-
PDF
- Split View
-
Views
-
Cite
Cite
James Gilbert, Nicole Pearcy, Rupert Norman, Thomas Millat, Klaus Winzer, John King, Charlie Hodgman, Nigel Minton, Jamie Twycross, Gsmodutils: a python based framework for test-driven genome scale metabolic model development, Bioinformatics, Volume 35, Issue 18, September 2019, Pages 3397–3403, https://doi.org/10.1093/bioinformatics/btz088
Close - Share Icon Share
Abstract
Genome scale metabolic models (GSMMs) are increasingly important for systems biology and metabolic engineering research as they are capable of simulating complex steady-state behaviour. Constraints based models of this form can include thousands of reactions and metabolites, with many crucial pathways that only become activated in specific simulation settings. However, despite their widespread use, power and the availability of tools to aid with the construction and analysis of large scale models, little methodology is suggested for their continued management. For example, when genome annotations are updated or new understanding regarding behaviour is discovered, models often need to be altered to reflect this. This is quickly becoming an issue for industrial systems and synthetic biotechnology applications, which require good quality reusable models integral to the design, build, test and learn cycle.
As part of an ongoing effort to improve genome scale metabolic analysis, we have developed a test-driven development methodology for the continuous integration of validation data from different sources. Contributing to the open source technology based around COBRApy, we have developed the gsmodutils modelling framework placing an emphasis on test-driven design of models through defined test cases. Crucially, different conditions are configurable allowing users to examine how different designs or curation impact a wide range of system behaviours, minimizing error between model versions.
The software framework described within this paper is open source and freely available from http://github.com/SBRCNottingham/gsmodutils.
Supplementary data are available at Bioinformatics online.
1 Introduction
Stoichiometric constraints based modelling for biological systems has been a mainstay of systems biology for several decades (Fell and Small, 1986; Varma and Palsson, 1994). Given its flexibility, low barrier to entry and requirement only on minimal knowledge regarding the stoichiometry of metabolic networks this structural approach has become an extremely popular method for modelling steady-state behaviour of large, biochemical networks (Kauffman et al., 2003). Such large scale reconstructions are often referred to as genome scale metabolic models (GSMMs), as the processes is significantly aided through the advent of relatively inexpensive genome sequencing (Land et al., 2015; O’Brien et al., 2015). Indeed, owing to their ability to model complex aspects of metabolism, GSMMs have been widely adopted as a standard to elucidate and optimize industrial biotechnology processes (Kim et al., 2017).
The reconstruction of GSMMs is a time consuming process of manual curation that follows a complex protocol to ensure model validity (Thiele and Palsson, 2010). Whilst many popular automated methods exist to construct GSMMs from reference genomes (Henry et al., 2010; Poolman, 2006), there is still a significant amount of manual curation. However, treating the creation of models as an isolated ‘one-off’ event ignores the significant amount of curation that is required for applications such as biotechnology.
As a consequence, a significant amount of work has gone into the management of genome scale models. The BiGG models database (King et al., 2016), for example, exists to provide a standardized repository of validated models that can be shared and reused. Perhaps one of the best examples of a well curated model developed in an iterative manner is yeast-GEM, a model for Saccharomyces cerevisiae under continuous development (Sánchez et al., 2018). Similarly, the MetaNetX (Moretti et al., 2016) system exists to provide a standardized namespace and toolchain for GSM analysis. However, in many cases too little focus is placed upon the collaborative design aspect of such models with few mechanisms existing to capture the differences between two model versions, model deltas.
Better tools for developing automated reconstructions of genome scale models are always under development. For example, a recent development in the domain of genome scale models is the EMBL-GEMS model repository for automated reconstructions of bacterial species created from NCBI annotations by the CarveMe tool (Machado et al., 2018). However, these tools will often add incorrect reactions, such as aerobic reactions in organisms that only survive in anaerobic environments (Norman et al., 2018b). Furthermore, we feel a specific advantage of genome scale models is that they encode domain specific knowledge that allows contradictions in understanding to be uncovered. For example, an entirely automated process is unlikely to find the correct cofactors involved in reactions under specific conditions.
