-
PDF
- Split View
-
Views
-
Cite
Cite
Elena Fasola, Riccardo Gazzola, Labia Majora Augmentation with Hyaluronic Acid Filler: Technique and Results, Aesthetic Surgery Journal, Volume 36, Issue 10, November/December 2016, Pages 1155–1163, https://doi.org/10.1093/asj/sjw083
Close - Share Icon Share
External female genitalia lose elasticity and volume with age. In the literature several techniques address the redundancy of the labia minora, but only few reports describe the augmentation of labia majora with fat grafting. At present, no studies describe the augmentation of the labia majora with hyaluronic acid.
This study aims to present our technique of infiltration of hyaluronic acid filler, analyzing effectiveness, patient satisfaction, and complications.
We retrospectively analyzed 54 patients affected by hypotrophy of the labia majora; they were treated with hyaluronic acid filler between November 2010 and December 2014. The Global Aesthetic Improvement Scale (GAIS) filled out by the doctor and the patients was used to evaluate the results 12 months after the infiltration. Complications were recorded.
A total of 31 patients affected by mild to moderate labia majora hypotrophy were treated with 19 mg/mL HA filler; 23 patients affected by severe labia majora hypotrophy were treated with 21 mg/mL HA filler. Among the first group of patients, one underwent a second infiltration 6 months later with 19 mg/mL HA filler (maximum 1 mL). A significant improvement (P < .0001) in GAIS score was observed, both in the scores provided by the patients and by the doctor. A greater relative improvement was observed in patients affected by severe hypotrophy. No complications were recorded.
Hyaluronic acid infiltration of the labia majora is able to provide a significant rejuvenation with a simple outpatient procedure. We achieved significant improvements with one infiltration in all cases. The treatment is repeatable, has virtually no complications and it is reversible.
4 Therapeutic
Therapeutic
Cosmetic surgery of the female external genitalia has gained increasing popularity over the past decade. The American Society for Aesthetic Plastic Surgery reported a total of 8,745 labiaplasty procedures performed in 2015, with a dramatic increase (more than 15%) compared to the previous year.1
Commonly these procedures address the morphological and structural modifications which arise as the effects of aging.
A clear example of aging of the female external genitalia is provided by the thickness of the vulvar epithelium, which reaches its peak during reproductive years and decreases with age. At a cellular level, estrogen induces nuclear modifications among the superficial epithelial cells during each menstrual cycle, thus influencing the thickness of the whole epithelium.2
Similarly, vascularization reaches a peak during pregnancy, while it rapidly decreases after menopause. Coloration reflects blood supply, thus explaining the pale appearance of external genitalia in advanced age.
Indeed, following the loss of follicular activity, the external genitalia and labia majora lose subcutaneous fat while connective tissue relaxes and becomes less elastic.3 Macroscopically, the labia majora decrease their tone and volume, while the labia minora increase in size.4
Tissues become more prone to inflammation; typical of aging is post-menopausal atrophic vulvovaginitis, characterized by reduced lubrication,5 skin hydration, friction and permeability.6 Progressive muscle fiber hypotrophy follows similarly in other tissues.7
The number of procedures that aim to rejuvenate the female genitalia is rising.8,9 The vast majority of these procedures address the cutaneous excess of the labia minora, with wedge resections,9,10 edge resections, Z-plasties, or modified resections.11
Rejuvenation of the labia majora is able to improve the aesthetic aspect of the external genitalia, producing a more youthful appearance, while augmentation of the mons pubis could improve discomfort during intercourse reducing trauma along the pubic bone.11,12
Few reports describe labia majora augmentation with fat grafting.4,13 De Lorenzi et al describe the use of hyaluronic acid injections to correct irregularities of the mons pubis, labia minora or labia majora.14 However, the literature lacks studies that investigate the effectiveness and complications of labia majora augmentation with hyaluronic acid injection and no systematic infiltration technique of this anatomical region is reported.
In this study we describe our infiltration technique for labia majora augmentation with hyaluronic acid. We conducted a retrospective analysis of 54 cases, considering the results and complications.
