-
PDF
- Split View
-
Views
-
Cite
Cite
Angela Noufaily, Roger A Morbey, Felipe J Colón-González, Alex J Elliot, Gillian E Smith, Iain R Lake, Noel McCarthy, Comparison of statistical algorithms for daily syndromic surveillance aberration detection, Bioinformatics, Volume 35, Issue 17, September 2019, Pages 3110–3118, https://doi.org/10.1093/bioinformatics/bty997
Close - Share Icon Share
Abstract
Public health authorities can provide more effective and timely interventions to protect populations during health events if they have effective multi-purpose surveillance systems. These systems rely on aberration detection algorithms to identify potential threats within large datasets. Ensuring the algorithms are sensitive, specific and timely is crucial for protecting public health. Here, we evaluate the performance of three detection algorithms extensively used for syndromic surveillance: the ‘rising activity, multilevel mixed effects, indicator emphasis’ (RAMMIE) method and the improved quasi-Poisson regression-based method known as ‘Farrington Flexible’ both currently used at Public Health England, and the ‘Early Aberration Reporting System’ (EARS) method used at the US Centre for Disease Control and Prevention. We model the wide range of data structures encountered within the daily syndromic surveillance systems used by PHE. We undertake extensive simulations to identify which algorithms work best across different types of syndromes and different outbreak sizes. We evaluate RAMMIE for the first time since its introduction. Performance metrics were computed and compared in the presence of a range of simulated outbreak types that were added to baseline data.
We conclude that amongst the algorithm variants that have a high specificity (i.e. >90%), Farrington Flexible has the highest sensitivity and specificity, whereas RAMMIE has the highest probability of outbreak detection and is the most timely, typically detecting outbreaks 2–3 days earlier.
R codes developed for this project are available through https://github.com/FelipeJColon/AlgorithmComparison
Supplementary data are available at Bioinformatics online.
1 Introduction
Epidemiological surveillance is becoming more important due to the increasing public health threats resulting from the quick spread of infections, especially as the world population increases and environmental risks augment. Public health authorities seek efficient algorithms that can detect unusual increases in infections quickly, so that they can investigate the sources of spread and ultimately take control measures. A main challenge for such algorithms is that they must be completely automated and must work across a range of different infections and syndromes encountered in real life in order to be useful in daily practice.
Most of the existing literature (Bédubourg and Stratt, 2017; Buckeridge and Burkom, 2010; Enki et al., 2016; Spreco et al., 2017; Texier et al., 2017; Unkel et al., 2012; Yang et al., 2018) considers and evaluates surveillance algorithms for weekly data. There is, however, a rising interest in daily surveillance (e.g. Abat et al., 2016; Mathes et al., 2017; Morbey et al., 2015; Smith et al., 2016; Vial et al., 2016). One of the initial motivations of daily surveillance was the early warning of bio-terrorist incidences. However, nowadays, daily surveillance is seen as important for various purposes such as situation awareness during events, reassurance about lack of incidents during mass gatherings and providing earlier detection for quicker control. Syndromic surveillance is generally performed daily based on diagnostic symptoms like cough, fever or diarrhoea, which are available before a laboratory-confirmed causal pathogen has been identified. Syndromic Surveillance has been in routine use at Public Health England (PHE) and its predecessors the Health Protection Agency and the Public Health Laboratory Service since 2001. Within PHE, syndromic surveillance is coordinated by the Real-time Syndromic Surveillance Team (ReSST). ReSST currently monitors general practitioner (GP) consultations using an in-hours syndromic system (GPIHSS) (Harcourt et al., 2012a,b) and an out-of-hours and unscheduled care system (GPOOHSS) (Harcourt et al., 2012a,b), calls to a national telephone health service (NHS 111) (Harcourt et al., 2016) and emergency department attendances (EDSSS) (Elliot et al., 2012).
The aim of this paper is to investigate the performance of three extensively used multi-purpose outbreak detection algorithms in monitoring daily syndromic data across a range of scenarios representing real-life syndromic activity. We base these scenarios on PHE’s syndromic surveillance system and the various syndromic data signals it encounters. We, therefore, compare: the multi-level regression approach known as the ‘rising activity, multilevel mixed effects, indicator emphasis’ (RAMMIE) method (Morbey et al., 2015) developed and currently used at PHE for syndromic daily surveillance; the improved quasi-Poisson regression-based (Noufaily et al., 2013) method (also known and referred to in this paper as Farrington Flexible) developed and currently used in PHE for weekly detection of infectious disease outbreaks; the ‘Early Aberration Reporting System’ (EARS) method (Hutwagner et al., 2003) based on Shewhart control charts, developed and used as the standard system (since 11 September 2001) at the United States CDC for conducting weekly syndromic surveillance (Fricker et al., 2008). In doing so, the study addresses key challenges in epidemiological surveillance. It provides a multi-purpose setting for evaluating algorithms based on simulations representing the range of real-life syndromes. It also addresses the challenge of monitoring daily counts for potential alarms, a theme that has not been thoroughly explored in the literature as the main focus has been on weekly surveillance. In addition, it presents the first formal evaluation of RAMMIE since its introduction in 2013.
