-
PDF
- Split View
-
Views
-
Cite
Cite
Maxat Kulmanov, Paul N Schofield, Georgios V Gkoutos, Robert Hoehndorf, Ontology-based validation and identification of regulatory phenotypes, Bioinformatics, Volume 34, Issue 17, September 2018, Pages i857–i865, https://doi.org/10.1093/bioinformatics/bty605
Close - Share Icon Share
Abstract
Function annotations of gene products, and phenotype annotations of genotypes, provide valuable information about molecular mechanisms that can be utilized by computational methods to identify functional and phenotypic relatedness, improve our understanding of disease and pathobiology, and lead to discovery of drug targets. Identifying functions and phenotypes commonly requires experiments which are time-consuming and expensive to carry out; creating the annotations additionally requires a curator to make an assertion based on reported evidence. Support to validate the mutual consistency of functional and phenotype annotations as well as a computational method to predict phenotypes from function annotations, would greatly improve the utility of function annotations.
We developed a novel ontology-based method to validate the mutual consistency of function and phenotype annotations. We apply our method to mouse and human annotations, and identify several inconsistencies that can be resolved to improve overall annotation quality. We also apply our method to the rule-based prediction of regulatory phenotypes from functions and demonstrate that we can predict these phenotypes with Fmax of up to 0.647.
1 Introduction
Although several definitions of what constitutes a phenotype have been proposed over time, a phenotype can be operationally defined as an observable characteristic of an organism arising from interactions between the organism’s genotype and the environment (Johannsen, 1909, 1911). Understanding the molecular and functional basis of phenotypes is an important factor in our understanding of disease mechanisms.
Abnormal phenotypes associated with loss of gene function provide valuable information for a variety of computational methods, such as identification of gene-disease associations (Hirschhorn et al., 2002), protein-protein interactions (Hu et al., 2011; Kahanda et al., 2015), disease causative variant prioritization (Boudellioua et al., 2017), finding orthologous genes (Hoehndorf et al., 2011), and drug discovery (Moffat et al., 2014) and repurposing (Hoehndorf et al., 2014). Identifying which phenotypes a gene may be associated with is challenging; even in the case of a complete loss of function of a gene, phenotypes may be highly variable (de Angelis et al., 2015).
Several consortia and research initiatives aim to systematically catalog the phenotypes associated with loss of function mutations in model organisms (Ring et al., 2015), and the experimental results produced by these initiatives provide valuable information for understanding gene function (Ring et al., 2015) or their role in disease (Meehan et al., 2017). In addition to high-throughput phenotyping, there are also ongoing efforts to identify genotype–phenotype relations from literature (Smith and Eppig, 2015), and to record phenotypes observed in a clinical setting which are associated with particular genotypes (Landrum et al., 2014).
There are several computational methods available for predicting the functions of proteins (Cozzetto et al., 2016; Gong et al., 2016; Kulmanov et al., 2017). Computational methods for function prediction have improved in predictive performance and, subsequently, in their utility, over recent years (Radivojac et al., 2013). Consequently, it is a reasonable question to ask whether the same or similar approaches may also work for phenotypes, i.e. whether we can build efficient methods to predict phenotypes from genotypes, and whether these methods can provide information that may be of clinical utility. While methods for protein function prediction are maturing, computational methods to predict phenotypes are still in their infancy.
There are many challenges in predicting phenotypes, both biologically and computationally. From a biological perspective, predicting the phenotypes that arise from a particular genotype is challenging due to the complex molecular and physiological interactions that give rise to phenotypes, open-ended environmental influences and determinants of phenotypes, incomplete penetrance and resilience of organisms to certain phenotypic manifestations, epigenetic regulation not detectable on the level of a genotype, and many other factors contributing to the variability and heterogeneity of phenotypes. The impact of pleiotropy and genetic background were themselves instrumental in motivating the very large scale knockout mouse project (IKMC), precisely because of the problems intrinsic to predicting phenotype from genotype (Austin et al., 2004; Tyler et al., 2016).
From a computational perspective, there are also several additional challenges. First, there is a substantial lack of potential training data that limits the application of machine learning approaches. The high variability in phenotypes and their descriptions (Gkoutos et al., 2005) makes it challenging to identify whether genotypes are involved in identical or similar phenotypes. There is also a lack of computationally represented background knowledge necessary to determine the relationship between phenotypes and their physiological and patho-physiological basis; in particular, there is no computationally accessible, qualitative representation of physiological interactions in mammals. Furthermore, representation of environmental influences is challenging, partly due to their heterogeneity, but also failure to capture environmental parameters in many phenotyping studies (Beckers et al., 2009; Schofield et al., 2016).
The premise underlying comprehensive phenotyping studies is that, uniquely, the phenotype of an organism lacking a functioning copy of a given gene provides definitive information on gene function; the primary goal of functional genomics. Here, we investigate the relationship between Gene Ontology (GO) (Ashburner et al., 2000) functions that are associated with gene products, and phenotypes associated with a loss of function in these gene products (either through targeted or random mutation, epigenetic modification or pharmaceutical effects). Our aim is to identify how much information functions of gene products carry about the phenotypes in which these gene products are involved. Specifically, we test the hypothesis that a loss of a regulatory function (i.e. the up- or down-regulation of some other process) will result in a regulatory phenotype. For example, if a protein is (unconditionally) involved in a positive regulation of B cell apoptosis, then a loss of function in that protein should lead to a phenotype in which the rate of B cell apoptosis is decreased. We first formalize our assumptions in meta-rules that relate axioms in the Web Ontology Language (OWL) (Grau et al., 2008). We then test how many function–phenotype pairs in the laboratory mouse (Mus musculus) and the human (Homo sapiens) satisfy these rules, how many annotations are consistent with our hypothesis, and how many annotations are not consistent with out hypothesis. We investigate some of the inconsistent pairs we identify, and characterize the reasons for the inconsistency; we find that they can be a result of incomplete or under-specified contextualization of function or phenotype annotations (such as by cell type), conflicting annotation derived from literature, or a consequence of inference over the ontology structure.
