-
PDF
- Split View
-
Views
-
Cite
Cite
Maxat Kulmanov, Robert Hoehndorf, DeepGOPlus: improved protein function prediction from sequence, Bioinformatics, Volume 36, Issue 2, January 2020, Pages 422–429, https://doi.org/10.1093/bioinformatics/btz595
Close - Share Icon Share
Abstract
Protein function prediction is one of the major tasks of bioinformatics that can help in wide range of biological problems such as understanding disease mechanisms or finding drug targets. Many methods are available for predicting protein functions from sequence based features, protein–protein interaction networks, protein structure or literature. However, other than sequence, most of the features are difficult to obtain or not available for many proteins thereby limiting their scope. Furthermore, the performance of sequence-based function prediction methods is often lower than methods that incorporate multiple features and predicting protein functions may require a lot of time.
We developed a novel method for predicting protein functions from sequence alone which combines deep convolutional neural network (CNN) model with sequence similarity based predictions. Our CNN model scans the sequence for motifs which are predictive for protein functions and combines this with functions of similar proteins (if available). We evaluate the performance of DeepGOPlus using the CAFA3 evaluation measures and achieve an Fmax of 0.390, 0.557 and 0.614 for BPO, MFO and CCO evaluations, respectively. These results would have made DeepGOPlus one of the three best predictors in CCO and the second best performing method in the BPO and MFO evaluations. We also compare DeepGOPlus with state-of-the-art methods such as DeepText2GO and GOLabeler on another dataset. DeepGOPlus can annotate around 40 protein sequences per second on common hardware, thereby making fast and accurate function predictions available for a wide range of proteins.
Supplementary data are available at Bioinformatics online.
1 Introduction
Prediction of protein functions is a major task in bioinformatics that is important in understanding the role of proteins in disease pathobiology, the functions of metagenomes, or finding drug targets. A wide range of methods have been developed for predicting protein functions computationally (Fa et al., 2018; Jiang et al., 2016; Kahanda and Ben-Hur, 2017; Kulmanov et al., 2018; Radivojac et al., 2013; You et al., 2018a,b; Zhou et al., 2019). Protein functions can be predicted from protein sequences (Fa et al., 2018; Jiang et al., 2016; Kulmanov et al., 2018; Radivojac et al., 2013; You et al., 2018a, b; Zhou et al., 2019), protein–protein interactions (PPI) (Kulmanov et al., 2018), protein structures (Yang et al., 2015), biomedical literature and other features (Kahanda and Ben-Hur, 2017; You et al., 2018a). Sequence-based methods employ sequence similarity, search for sequence domains, or multi-sequence alignments to infer functions. As proteins rarely function on their own, protein–protein interactions can be a good predictor for complex biological processes to which proteins contribute. Although it is experimentally challenging to identify protein structures, they are crucial in understanding what proteins are capable of doing. Literature may contribute to function predicting because it may contain explicit descriptions of protein functions or describe properties of proteins that are predictive of protein functions indirectly. Overall, many of these features are available only for a small number of proteins, while a protein’s amino acid sequence can be identified for most proteins. Therefore, methods that accurately predict protein functions from sequence alone may be the most general and applicable to proteins that have not been extensively studied.
Proteins with similar sequence tend to have similar functions (Radivojac et al., 2013). Therefore, a basic way of predicting functions for new sequences is to find the most similar sequences with known functional annotations and transfer their annotations. Another approach is to search for specific sequence motifs which are associated with some function; for example, InterProScan (Mitchell et al., 2014) is a tool which can help to find protein domains and families. The domains and families can be used to infer protein functions.
Recent developments in deep feature learning methods brought many methods which can learn protein sequence features. In 2017, we developed DeepGO (Kulmanov et al., 2018) as one of first deep learning models which can predict protein functions using the protein amino acid sequence and interaction networks. Since 2017, many successor methods became available that achieve better predictive performance (You et al., 2018a, b).
DeepGO suffers from several limitations. First, it can only predict functions for proteins with a sequence length less than 1002 and which do not contain ‘ambiguous’ amino acids such as unions or unknowns. While around 90% of protein sequences in UniProt satisfy these criteria, it also means that DeepGO could not predict functions for about 10% of proteins. Second, due to computational limitations, DeepGO can only predict around 2000 functions out of more than 45 000 which are currently in the Gene Ontology (GO) (Ashburner et al., 2000). Third, DeepGO uses interaction network features which are not available for all proteins. Specifically, for novel or uncharacterized proteins, only the sequence may be known and not any additional information such as the protein’s interactions or mentions in literature. Finally, DeepGO was trained and evaluated on randomly drawn training, validation and testing sets. However, such models may overfit to particular features in the training data and may not yield adequate results in real prediction scenarios. Consequently, challenges such as the Critical Assessment of Function Annotation (CAFA) (Jiang et al., 2016; Radivojac et al., 2013; Zhou et al., 2019) use a time-based evaluation where training and predictions are fixed and evaluated after some time has elapsed on predictions that became available in that time. DeepGO did not achieve the same performance in the CAFA3 (Zhou et al., 2019) challenge as it had in our own experiments.
