel differentiation). The protophloem sieve tubes start to function before the protoxylem vessels. Functioning sieve tubes (Fig. 2A) are characterized by a rapid non-polar IAA transport (Morris et al., 1973; Goldsmith et al., 1974).
There is a linear correlation between the number of protoxylem strands and the circumference of the vascular cylinder (Sachs 1981, fig. 30; Fahn, 1990). It is possible that auxin regulates the diameter of the vascular cylinder by regulating the size of the root apical meristems (Torrey, 1957). We suggest that the number of protoxylem strands is determined by the free available space at the circumference of the vascular cylinder. An increase in the surface circumference enables more free space for additional separate IAA streams, which results in an increased number of protoxylem strands and protophloem strands among them. Thus, for example, the primary monocotyledonous root has only a few protoxylem strands, whereas the adventitious roots, induced later during plant development by more auxin from larger leaves, are wider and their induced, wide vascular cylinder enables many more IAA streams, resulting in many strands of protoxylem and protophloem in the typical polyarch monocot adventitious root. Pattern analysis of phloem and xylem strands in adventitious monocot roots has revealed modifications (e.g. differentiation of phloem and xylem strands on the same radius) in the typical radial pattern of roots (R. Aloni, unpublished data), indicating that each phloem strand, or xylem strand, is induced by a separate independent IAA stream. In Arabidopsis thaliana, which has a narrow vascular cylinder, usually two protoxylem strands can differentiate (a diarch root).
The primary xylem in the root matures centripetally and therefore the root is characterized by an exarch xylem, i.e. the metaxylem is situated inside the protoxylem. It has been suggested (Aloni, 2004) that IAA flow in the pericycle influences the width of primary vessels in the root vascular cylinder. Accordingly, in primary roots, although the metaxylem and protoxylem vessels begin differentiation almost simultaneously, auxin flow in the pericycle (Fig. 2B) enhances the rate of vessel differentiation in the neighbouring protoxylem elements, which therefore deposit their secondary walls earlier, resulting in narrow vessels. By contrast, the metaxylem vessels, which differentiate away from the pericycle, have more time to expand before secondary wall deposition and therefore become wide vessels. This explanation follows ideas suggested by Aloni and Zimmermann (1983). Here we explain (see Fig. 2B) how one IAA pathway (auxin transport through the pericycle) may accelerate the differentiation in a nearby IAA pathway (the inducing IAA transport in a differentiating-protoxylem vessel) in the centripetal direction. By contrast, in the model of lateral root initiation (Fig. 3) we discuss the possible influence in the centrifugal direction, where the IAA transport in a differentiating-protoxylem vessel might affect IAA transport in the pericycle.
Dicotyledonous roots have few primary xylem strands and their lateral root initiation typically occurs in pericycle cells located immediately outside the protoxylem point (Torrey, 1986; Dubrovsky et al., 2001; Casimiro et al., 2001). This observation raises the question of what is the internal signal produced in the differentiating protoxylem vessels and how it controls the site of lateral root initiation, which is discussed further below. However, lateral roots may also develop in various positions between the protoxylem strands, e.g. near the phloem in polyarch roots. Nevertheless, there are no indications that either a low-concentration IAA stream that induces a sieve tube (Aloni, 1995) or the non-polar IAA transport through intact sieve tubes (Morris et al., 1973; Goldsmith et al., 1974) could induce morphogenetic effects on their surrounding cells. In all cases, the vascular tissues of the main root are continuous with those of a lateral root because the root tip is a sink for the IAA-inducing signal (Sachs, 1968).
CONTROL OF LATERAL ROOT INITIATION – A NEW HYPOTHESIS
CK and IAA have antagonistic roles in root development; auxin promotes the formation of lateral roots (Malamy and Benfey, 1997; Zhang and Hasenstein, 1999; Casimiro et al., 2001; Guo et al., 2005; Woodward and Bartel, 2005) and adventitious roots (Falasca et al., 2004; Sorin et al., 2005), whereas CK application at physiological concentrations inhibits root formation and reverses the IAA effect (Torrey, 1986; Zhang and Hasenstein, 1999; Lloret and Casero, 2002). A low cytokinin content in CK-deficient transgenic plants, which overexpress the cytokinin oxidase/dehydrogenase (CKX) genes, results in an enlarged root meristem, formation of lateral roots closer to the root apical meristem, increased root branching and promotion of adventitious root formation (Werner et al., 2001, 2003; Schmülling, 2002; Lohar et al., 2004).