After validating and characterizing possible inconsistent annotations, we apply our hypothesis predictively and predict regulatory phenotypes associated with loss of function mutations in 11 987 gene products in the mouse and 15 680 in the human. We validate our predictions by predicting protein-protein interactions using phenotype similarity and demonstrate that our rules result in predictions that can reproduce known associations.
2 Materials and methods
2.1 Data sources
We use functional and phenotypic annotations for mouse and human. We downloaded Gene Ontology (GO) (Ashburner et al., 2000) annotations from http://geneontology.org/ on December 15, 2017. The file contains 439 128 distinct annotations to 19 452 human gene products, and 376 532 distinct annotations to 24 526 mouse gene products. We use the phenotype annotations for mouse downloaded from the Mouse Genome Informatics (MGI) (Smith and Eppig, 2015) database (http://www.informatics.jax.org/downloads/reports/index.html) on December 5, 2017. We use the MGI_Gene_Pheno.rpt file which contains phenotypes for non-conditional loss of function mutations in single genes; the file contains phenotypes for 11 887 mouse genes and 206 272 distinct associations between a gene and a Mammalian Phenotype Ontology (MP) (Smith and Eppig, 2015) class. For human, we downloaded annotations provided by the Human Phenotype Ontology (HPO) database (Robinson et al., 2008) on December 5, 2017. We use the file containing phenotypes from ‘all sources’ and ‘all frequencies’; the file contains phenotype associations for 3682 human genes and 120 289 distinct associations between human genes and HPO classes.
For reasoning and processing formal definitions of phenotypes, we use the multi-species integrated PhenomeNET ontology (Hoehndorf et al., 2011; Rodríguez-García et al., 2017). We downloaded the latest version of the PhenomeNET ontology from the AberOWL (Hoehndorf et al., 2015) ontology repository http://aber-owl.net/ontology/PhenomeNET/. We also downloaded the GO in its OWL format, released on December 2, 2017, from the AberOWL ontology repository.
2.2 Filtering GO annotations
To obtain only experimental GO annotations, we filtered all GO annotations by their evidence codes so that we only retain annotations with an experimental evidence. Specifically, we only keep annotations with evidence codes EXP, IDA, IPI, IMP, IGI, IEP, TAS and IC. We removed all annotations which are negated (i.e. using a NOT qualifier); we also excluded all annotations that are context specific, i.e. which are explicitly conditional on a particular environment or other restrictions (such as occurring only in particular cell types, or tissues, or during certain developmental stages).
After filtering all annotations, our GO annotation set contains 100 336 annotations to 11 987 mouse gene products and 295 357 annotations to 15 680 human gene products. We mapped all protein identifiers to MGI identifiers for mouse proteins, and to HUGO (Yates et al., 2017) standard human gene names.
2.3 Protein–protein interactions
For further validation of our predictions, we use protein-protein interactions provided by the STRING Database (Szklarczyk et al., 2015). STRING database uses different data sources such as high throughput lab experiments, conserved co-expressions, text mined, computationally predicted interactions and indirect functional associations and provide a confidence score for each pair of proteins. We downloaded all mouse and human protein-protein interactions from STRING version 10.5 and filtered the interactions by a confidence score higher or equal to 300. We use the protein.aliases file provided by the STRING database to map STRING protein identifiers to MGI identifiers (for mouse genes and proteins) and HUGO gene names (for human genes and proteins).
2.4 Computing semantic similarity
We measure the similarity between sets of MP and HPO classes by computing Resnik’s pairwise similarity measure using the PhenomeNET Ontology (Hoehndorf et al., 2011), and using the Best-Match-Average (BMA) (Pesquita et al., 2009) strategy to combine pairwise similarities into a single similarity score between two sets of annotations. We use the normalized similarity value as a prediction score for interactions between proteins and compute the area under the receiver operating characteristic (ROC) curve (Fawcett, 2006) as a quantitative measure of predictive performance.
2.5 Evaluation metrics
In these measures, p is a phenotype class, is a set of predicted classes for a gene i using a threshold t, and Ti is a set of annotated classes for a gene i. Precision is averaged over the genes where we at least predict one term and m(t) is the total number of such genes. n is a number of all genes in a evaluation set. We evaluate predictions for mouse genes with experimental annotations where we at least make one prediction.
2.6 Predicting protein functions with DeepGO
In order to evaluate our method for predicting phenotypes from functions for gene products without experimental annotations, we predicted GO function annotations using the DeepGO function prediction system (Kulmanov et al., 2017). We downloaded SwissProt reviewed human and mouse protein sequences from the UniProt database (The UniProt Consortium, 2017) on January 28, 2018.