Here, we extend and improve DeepGO overcoming its main limitations related to sequence length, missing features and number of predicted classes. We increased the model’s input length to 2000 amino acids and now cover more than 99% of sequences in UniProt. Furthermore, our new model’s architecture allows us to split longer sequences and scan smaller chunks to predict functions. We also remove features derived from interaction networks because only a small number of proteins have such network information. Instead, we combine our neural network predictions with methods based on sequence similarity to capture orthology and, indirectly, some interaction information. Through this step we also overcome the limitation in the number of classes to predict and we can, in theory, predict any GO class that has ever been used in an experimental annotation. To avoid overfitting of our model, we substantially decreased our model’s capacity by replacing the amino acid trigram embedding layer with a one-hot encoding and removing our hierarchical classification layer.
In our evaluation we exactly reproduce the CAFA3 evaluation by training our model using only data provided by CAFA3 as training data and evaluating on the CAFA3 testing data. Using the publicly available CAFA Assessment Tool, DeepGOPlus achieves an Fmax of 0.390, 0.557 and 0.614 for BPO, MFO and CCO evaluations, respectively. These results would have made DeepGOPlus the one of three best predictors in CCO and the second best performing method in the BPO and MFO evaluations.
We also compare DeepGOPlus with our baseline methods including DeepGO and two of the best-performing protein function prediction methods, GOLabeler (You et al., 2018b) and DeepText2GO (You et al., 2018a), on another dataset. GOLabeler mainly uses sequence-based features, DeepGO uses interaction network features, and DeepText2GO uses features extracted from literature in addition to sequence-based ones. In terms of Fmax measure, we outperform all methods in predicting biological processes and cellular components. Notably, our model significantly improves predictions of biological process annotations with an Fmax of 0.474.
To provide an insight into what kind of features our model uses to predict functions, we analyze the convolutional filters of our model to understand what type of feature they recognize. We found that sequence regions that activate our filters are very similar to seed sequences of protein families and domains in the Pfam database (El-Gebali et al., 2019). We were able to associate protein sequences in our test set with almost half of their InterPro (Finn et al., 2017) annotations by using sequence regions which activate our convolutional filters.
By using a single model with few parameters, we also significantly improved the runtime of the model. In average, DeepGOPlus can annotate 40 proteins per second on ordinary hardware. Overall, with these improvements, our model can now rapidly perform function prediction for any protein with available sequence. Our online predictor is available at http://deepgoplus.bio2vec.net and DeepGOPlus.
2 Materials and methods
2.1 Datasets and gene ontology
We use two datasets to evaluate our approach. Firstly, we downloaded CAFA3 challenge training sequences and experimental annotations published on September, 2016 and test benchmark published on November 15, 2017 which was used to evaluate protein function prediction methods submitted to the challenge. According to CAFA3, the annotations with evidence codes: EXP, IDA, IPI, IMP, IGI, IEP, TAS, or IC are considered to be experimental. The training set includes all proteins with experimental annotations known before September, 2016 and the test benchmark contains no-knowledge proteins which gained experimental annotation between September, 2016 and November 2017. Similar time based splits were used in all previous CAFA challenges.
We propagate annotations using the hierarchical structure of the Gene Ontology (GO) (Ashburner et al., 2000). We use the version of GO released on June 1, 2016. The version has 10 693 molecular function (MFO), classes, 29 264 biological process (BPO) classes and 4034 cellular component (CCO) classes. This version is also used to evaluate CAFA3 predictions. While propagating annotations, we consider all types of relations between classes. For instance, if a protein P is annotated with a class C which has a part-of relation to a class D, then we annotate P with the class D. This procedure is repeated until no further annotation can be propagated. After this step, we count the number of annotated proteins for each GO class and select all classes with 50 or more annotations for our prediction model. The statistics with the number of classes in Table 1 represent how many classes we can predict using our deep neural network model.