Furthermore, as with many areas of bioinformatic study the number of available computational tools has become vast. This covers a huge variety of software platforms including the COBRA toolbox for MATLAB (Schellenberger et al., 2011), ScrumPy and COBRApy in python (Ebrahim et al., 2013; Poolman, 2006) with additional tools and libraries such as cameo (Cardoso et al., 2018), OptFlux in Java (Rocha et al., 2010) and SurreyFBA (Gevorgyan et al., 2011). Whilst most of these tools are Open Source and follow standards, such as SBML (Finney and Hucka, 2003), it is often challenging to replicate the initial modelling efforts conducted by authors of papers. Consequently, we feel that software tools are urgently needed to address this issue. Similarly, the archetype design, build, test and learn cycle of synthetic biology heavily relies on bioinformatics software and modelling to improve the production of natural products (Carbonell et al., 2016). In order to speed up the use of bioinformatics tools to produce high value platform chemicals, genome scale models are often used to discover methods for process optimization in silico.
For example, many tools such as RetroPath (Carbonell et al., 2014a), XTMS (Carbonell et al., 2014b) and GEM-Path (Campodonico et al., 2014) suggest thousands of potential heterologous pathways. Many of these tools significantly increase the value of genome scale models, for example by coupling commodity production to an organism’s growth (Feist et al., 2010). These tools all suggest major changes to wild-type strains must be tracked and compared to allow models to remain relevant. In effect, mechanisms are required to relate to modified test and production strains.
Similarly, many conventional applications of genome scale models in systems biology have often suffered from unnecessary replication of work due to a lack of adherence to standards (Monk et al., 2014). For example, there are now many independently developed models of Clostridium acetobutylicum (Dash et al., 2014; Lee et al., 2008; McAnulty et al., 2012; Senger and Papoutsakis, 2008; Yoo et al., 2015), an organism used in the production of solvents for around a century (Moon et al., 2016; Weizmann, 1919). These models all exist to solve similar biological problems some being updates to the initial base models. However, there has been disagreement over fundamental biochemical properties of this anaerobic organism, notably with the focus on redox balancing (Dash et al., 2014). Such models also include updates based on improved genome annotations and the inclusion of fluxomic, transcriptomic and metabolomic characterizations (Yoo et al., 2015).
Unfortunately, many of the results reported in (Dash et al., 2014; Lee et al., 2008; McAnulty et al., 2012; Senger and Papoutsakis, 2008; Yoo et al., 2015) are difficult to compare or reproduce as the result of a number of issues. Often, model authors do not use a standardized set of identifiers for reaction names [such as the MetaNetX namespace (Moretti et al., 2016)], which makes direct comparison of model structure as well as differences between reactions a challenge. Where models are shared, it is often in non-standard spreadsheet formats, rather than SBML models. Indeed, even in the case of valid SBML models being made available at the time of publication few details are given as to how to run such models for conditions discussed in original articles.
In this paper, we present a software framework geared towards test-driven genome scale model development, a concept that is taken directly from good software development practices (Martin, 2002). By this we mean the notion that, as a model is curated to represent biological phenomena, much of the validation can be turned into specific test cases that can be repeated between model versions. We provide an example test case for Clostridium autoethanogenum, an organism that has had considerable focus in terms of genome scale models and how a working methodology using the software presented here can reduce repetition of work and improve the reproducibility of results. This article aims to summarize the main objectives of the gsmodutils software and we refer the reader to the software user guide for a more detailed exploration of features.
2 Improving the design phase of industrial biotechnology
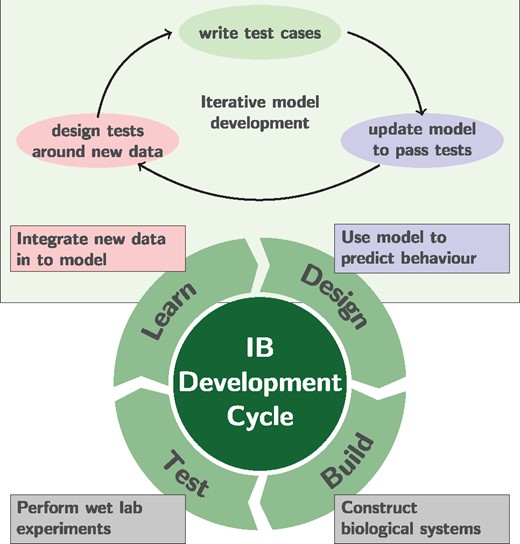
Recent efforts in systems and synthetic biology have been based around a form of iterative, design, build, test and learn cycle (Carbonell et al., 2016) (see Fig. 1). In terms of computational models, this iterative strategy requires adapting and updating models to integrate new biological knowledge (Reed et al., 2006). However, the conventional processes of scientific literature often coalesces to a point at which models are published. In reality, an iterative process means that it is essential that digital experiments can be repeated in a reproducible manner (Cooper et al., 2015). Future changes to models, borne out of a need to meet new challenges and integrate new knowledge, should reflect this.