Initially, our dataset had 16 950 mouse and 20 244 human proteins. To meet the DeepGO requirements and limitations, we filtered this set of proteins and removed all sequences with ambiguous amino acid symbols (i.e. B, O, J, U, X and Z); we also removed all proteins with >1002 amino acids. After filtering, we retained 14 916 mouse and 17 837 human proteins for which we could predict functions using DeepGO. We mapped UniProt identifiers to MGI identifiers and HUGO gene names.
2.7 Implementation
We implemented our approach using the OWL API (Horridge and Bechhofer, 2011) version 4.1.0 and used the Similarity Measures Library (Harispe et al., 2014) for measuring semantic similarities. The source code, documentation and data files are freely available at https://github.com/bio-ontology-research-group/phenogocon.
3 Results
3.1 The correspondence between regulation and regulatory phenotypes
Our main hypothesis is that there should be a close relationship between some functions to which gene products are annotated and some phenotypes. In particular, if a gene product is involved in the up- or down-regulation of a process P, then a loss-of-function of that gene product (introduced, for example, through a pathogenic variant, a targeted mutation, or an epigenetic interference) will usually lead to a phenotype in which the rate or intensity of P is decreased or increased.
Specifically, we assume that, if a phenotype is defined as a change of some biological process (such as an increased or decreased rate or turnover of the process), then we can annotate the gene products which negatively or positively regulate or contribute to P biological process with the given phenotype. For example, when a protein that is normally involved in positive regulation of B cell apoptotic process (GO: 0002904) is inhibited (for example through a genetic mutation, or through a small molecule which inhibits the protein), we would expect the rate with which processes of the type B cell apoptotic process (GO: 0001783) occur to decrease.
We formalize this hypothesis in the form of rules that assign a new annotation to a protein with a particular function annotation. Let X be a protein involved in (i.e. annotated with) the function P. We then implement our hypothesis through the following three (meta-)rules:
Increased Function–Decreased Phenotype: If P SubClassOf ‘positively regulates’ some P2, then a loss of function of X results in the phenotype ‘phenotype of’ some (P2 and ‘has quality’ some ‘decreased quality’).
Decreased Function–Increased Phenotype: If P SubClassOf ‘negatively regulates’ some P2, then a loss of function of X results in the phenotype ‘phenotype of’ some (P2 and ‘has quality’ some ‘increased quality’).
Abnormal Function–Abnormal Phenotype: A loss of function of X results in the phenotype ‘phenotype of’ some [P and ‘has quality’ some (‘has modifier’ some abnormal)].
While the first two rules directly implement our hypothesis, the third rule establishes a correspondence between a loss of GO function and the resulting phenotype; it is, in a sense, more general than the previous two rules which establish a correspondence between regulatory functions and phenotypes. The inverse of the abnormality rule has previously been used to predict GO functions from phenotypes (Hoehndorf et al., 2013).
To determine whether a pair of classes in GO and a phenotype ontology class match our hypothesis and subsequent rules, we use the formal definitions and axioms that constrain the GO classes and the classes in phenotype ontologies. Over the past years, many classes in phenotype ontologies have been formally defined using definition patterns based on the Entity–Quality (EQ) method (Gkoutos et al., 2005, 2017; Mungall, 2009). In the EQ method, phenotypes are decomposed into an entity—either an anatomical entity or a biological process or function—and a quality. We identify the GO class underlying each phenotype in MP and HPO based on these EQ-based definition patterns, and we also identify for each phenotype the direction (i.e. increased or decreased) in which the process or function is modified. As a result, we obtain, for each phenotype class in HPO or MP that is based on an abnormal function or process, a pair of a GO class and a direction (i.e. increased or decreased) in which the rate of the process is changed. For example, the class Increased thymocyte apoptosis (MP: 0009541) is defined using the Entity Thymocyte apoptotic process (GO: 0070242) and the Quality Increased rate (PATO: 0000912); the Quality is further constrained by adding the Abnormal (PATO: 0000460) quality (in order to distinguish the abnormal phenotype from a physiological increase in thymocyte apoptotic rate). From the definitions we obtain the pair Thymocyte apoptotic process (GO: 0070242) and Increased as characteristic of the Increased thymocyte apoptosis phenotype.
In total, there are 1543 classes in MP which are based on GO processes or functions; of these, 272 classes are increased in rate, 342 classes are decreased in rate and 929 classes are abnormalities of a process or function. In HPO, 287 phenotype classes are based on GO processes or functions, of which 17 are increased in rate, 54 are decreased in rate and 216 are abnormalities of a process or function.
As next step in our workflow, we identify all GO processes that up- or down-regulate other processes. For this purpose, we use the Elk OWL reasoner (Kazakov et al., 2012) to query GO for all equivalent classes of ‘Biological regulation’ and ‘positively regulates’ some X and ‘Biological regulation’ and ‘negatively regulates’ some X, for all classes X. In total, we identify 3013 processes that positively regulate another biological process, and 3043 processes that negatively regulate another biological process.
We then match the processes that are known to up- or down-regulate other process according to GO and the processes used to define phenotype classes to find corresponding pairs. In total, we identify 1570 correspondence rules between GO and phenotype classes of which 1328 classes are from MP and 242 classes are from HPO. The complete set of correspondences between a GO class and phenotype class is available on our project website. We use the correspondences between regulatory phenotypes and GO functions in two ways: first, we evaluate how many annotations are inconsistent with these rules, and determine why they are inconsistent; second, we use these rules to predict phenotypes from GO functions.