The number of protein sequences with experimental annotations in CAFA3 and 2016 datasets grouped by sub-ontologies
| Dataset . | Statistic . | MFO . | BPO . | CCO . | All . |
|---|---|---|---|---|---|
| CAFA3 | Training size | 36 110 | 53 500 | 50 596 | 66 841 |
| CAFA3 | Testing size | 1137 | 2392 | 1265 | 3328 |
| CAFA3 | Number of classes | 677 | 3992 | 551 | 5220 |
| 2016 | Training size | 34 488 | 51 716 | 49 346 | 65 028 |
| 2016 | Testing size | 679 | 1434 | 1148 | 1788 |
| 2016 | Number of classes | 652 | 3904 | 545 | 5101 |
| Dataset . | Statistic . | MFO . | BPO . | CCO . | All . |
|---|---|---|---|---|---|
| CAFA3 | Training size | 36 110 | 53 500 | 50 596 | 66 841 |
| CAFA3 | Testing size | 1137 | 2392 | 1265 | 3328 |
| CAFA3 | Number of classes | 677 | 3992 | 551 | 5220 |
| 2016 | Training size | 34 488 | 51 716 | 49 346 | 65 028 |
| 2016 | Testing size | 679 | 1434 | 1148 | 1788 |
| 2016 | Number of classes | 652 | 3904 | 545 | 5101 |
The number of protein sequences with experimental annotations in CAFA3 and 2016 datasets grouped by sub-ontologies
| Dataset . | Statistic . | MFO . | BPO . | CCO . | All . |
|---|---|---|---|---|---|
| CAFA3 | Training size | 36 110 | 53 500 | 50 596 | 66 841 |
| CAFA3 | Testing size | 1137 | 2392 | 1265 | 3328 |
| CAFA3 | Number of classes | 677 | 3992 | 551 | 5220 |
| 2016 | Training size | 34 488 | 51 716 | 49 346 | 65 028 |
| 2016 | Testing size | 679 | 1434 | 1148 | 1788 |
| 2016 | Number of classes | 652 | 3904 | 545 | 5101 |
| Dataset . | Statistic . | MFO . | BPO . | CCO . | All . |
|---|---|---|---|---|---|
| CAFA3 | Training size | 36 110 | 53 500 | 50 596 | 66 841 |
| CAFA3 | Testing size | 1137 | 2392 | 1265 | 3328 |
| CAFA3 | Number of classes | 677 | 3992 | 551 | 5220 |
| 2016 | Training size | 34 488 | 51 716 | 49 346 | 65 028 |
| 2016 | Testing size | 679 | 1434 | 1148 | 1788 |
| 2016 | Number of classes | 652 | 3904 | 545 | 5101 |
Secondly, to compare with other methods for function prediction such as DeepText2GO (You et al., 2018a) and GoLabeler (You et al., 2018b) we downloaded SwissProt reviewed proteins published on January, 2016 and October, 2016. We use all experimental annotations before January 2016 as a training set and experimental annotations collected between January and October 2016 as testing set. We filter the testing set with 23 target species which are in CAFA3 evaluation set. Table 1 summarizes both datasets.
2.2 Baseline comparison methods
2.2.1 Naive approach
2.2.2 DiamondBLAST
2.2.3 DiamondScore
2.2.4 DeepGO
DeepGO (Kulmanov et al., 2018) was developed by us previously and it is one of the first methods which learns sequence features with a deep learning model and combines it with PPI network features to predict protein functions. It also uses a hierarchical classifier to output predictions consistent with structure of GO. Here we trained three separate models for three parts of GO mainly because of the computational costs involved in training larger models. We use our previously reported optimal parameters and set of functions to train new models with our current datasets. With DeepGO, we trained and predicted 932 BPO, 589 MFO and 436 CCO classes.
2.2.5 GOLabeler and DeepText2GO
Currently the best performing methods for function prediction task are GOLabeler (You et al., 2018b) and DeepText2GO (You et al., 2018a), both developed by the same group. GOLabeler achieved some of the best results in the preliminary evaluation for all three subontologies of GO in the CAFA3 challenge. It is an ensemble method which combines several approaches and predicts functions mainly from sequence features. DeepText2GO improves the results achieved by GOLabeler by extending their ensemble with models that predict functions from literature.
Our second dataset is specifically designed to compare our results with these two methods. Since we use same training and testing data, we directly compare our results with the results reported in their papers.
2.3 Model training and tuning
We use Tensorflow (Abadi et al., 2016) to build and train our neural network model. Our model was trained on Nvidia Titan X and P6000 GPUs with 12–24 Gb of RAM.
Our neural network model has many hyperparameters such as convolutional filter lengths, number of convolutional filters, depth of fully connected layers, loss functions, activation functions, optimizers and learning rate. In addition, we use weighted sum model to combine sequence similarity method score with neural network model score which has α parameter to be tuned. In general, all parameters were tuned depending on their performance on a validation set which is a randomly split 10% of our training set. Since the parameter search space is quite large we evaluated several loss functions, activation functions, optimizers and learning rate on a simple model and selected binary cross-entropy loss and Adam (Kingma and Ba, 2014) with learning rate of 0.0003. We selected ReLU (Nair and Hinton, 2010) activations for intermediate layers and used Sigmoid function for our final classification layer. Then, we ran an extensive search for the other parameters. Our model uses multiple 1D convolutional layers with different filter lengths where the smallest filter starts from length 8 and the following filter is increased by 8 units. The tested settings were {{8, 16, 24, 32}, {8, 16, 24,…, 64}, {8, 16, 24,…, 128}, {8, 16, 24,…, 256}, {8, 16, 24,…, 512}} where each layer’s number of filters were selected from 32, 64, 128, 256, 512. The depth of fully connected layers were selected from {1, 2, 3}. We tested all combinations of these parameters (in total 75) and the best performing parameters were convolutional layers with filter lengths {8, 16, 24,…, 128} with 512 filters each and 1 fully connected layer. This setting generated 8192 (16 × 512) convolutional filter outputs which were used as a sequence features. The α parameter values which give best performance on a validation set are 0.55, 0.59 and 0.46 for MFO, BPO and CCO evaluations respectively.