METHODS
Indications and Patient Consultation
Cosmetic procedures on external genitalia have been widely debated. Ethical concerns arise, especially when these procedures, as advertised or proposed by practitioners, may induce the unjustified belief that genital morphology is abnormal and that surgery can correct this “abnormality.”15 In fact a great variability in female external genitalia appearance has been described. This issue should always be borne in mind before any procedure.16
Infiltration of hyaluronic acid dermal filler is a minimally invasive procedure. Nonetheless, a thorough and detailed consultation is necessary. Motivations and expectations should be investigated; the practitioner must properly inform the patient, allowing an autonomous decision making.15 Shaw et al17 underline the necessity to rule out the possibility that a partner or parent is playing a role in coercing the patient to undergo the procedure.
This procedure aims to rejuvenate the female external genitalia, donating more volume and hydration to labia majora, with a possible positive influence on the psychological sphere.14
The consultation should yield certain essential information such as allergies and previous reactions to fillers. Moreover it is essential to investigate those symptoms that could reveal a previous herpetic outbreak and localization.
The patient must be informed about possible complications, although the literature is lacking concerning complications of HA on external female genitalia.
Generic complications of HA infiltration include herpes simplex and herpes zoster reactivation after filler injection. This issue should be prevented with an adequate prophylactic therapy.18 Furthermore, when intravascular infiltration of hyaluronic acid is administered, cutaneous necrosis and distant embolization could occur.19,20 On the other hand, the risk of infection is low and could be minimized by accurate disinfection of the area.21 However there are no data in the literature concerning the infective risk for HA infiltration in this region.
Erythema, edema and bruising may be observed immediately after infiltration, which resolve spontaneously. Allergies are rare but could occur within hours from injection, although late allergic reactions, granulomas and chronic inflammations are described.21
Bumps could occur in cases of superficial infiltration or injection of excessive quantities in a single location.
Regional Anatomy: Important Warnings and Infiltration Rules
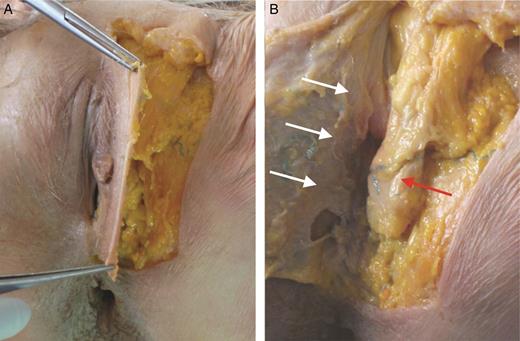
Dissection of labia majora (LM) in a 61-year-old female cadaver. (A) Incision is performed on the lateral margin of LM and the skin is gently retracted medially. (B) On the posterior side of this flap, the lip dartos could be observed (white arrows). This layer is composed of smooth muscle and is more represented on the lateral and inferior sides of the LM. The fibrous tunic (or elastic sack) contains the fat body of LM (red arrow) and fan fibers of round ligament. The caudal third of the sack is close to the fork. These images were acquired during a conventional cadaver dissection for educational purposes. Institutional review board (IRB) approval was not required.
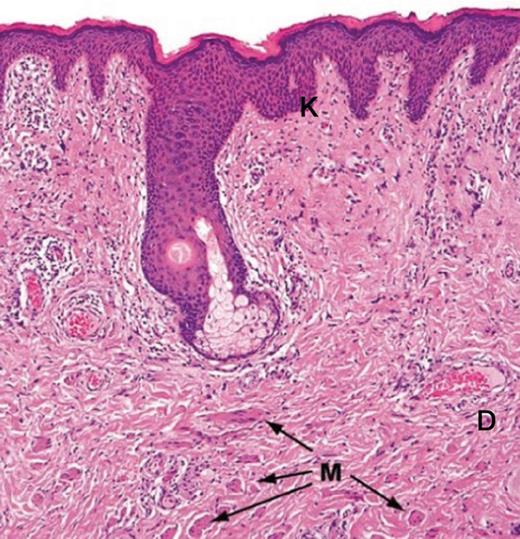
This section (hematoxylin and eosin staining) illustrates the keratinized and stratified squamous epithelium (K) of the labia majora. This epithelium includes hair follicles, sebaceous and apocrine glands. The dartos layer (D) is rich of connective tissue and smooth muscle (M).