3.2 Determining consistency between function annotations and phenotype annotations
We consider a regulation function annotation and regulatory phenotype annotation as consistent if they do not contradict our rules. An inconsistent pair of annotations is a pair of function and phenotype annotations which contradict our rules (i.e. the function annotation is to the up- or down-regulation of a process and the phenotype of the loss of function is an increased or decreased rate of that process). We generated 423 GO–phenotype pairs that could represent an inconsistency; of these 423 pairs, 398 pairs are GO–MP classes and 25 pairs are GO–HPO classes.
We determine whether the function and phenotype annotations in the Mouse Genome Informatics (MGI) (Smith and Eppig, 2015) model organism database are consistent with our hypothesis, and whether the function annotations for human proteins provided by UniProt (The UniProt Consortium, 2017) and the phenotypes associated with these proteins provided by the HPO database (Robinson et al., 2008; Köhler et al., 2017) are consistent. In the first instance, and to identify only unambiguously matching pairs, we ignore inferences over the ontology and consider only exactly matching phenotypes, i.e. only the annotations in which the direct annotation to the phenotype matches our rule. We find 105 function–phenotype annotation pairs for mouse and one annotation for human which are inconsistent according to our set of inconsistent pairs.
We manually analyzed some of the annotations we tagged as inconsistent with our rules. In many cases, inconsistency with our rules may arise from conflicting GO or phenotype annotations. For example, folliculin interacting protein 1 (Fnip1, MGI: 2444668) is annotated with the GO function Positive regulation of B cell apoptotic process (GO: 0002904), and the loss of function of Fnip1 is annotated with the phenotype increased B cell apoptosis (MP: 0008782). Using our rule (Increased Function–Decreased Phenotype), we flagged this pair of annotations as inconsistent. Both annotations are asserted based on evidence from the same publication (Park et al., 2012), which reports a negative regulatory role for Fnip1 in B cell apoptosis and uses as experimental evidence that B cell apoptosis is increased in response to metabolic stress in mice lacking Fnip1 function. The reports in the paper, together with our rule-based identification of the possible inconsistency, indicates that the GO annotation of Fnip1 to Positive regulation of B cell apoptotic process may not be correct and should be replaced by an annotation to Negative regulation of B cell apoptotic process.
Another example involved glypican 3 (Gpc3, MGI: 104903), which is annotated with the function Negative regulation of growth and the phenotype Postnatal growth retardation. Here, the asserted annotation to postnatal growth retardation is based on Chiao et al. (2002). The postnatal growth catch-down and catch-up seen in homozygote nulls was subject to extensive analysis in the paper and the authors conclude that the normal, growth suppressing, function of Gpc3 is restricted to the embryonic period. The knockout phenotype should therefore have been annotated as Increased embryo size, not Postnatal growth retardation as the closest description to the phenotype described in the paper.
The complexity of phenotypic annotations is well demonstrated by the inconsistency we detect for an annotation of the CD28 cell surface receptor. Annotated in GO to Positive regulation of T cell proliferation, the knockout strain phenotype is annotated in MGI to Increased T cell proliferation (Bour-Jordan et al., 2004). Regulatory T cells (Tregs; CD4 + CD25+) depend on CD28 for activation and proliferation. Effector T cells are suppressed in non-obese diabetic (NOD) mice by active Tregs. In the absence of CD28, Tregs do not proliferate, thereby permitting effector cells to proliferate. This proliferation of effector T cells is reported in the manuscript on which the phenotype annotation is based, and leads to the phenotype annotation of the knockout. Formally this is accurate, but the phenotype reported is dependent on the function of a cell type whose own function is affected by the loss of CD28 in a different cell. This ‘russian doll’ effect is likely to be a significant confounder in relating phenotype to function, particularly at a high level of phenotypic granularity.
We also experimented with extending the scope of our method and included inferred phenotype annotations (we consider a phenotype annotation to phenotype class C as inferred if and only if the annotation is made to a subclass of C in the phenotype ontology). This allows us to identify significantly more potentially inconsistent function–phenotype pairs. We find, for example, the inconsistent annotation pair in BCL2-associated athanogene 6 (BAG6) between the GO process Negative regulation of apoptotic process (GO: 0043066) and Decreased apoptosis (MP: 0006043). However, the directly asserted annotation of BAG6 is to Decreased susceptibility to neuronal excitotoxicity (MP: 0008236), a subclass of Decreased apoptosis in MP. While a direct annotation to Decreased apoptosis would likely have implied that apoptotic processes are, in general, decreased in rate, an annotation to Decreased susceptibility to neuronal excitotoxicity does not have the same implications: apoptotic processes occurring in neurons under certain conditions are decreased in rate, but most apoptotic processes are unaffected. Due to these implications, we do not apply our rules to phenotypes that are inferred over a phenotype ontology.
3.3 Predicting phenotypes from functions
We can also use our rules to predict phenotypes from function annotations. In this case, we take function annotations of a gene product as input, and predict a phenotype that satisfies the definition in our rules. Not all function annotations readily imply a phenotype; therefore, we cannot generate phenotype annotations for all proteins. We generated 78 298 phenotype annotations for 10 041 human genes, and 61 875 phenotype annotations for 7314 mouse genes. Of the generated annotations, 116 human gene annotations and 3170 mouse gene annotations are already present in our data while the remaining predictions are novel. Notably, we predict phenotype annotations for 1986 genes that have no phenotype annotations at all in the mouse, and for 7301 genes without any phenotype annotations in the human. Table 1 summarizes our findings.