Iterative cycle for systems and synthetic biology development, prevalent in industrial biotechnology applications. This approach captures an iterative mode of development, where models are used to inform wet lab decision making and the information is fed back into future modelling decisions. By integrating test-driven model development (top section) the objective is to simultaneously capture research questions, model validation criteria and minimize the impact of changes on previously completed models
An agile methodology for the development of models places the focus on adapting work to new requirements (Martin, 2002). Such an approach best fits genome scale models because they are rarely created to investigate individual processes and, instead, capture the complexity of large systems. Genome scale models are intrinsically related to available genome annotations. Such annotations rely heavily on automated matches to related species, with the characterizations of individual genes or changes in cofactors and substrates for specific reactions often being left to a few of critical interest (Seemann, 2014). This modelling formation costs an in-depth understanding of dynamic behaviour. However, capturing steady-state phenomena still provides a good understanding of system properties (O’Brien et al., 2015).
As such, approaches often leave models with missing reactions, incorrect gene-reaction rules (Thiele and Palsson, 2010) or with pathways based on gap filling methods that add reactions that may not actually be catalyzed by the organism in question (Benedict et al., 2014). When attempting to understand specific natural phenomena, genome annotations are frequently updated and models are often corrected in an ad hoc manner.
Therefore, models undergo significant manual annotation and curation; a process which has a high chance of error. In this work, we advocate a test-driven approach to model development highlighted in Figure 1 (top). Here, the model is changed to achieve research goals that are dynamic in response to the changes of a project. In order to meet this objective, validation criteria for a model such as growth conditions or the impact of gene knock-outs, should be formally set. When a model is changed, all such validation criteria should be retested to ensure that models do not regress to previous states.
We feel that many of the current software tools for genome scale models do an excellent job of facilitating answers to crucial research and design questions. However, there is a major gap in terms of the reliability and re-usability of models due to a lack of standardization and software tools to aid such processes. The following sections provide an overview of the gsmodutils software framework. gsmodutils aims to provide a basis for test-driven, version controlled agile model development. All software and packages are open source and is designed to be interoperable with platforms widely used in the domain of constraints based modelling.
3 Software
3.1 Outline and features
Test-driven development is motivated by the idea of clearly defined test cases written before significant changes are made to any underlying architecture. In the case of genome scale models, errors can easily occur as a product of human curation designed to better represent newly discovered aspects of metabolism.
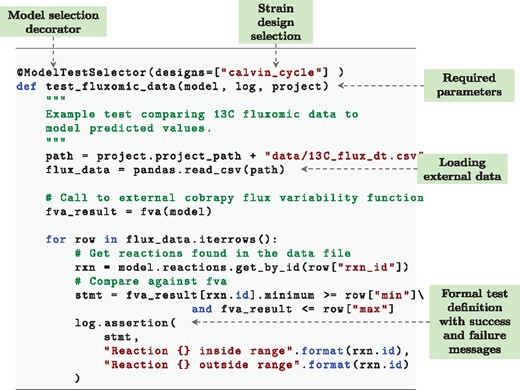
By automatically integrating COBRApy (Ebrahim et al., 2013) users can easily write convenient test cases following examples given in the user guide. A standard test case, ensuring that a given model grows on media is given in Figure 2. When a new model repository is created with the gsmodutils tool, a number of pre-written test cases are automatically added to a file. However, we stress that the vast majority of individual use cases for a model must be specific to a given biological problem.
An example gsmodutils test case written in python. In this test, flux variability analysis is used to compare a model against 13C carbon flux tracking data. The test also demonstrates how designs can be integrated into a test workflow by specifying the identifier in the ‘ModelTestSelector’ function decorator
The software provides a number of features such as import and export of models in different formats and the generation of test reports through use of the command line. The use of flat files enables easy integration with version control software such as git and mercurial. In addition, projects are easy to export using portable standardized docker images (Merkel, 2014), the idea being to allow users to share models as quickly and easily as possible without concern for custom system configurations (see software documentation for more details).