To avoid overfitting we use an early stopping strategy depending on the validation loss. Our CNN layers do not use any activation function or dropout because we use MaxPooling layer with maximum pool size. This means that every filter will return only a single value. The aim is to force the CNN filters to learn set of similar patterns (motifs) and if the filter finds the pattern in the sequence it returns a high value which is pooled with the MaxPooling layer. We obtained our best model with only one fully connected layer after the MaxPooling layer. This makes our model relatively simple and less prone to overfitting.
2.4 DeepGOPlus versus DeepGO
There are three main differences of our model from the original DeepGO model (Kulmanov et al., 2018). First, DeepGO uses a trigram embedding layer to represent the sequence. The embedding layer has vectors of size 128 for each trigram (20 × 20 × 20 in total). This layer adds 128 × 8000 parameters to the model. We replaced this representation with a parameter-free one-hot encoding which allowed us to significantly reduce the number of parameters of the new model. We noticed during our experiments that the models with embedding layer easily memorize the training data and overfit to it. Using one-hot encoding helped to avoid this problem. Second, DeepGO has one CNN layer with fixed a filter length which was extended to several CNN layers with different filter lengths. Finally, in DeepGOPlus, we use a flat classification layer instead of hierarchical classifier in DeepGO. The reason for this choice is that we built a single model for all three ontologies with more than 5000 classes and we were not able to build a hierarchical classifier due to memory limitations and time complexities.
2.5 Evaluation
To evaluate our predictions we use the CAFA (Radivojac et al., 2013) evaluation metrics Fmax and Smin (Radivojac and Clark, 2013). In addition, we report the area under the precision–recall curve (AUPR) which is a reasonable measure for evaluating predictions with high class imbalance (Davis and Goadrich, 2006).
In our evaluation, we consider the complete GO ontology when computing parent and child classes, and then separate classes into their individual sub-ontologies (MFO, BPO, CCO) based on the namespace attribute associated with classes in GO. This method is also used by GOLabeler (You et al., 2018b) and DeepText2GO (You et al., 2018a). In the CAFA3 evaluation (Zhou et al., 2019), individual classes are first separated by their sub-ontology and parent and child classes are then computed locally within the sub-ontology. GO has relations between classes in the three sub-ontologies, and many MFO classes stand in a ‘part-of’ relation to BPO classes or in ‘occurs-in’ relations with CCO classes. For example, the MFO class acyl carrier activity (GO: 0000036) stands in a ‘part-of’ relation to fatty acid biosynthetic process (GO: 0006633) in the BPO ontology, and while we take this class into account when computing our evaluation measures, the CAFA3 evaluation does not. To compare our results with the CAFA3 evaluation results (Zhou et al., 2019), we perform the evaluation twice, using the complete ontology and using the separate evaluation as in CAFA3 (using the publicly available CAFA3 evaluation tool).
3 Results
3.1 DeepGOPlus learning model
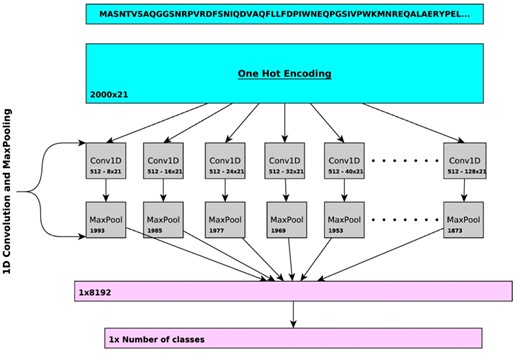
In DeepGOPlus, we combine sequence similarity and sequence motifs in a single predictive model. To learn sequence motifs that are predictive of protein functions, we use one-dimensional convolutional neural networks (CNNs) over protein amino acid sequence to learn sequence patterns or motifs. Figure 1 describes the architecture of our deep learning model. First, the input sequence is converted to a one-hot encoded representation of size 21 × 2000, where a one-hot vector of length 21 represents an amino acid (AA) and 2000 is the input length. Sequences with a length less than 2000 are padded with zeros and longer sequences are split into smaller chunks with less than 2000 AAs. This input is passed to a set of CNN layers with different filter sizes of 8, 16, …, 128. Each of the CNN layers has 512 filters which learn specific sequence motifs of a particular size. Each filter is scanning the sequence and their maximum score is pooled using a MaxPooling layer. In total, we generate a feature vector of size 8192 where each value represents a score that indicates the presence of a relevant sequence motif. This vector is passed to the fully connected classification layer which outputs the predictions. To select the best parameters and hyperparameters for our deep learning model, we extensively searched for optimal combinations of parameters such as filter sizes, number of filters and depth of dense layers based on a validation set loss. We report the list of parameters and validation losses in Supplementary Table S1.