The vulva is supplied by the anterior labial arteries, branches of the external pudendal arteries, posterior labial arteries, and branches of the internal pudendal arteries. An adequate knowledge of their localization is fundamental to avoid inadvertent intraluminal infiltration with potential severe complications (vascular occlusion, embolization).19
Innervation is provided by the anterior labial nerves while posteriorly by the posterior pudendal branches. The clitoris is innervated at the same time by the pelvic plexus, pudendal nerve and dorsal clitoral nerve. Perineal nerves innervate the vestibular bulb.11
Infiltration Technique
The area selected for the entrance of the needle or cannula is anesthetized with 1-2 mL of mepivacain 2% with epinephrine 1/100,000 on each side. This area is usually located on the vestibular vaginal edge of the LM.
Syringes pre-filled with cross-linked, non-animal hyaluronic acid with mannitol are employed. Mannitol is an effective antioxidant and is able to counteract the degradation of hyaluronic acid.22,23 Two types of filler are indicated for the procedure. When there is mild hypotrophy, a filler with a concentration of 19 mg/mL of hyaluronic acid is selected (from here on termed “19 mg/mL HA filler”). With moderate to severe hypotrophy, a filler with a concentration of 21 mg/mL of hyaluronic acid (“21 mg/mL HA filler”), in addition to mannitol, is preferred. Both fillers are approved for gynecological indication in Italy. The first filler is injected with a 30- gauge needle and the second with a 18- or 21- gauge cannula (in this case the access for the cannula is made first with a 19G needle).
The infiltration is performed in two layers. Approximately one-third is placed in the subcutaneous layer, while two-thirds are injected between the lip dartos (layer composed of smooth muscle cells immediately beneath the skin) and the fibrous tunic in a fan-shaped fashion, oriented along the longitudinal axis of the LM. Once infiltration is complete, the operator can homogeneously distribute the filler with a mild massage (Videos 1 and 2, available as Supplementary Data at www.aestheticsurgeryjournal.com).
If more than 2 mL of filler are required to achieve a satisfactory volume, we suggest that a subsequent appointment be arranged for a second infiltration at least 4 months later with 19 mg/mL HA filler. On this occasion a small subcutaneous infiltration could be performed.
However, in most cases, 2 mL are sufficient to achieve a satisfactory appearance. Hyaluronic acid is a hygroscopic molecule and the volume reached in living tissues is greater than the injected one. Infiltration of an excessive volume could lead to ischemic complications, granulomas, and raise the risk of infection.
Topical antimicrobial and antimycotic treatment with phenethyl alcohol is regularly administered for one week.24 Antibiotic prophylaxis with amoxicillin clavulanate is administered for six days to those patients infiltrated with 21 mg/mL HA filler via an 18 or 21 Gauge cannula.
We prefer to administer a topical prophylactic treatment due to the frequent contamination of the vulvar area by yeast and bacteria from the vagina and anus.
Concerning the vagina, yeast and fungi are present in the vaginal flora in 21% of women with an absence of symptoms. Candida albicans is commonly associated with vulval itching, Trichomonas vaginalis and gram-negative bacteria are usually associated with vaginal discharge. However, other than those mentioned above, bacteria and fungi do not produce any symptoms or vaginal discharge abnormalities.25
Similarly, antibiotic prophylaxis is administered to those patients infiltrated with 21 mg/mL HA filler since the larger cutaneous access heals without sutures.
In order to avoid intravascular injection, hyaluronic acid should be injected slowly and gently. As a general rule, it is always necessary to aspirate so as to verify any inadvertent vessel incannulation.26 When injecting with a cannula, a blunt tip should be preferred.
In the case of excessive, too superficial, non-homogeneous infiltration or if there is rapid cutaneous discoloration, or pain and reticulated erythema occurs, the use of hyaluronidases should be considered.26,27
Data Collection and Analysis
We retrospectively analyzed 54 patients affected by hypotrophy of the LM, treated with hyaluronic acid filler between November 2010 and December 2014.
All patients included in the study provided written informed consent, and the procedures conformed to the ethical guidelines of the Declaration of Helsinki.
Inclusion criteria were: hypotrophy of LM and cosmetic indication to treatment. Exclusion criteria were: previous surgery on external genitalia, history of vulvar cancer, previous regional radiotherapy, active local herpes simplex /or herpes zoster infection, active herpes zoster infection in other sites, local dermatitis, vulvar squamous papillomas, mycosis, bacterial infections, autoimmune diseases, and history of adverse reactions to hyaluronic acid fillers.