The third hormonal signal involved in lateral root formation is ethylene. Treatments that induce C2H4 production (i.e. flooding) promote adventitious and lateral root formation (Yamamoto et al., 1995; Visser et al., 1996; Aloni et al., 1998; Lorbiecke and Sauter, 1999; Mergemann and Sauter, 2000). A decrease in either endogenous auxin or ethylene concentrations induced by application of inhibitors of either IAA transport or C2H4 biosynthesis reduced the number of adventitious roots produced by flooded plants (Visser et al., 1996).
Naturally occurring xylem regeneration around adventitious roots in hypocotyls of Luffa cylindrica seedlings (Aloni and Baum, 1991) marks the sites where the polar IAA transport is interrupted inside the vascular bundles by a naturally occurring signal. The combination of both adventitious root initiation and xylem regeneration at the same site of a vascular bundle can be promoted by main root decapitation (Aloni and Baum, 1991), or by external ethylene application (R. Aloni, unpublished data), indicating that wound-induced ethylene (following root decapitation) or elevated ethylene concentration might interrupt the polar IAA transport along two naturally occurring pathways inside a vascular bundle. The adventitious roots are induced by the interruption of IAA transport in the bundle sheath, whereas xylem regeneration is induced by the disturbance of IAA movement through the vascular meristem (see below).
We emphasize the role of ethylene in inducing lateral root initiation and the influence of air spaces on ethylene movement. Regardless of the degree of differentiation, the root's cortical tissue contains numerous intercellular spaces, which form continuous air spaces essential for aeration of the root cells. Conversely, the innermost layer of the cortex, the endodermis, is compactly arranged and lacks air spaces (Raven et al., 2005). Therefore, we propose that the endodermis slows down the transport of any ethylene emission from differentiating-protoxylem cells outwards to the cortex, thus locally boosting ethylene concentration in the pericycle (which is located just inside the endodermis). We further suggest that when ethylene reaches the pericycle, either from inside the vascular cylinder (from differentiating-protoxylem vessel elements) or from the cortex direction (following wounding, flooding or external ethylene application), the elevated ethylene concentration inhibits locally the polar IAA transport through the pericycle, which induces lateral root formation as will be clarified below.
The architecture of the root system is shaped by environmental and hormonal stimuli (Torrey, 1986; López-Bucio et al., 2003; Sorin et al., 2005). The root structure shows extreme developmental plasticity, where the site and amount of lateral roots are influenced by complexes of diverse external and internal signals (Malamy, 2005). Gene and microRNA-mediated regulation suggest molecular models to explain lateral root initiation (Malamy and Ryan, 2001; Guo et al., 2005; Vanneste et al., 2005). However, owing to the regulation complexity of lateral root development, the mechanisms inducing lateral roots remain obscure (Lloret and Casero, 2002). In spite of the increasing physiological and molecular information on lateral root formation, the hormonal mechanism that controls lateral root initiation and orientation remains poorly understood but is definitely required for interpretation of molecular and genetic findings.
Therefore, to clarify the hormonal mechanism that regulates lateral root initiation, a new hypothesis, the ‘lateral root initiation hypothesis’, is proposed which explains how the IAA, ethylene and CK signals regulate lateral root initiation in the differentiation zone of growing dicotyledonous roots. The primary hormonal signal that promotes lateral root initiation is IAA. The IAA which induces lateral roots descends from the young leaves and moves in a polar manner toward the root tip along the vascular cylinder in distinct pathways: through the pericycle (Pe) and the differentiating xylem (X) cells (Fig. 2B). The polar movement of IAA through the pericycle maintains its tissue identity. The CK that inhibits lateral root initiation originates in the root cap and moves upward mainly through the vascular cylinder of the root (Aloni et al., 2005). The ethylene, which is produced locally in differentiating-protoxylem vessel (DPV) cells, determines the site of lateral root initiation (LRi) (Fig. 3). The earliest stage of lateral root initiation is probably induced by local ethylene production in the differentiating-protoxylem vessel elements as a result of the elevated IAA concentrations that induce these vessel elements. The C2H4 is released from the differentiating-protoxylem vessel elements and diffuses to the neighbouring tissue; in the centrifugal direction it locally inhibits IAA movement in the pericycle. Therefore, immediately above this IAA inhibition site, newly arriving IAA from young leaves is locally accumulated in the pericycle; this fast IAA build-up stimulates cell divisions in the pericycle founder cells (Dubrovsky et al., 2000, 2001; Casimiro et al., 2003) and the initiation of a lateral root primordium in the xylem pole. This basic hormonal stimulation of auxin–ethylene–auxin signalling (Fig. 3) regulates root architecture by inducing lateral roots along longitudinal lines adjacent to the differentiating protoxylem vessels (from where the lateral root-inducing ethylene originates). Overproduction of IAA or C2H4 may induce additional lateral roots also between the protoxylem strands. The distance of lateral root initiation from the root tip is regulated by CK concentration. The high CK concentrations at the root cap antagonize IAA and inhibit lateral root initiation in the vicinity of the tip, which is crucial for enabling uninterrupted elongation of the root tip. Therefore, lateral roots initiate further away from the CK-synthesizing cap, occurring above the elongation zone (Van Staden and Ntigane, 1996), thus ensuring the elongation of a smooth primary root tip free from lateral roots. Above the elongation zone, where concentrations of CK decrease, lateral roots can initiate.