Number of predicted annotations using rules inferred with ontology structure, and the number of annotations that are already asserted
| . | Predictions . | Increased . | Decreased . | Abnormal . |
|---|---|---|---|---|
| Mouse | ||||
| Predicted | 61875 | 11656 | 4591 | 45628 |
| Found | 42175 | 370 | 503 | 41302 |
| Human | ||||
| Predicted | 78298 | 18114 | 9588 | 50596 |
| Found | 13142 | 6 | 89 | 13047 |
| . | Predictions . | Increased . | Decreased . | Abnormal . |
|---|---|---|---|---|
| Mouse | ||||
| Predicted | 61875 | 11656 | 4591 | 45628 |
| Found | 42175 | 370 | 503 | 41302 |
| Human | ||||
| Predicted | 78298 | 18114 | 9588 | 50596 |
| Found | 13142 | 6 | 89 | 13047 |
Note: For inferred matches we assume that genotypes are annotated to all superclasses of their annotated classes and propagate both functional and phenotypic annotations. For example, if a genotype has the phenotype Increased B cell apoptosis and application of our rule predicts increased apoptosis, we will also consider this as a match.
Number of predicted annotations using rules inferred with ontology structure, and the number of annotations that are already asserted
| . | Predictions . | Increased . | Decreased . | Abnormal . |
|---|---|---|---|---|
| Mouse | ||||
| Predicted | 61875 | 11656 | 4591 | 45628 |
| Found | 42175 | 370 | 503 | 41302 |
| Human | ||||
| Predicted | 78298 | 18114 | 9588 | 50596 |
| Found | 13142 | 6 | 89 | 13047 |
| . | Predictions . | Increased . | Decreased . | Abnormal . |
|---|---|---|---|---|
| Mouse | ||||
| Predicted | 61875 | 11656 | 4591 | 45628 |
| Found | 42175 | 370 | 503 | 41302 |
| Human | ||||
| Predicted | 78298 | 18114 | 9588 | 50596 |
| Found | 13142 | 6 | 89 | 13047 |
Note: For inferred matches we assume that genotypes are annotated to all superclasses of their annotated classes and propagate both functional and phenotypic annotations. For example, if a genotype has the phenotype Increased B cell apoptosis and application of our rule predicts increased apoptosis, we will also consider this as a match.
We evaluate the performance of our predictions using Fmax measure, which is a main evaluation metric of CAFA (Radivojac et al., 2013) challenge. The Fmax measure provides a similarity for sets of annotations which is computed over the ontology structure. For predictions with experimental GO annotations we use a prediction score of 1.0. Table 2 provides a summary of the evaluation results.
Evaluation of phenotype annotation predictions
| Rules . | Number of genes . | . |
|---|---|---|
| Mouse—Experimental GO Annotations | ||
| Increase/Decrease | 2137 | 0.371 |
| Abnormal | 6753 | 0.367 |
| All | 6974 | 0.361 |
| Mouse—DeepGO Annotations | ||
| Increase/Decrease | 2030 | 0.424 |
| Abnormal | 6956 | 0.313 |
| All | 7675 | 0.189 |
| Human—Experimental GO Annotations | ||
| Increase/Decrease | 242 | 0.356 |
| Abnormal | 2453 | 0.252 |
| All | 2492 | 0.248 |
| Human—DeepGO Annotations | ||
| Increase/Decrease | 1290 | 0.647 |
| Abnormal | 2891 | 0.442 |
| All | 2891 | 0.439 |
| Rules . | Number of genes . | . |
|---|---|---|
| Mouse—Experimental GO Annotations | ||
| Increase/Decrease | 2137 | 0.371 |
| Abnormal | 6753 | 0.367 |
| All | 6974 | 0.361 |
| Mouse—DeepGO Annotations | ||
| Increase/Decrease | 2030 | 0.424 |
| Abnormal | 6956 | 0.313 |
| All | 7675 | 0.189 |
| Human—Experimental GO Annotations | ||
| Increase/Decrease | 242 | 0.356 |
| Abnormal | 2453 | 0.252 |
| All | 2492 | 0.248 |
| Human—DeepGO Annotations | ||
| Increase/Decrease | 1290 | 0.647 |
| Abnormal | 2891 | 0.442 |
| All | 2891 | 0.439 |
Evaluation of phenotype annotation predictions
| Rules . | Number of genes . | . |
|---|---|---|
| Mouse—Experimental GO Annotations | ||
| Increase/Decrease | 2137 | 0.371 |
| Abnormal | 6753 | 0.367 |
| All | 6974 | 0.361 |
| Mouse—DeepGO Annotations | ||
| Increase/Decrease | 2030 | 0.424 |
| Abnormal | 6956 | 0.313 |
| All | 7675 | 0.189 |
| Human—Experimental GO Annotations | ||
| Increase/Decrease | 242 | 0.356 |
| Abnormal | 2453 | 0.252 |
| All | 2492 | 0.248 |
| Human—DeepGO Annotations | ||
| Increase/Decrease | 1290 | 0.647 |
| Abnormal | 2891 | 0.442 |
| All | 2891 | 0.439 |
| Rules . | Number of genes . | . |
|---|---|---|
| Mouse—Experimental GO Annotations | ||
| Increase/Decrease | 2137 | 0.371 |
| Abnormal | 6753 | 0.367 |
| All | 6974 | 0.361 |
| Mouse—DeepGO Annotations | ||
