-
PDF
- Split View
-
Views
-
Cite
Cite
Phu Van, Wenxin Jiang, Raphael Gottardo, Greg Finak, ggCyto: next generation open-source visualization software for cytometry, Bioinformatics, Volume 34, Issue 22, November 2018, Pages 3951–3953, https://doi.org/10.1093/bioinformatics/bty441
Close - Share Icon Share
Abstract
Open source software for computational cytometry has gained in popularity over the past few years. Efforts such as FlowCAP, the Lyoplate and Euroflow projects have highlighted the importance of efforts to standardize both experimental and computational aspects of cytometry data analysis. The R/BioConductor platform hosts the largest collection of open source cytometry software covering all aspects of data analysis and providing infrastructure to represent and analyze cytometry data with all relevant experimental, gating and cell population annotations enabling fully reproducible data analysis. Data visualization frameworks to support this infrastructure have lagged behind.
ggCyto is a new open-source BioConductor software package for cytometry data visualization built on ggplot2 that enables ggplot-like functionality with the core BioConductor flow cytometry data structures. Amongst its features are the ability to transform data and axes on-the-fly using cytometry-specific transformations, plot faceting by experimental meta-data variables and partial matching of channel, marker and cell populations names to the contents of the BioConductor cytometry data structures. We demonstrate the salient features of the package using publicly available cytometry data with complete reproducible examples in a Supplementary Material.
https://bioconductor.org/packages/devel/bioc/html/ggcyto.html
Supplementary data are available at Bioinformatics online.
1 Introduction
Cytometry (FCM) is the primary assay for immune monitoring in clinical and research applications (Maecker et al., 2012). Pipelines must handle preprocessing, quality control, analysis (i.e. cell clustering or manual partitioning into homogeneous groups) (O(oups)eet al., 2013; Saeys et al., 2016) and visualization. Proprietary platforms, including FlowJo (Ashland, OR), WinList, FCSExpress and DIVA are the de-facto standards for end-to-end FCM data analysis. Other programming frameworks like Matlab (Matlab 7.0.4, Natick, MA: MathWorks) and Mathematica (Mathematica 9.0, Champaign, IL: Wolfram Research) provide functionality for data import and exploration [indeed, SPADE (Qiu et al., 2011) was initially developed for MATLAB], but lack the general abstraction of cytometry-specific data structures helpful for data analysis. Open-source projects like R/BioConductor (R/BioC) (Gentleman et al., 2004; Ihaka and Gentleman, 1996) and Python provide FCM functionality through user-contributed packages (Frelinger et al., 2012). Currently 47 open source software packages in BioConductor are tagged for ‘FlowCytometry’ (http://bioconductor.org/packages/release/BiocViews.html) but only flowViz (Sarkar et al., 2008) is visualization-centric and doesnli support the core BioConductor data structures used to store analyzed, gated and annotated, single-cell FCM data (see Supplementary Material). Other packages focus on different aspects of automated analysis.
We introduce ggcyto, a BioConductor package for building reproducible FCM visualizations programmatically. It is built on ggplot2 (Wickham, 2009) and supports the core BioConductor cytometry data structures making it compatible with any package using those structures (see Supplementary Material).
2 ggCyto
2.1 Basic principles
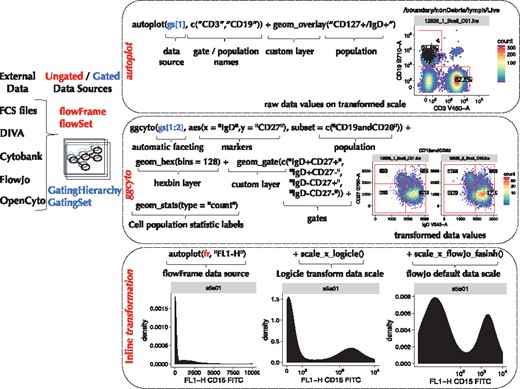
To construct a plot with ggcyto users specify a data source (Fig. 1), and, analogous to ggplot2, they map plot elements to variables in the data source. With ggcyto however, users map plot axes to flow parameters (e.g. channels or markers), specify the cell population to plot, specify cytometry-specific axis transformations and potentially specify gates (e.g. elements defining cell populations) to add to the plot. These elements are built up via layers and are referred by name, mapping directly to quantities (i.e. data) in the data source. For ease of use, ggcyto supports partial string matches (Fig. 1 and Supplementary Material), particularly useful for identifying complex channel names or cell populations.
ggcyto is compatible with ungated and gated data sources represented by the core BioConductor FCM data structures (flowSet/flowFrame and GatingSet/GatingHierarchy). Plots can be constructed using the (1) autoplot or (2) ggcyto APIs, giving users more control. Custom layers control cytometry-specific plot elements including 3) data transformation
2.2 Availability
ggcyto is open-source and available on GitHub and BioConductor (https://github.com/RGLab/ggcyto/releases/tag/v1.9.5 and https://bioconductor.org/packages/devel/bioc/html/ggcyto.html).