Demographic data, menopause, and pharmacologic treatments were recorded. Hypotrophy was preoperatively staged according to our classification into three grades (mild, moderate, and severe) considering both the adipose tissue and the cutaneous layer (Table 1).
Classification of Labia Majora Hypotrophy
| . | Subcutaneous Layers . | Cutaneous Layer . | Symptoms . |
|---|---|---|---|
| Stage I - Mild (Early) | Mild hypotrophy. Distribution of adipose tissue is usually symmetrical. | None to mild cutaneous hypotrophy. Thin wrinkles may be visible. | Usually asymptomatic. Possible consequence of weight loss. |
| Stage II - Moderate | Moderate hypotrophy. Possible asymmetric distribution of adipose tissue. | Moderate cutaneous laxity, dermatochalasis. Wrinkles are visible. | Dryness, dispareunia, soreness could be observed. |
| Stage III - Severe | Severe hypotrophy. Frequent asymmetric distribution of adipose tissue. | Severe dermatochalasis and deep wrinkles. | Usually associated to symptoms like dryness, dispareunia, soreness. |
| . | Subcutaneous Layers . | Cutaneous Layer . | Symptoms . |
|---|---|---|---|
| Stage I - Mild (Early) | Mild hypotrophy. Distribution of adipose tissue is usually symmetrical. | None to mild cutaneous hypotrophy. Thin wrinkles may be visible. | Usually asymptomatic. Possible consequence of weight loss. |
| Stage II - Moderate | Moderate hypotrophy. Possible asymmetric distribution of adipose tissue. | Moderate cutaneous laxity, dermatochalasis. Wrinkles are visible. | Dryness, dispareunia, soreness could be observed. |
| Stage III - Severe | Severe hypotrophy. Frequent asymmetric distribution of adipose tissue. | Severe dermatochalasis and deep wrinkles. | Usually associated to symptoms like dryness, dispareunia, soreness. |
Classification of Labia Majora Hypotrophy
| . | Subcutaneous Layers . | Cutaneous Layer . | Symptoms . |
|---|---|---|---|
| Stage I - Mild (Early) | Mild hypotrophy. Distribution of adipose tissue is usually symmetrical. | None to mild cutaneous hypotrophy. Thin wrinkles may be visible. | Usually asymptomatic. Possible consequence of weight loss. |
| Stage II - Moderate | Moderate hypotrophy. Possible asymmetric distribution of adipose tissue. | Moderate cutaneous laxity, dermatochalasis. Wrinkles are visible. | Dryness, dispareunia, soreness could be observed. |
| Stage III - Severe | Severe hypotrophy. Frequent asymmetric distribution of adipose tissue. | Severe dermatochalasis and deep wrinkles. | Usually associated to symptoms like dryness, dispareunia, soreness. |
| . | Subcutaneous Layers . | Cutaneous Layer . | Symptoms . |
|---|---|---|---|
| Stage I - Mild (Early) | Mild hypotrophy. Distribution of adipose tissue is usually symmetrical. | None to mild cutaneous hypotrophy. Thin wrinkles may be visible. | Usually asymptomatic. Possible consequence of weight loss. |
| Stage II - Moderate | Moderate hypotrophy. Possible asymmetric distribution of adipose tissue. | Moderate cutaneous laxity, dermatochalasis. Wrinkles are visible. | Dryness, dispareunia, soreness could be observed. |
| Stage III - Severe | Severe hypotrophy. Frequent asymmetric distribution of adipose tissue. | Severe dermatochalasis and deep wrinkles. | Usually associated to symptoms like dryness, dispareunia, soreness. |
The patient and a single independent medical observer rated the aesthetic improvement on the Global Aesthetic Improvement Scale (GAIS) ranging from 1 (no change) to 10 (great improvement) at baseline (preoperative condition) and 12 months after treatment. During the last visit the patient and the medical observer rated their improvement on the GAIS compared with a preoperative digital picture. In order to allow an objective evaluation, each patient was identified by a serial number. The patient's name remained anonymous during the evaluation by the independent medical observer.
Patients were asked to write their score on a form, analogously identified by a serial number. The forms were then collected and analyzed once all evaluations had been completed. Adverse reactions and complications were accurately recorded at the periodic follow-up visit at 1, 3, 6, and 12 months.