3.2 Strain designs
A core aspect behind the implementation of gsmodutils is the concept of a design, this encompasses a simple set of changes to a ‘wild-type’ model that are required for analysis. However, it is often the case that such deletions are of scientific or industrial interest and, as such, the strain will be used in future work. Consequently, such designs are hereditary in nature. By taking the difference between the constraints applied to an initial model and subsequent modifications, gsmodutils allows users to easily reuse and export models with this design delta.
Formally, we consider a design delta to be the set of differences between any reactions, metabolites and genes stored within the COBRApy object. This should not be considered the same as a diff in version control systems such as git. Instead, designs of this nature are stored as JSON objects within the gsmodutils project and can be tracked by version control systems.
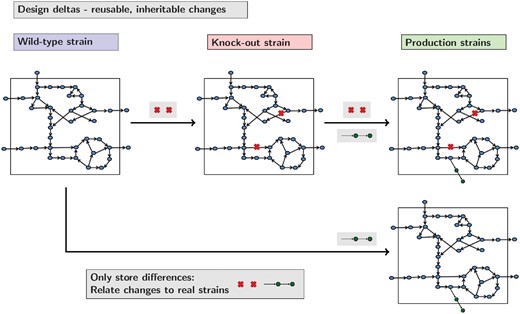
As designs inherit from a base model, future curation to a wild-type base model is automatically included in the resulting models. Similarly, designs are self contained and will not interfere with one another allowing project management and annotation as to the function of each design. Figure 3 shows how this could work in a practical situation. Here we consider how functional gene knock-outs can be combined with heterologous genes to create production strains. As designs can be inherited common knock-outs or changes to designs can be combined.
Examples of gsmodutils design inheritance. Each design stores the delta between the wild-type base model, any parents and the changes to constraints the design contains. In the example presented above, a heterologous production pathway is combined with a reusable set of knock-outs. Rather than keeping redundant copies of models, designs make projects easier to maintain and understand by only storing annotated differences between models. Designs can then be loaded in a hierarchical manner. In practice, ideally, these designs should relate to experimentally evaluated constructs and strains
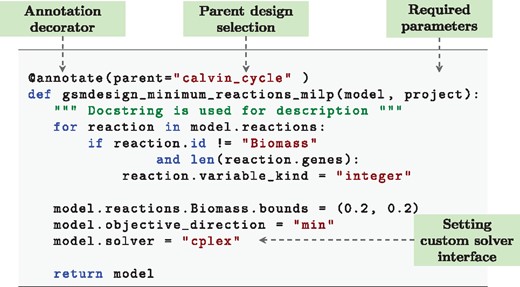
Designs of this nature can also be programmatic, allowing the implementation of features such as non-standard constraints that can be dynamically loaded. An example of this is shown in Figure 4. This example converts an existing model to one based on a Mixed Integer Linear Program (MILP) and sets the objective to minimize the number of enzymatic reactions used with a fixed biomass constraint. This relates to minimizing the number of active genes within a system. As the reaction names do not need to be specified, should reactions be altered within the base model the design will remain functionally the same. Alternative examples could include reductions of models through methods such as elementary flux modes or minimum cut sets, which can change dramatically with only small changes to stoichiometry. Furthermore, functionality of all strain designs is automatically included in tests as part of the default gsmodutils testing framework.
An example gsmodutils programmatic design written in python. This design converts reactions to integer type, allowing an MILP formation. The above example seeks to utilize the MILP problem in order to minimize the number of reactions to produce the required biomass components. Loading a model of this form dynamically, as opposed to storing it as an SBML model, allows any underlying reactions to be changed. Designs of this form can also easily be exported to model files via the command line utility
3.3 Development workflow
In this section, we propose a method for the development of genome scale models that integrates gsmodutils with version control systems. The basic workflow is that the user writes a formal test case for some modelling goal, perhaps driven by captured experimental data, that fits a specific form of validation criteria. We note that, in principle, test cases should be written before changes to a model are made.
Figure 2 highlights the notion of test cases, taken from test-driven development. In this example, a reusable test is written that incorperates data from 13C metabolic flux tracking. Flux variability analysis is then used to compare the expected flux ranges of a model against the experimental data. The test assertions will pass or fail based upon the models flux values when compared to the experimentally observed data.
4 Case study usage Clostridium autoethanogenum
Clostridium autoethanogenum is a bacterial species used for the production of commodity chemicals at industrial scale (Abrini et al., 1994; Norman et al., 2018a). A new GSMM of C.autoethanogenum, ‘MetaCLAU’, has been analyzed to improve this bioprocess (Norman et al., 2018b) (submitted for peer review). In this section we describe how gsmodutils has been utilized to ensure that future versions of MetaCLAU will remain functionally relevant from the perspective of industrial biotechnology.