Overview of the CNN in DeepGOPlus. The CNN uses multiple filters of variable size to detect the presence of sequence motifs in the input amino acid sequence
3.2 Evaluation and comparison
We evaluate DeepGOPlus using two datasets. First, we use the latest CAFA3 (Radivojac et al., 2013) challenge dataset and compare our method with baseline methods such as Naive predictions, BLAST and our previous deep learning model DeepGO. We use two strategies for predicting functions based on sequence similarity computed with the Diamond tool (Buchfink et al., 2015) (which is a faster implementation of the BLAST algorithm). We call them DiamondBLAST and DiamondScore. DiamondBLAST considers only the most similar sequence whereas DiamondScore predicts functions using all similar sequences returned by Diamond. We also report the performance of using only our neural network model (labeled as DeepGOCNN). We find that with the DiamondScore approach, we can outperform DeepGO predictions in MFO and achieve comparable results in BPO and CCO evaluations while DeepGOCNN gives better predictions in CCO. We achieve the best performance in all three subontologies with our DeepGOPlus model which combines the DiamondScore and DeepGOCNN. Table 2 summarizes the performance of the models.
The comparison of performance on the first CAFA3 challenge dataset
| Method . | F max . | S min . | AUPR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . |
| Naive | 0.290 | 0.357 | 0.562 | 10.733 | 25.028 | 8.465 | 0.130 | 0.254 | 0.456 |
| DiamondBLAST | 0.431 | 0.399 | 0.506 | 10.233 | 25.320 | 8.800 | 0.178 | 0.116 | 0.142 |
| DiamondScore | 0.509 | 0.427 | 0.557 | 9.031 | 22.860 | 8.198 | 0.340 | 0.267 | 0.335 |
| DeepGO | 0.393 | 0.435 | 0.565 | 9.635 | 24.181 | 9.199 | 0.303 | 0.385 | 0.579 |
| DeepGOCNN | 0.420 | 0.378 | 0.607 | 9.711 | 24.234 | 8.153 | 0.355 | 0.323 | 0.616 |
| DeepGOPlus | 0.544 | 0.469 | 0.623 | 8.724 | 22.573 | 7.823 | 0.487 | 0.404 | 0.627 |
| Method . | F max . | S min . | AUPR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . |
| Naive | 0.290 | 0.357 | 0.562 | 10.733 | 25.028 | 8.465 | 0.130 | 0.254 | 0.456 |
| DiamondBLAST | 0.431 | 0.399 | 0.506 | 10.233 | 25.320 | 8.800 | 0.178 | 0.116 | 0.142 |
| DiamondScore | 0.509 | 0.427 | 0.557 | 9.031 | 22.860 | 8.198 | 0.340 | 0.267 | 0.335 |
| DeepGO | 0.393 | 0.435 | 0.565 | 9.635 | 24.181 | 9.199 | 0.303 | 0.385 | 0.579 |
| DeepGOCNN | 0.420 | 0.378 | 0.607 | 9.711 | 24.234 | 8.153 | 0.355 | 0.323 | 0.616 |
| DeepGOPlus | 0.544 | 0.469 | 0.623 | 8.724 | 22.573 | 7.823 | 0.487 | 0.404 | 0.627 |
Best performance in bold. Fmax and AUPR, highest; Smin, lowest.
The comparison of performance on the first CAFA3 challenge dataset
| Method . | F max . | S min . | AUPR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . |
| Naive | 0.290 | 0.357 | 0.562 | 10.733 | 25.028 | 8.465 | 0.130 | 0.254 | 0.456 |
| DiamondBLAST | 0.431 | 0.399 | 0.506 | 10.233 | 25.320 | 8.800 | 0.178 | 0.116 | 0.142 |
| DiamondScore | 0.509 | 0.427 | 0.557 | 9.031 | 22.860 | 8.198 | 0.340 | 0.267 | 0.335 |
| DeepGO | 0.393 | 0.435 | 0.565 | 9.635 | 24.181 | 9.199 | 0.303 | 0.385 | 0.579 |
| DeepGOCNN | 0.420 | 0.378 | 0.607 | 9.711 | 24.234 | 8.153 | 0.355 | 0.323 | 0.616 |
| DeepGOPlus | 0.544 | 0.469 | 0.623 | 8.724 | 22.573 | 7.823 | 0.487 | 0.404 | 0.627 |
| Method . | F max . | S min . | AUPR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . |
| Naive | 0.290 | 0.357 | 0.562 | 10.733 | 25.028 | 8.465 | 0.130 | 0.254 | 0.456 |
| DiamondBLAST | 0.431 | 0.399 | 0.506 | 10.233 | 25.320 | 8.800 | 0.178 | 0.116 | 0.142 |
| DiamondScore | 0.509 | 0.427 | 0.557 | 9.031 | 22.860 | 8.198 | 0.340 | 0.267 | 0.335 |
| DeepGO | 0.393 | 0.435 | 0.565 | 9.635 | 24.181 | 9.199 | 0.303 | 0.385 | 0.579 |
| DeepGOCNN | 0.420 | 0.378 | 0.607 | 9.711 | 24.234 | 8.153 | 0.355 | 0.323 | 0.616 |
| DeepGOPlus | 0.544 | 0.469 | 0.623 | 8.724 | 22.573 | 7.823 | 0.487 | 0.404 | 0.627 |
Best performance in bold. Fmax and AUPR, highest; Smin, lowest.