Preoperative and postoperative results were studied with the t-test for paired samples.28
RESULTS
A total of 54 women affected by LM hypotrophy, with a mean age of 45 years (range, 36 to 68 years), were included in the study. There were 19 patients in menopause, with at least one of the following symptoms: dryness, dyspareunia, soreness. Six patients in this group were under Hormonal Replacement Therapy (HRT). There were 12 patients who were not in menopause but presented at least one of the previous symptoms while 22 patients were of fertile age without any symptoms. None of the patients were taking any other medications.
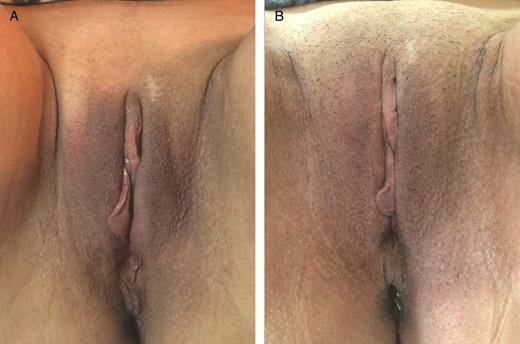
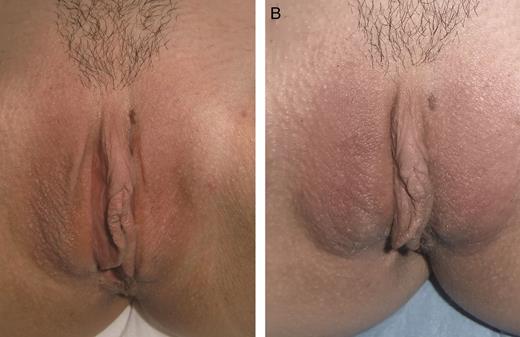
A 56-year-old woman affected by mild hypotrophy of labia majora. The patient received 19 mg/mL hyaluronic acid, 0.7 mL on the right labium and 1.3 mL on the left labium. (A) A preoperative photograph and the result at 12 months (B) from the first treatment are shown.
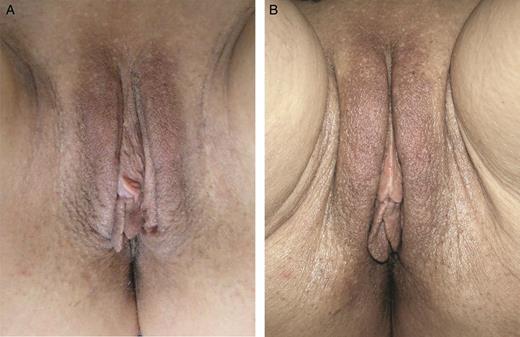
A 63-year-old woman affected by moderate hypotrophy of labia majora. The patient received 19 mg/mL hyaluronic acid, 1 mL per part. At 6 months later a second treatment with 19 mg/mL hyaluronic acid was carried out (1 mL per part). (A) A preoperative photograph and the result at 12 months (B) from the first treatment are shown.
A 34-year-old woman affected by severe hypotrophy of labia majora. The patient was infiltrated with 21 mg/mL hyaluronic acid, 1 mL per part. At 7 weeks later, a second infiltration with 19 mg/mL hyaluronic acid was carried out (1 mL per part). (A) A preoperative photograph and the result at 12 months (B) from the first infiltration are shown.
Among the first group, one patient underwent a second infiltration 6 months later with 19 mg/mL HA filler (maximum 1 mL). Among the second group (severe hypotrophy), 3 patients underwent a second infiltration with 19 mg/mL HA filler (maximum 1 mL) (Table 2).