| Increase/Decrease | 2030 | 0.424 |
| Abnormal | 6956 | 0.313 |
| All | 7675 | 0.189 |
| Human—Experimental GO Annotations | ||
| Increase/Decrease | 242 | 0.356 |
| Abnormal | 2453 | 0.252 |
| All | 2492 | 0.248 |
| Human—DeepGO Annotations | ||
| Increase/Decrease | 1290 | 0.647 |
| Abnormal | 2891 | 0.442 |
| All | 2891 | 0.439 |
Phenotype annotations have many applications; in particular, it is accepted that phenotypes reflect underlying physiological interactions and networks (Costanzo et al., 2016) and phenotype annotations are widely used to investigate the molecular basis of diseases (Köhler et al., 2009; Singleton et al., 2014). To validate our phenotype predictions, we performed a set of experiments that provide an indirect, external validation of our predictions. Specifically, we apply a measure of semantic similarity to compute the pairwise similarity between phenotypes associated with genes, and we use the gene–gene phenotypic similarity to predict interactions between the genes [combining the different interaction types aggregated in the STRING database (Szklarczyk et al., 2015), including genetic interactions and protein–protein interactions]. We evaluate our performance using a receiver operating characteristic (ROC) curve (Fawcett, 2006). A ROC curve is a plot of a classifiers true positive rate as a function of the false positive rate, and the area under the ROC curve (ROCAUC) is a quantitative measure of a classifier’s performance (Fawcett, 2006). In our evaluation, we rank pairs of genes based on their phenotype similarity and treat interacting pairs (according to STRING) as positive instances and all other pairs as negative instances.
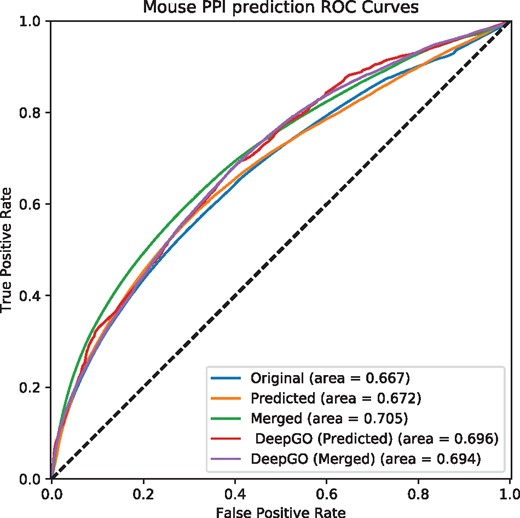
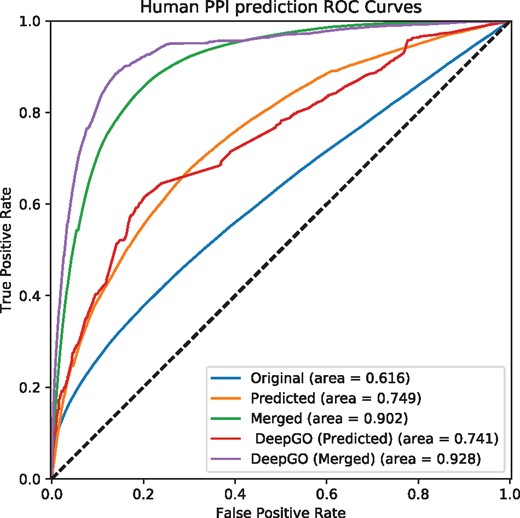
We observe that the performance for predicting interactions improved even over the performance achieved with the original annotations when using the phenotypes generated by our method. Performance further improved when merging original and predicted phenotype annotations, demonstrating that there is significant complimentary information in both (see Table 3 and Figs 1 and 2). We also observe a significant difference in predictive performance between human and mouse; this is likely due to different protocols and standards used in generated both phenotype annotations and function annotations, as well as the very low number of regulatory phenotype annotation that are available for human genes and coded through the HPO. In particular, since human phenotypes in the HPO database predominantly focus on morphological abnormalities (in contrast to mouse phenotypes encoded using the MP which balance morphological and physiological abnormalities), our predictive approach can generate significant volumes of additional annotations that drastically improve predictive performance in our indirect evaluation setting.
Predicting interactions using predicted phenotypes for mouse. Original uses asserted phenotype annotations, Predicted uses only predicted phenotype annotations, and Merged combine asserted and predicted phenotype annotations. DeepGO (Predicted) uses only predicted phenotype annotations based on DeepGO’s predicted GO function annotations, and DeepGO (Merged) combines them with asserted phenotype annotations
Predicting interactions using predicted phenotype annotations for human. Original uses asserted phenotype annotations, Predicted uses only predicted phenotype annotations, and Merged combine asserted and predicted phenotype annotations. DeepGO (Predicted) uses only predicted phenotype annotations based on DeepGO’s predicted GO function annotations, and DeepGO (Merged) combines them with asserted phenotype annotations
3.4 Predicting functions, predicting phenotype annotations
Our method mainly relies on functional annotations of gene products. However, not all genes and gene products have experimental functional annotations. Furthermore, the manual annotations are often derived from mutant phenotypes, thereby limiting the scope of our approach. However, with the recent advances in methods for computational prediction of protein functions (Cozzetto et al., 2016; Gong et al., 2016; Kulmanov et al., 2017; Radivojac et al., 2013), we can experiment with a two-step process: first, we predict GO functions for proteins, and, second, we predict phenotype annotations arising from a loss of function in the protein using our rules.