2.3 Quick plotting with the autoplot API
The autoplot API is a quick way to build plots. It makes most of the plot decisions for the user based on domain knowledge and information encoded in the data source (Fig. 1 and Supplementary Material). For example, passing a GatingHierarchy and a vector of cell population names (defined by gated cell populations in the GatingHierarchy) creates a faceted array (one panel for each sample) of two-dimensional density plots (using hexagonal binning) of the parent cell population projected onto the dimensions of any gates defining those cell subsets (Fig. 1, Supplementary Material). The ‘CD3’ and ‘CD19’ populations shown in Figure 1 are named cell populations defined by gates in the GatingHierarchy. They should not to be confused with markers of the same name. Two-dimensional densities are chosen by autoplot because the gates defining the CD3 and CD19 cell populations are two dimensional. In cases where gates defining a cell population are one dimensional, a one-dimensional density would be plotted. In this sense, autoplot is context aware, selecting geoms appropriate for visualizing the desired cell population.
Analogously, autoplot can be used to create plots from flowSet and flowFrame objects (for ungated data) or GatingHierarchy and GatingSet objects (for gated data, Fig. 1 and Supplementary Material). In the case of ungated data, the user specifies the channels/markers to visualize, rather than the cell population (since the latter is not defined).
2.4 Customizing plots with cytometry-specific layers
The ggcyto() API provides greater flexibility and customization than autoplot (Fig. 1). When using ggcyto, the layers and defaults selected by autoplot are decisions left to the user. Leveraging ggcytogs cytometry-specific layers and geoms, the user builds the plot (Fig. 1 and Supplementary Material) to include the gates, overlays (e.g. backgating), data or axis transformations, cell subpopulations and cell subpopulation statistics of interest, and specifies the faceting of plots by metadata annotations (see Supplementary Material). The ggcyto API can be particularly useful to project cell populations onto other markers (i.e. not necessarily those on which the populations are defined). The support for data transformations in ggcyto is 2-fold: ggcyto can transform the underlying data (Fig. 1), or it can transform the axes using the transformation stored in the data source (Fig. 1). These approaches are demonstrated in the Supplementary Material.
3 Examples
The functionality of ggcyto is demonstrated using the Lyoplate dataset from FlowCAP 4 (Finak et al., 2016) available in the flowWorkspaceData R/BioConductor package and on the ImmuneSpace portal (Brusic et al., 2014) (see the Supplementary Material for link to this data on ImmuneSpace), as well as the graft versus host disease (GvHD) data available in the flowCore R/BioConductor package. Reproducible examples with R code are in the Supplementary Material and available at http://rglab.org/ggcyto/. In future, additional cytometry data may be available via the more modern AnnotationHub or ExperimentHub resources (Morgan et al., 2016; Pasolli et al., 2017).
4 Conclusion
The ggcyto package provides a powerful and unified visualization interface to complex, ungated or gated, annotated cytometry data structures and provides a key component of a reproducible research workflow. Specifically, the package allows for easy visualization of specific cytometry cell populations and gates, on the fly data and axis transformation, back-gating visualization and easy faceting by study metadata in order to explore variability in an experiment. User-friendliness is made possible through fuzzy name matching, lazy data loading and context-sensitive behavior that aims to capture ‘what the user means to do’ most frequently. Areas for future developments are highlighted in the Supplementary Material.
Acknowledgements
The authors wish to acknowledge the contributions of the computational flow community for testing and feedback on this software package.
Funding
This work was supported by an NIGMS grant [R01 GM118417-01A1 to G.F.], and a grant from the Bill and Melinda Gates Foundation, [OPP1032317 to R.G.].
Conflict of Interest: none declared.
References
Author notes
The authors wish it to be known that, in their opinion, the Phu Van and Wenxin Jiang authors should be regarded as Joint First Authors.
The authors wish it to be known that, in their opinion, the Raphael Gottardo and Raphael Gottardo authors should be regarded as Joint Senior Authors.