4.1 Scientific background and model integration
C.autoethanogenum is a strictly anaerobic, acetogenic bacterium which naturally produces ethanol and trace amounts of 2,3-butanediol (2,3-BD) from carbon monoxide and water (Abrini et al., 1994; Norman et al., 2018a; Schuchmann and Müller, 2014). Since carbon monoxide is readily available in the form of industrial waste gas, and 2,3-BD has a global market value of $43 billion (Köpke et al., 2011), the optimization of yields of 2,3-BD from carbon monoxide is highly desirable in the context of industry (Norman et al., 2018a). MetaCLAU was built using Pathway Tools (Karp et al., 2002) and ScrumPy (Poolman, 2006), and is based on a manually annotated genome sequence of C.autoethanogenum (Humphreys et al., 2015). The resulting model consisted of 758 reactions, 773 metabolites and 518 genes. For full details of the model (see Norman et al., 2018b) (submitted for peer review). The model has been integrated with the gsmodutils modelling framework as a test-driven project. The following section details specific tests used to evaluate the model at each stage of its continued development.
4.2 Evaluation of model validation criteria
In this section we outline specific test criteria that have been applied for the C.autoethanogenum model discussed in this study. All of the examples discussed here are available in more detail in the supplementary repository Supplementary Material File S1.
Energetic consistency: An important limitation of FBA is that optimal solutions may be thermodynamically infeasible if appropriate constraints are not applied (Fell et al., 2010). In order to identify these unwanted flux distributions and to constrain the model such that they are not in the feasible solution space, a diagnostic FBA is applied with the following constraints: i) All transport reactions are constrained to allow no uptake, and ii) the ATPase reaction is given a fixed flux of one. If a (non-zero) solution to this problem exists, it must contain a thermodynamic inconsistency, which can be dealt with by manual inspection of the solution and modification of one or more of the involved reactions (Fell et al., 2010).
Flux-minimization tests: One conventional approach in FBA is to set an optimization criterion of minimizing flux across enzymatic reactions with a fixed biomass constraint (Holzhütter, 2006). The solution to this FBA problem represents minimal protein investment (Holzhütter, 2006). Execution of flux minimization in COBRApy requires a model in which reversible reactions are split into two irreversible reactions, representing forward and backward reactions. A gsmodutils strain design was created in which all reversible reactions are split using a programmatic python based design.
In the case of MetaCLAU, the minimal-flux solution includes both ethanol and acetate production, which represents good qualitative agreement with experimental data (Norman et al., 2018a, under review). Since any changes to this predicted phenotype must be investigated, the flux minimization analysis has been formulated as a gsmodutils test which utilizes the above mentioned design.
Product scans: Of interest to this project were changes in the product spectrum of C.autoethanogenum under conditions where the organism can and cannot produce molecular hydrogen (with carbon monoxide as sole carbon and energy source). The hypothesis tested in (Norman et al., 2018a, under review), was that in the case where hydrogen production is infeasible, alternative electron sinks like lactate and 2,3-BD would be produced. As in the previous case, the model-predicted behaviour showing both lactate and 2,3-BD was deemed an important result, which model curators should be notified of if lost during model development. Thus the analysis was built into a gsmodutils test.
Lethal knock-out mutants: The prediction of lethal single-gene KO mutants through FBA of a GSMM is useful in two ways: i) the identification of essential genes is an important first step for metabolic engineering strategies, and ii) with the advent of high-throughput TraDIS gene-essentiality datasets (Langridge et al., 2009), GSMMs can be validated by their ability to predict essential genes. Furthermore, any change in the set of essential genes (particularly an increase in their number) represents important information for metabolic engineering. For these reasons, a test has been built into the MetaCLAU project which enables the computation of the set of essential genes and their comparison with TraDIS datasets.
5 Related software
The reproducibility of computational based research has achieved more and more attention within the last decade (Cooper et al. 2015; Peng, 2011; Sandve et al., 2013). Consequently, there has been a proliferation of tools to support researchers in this endeavour. In this section we briefly review a number of tools that exist, both for genome scale models and from the wider mathematical and computational biology community.