We recently developed DeepGO (Kulmanov et al., 2017), a computational method for function prediction which uses a deep neural network algorithm to predict functions from protein sequence and (when available) a cross-species interaction network. Using DeepGO, we can predict functions for gene products with known amino-acid sequences. From the predicted function, we can predict phenotype annotations using our rules.
The DeepGO model can only predict annotations to 932 distinct biological process classes in GO (Kulmanov et al., 2017). Of the 932 classes that DeepGO can predict, 443 classes are covered by our rules, and 28 classes are negative regulations and 55 classes are positive regulations. We used DeepGO to predict at least one function for 14 916 mouse and 17 837 human proteins, and based on them, we generated phenotype annotations for 13 225 mouse and 14 187 human genes. 6033 mouse genes and 11 570 human genes for which we predicted phenotypes do not currently have any experimental phenotype annotations.
We evaluated our predictions using Fmax measure and by predicting interactions from the STRING database, similarly to our evaluation of phenotypes predicted from experimental GO annotations. For computing Fmax measure, we used the prediction score of DeepGO annotations as a prediction score for corresponding phenotype in our rules. We find that phenotype predictions with DeepGO annotations for regulatory phenotypes performs better than predictions with experimental GO annotations. Table 2 provides evaluation results with Fmax measure.
Furthermore, we used predicted phenotype annotations for predicting protein-protein interactions. Figures 1 and 2 show the performance of predicting interactions in mouse and human, respectively. We find that predicting phenotype annotations based on DeepGO’s predicted functions allows us to further improve our ability to predict interactions in humans. For the mouse, however, the performance of predicting interactions using phenotype annotations generated from DeepGO’s predicted functions is slightly lower than predictions based on experimental GO annotations, likely due to phenotype annotations already being more complete in the mouse. Table 3 provides a summary of the results.
Summary of evaluation of prediction phenotype annotations for mouse and human
| Method . | AUC (original) . | AUC (predicted) . | AUC (merged) . |
|---|---|---|---|
| Mouse | |||
| Interactions with experimental GO annotations | 0.667 | 0.672 | 0.705 |
| Interactions with DeepGO annotations | 0.667 | 0.696 | 0.694 |
| Human | |||
| Interactions with experimental GO annotations | 0.616 | 0.749 | 0.902 |
| Interactions with DeepGO annotations | 0.616 | 0.741 | 0.928 |
| Method . | AUC (original) . | AUC (predicted) . | AUC (merged) . |
|---|---|---|---|
| Mouse | |||
| Interactions with experimental GO annotations | 0.667 | 0.672 | 0.705 |
| Interactions with DeepGO annotations | 0.667 | 0.696 | 0.694 |
| Human | |||
| Interactions with experimental GO annotations | 0.616 | 0.749 | 0.902 |
| Interactions with DeepGO annotations | 0.616 | 0.741 | 0.928 |
Note: Original uses asserted phenotype annotations, Predicted uses only predicted phenotype annotations, and Merged combine asserted and predicted phenotype annotations.
Summary of evaluation of prediction phenotype annotations for mouse and human
| Method . | AUC (original) . | AUC (predicted) . | AUC (merged) . |
|---|---|---|---|
| Mouse | |||
| Interactions with experimental GO annotations | 0.667 | 0.672 | 0.705 |
| Interactions with DeepGO annotations | 0.667 | 0.696 | 0.694 |
| Human | |||
| Interactions with experimental GO annotations | 0.616 | 0.749 | 0.902 |
| Interactions with DeepGO annotations | 0.616 | 0.741 | 0.928 |
| Method . | AUC (original) . | AUC (predicted) . | AUC (merged) . |
|---|---|---|---|
| Mouse | |||
| Interactions with experimental GO annotations | 0.667 | 0.672 | 0.705 |
| Interactions with DeepGO annotations | 0.667 | 0.696 | 0.694 |
| Human | |||
| Interactions with experimental GO annotations | 0.616 | 0.749 | 0.902 |
| Interactions with DeepGO annotations | 0.616 | 0.741 | 0.928 |
Note: Original uses asserted phenotype annotations, Predicted uses only predicted phenotype annotations, and Merged combine asserted and predicted phenotype annotations.
4 Discussion
4.1 Rules and statistical approaches for predicting phenotypes
Accurate prediction of the phenotypes of an organism from its genotype, and possibly some environmental features, is probably unachievable in the foreseeable future. However, some phenotypes associated with some genes are sufficiently fundamental that they can be predicted reliably given some basic knowledge about a gene and the gene products it encodes. We identify three rules that establish a correspondence between functions of gene products and the phenotypes that a loss of function in these gene products would entail. The main limitation in applying our rules predictively is the precision with which function annotations are contextualized, i.e. how universally a function annotation without any context constraints should be interpreted. However, we focus on three rules which we believe to be sufficiently robust to hold universally, almost as a consequence of the definition of the corresponding phenotypes.
There are likely more rules that can be used to reliably predict phenotypes from functions; some may be as simple as the rules we propose, while others may require complex combinations of functions, and additional constraints, to be applied. Rule mining techniques (Bodenreider et al., 2005), in particular those that can utilize axioms and rules in OWL (Lehmann, 2009), could identify more rules of varying strength and may provide an opportunity to further extend our approach.