The concept suggested in the lateral root initiation hypothesis can also be used to explain adventitious root initiation. The difference between the initiations of the two types of roots is that in the shoot the ethylene, which induces adventitious root initiation, is released from a vascular bundle (consisting of both xylem and phloem); this ethylene, which diffuses in the centrifugal direction, locally inhibits polar IAA transport in the bundle sheath (see the IAA structural pathways in Aloni, 2004, fig. 4I), whereas in the root the ethylene originates from a protoxylem strand and locally inhibits IAA movement in the pericycle.
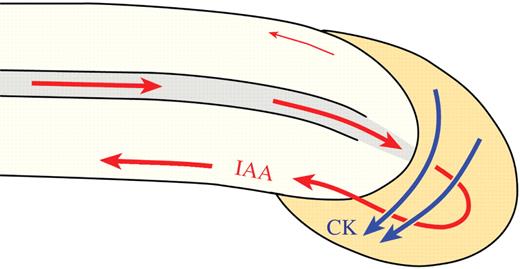
Model of CK- and IAA-regulated root gravitropiosm. When roots are turned to the horizontal position, both CK and IAA are transported laterally downward and become concentrated in the lower root side (sites of high concentrations of CK and IAA are marked). The model shows the direction of the lateral movement of free CK (marked by blue arrows) from the new upper side of the root cap to its lower side, where the high free CK concentration inhibits elongation of the lower side at the distal elongation zone (closely behind the root cap). The polar movement of IAA (red arrows) from the shoot to the root tip occurs through the central vascular cylinder, and at the cap it is laterally distributed mainly to the lower side and then transported along the lower root side to the central elongation zone, where it inhibits elongation. The asymmetric distributions of both CK and IAA inhibit the lower root side and promote elongation of the upper side, resulting in downward root bending.
These ideas can be tested in stems of the Never ripe (Nr) tomato mutant in comparison with wild-type tomato stems, on which adventitious roots appear normally along the vascular bundles, from which the ethylene is probably released and starts adventitious root initiation. However, on the Nr mutant only very few adventitious roots developed, because the Nr cells are almost completely insensitive to ethylene, indicating that the C2H4 is ineffective in inhibiting polar IAA transport in the Nr bundle sheath, therefore resulting in almost complete absence of adventitious root initiation on the Nr stems (see Aloni et al., 1998, fig. 1d).
External ethylene application or treatments that induce C2H4 production (Yamamoto et al., 1995; Visser et al., 1996; Lorbiecke and Sauter, 1999; Mergemann and Sauter, 2000) cause local inhibitions of IAA transport in a root pricycle or in a stem bundle sheath; immediately above these IAA-inhibition sites, the IAA accumulation in these two IAA-transporting tissues (Aloni, 2004, fig. 4) induces the initiation of lateral and adventitious roots, respectively.
CYTOKININ-DEPENDENT ROOT APICAL DOMINANCE
Actively growing primary roots of dicot plants may exhibit apical dominance, where the primary root inhibits lateral root initiation (Zhang and Hasenstein, 1999; Lloret and Casero, 2002). The phenomenon of root apical dominance can be compared with the well-known shoot apical dominance; in both cases the actively growing leader inhibits lateral organ initiation and development. Decapitation of a shoot results in rapid growth of one or more axillary buds below the cut, suggesting that the polar flow of IAA produced in the apical bud and young leaves inhibits the outgrowth of axillary buds (Thimann and Skoog, 1933, 1934; Taiz and Zeiger, 2002). Similarly, in lettuce (Lactuca sativa) seedlings, the removal of the primary root tip stimulates rapid formation of lateral root primordia, which normally are not produced by the intact lettuce root (Zhang and Hasenstein, 1999). As auxin and cytokinin concentrations along the parent root are thought directly to control lateral root initiation and emergence, they could regulate root apical dominance (Lloret and Casero, 2002).