PSAMM: PSAMM (Steffensen et al., 2016) is a tool that has similar goals to gsmodutils in aiding the portability of genome scale models. PSAMM uses a custom YAML format which allows the annotation of models in a simpler manner than the conventional SBML standard. This, simultaneously allows model settings to be easily curated in a human friendly manner as well as allowing changes to be tracked in version control software such as git. This format relates, directly, to a gsmodutils design object, which captures the difference between cobra objects. Both approaches allow export to standardized SBML, MATLAB and JSON models for usage in other tools. A core difference between the two frameworks, however, is that PSAMM includes much more functionality for working with genome scale models including gap filling and even its own interface to linear programming solvers. In contrast, gsmodutils is designed to sit on top of the already existing COBRApy stack, with tools such as cameo (Cardoso et al., 2018) providing additional functionality for more complex analysis. To this end, gsmodutils also has a full python API allowing models and designs to be loaded from within external scripts or jupyter notebooks.
Memote: Lieven et al. (2018) is an excellent tool with similar ambitions to gsmodutils for making reusable genome scale models. It features a fully specified set of tests, including custom test cases and has strong version control integration with git. The core functionality of memote is to provide a standardized, community driven set of tests that check model consistency as well as annotations. Along with user defined tests for experimental data, these tests allow continuous integration as models are updated within a git repository. One of memotes strongest aspects is providing human readable reports between model versions, this allows one to easily track the changes between annotations in reconstructions.
A major difference between these projects is that gsmodutils has a stronger focus on reducing the redundancy in model storage through the use of design deltas, as described above. Similarly, a core goal of gsmodutils is to allow easy import and export outside of the framework for compatibility with other modelling suites. It should be noted that, as memote is written in python, utilizes COBRApy and, at the time of writing, is fully compatible with gsmodutils.
Model repositories: Models are frequently shared, at the time of publication through services such as BiGG (King et al., 2016) and BioModels (Chelliah et al., 2013). Whilst these repositories encourage the reuse of models and the reproducibility of in silico predictions, they are not designed to improve collaboration. The software presented here is designed with the notion that genome scale models are never finished, per se, but under continuous development. The cornerstone of this is the use of test cases, which formalize modelling validation criteria. Model repositories such as EMBL-GEMs, based on automated reconstructions generated by CarveMe (Machado et al., 2018), could greatly benefit from an ever evolving set of tests that better capture biological understanding. Indeed, adding more features to control the future management of the BioModels repository has achieved recent attention with tools such as JUMMP (JUst a Model Management Platform (JUMMP) is available at https://bitbucket.org/jummp/jummp/, Accessed: 2018-12-13) that aim to add version control to the management of repositories.
Tools such as BiVes and BudHat (Scharm et al., 2016) also exist and apply more generally than gsmodutils to capture the changes to models between versions. In a similar manner to gsmodutils, this utility lends itself to version control between model versions by capturing changes to parameters that impact a models performance. As with gsmodutils, this type of approach ignores irrelevant properties such as changes to the white space within XML files captured by a conventional unix diff.
6 Discussion
In order to facilitate the sharing and dissemination of high quality computational research, good standards and software are required (Jimenez et al., 2017). Naturally a great deal of effort has gone into producing high quality systems and synthetic biology standards (Cox et al., 2018; Hucka et al., 2003). Furthermore, when research projects end it is common for important, large models to be published and become relics lost within the literature, forgotten to all but the most dedicated of individuals. As GSMMs grow in terms of the information about metabolism they contain as well as the biological problems they are used to solve, problems with annotation and curation naturally accumulate as a product of human error. Software that facilitates actively improving how researchers develop and apply models to new phenomena is required.
We have presented a framework with a number of features taken from the software development world specifically designed to improve collaboration and minimize such error. However, it is important to stress the difference between defined behaviour expected from pre-written test cases and novel predictions made by a model. Indeed, a core objective of this framework is to ensure that good practices are followed in model development that help scientists to better trust the results discovered by their models. In an ideal world, we would envision a methodology such as ours becoming a pre-requisite for GSMMs to pass peer review.
As with most software development projects, gsmodutils will see expanded features. Initially this will include tighter integration with version control systems such as git and mercurial. Furthermore, the objective of the project is to cultivate collaboration by simplifying the process of distributing large models to different users.
Acknowledgements
We would like to thank the Oxford Brookes Cell Systems Modelling group for helpful discussions regarding this work and for scientific advice.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council [grant numbers BB/L013940/1, BB/K00283X/1, BB/L502030/1]; and the Engineering and Physical Sciences Research Council.
Conflict of Interest: none declared.
References