We demonstrated that we could not only apply our rules to experimentally determined function predictions, but we were also able to use a function prediction method to predict GO functions, then apply our rules and predict phenotypes. While this approach already yields phenotypes that are useful in computational methods (such as similarity-based prediction of protein–protein interactions), some technical modifications could further improve the accuracy and coverage of predicting phenotypes. A main limitation is that both parts of the method are trained and generated separately; an end-to-end learning approach in which phenotypes are predicted directly (and in which the DeepGO model—or another function prediction method—is used as intermediate, pre-trained part) may further improve the performance as well as coverage of our method.
4.2 Morphological and physiological abnormalities
We found that the performance of our methods is significantly lower in human proteins compared to mouse proteins. However, the number of physiological phenotypes in the HPO, i.e. phenotypes that are defined as an abnormality of a process or function, is much smaller in the HPO than it is in the MP; while HPO mainly contains morphological and developmental abnormalities, MP has a rich classification of abnormal processes.
Our method uses the PhenomeNET ontology (Rodríguez-García et al., 2017) for prediction; PhenomeNET integrates the MP and HPO and therefore predicts many physiological abnormalities for human proteins which cannot currently be captured using the HPO, but which could be captured using the MP. We observe that the under-representation of physiological abnormalities in the HPO results in a low predictive performance (e.g. Fmax measure) when comparing against annotations of human proteins, because our method over-predicts many phenotype annotations. However, our external validation using prediction of interactions between proteins demonstrates that our predictions are highly useful and complementary to the existing phenotype annotations of human proteins; adding our predictions leads to a high increase in ROCAUC when predicting interactions between proteins. In the future, more human physiological abnormalities could be added to and defined in the HPO so that such information can be captured about human genes.
4.3 What do phenotype annotations mean?
Our method can be used both to identify possibly conflicting annotations as well as to suggest phenotypes that may arise from a particular genotype. One observation from our experiments is that the meaning of the annotation relation can be different depending on whether the annotation is asserted or inferred using the ontology structure. Specifically, there seems to be a difference between annotations to a phenotype such as Increased apoptosis, depending on whether the annotation is inferred from the ontology hierarchy (as in the case of an annotation to Increased B cell apoptosis), or asserted. If the annotation is asserted at the level of the class Increased apoptosis, we would usually expect all types of apoptosis processes in the organism to be increased in rate, including apoptosis of B cells and other specific cell types. However, if the annotation is to a more specific class, such as increased B cell apoptosis from which an annotation to Increased apoptosis can be inferred, this no longer holds true.
We can use OWL to provide the outlines of a data model in which these considerations are made explicit. Let us assume that X is annotated with the phenotype P, and, without loss of generality, that P is defined as an increased rate of process F. There are multiple different options for formalizing the meaning of this annotation. The ‘weakest’ form of interpretation (i.e. the form from which the least amount of information can be derived) would be that an organism with X (e.g. an organism with a loss of function mutation in X) would have a part in which at least one process of type F can be observed to be increased in rate; formally, the organism with X would be a subclass of has-part some [(inverse occurs-in) some (F and has-quality some ‘increased rate’)]. A stronger interpretation could be that all processes of type F occurring in an organism with X would be increased in rate. In this case, processes of type F that occur in an organism with X would be come a subclass of things with increased rate, i.e, (F and occurs-in some X) SubClassOf: has-quality some ’increased rate’.
From the first interpretation and its formal representation, we cannot conclude that processes of type F will always, or usually, be increased in rate. We can also not infer much information about subclasses of the phenotype P; we can only infer that the organism with X would also be annotated to any superclass of P. In the second case, however, we can infer that X would also be annotated with all subclasses of P (but not with its superclasses).
To avoid ambiguity in interpretation of phenotype annotations, it would be beneficial to make their intended meaning clear, in particular as the inferences that can be drawn from the interpretations are different. There have already been some efforts to integrate annotations and ontologies in a single knowledge-based model (Hoehndorf et al., 2016; Santana da Silva et al., 2017) which can be used as a formalized data model. Future work on formalizing the intended meaning of annotations, and the adoption of a semantic model, would further improve interoperability and reuse of these annotations and thereby improving their compliance with FAIR standards (Wilkinson et al. 2016).
5 Conclusions
We have developed a novel rule-based method for predicting phenotypes from functions. Our approach can be used as a method to validate phenotype annotations in literature-curated databases, and also to predict phenotypes from a loss of function genotype in a reverse genetics manner (Gilchrist and Haughn, 2010). While the prediction of phenotypes from genotypes is going to remain a challenge, our approach has implications for computational methods that utilize phenotypes. We demonstrated that the phenotypes we predict are predictive of interactions; using a multi-step method in which we first predict protein functions from sequence and then phenotypes from the functions, we could predict phenotypes for genes which have not yet been investigated using a reverse genetic screen. Our approach can therefore extend the scope of phenotype-based methods, including methods for predicting variants, disease genes, or candidate drugs, to cover a significantly larger portion of the mammalian phenome.
Funding
This work was supported by funding from King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) under Award No. URF/1/3454-01-01 and FCC/1/1976-08-01. GVG acknowledges support from H2020-EINFRA (731075) and the National Science Foundation (IOS: 1340112) as well as support from the NIHR Birmingham ECMC, NIHR Birmingham SRMRC and the NIHR Birmingham Biomedical Research Centre and the MRC HDR UK. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, the Medical Research Council or the Department of Health.
Conflict of Interest: none declared.
References