Summary of Hyaluronic Acid Filler Infiltration for Labia Majora Atrophy
| . | First Treatment . | Second Treatment at 6 Months . |
|---|---|---|
| . | No. of patients (HA filler concentration) . | |
| Stage I | 11 (19 mg/mL) | – |
| Stage II | 20 (19 mg/mL) | 1 (19 mg/mL) |
| Stage III | 23 (21 mg/mL) | 3 (19 mg/mL) |
| Total | 54 | 4 |
| . | First Treatment . | Second Treatment at 6 Months . |
|---|---|---|
| . | No. of patients (HA filler concentration) . | |
| Stage I | 11 (19 mg/mL) | – |
| Stage II | 20 (19 mg/mL) | 1 (19 mg/mL) |
| Stage III | 23 (21 mg/mL) | 3 (19 mg/mL) |
| Total | 54 | 4 |
Summary of Hyaluronic Acid Filler Infiltration for Labia Majora Atrophy
| . | First Treatment . | Second Treatment at 6 Months . |
|---|---|---|
| . | No. of patients (HA filler concentration) . | |
| Stage I | 11 (19 mg/mL) | – |
| Stage II | 20 (19 mg/mL) | 1 (19 mg/mL) |
| Stage III | 23 (21 mg/mL) | 3 (19 mg/mL) |
| Total | 54 | 4 |
| . | First Treatment . | Second Treatment at 6 Months . |
|---|---|---|
| . | No. of patients (HA filler concentration) . | |
| Stage I | 11 (19 mg/mL) | – |
| Stage II | 20 (19 mg/mL) | 1 (19 mg/mL) |
| Stage III | 23 (21 mg/mL) | 3 (19 mg/mL) |
| Total | 54 | 4 |
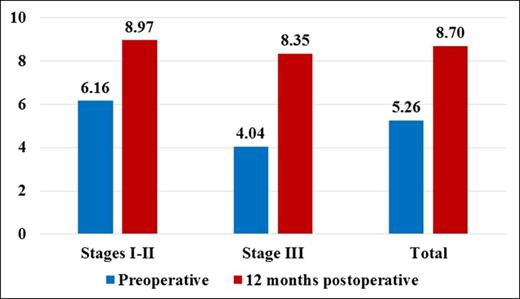
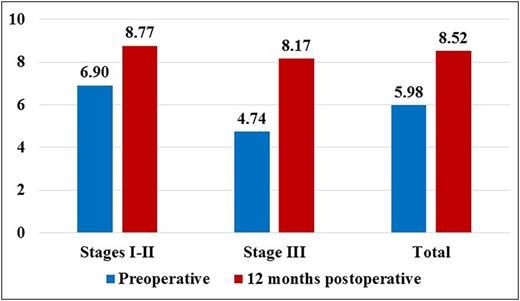
Global Aesthetic Improvement Scale (GAIS) Preoperatively and 12 Months Postoperatively
| . | Preoperative – GAIS Average (Range) . | 12 Months – GAIS Average (Range) . | ||
|---|---|---|---|---|
| . | Patient . | Doctor . | Patient . | Doctor . |
| Stage I-II | 6.16 (4-9) | 6.90 (5-9) | 8.97 (6-10) | 8.77 (6-10) |
| Stage III | 4.04 (2-6) | 4.74 (2-6) | 8.35 (6-10) | 8.17 (6-10) |
| All | 5.26 (2-9) | 5.98 (2-9) | 8.70 (6-10) | 8.52 (6-10) |
| . | Preoperative – GAIS Average (Range) . | 12 Months – GAIS Average (Range) . | ||
|---|---|---|---|---|
| . | Patient . | Doctor . | Patient . | Doctor . |
| Stage I-II | 6.16 (4-9) | 6.90 (5-9) | 8.97 (6-10) | 8.77 (6-10) |
| Stage III | 4.04 (2-6) | 4.74 (2-6) | 8.35 (6-10) | 8.17 (6-10) |
| All | 5.26 (2-9) | 5.98 (2-9) | 8.70 (6-10) | 8.52 (6-10) |
Global Aesthetic Improvement Scale (GAIS) Preoperatively and 12 Months Postoperatively
| . | Preoperative – GAIS Average (Range) . | 12 Months – GAIS Average (Range) . | ||
|---|---|---|---|---|
| . | Patient . | Doctor . | Patient . | Doctor . |
| Stage I-II | 6.16 (4-9) | 6.90 (5-9) | 8.97 (6-10) | 8.77 (6-10) |
| Stage III | 4.04 (2-6) | 4.74 (2-6) | 8.35 (6-10) | 8.17 (6-10) |
| All | 5.26 (2-9) | 5.98 (2-9) | 8.70 (6-10) | 8.52 (6-10) |
| . | Preoperative – GAIS Average (Range) . | 12 Months – GAIS Average (Range) . | ||
|---|---|---|---|---|
| . | Patient . | Doctor . | Patient . | Doctor . |
| Stage I-II | 6.16 (4-9) | 6.90 (5-9) | 8.97 (6-10) | 8.77 (6-10) |
| Stage III | 4.04 (2-6) | 4.74 (2-6) | 8.35 (6-10) | 8.17 (6-10) |
| All | 5.26 (2-9) | 5.98 (2-9) | 8.70 (6-10) | 8.52 (6-10) |
Observed Variation in Global Aesthetic Improvement Scale (GAIS)
| . | Patient GAIS Δ (%a) . | Pb . | Doctor GAIS Δ (%a) . | Pb . |
|---|---|---|---|---|
| Stage I-II | 2.81 (45.61) | <.0001 | 1.87 (27.10) | <.0001 |
| Stage III | 4.30 (106.43) | <.0001 | 3.43 (72.36) | <.0001 |
| All | 3.44 (65.40) | <.0001 | 2.53 (42.31) | <.0001 |
| . | Patient GAIS Δ (%a) . | Pb . | Doctor GAIS Δ (%a) . | Pb . |
|---|---|---|---|---|
| Stage I-II | 2.81 (45.61) | <.0001 | 1.87 (27.10) | <.0001 |
| Stage III | 4.30 (106.43) | <.0001 | 3.43 (72.36) | <.0001 |
| All | 3.44 (65.40) | <.0001 | 2.53 (42.31) | <.0001 |
aPercentage of relative variation compared to the initial score is reported.