Free bioactive CK can be visualized by the expression of ARR5::GUS (a CK-activated promoter sequence of a response regulator fused to β-glucuronidase), which reflects the transcriptional activation of a CK-sensitive promoter fused to the GUS reporter gene. The construct reacts with free bioactive CK in a concentration-dependent manner (D'Agostino et al., 2000; Aloni et al., 2004). This construct shows that the cap of the primary root of a soil-grown Arabidopsis plant produces elevated concentrations of free bioactive CK (Aloni et al., 2005, fig. 1A), which is usually higher than free CK concentrations of lateral roots (Aloni et al., 2005, fig. 1D, G). When primary root tips are excised, one or more lateral roots become dominant and their tip may start to produce higher concentrations of free CK (Forsyth and Van Staden, 1981; Lloret and Casero, 2002).
Root apical dominance may occur in wild-type plants with an actively growing primary root that inhibits lateral root initiation by the root-cap-synthesized CK, and their lateral roots develop further away from the root tip. By contrast, a low CK content in CK-deficient transgenic plants (overexpressing the CKX genes; Werner et al., 2001, 2003), or almost CK insensitivity [in the double and triple loss-of-function CK receptor, of the ahk (Arabidopsis histidine kinase) mutants, which are almost insensitive to cytokinin], result in the formation of lateral roots closer to the root tip and an increase in root branching (Schmülling, 2002; M. Riefler and T. Schmülling, personal communication). Here we emphasize and extend the previously formulated concept of root apical dominance (Zhang and Hasenstein, 1999; Lloret and Casero, 2002) by focusing on CK mediation. The recent evidence that the root cap predominantly produces CK by expressing the IPT genes (Miyawaki et al., 2004, Takei et al., 2004) and that the highest concentration of free CK in a root is found in the root tip (Aloni et al., 2004, 2005) justify a more precise term for this instance of apical dominance, namely cytokinin-dependent root apical dominance. From an ecological point of view, this CK-dependent root apical dominance gives priority to the primary root in competition with its own lateral roots as well as neighbouring root systems and enables the main root to reach water in deeper soil layers, which might be vital for plants before a dry period. CK regulates root architecture by balancing the promoting role of IAA in lateral root development. CK produced in the active cap of a primary root is the hormonal signal which enables maximum development of an actively growing primary root by retarding lateral root initiation. This reduces the quantity of lateral roots, development and requirements of which would be at the expense of the primary root growth. However, local stimulation by low
THE REGULATION OF ROOT GRAVITROPISM
In the root cap, sedimentation of starch grains (amyloplasts) in gravity-sensing cells (statocytes) enables gravity perception and response (Sievers and Volkmann, 1972). The root cap cells produce CKs, which appear to regulate growth and gravitropism (Aloni et al., 2004). The findings that there is no gravity response after root cap removal (Shaw and Wilkins, 1973) and that CK production occurs in the root cap, which is detected by both IPT5 expression (Miyawaki et al., 2004; Takei et al., 2004) and ARR5::GUS expression (Aloni et al., 2004), suggest that it is likely that the CK produced in the cap is the primary signal of the root statocytes. These cap cells produce the hormone continuously so that it is available for regulating plant growth and development and for lateral flow immediately upon gravity stimulation (Aloni et al., 2004).
Surgical removal of half of the cap from a vertical root results in root bending towards the side with the remaining half-cap. This suggests that in a horizontally positioned root the cap supplies a root growth inhibitor to the lower side of the root during gravitropic bending (Shaw and Wilkins, 1973; Taiz and Zeiger, 2002, fig. 19.31). Similarly, lateral exogenous application of CK to a vertical root induced root bending towards the site of application, confirming the inhibitory effect of CK on root elongation during gravitropism (Aloni et al., 2004).