To compare our approach with the state of the art methods GOLabeler (You et al., 2018b) and DeepText2GO (You et al., 2018a), we generate a second dataset which uses data obtained at the same dates as the other methods so that we can generate a time-based split of training and testing data. Both methods train on experimental function annotations that appeared before January 2016 and test on annotations which were asserted between January 2016 and October 2016. Furthermore, we use the same version of GO and follow the CAFA3 challenge procedures to process the data. As a result, we can directly compare our evaluation results with the other methods. In this evaluation, DeepGOPlus gives the best results for BPO and CCO in terms of Fmax measure and ranks second in the MFO evaluation (after DeepText2GO). However, it is important to note that DeepText2GO uses features extracted from literature in addition to sequence based features while DeepGOPlus predictions are only based on protein sequence. Notably, our method significantly increased performance of predictions of BPO classes in both evaluation datasets (Table 3).
The comparison of performance on the second dataset generated by a time-based split
| Method . | F max . | S min . | AUPR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . |
| Naive | 0.306 | 0.318 | 0.605 | 12.105 | 38.890 | 9.646 | 0.150 | 0.219 | 0.512 |
| DiamondBLAST | 0.525 | 0.436 | 0.591 | 9.291 | 39.544 | 8.721 | 0.101 | 0.070 | 0.089 |
| DiamondScore | 0.548 | 0.439 | 0.621 | 8.736 | 34.060 | 7.997 | 0.362 | 0.240 | 0.363 |
| DeepGO | 0.449 | 0.398 | 0.667 | 10.722 | 35.085 | 7.861 | 0.409 | 0.328 | 0.696 |
| DeepGOCNN | 0.409 | 0.383 | 0.663 | 11.296 | 36.451 | 8.642 | 0.350 | 0.316 | 0.688 |
| DeepText2GO | 0.627 | 0.441 | 0.694 | 5.240 | 17.713 | 4.531 | 0.605 | 0.336 | 0.729 |
| GOLabeler | 0.580 | 0.370 | 0.687 | 5.077 | 15.177 | 5.518 | 0.546 | 0.225 | 0.700 |
| DeepGOPlus | 0.585 | 0.474 | 0.699 | 8.824 | 33.576 | 7.693 | 0.536 | 0.407 | 0.726 |
| Method . | F max . | S min . | AUPR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . |
| Naive | 0.306 | 0.318 | 0.605 | 12.105 | 38.890 | 9.646 | 0.150 | 0.219 | 0.512 |
| DiamondBLAST | 0.525 | 0.436 | 0.591 | 9.291 | 39.544 | 8.721 | 0.101 | 0.070 | 0.089 |
| DiamondScore | 0.548 | 0.439 | 0.621 | 8.736 | 34.060 | 7.997 | 0.362 | 0.240 | 0.363 |
| DeepGO | 0.449 | 0.398 | 0.667 | 10.722 | 35.085 | 7.861 | 0.409 | 0.328 | 0.696 |
| DeepGOCNN | 0.409 | 0.383 | 0.663 | 11.296 | 36.451 | 8.642 | 0.350 | 0.316 | 0.688 |
| DeepText2GO | 0.627 | 0.441 | 0.694 | 5.240 | 17.713 | 4.531 | 0.605 | 0.336 | 0.729 |
| GOLabeler | 0.580 | 0.370 | 0.687 | 5.077 | 15.177 | 5.518 | 0.546 | 0.225 | 0.700 |
| DeepGOPlus | 0.585 | 0.474 | 0.699 | 8.824 | 33.576 | 7.693 | 0.536 | 0.407 | 0.726 |
Best performance in bold. Fmax and AUPR, highest; Smin, lowest.