bSignificance at t-test for paired samples.
Observed Variation in Global Aesthetic Improvement Scale (GAIS)
| . | Patient GAIS Δ (%a) . | Pb . | Doctor GAIS Δ (%a) . | Pb . |
|---|---|---|---|---|
| Stage I-II | 2.81 (45.61) | <.0001 | 1.87 (27.10) | <.0001 |
| Stage III | 4.30 (106.43) | <.0001 | 3.43 (72.36) | <.0001 |
| All | 3.44 (65.40) | <.0001 | 2.53 (42.31) | <.0001 |
| . | Patient GAIS Δ (%a) . | Pb . | Doctor GAIS Δ (%a) . | Pb . |
|---|---|---|---|---|
| Stage I-II | 2.81 (45.61) | <.0001 | 1.87 (27.10) | <.0001 |
| Stage III | 4.30 (106.43) | <.0001 | 3.43 (72.36) | <.0001 |
| All | 3.44 (65.40) | <.0001 | 2.53 (42.31) | <.0001 |
aPercentage of relative variation compared to the initial score is reported.
bSignificance at t-test for paired samples.
Patient evaluation of the treatment on the Global Aesthetic Improvement Scale (GAIS), preoperatively and 12 months postoperatively.
Doctor evaluation of the treatment on the Global Aesthetic Improvement Scale (GAIS) preoperatively and 12 months postoperatively.
Interestingly, the t-test revealed that the scores provided by the doctor was significantly higher than those provided by patients in the preoperative (P < .0001) and significantly lower in the postoperative (P < .011).
Improvement of dyspareunia was not observed (in fact vaginal introitus was not infiltrated). Nonetheless some patients reported improvements in hydration.
In our case series we did not observe granulomas, ischemic complications, and other complications. Transient hyperemia was observed in half of the patients after treatment lasting usually 30 minutes. One patient, treated with the needle technique, reported edema for 5 days. Mild ecchymosis were observed in two patients treated with the needle. A small amount of product accumulation, appearing as a small bump, was observed in the patients after HA injection with the needle. A light massage of the area was sufficient to correct the defect.
DISCUSSION
The demand for cosmetic procedures of female genitalia is increasing. Labiaplasty, vaginoplasty and clitoral hood reduction are widespread and have excellent outcomes. In particular, labiaplasty demonstrates excellent results in 96.6% of patients, enhancing sexual satisfaction in 64.7%.29
Labiaplasty addresses the redundancy, laxity and hypertrophy of the labia minora. Several techniques have been described, including wedge resections, edge resections, Z-plasties, and modified de-epithelization techniques.9 However the great majority of these procedures leaves labia majora hypotrophy an unsolved issue.
The literature is scant concerning techniques for perform labia majora augmentation and rejuvenation, whilst there are no studies on labia majora augmentation with hyaluronic acid fillers.12 Salgado et al4 report one case of labia majora augmentation with autologous fat grafting, obtaining a stable volume-restoration. Vogt et al describe one case of augmentation after vulvar reconstruction, performed with fat grafting, injected with 1.5 mm cannulas with 5 mL syringes.13
Karabagli et al describe an interesting technique that aims to treat simultaneously labia minora redundancy and labia majora hypotrophy.30 This technique is based on de-epithelization of the protruding segment of the labia minora. This area is dissected as a flap and transposed in a subcutaneous pocket under the labia majora.