Models explaining root gravitropism have proposed that the growth response of plants to gravity is regulated by an asymmetric distribution of IAA (Cholodny, 1926; Went and Thimann, 1937; Wolverton et al., 2002; Ottenschläger et al., 2003; Morris et al., 2004). This was also suggested for CK (Aloni et al., 2004), which becomes asymmetrically distributed in response to gravity (Fig. 4). Roots of the triple loss-of-function CK receptor, the ahk mutant, are almost insensitive to CK but respond to gravity (M. Riefler and T. Schmülling, personal communication), indicating that in CK-insensitive mutants the gravity response could be regulated only, or mainly, by IAA. Ethylene, probably induced by high IAA and CK concentrations in the lower root side of horizontally orientated roots, might retard elongation of cells in the lower side by modifying their cytoskeleton. Hydrotropism research suggests a possible role for abscisic acid (ABA) in controlling the direction of tropic root growth (Eapen et al., 2005).
Detailed studies of the root's gravitropic curvature (Wolverton et al., 2002) have revealed that the downward curvature is initiated in the region just behind the root meristem defined as the distal elongating zone (DEZ), and not in the central elongation zone (CEZ) of the root which is inhibited by external auxin application (Ishikawa and Evans, 1993). Unlike the CEZ cells located further basal in the root, the cells in the DEZ are not susceptible to inhibition by elevated concentrations of auxin (Ishikawa and Evans, 1993), but they do respond to external cytokinin application (Aloni et al., 2004). It therefore seems unlikely that IAA mediates the early phase of gravitropic curvature at the root tip (Wolverton et al., 2002) and that the early rapid bending at the DEZ is regulated by CK (Aloni et al., 2004). The late, slower phase of gravity root bending occurs in the CEZ and is probably regulated by IAA (Wolverton et al., 2002).
In vertically growing roots, free CK (detected by ARR5::GUS expression) is distributed symmetrically. When roots are turned to the horizontal position, the free CK is transported radially (Fig. 4), becomes asymmetrically distributed and concentrates in the new lower side of the root cap. This rapidly induced asymmetric activation pattern, detected within less than 30 min, was visualized as a lateral spot of distinctly blue GUS staining appearing in the new lower root side of the lateral cap cells (Aloni et al., 2004). The downward curvature starts near the root apex during the early phase of gravity response by inhibiting growth at the lower root side and promoting elongation of the upper side at the distal elongation zone closely behind the root cap (Aloni et al., 2004).
In CK-deficient transgenic Arabidopsis plants, which overexpress the CKX genes and have only 30–45 % of the wild-type CK content (Werner et al., 2003), the roots grow faster. In AtCKX1 and 3 plants the rates of primary root elongation were up to 70 and 90 % greater, respectively, than that of wild-type seedlings (Werner et al., 2003), indicating that CK retards root elongation in wild-type plants. Accordingly, as a result of CK movement to the new lower side of the cap in horizontally orientated roots (Fig. 4), the local increase of the bioactive free-CK concentration in the lower root side vs. the decreased CK level in the upper side (Aloni et al., 2004) are crucial in regulating tropic root elongation during gravity response, by inhibiting growth at the lower root side and promoting elongation at the upper side.
CKs produced in the shoot (Miyawaki et al., 2004; Nordström et al., 2004; Takei et al., 2004) are not involved in the regulation of root gravitropism. This was evident in horizontally orientated roots of young Arabidopsis plants, in which the cytokinin in the vascular cylinder was retained by the endodermis and therefore was not involved in regulating root gravitropism (Aloni et al., 2004).
In summary, the extreme developmental plasticity of roots is regulated by complexes of diverse external and internal signals, in which hormones may mediate the external stimulation and adapt the root to changing environment, e.g. response to gravity. Our focus here was on root vascular differentiation, lateral root initiation, root development, architecture and root gravitropism, and how they are regulated by the three hormonal signals: CK, IAA and ethylene. A detailed understanding of these hormonal interactions in the control of root orientation, growth and lateral root initiation is clearly required for the interpretation of molecular findings and a better understanding how roots develop, respond and function.
Present address: Department of Philosophy, The Hebrew University, Jerusalem 91905, Israel.
Present address: Department of Cell Biology, University of Heidelberg, Im Neuenheimer Feld 230, 69120 Heidelberg, Germany.
This paper is dedicated to the memory of Arie Blachmann (deceased 10 September, 2005), brother-in-law of the senior author, for encouragement, advice and generosity throughout the years.
We thank Professors Thomas Schmülling (Freie Universität Berlin, Germany) and Joseph J. Kieber (University of North Carolina, USA) for kindly providing seeds of ARR5::GUS transformants of Arabidopsis.
LITERATURE CITED
Aloni R.
Aloni R.
Aloni R.
Aloni R.