The comparison of performance on the second dataset generated by a time-based split
| Method . | F max . | S min . | AUPR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . |
| Naive | 0.306 | 0.318 | 0.605 | 12.105 | 38.890 | 9.646 | 0.150 | 0.219 | 0.512 |
| DiamondBLAST | 0.525 | 0.436 | 0.591 | 9.291 | 39.544 | 8.721 | 0.101 | 0.070 | 0.089 |
| DiamondScore | 0.548 | 0.439 | 0.621 | 8.736 | 34.060 | 7.997 | 0.362 | 0.240 | 0.363 |
| DeepGO | 0.449 | 0.398 | 0.667 | 10.722 | 35.085 | 7.861 | 0.409 | 0.328 | 0.696 |
| DeepGOCNN | 0.409 | 0.383 | 0.663 | 11.296 | 36.451 | 8.642 | 0.350 | 0.316 | 0.688 |
| DeepText2GO | 0.627 | 0.441 | 0.694 | 5.240 | 17.713 | 4.531 | 0.605 | 0.336 | 0.729 |
| GOLabeler | 0.580 | 0.370 | 0.687 | 5.077 | 15.177 | 5.518 | 0.546 | 0.225 | 0.700 |
| DeepGOPlus | 0.585 | 0.474 | 0.699 | 8.824 | 33.576 | 7.693 | 0.536 | 0.407 | 0.726 |
| Method . | F max . | S min . | AUPR . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . | MFO . | BPO . | CCO . |
| Naive | 0.306 | 0.318 | 0.605 | 12.105 | 38.890 | 9.646 | 0.150 | 0.219 | 0.512 |
| DiamondBLAST | 0.525 | 0.436 | 0.591 | 9.291 | 39.544 | 8.721 | 0.101 | 0.070 | 0.089 |
| DiamondScore | 0.548 | 0.439 | 0.621 | 8.736 | 34.060 | 7.997 | 0.362 | 0.240 | 0.363 |
| DeepGO | 0.449 | 0.398 | 0.667 | 10.722 | 35.085 | 7.861 | 0.409 | 0.328 | 0.696 |
| DeepGOCNN | 0.409 | 0.383 | 0.663 | 11.296 | 36.451 | 8.642 | 0.350 | 0.316 | 0.688 |
| DeepText2GO | 0.627 | 0.441 | 0.694 | 5.240 | 17.713 | 4.531 | 0.605 | 0.336 | 0.729 |
| GOLabeler | 0.580 | 0.370 | 0.687 | 5.077 | 15.177 | 5.518 | 0.546 | 0.225 | 0.700 |
| DeepGOPlus | 0.585 | 0.474 | 0.699 | 8.824 | 33.576 | 7.693 | 0.536 | 0.407 | 0.726 |
Best performance in bold. Fmax and AUPR, highest; Smin, lowest.
Due to large number of available sequences, analyzing sequences require both accurate and fast prediction methods. Specifically, function prediction is a crucial step in interpretation of newly sequenced genomes or meta-genomes. While we have compared DeepGOPlus in terms of prediction performance, we could not compare the running time of the models because the runtime of prediction models is rarely reported. With DeepGOPlus, 40 protein sequences can be annotated per second using a single Intel(R) Xeon(R) E5-2680 CPU and Nvidia P6000 GPU.
3.3 Comparison with CAFA3 methods
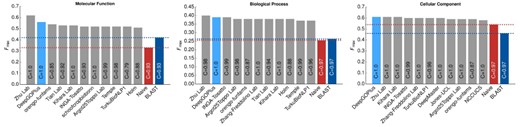
The CAFA3 challenge results (Zhou et al., 2019) became available recently which allowed us to evaluate our method on the same dataset used in CAFA3. We use the method used by CAFA3 to evaluate DeepGOPlus (see Methods) and compare against other methods that were evaluated in CAFA3. According to the CAFA3 evaluation measures, DeepGOPlus achieves an Fmax of 0.390, 0.557 and 0.614 for BPO, MFO and CCO evaluations, respectively. These results would have made DeepGOPlus one of the three best predictors in CCO and the second best performing method in the BPO and MFO evaluations. Figure 2 shows the comparison of DeepGOPlus with all CAFA3 top performing methods.
Using our own evaluation (based on inferring parent and child classes over the complete GO ontology instead of separately in the sub-ontologies), we obtain similar results for MFO and CCO as with the CAFA3 evaluation method while the difference in the BPO evaluation is quite large.
3.4 Smin performance analysis
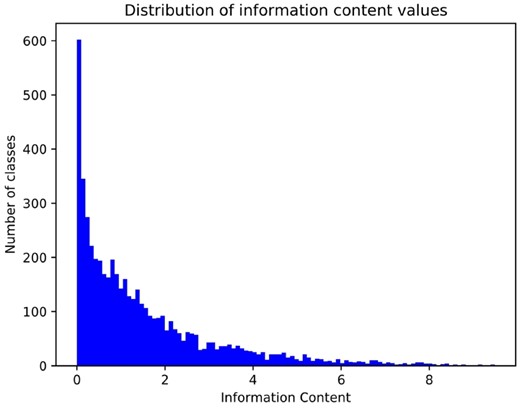
Although our method performed among the top-ranking methods in several Fmax evaluations, both GOLabeler and DeepText2GO perform significantly better when considering the evaluation based on the Smin measure. DeepGOPlus can potentially predict any GO class, including classes that are very specific. The Smin evaluation depend on the number of false negatives, false positives and the information content (IC) of GO classes. Figure 3 shows the distribution of IC values for the classes that are predicted by the DeepGOPlus model. The IC for general classes is close to zero and more specific classes have IC values of close to 10. Consequently, the methods which attempt to predict many specific classes will, in general, have a higher Smin. To test if this is true for our method, we evaluated the false positive predictions for the CAFA3 test set. In average, our method predicts 6.1 false positive classes per protein with a total IC of 8.3 for MFO, demonstrating that our false positive predictions are quite specific.