These techniques are able to produce an augmentation of labia majora with a surgical procedure.
However, there are no studies in the literature concerning the effectiveness and safety of hyaluronic acid infiltration, although hyaluronic acid fillers allow a significant rejuvenation with a simple outpatient procedure.
We achieved significant improvements with one infiltration in all cases (P < .0001). A greater improvement is observed in those patients affected by stage III hypotrophy.
We recommend not to exceed the quantity of 1 to 2 mL per labia in the same session in order to avoid accumulations of filler, granulomas and ischemic complications.
Mannitol, present in the injected filler, is a well-known scavenger for free radicals. Several beneficial effects have been studied, such as the capacity to reduce skeletal muscle necrosis after postischaemic damage,31 to lower the ischemic damage during partial nephrectomy,32 to limit the damage of cerebral ischemia, to decrease the concentration of free radicals during cardio-pulmonary bypass33 and recently, to protect against ischemic damage in an experimental model of testicular torsion.34
Concerning fillers, the needle employed for the injection triggers an inflammatory process that promotes the production of free radicals and thus a faster degradation of hyaluronic acid. The inclusion of mannitol could reduce HA degradation.35 Experimental models of cataract surgery have demonstrated the effectiveness of mannitol along with HA in reducing the oxidative damage,22 although not specifically studied for dermal fillers.
Moreover, the presence of mannitol increases the osmolarity of the injected filler and the final volume achieved in vivo. This needs to be taken into consideration to avoid infiltration of excessive quantities.
When administering hyaluronidase treatment, the ischemic complications of filler accumulation, a treatment with hyaluronidase need to be thoroughly taken into account.20,27
The dosage of hyaluronidase should be adjusted according to the extent of infiltration, the injected quantity, HA concentration and cross-linking. The dosage of enzyme used for this purpose ranges in the literature from 1.5 to 75 International Units (IU).36,37 Although the toxicity of high dosages of enzymes for fibroblast proliferation or human skin viability has not been demonstrated, we recommend not to exceed 14 IU per infiltration.38
Hyaluronidase could also be used if the patient requests that the treatment be reversed, thereby reverting to, and re-establishing, the previous aspect of the labia majora, although we received no such request in our case series. In such cases, the dosages of hyaluronidases are the same as those described earlier.
Infiltration of HA in labia majora has few and theoretical complications, which have not been described in the literature for labia majora augmentation nor were experienced in our case series. Conversely, a surgical approach on external genitalia could have complications in 7.3% of cases.29
We therefore recommend labia majora rejuvenation even in severe hypotrophies. The treatment is repeatable, has virtually no complications and is reversible.
The main limitations of this study are the size of the sample and the evaluation method. In particular, the Global Aesthetic Improvement Scale (GAIS) is a subjective evaluation and does not evaluate volume enhancement, filler resorption, nor duration of the result. Light could be shed on these issues in further studies involving imaging (eg, ultrasonography).
CONCLUSION
Hyaluronic acid infiltration of the labia majora is able to bring about a significant rejuvenation via a simple outpatient procedure. At present, this is the first study to describe the augmentation of labia majora with hyaluronic acid.
Two types of filler are indicated for the procedure. For mild hypotrophy, a filler with a concentration of 19 mg/mL of hyaluronic acid is selected while for moderate to severe hypotrophy, a filler with a concentration of 21 mg/mL of hyaluronic acid in addition to mannitol, is preferred. The infiltration is performed firstly in the subcutaneous layer, then between the lip and the fibrous tunic.
A total of 54 women affected by labia majora hypotrophy underwent this procedure. A significant improvement (P < .0001) on the Global Aesthetic Improvement Scale was observed, both in the scores provided by patients and by the doctor. A greater relative improvement was observed in patients affected by severe hypotrophy of labia majora.
In our case series we did not observe any complications. The treatment is repeatable, reversible and has less complications than a cosmetic labiaplasty.
Further studies will be necessary to investigate the influence of the psychological sphere and sexual enhancement.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Acknowledgment
The authors would like to thank Dr Nicoletta Di Mari (Pathology Service, Centro Diagnostio Italiano) for assistance with histology and comments that greatly improved the manuscript.
REFERENCES