Aloni R.
Aloni R.
Aloni R, Baum SF.
Aloni R, Plotkin T.
Aloni R, Zimmermann MH.
Aloni R, Baum SF, Peterson CA.
Aloni R, Wolf A, Feigenbaum P, Avni A, Klee HJ.
Aloni R, Schwalm K, Langhans M, Ullrich CI.
Aloni R, Langhans M, Aloni E, Ullrich CI.
Aloni R, Aloni E, Langhans M, Ullrich CI.
Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI.
Balušká F, Šamaj J, Menzel D.
Bangerth F, Li C-J, Gruber J.
Baum SF, Aloni R, Peterson CA.
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jürgens G, Friml J.
Berleth T, Mattsson J, Hardtke CS.
Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al.
Booker J, Chatfield S, Leyser O.
Bürkle L, Cedzich A, Döpke C, Stransky H, Okumoto S, Gillissen B, et al.
Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, et al.
Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, et al.
Cholodny N.
Coenen C, Lomax TL.
D'Agostino IB, Deruère J, Kieber JJ.
Dubrovsky JG, Doerner PW, Colon-Carmona A, Rost TL.
Dubrovsky JG, Rost TL, Colon-Carmona A, Doerner P.
Eapen D, Barroso ML, Ponce G, Campos ME, Cassab GI.
Emery RJN, Atkins CA.
Falasca G, Zaghi D, Possenti M, Altamura MM.
Forsyth C, Van Staden J.
Friml J, Palme K.
Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, et al.
Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, et al.
Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K.
Gessler A, Kopriva S, Rennenberg H.
Goldsmith MHM, Catealdo DA, Karn J, Brenneman T, Trip P.
Guo HS, Xie Q, Fei JF, Chua NH.
Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM.
Horner HT, Lersten NR, Wirth CL.
Howell SH, Lall S, Che P.
Ishikawa H, Evans ML.
Jacobs WP.
Jiang K, Feldman LJ.
Kerk NM, Jiang K, Feldman LJ.
Kim I, Zambryski PC.
Leitch MA.
Lejeune P, Prinsen E, Van Onckelen H, Bernier G.
Letham DS.
Leyser O.
Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg. G.
Lloret PG, Casero PJ.
Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM.
López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L.
Lorbiecke R, Sauter M.
Malamy JE.
Malamy JE, Benfey PN.
Malamy JE, Ryan KS.
Mergemann H, Sauter M.
Miyawaki K, Matsumoto-Kitano M, Kakimoto T.
Morris DA, Kadir GO, Barry AJ.
Morris DA, Friml J, Zažimalová E.
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G.
Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, et al.
Paponov IA, Teale WD, Trebar M, Blilou I, Palme K.
Ponce G, Barlow PW, Feldman LJ, Cassab GI.
Rahayu YS, Walch-Liu P, Neumann G, Römheld V, von Wirén N, Bangerth F.
Robbertse PJ, McCully ME.
Roberts LW, Gahan PB, Aloni R.
Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, et al.
Sachs T.
Sachs T.
Saks Y, Feigenbaum P, Aloni R.
Scheres B, Benfey P, Dolan L.
Schmülling T.
Shaw S, Wilkins MB.
Sievers A, Volkmann D.
Sorin C, Bussell JD, Camus I, Ljung K, Kowalczyk M, Geiss G, et al.
Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K, et al.
Tanaka M, Mori H, Takei K, Sakakibara H.
Teale WD, Paponov IA, Ditengou F, Palme K.
Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, et al.
Thimann KV, Skoog F.
Thimann KV, Skoog F.
Ticconi CA, Abel S.
Torrey JG.
Torrey JG.
Torrey JG, Fosket DE, Hepler PK.
Tirlapur UK, König K.
Turner S, Sieburth LE.
Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, et al.
Van Staden J, Ntigane BM.
Visser E, Cohen JD, Barendse G, Blom C, Voesenek L.
Werner T, Motyka V, Strnad M, Schmülling T.
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen HV, Schmülling T.
Wolverton C, Ishikawa H, Evans ML.
Woodward AW, Bartel B.
Yamamoto F, Sakata T, Terazawa K.
Yong JW, Wong SC, Letham DS, Hocart CH, Farquhar GD.
Zhang H, Forde BG.
Author notes
1Department of Plant Sciences, Tel Aviv University, Tel Aviv 69978, Israel and 2Institute of Botany, Darmstadt University of Technology, Schnittspahnstrasse 3, 64287 Darmstadt, Germany