Distribution of Information Content (IC) values for 5220 GO classes that are predicted for CAFA3 dataset
3.5 Convolutional filters
To understand what is being learned by the convolutional filters of the CNN model we performed an experiment where we analyze sequence regions which activate our filters. We selected the specific molecular function class ‘enzyme activator activity’ (GO: 0008047) and filtered out all proteins annotated to this class. In total, 623 proteins have been experimentally annotated to this class. The reason we selected this class is that this class is very specific and it is referenced by multiple InterPRO functional domains and families. Our hypothesis is that our CNN filters recognize sequence regions that are similar to sequence functional domains.
First, we extracted the scores of all 8192 filters and ordered them in descending order for all sequences. We found that filter number 8048 (0-based) gives the highest score for all sequences and many other top 10 filters are active in more than 600 sequences. This shows that the filters learned similar motifs that are related to our selected function. We then extracted sequence regions which give the highest score for top 10 CNN filters and compare them to Pfam (El-Gebali et al., 2019) protein families database seeds. We use Diamond BLAST (Buchfink et al., 2015) with an e-value of 0.001 and associate similar sequence regions to Pfam families. Furthermore, we map Pfam family IDs to InterPRO IDs. Through this process, we associated protein sequences to InterPRO domains using sequence regions recognized by our CNN filters. In total, 563 sequences have been associated to at least one InterPRO ID. Finally, we compare the InterPRO associations with InterPRO annotations in UniProt (The Uniprot Consortium, 2007) database using a protein-centric F measure and obtain an F-score of 0.62 with precision 0.9 and recall of 0.47. This experiment demonstrates that our CNN filters are learning meaningful sequence motifs and can, for enzyme activators, accurately recognize almost half of the currently known functional domain annotations.
3.6 Implementation and availability
DeepGOPlus is available as free software at https://github.com/bio-ontology-research-group/deepgoplus. We also publish training and testing data used to generate evaluation and results at http://deepgoplus.bio2vec.net/data/. Furthermore, DeepGOPlus is available through a web interface and REST API at http://deepgoplus.bio2vec.net.
4 Discussion
DeepGOPlus is a fast and accurate tool to predict protein functions from protein sequence alone. Our model overcomes several limitations of other methods and our own DeepGO model (Kulmanov et al., 2018). In particular, DeepGOPlus has no limits on the length of the amino acid sequence and can therefore be used for the genome-scale annotation of protein functions, in particular in newly sequenced organisms. DeepGOPlus also makes no assumptions on the taxa or kingdom to which a protein belongs, therefore enabling, for example, function prediction for meta-genomics in which proteins from different kingdoms may be mixed. Furthermore, DeepGOPlus is fast and can annotate several thousand proteins in minutes even on single CPUs, further enabling its application in metagenomics or for projects in which a very large number of proteins with unknown functions are identified. While we initially expected the absence of features derived from interaction networks to impact predictive performance, we found that we can achieve even higher prediction accuracy with our current model; additionally, our model is not limited by unbalanced or missing information about protein–protein interactions.
In DeepGOPlus, we combine similarity-based search to proteins with known functions and motif-based function prediction, and this combination gives us overall the best predictive performance. However, DeepGOPlus can also be applied using only sequence motifs; in particular when annotating novel proteins for which no similar proteins with known functions exist, our motif-based model would be most suitable.
In the future, we plan to incorporate additional features and test other types of deep neural network models. While related methods use features that can be derived only for known proteins, such as information obtained from literature or interaction networks, DeepGOPlus will rely primarily on features that can be derived from amino acid sequences to ensure that the model can be applied as widely as possible. Possible additional information that may improve DeepGOPlus in the future is information about protein structure, in particular as structure prediction methods are improving significantly (Wang et al., 2017). We have already experimented with several types of neural networks such as recurrent neural networks, long-short term memory networks and autoencoders to learn seqeunce features. However, our attempts were unsuccessful and CNNs gave us the best results. Recently, attention networks have been successfully applied to protein sequences (Rives et al., 2019) and we plan to test them on function prediction.
Acknowledgements
We acknowledge the use of computational resources from the KAUST Supercomputing Core Laboratory.
Funding
This work was supported by funding from King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) under Award No. URF/1/3454-01-01, URF/1/3790-01-01, FCC/1/1976-08-01 and FCS/1/3657-02-01.
Conflict of Interest: none declared.
References
The Uniprot Consortium (