Farrington Flexible and EARS are methods usually applied to weekly data, therefore we adapted them for daily surveillance by using 7-day moving totals. We tested all four variants of the EARS method (called C1, C2, C3 and NB). Also, we developed a modified version of RAMMIE which includes testing for long-term trends. Our comparison thus involves the two major surveillance algorithms (regression-based RAMMIE and Farrington Flexible) used at PHE and arguably the most commonly used surveillance algorithm, EARS, which includes non-regression-based variants, and so we provide a variety of approaches to contrast. The main challenge facing such algorithms is to control the false alarms whilst keeping a good power of detection, i.e. producing high sensitivity and specificity at the same time. We evaluate the performance of RAMMIE, Farrington Flexible and EARS by comparing the power of detection, sensitivity, specificity and timeliness using extensive simulations based on various scenarios that reflect the range of different data structures encountered in PHE’s syndromic surveillance system and seen in the real world including volume, trend, seasonality and day-of-the-week effects.
In Section 2, we describe the RAMMIE, Farrington Flexible and EARS algorithms. Section 3 explains the simulation study design used to compare the three algorithms and Section 4 introduces the measures used for this evaluation. Section 5 displays the results. We conclude with a final discussion and interpretation of the results in Section 6.
2 The algorithms
In this section, we provide a description of the algorithms we compared. These algorithms offer public health bodies a first indication of unusual activity or aberrations in the form of statistical alarms. Within PHE, a risk assessment process follows the identification of statistical alarms after which a smaller proportion of the alarms will be acted upon as required (Smith et al., 2016).
2.1 The ‘rising activity, multilevel mixed effects, indicator emphasis’ (RAMMIE) method
RAMMIE (Morbey et al., 2015) fits a multilevel mixed effects negative binomial regression model to historical daily syndromic data counts and provides estimates for current counts at a local, regional and national level in England. The model controls for the days of the week/month and bank holidays which can impact on health care consulting behaviour. Upper prediction intervals for the estimates are used to create thresholds and generate statistical alarms whenever actual counts exceed the thresholds. ReSST uses RAMMIE as the first stage in its risk assessment process for decision-making (Smith et al., 2016). For this study, data was not stratified into different geographies so the RAMMIE models are not multilevel, instead a simplified negative binomial model was used.
An alarm is signalled if the current expected count is larger than three and at the same time higher than the current observed count plus three times the standard deviation; details can be found in Morbey et al. (2015).
RAMMIE does not include an independent variable for trends. However, for this study, a modified version of RAMMIE was also created with an added simple linear trend to account for potential long-term trends in the data. This version provided better detection and allowed a fairer comparison with the other algorithms.
2.2 The quasi-Poisson regression-based exceedance algorithm
To reduce the effect of baseline outbreaks on current predictions, the algorithm reweights baseline data. As explained in Noufaily et al. (2013), the baseline at week ti is down-weighted by a factor of the Anscombe residual when the latter is greater than 2.58 at that week.
This algorithm is implemented in R Development Core Team (2018) and is available via the function Farrington Flexible within the package surveillance (Höhle, 2007; Salmon et al., 2016).
2.3 The Early Aberration Reporting System (EARS)
Alarms for the different variants are produced when corresponding statistics C1 or C2 exceed three sample standard deviations above the sample mean or if C3 exceeds two sample standard deviations above the sample mean.
EARS-NB implements Shewhart regression Poisson and negative binomial charts based on the generalized likelihood ratio statistic and is described in Höhle and Paul (2008). The method is implemented via the algo.glrnb function within the R Development Core Team (2018) surveillance package (Höhle, 2007; Salmon et al., 2016).
3 Simulation study
We first describe how baseline data is simulated and second how outbreaks are generated. The simulations reflect the real world experience of the syndromic surveillance systems. A novel and key feature in this paper is the way simulations take into account the day-of-the-week effects based on health-seeking behaviour. These effects are also applied to the outbreaks before combining them with the synthetic baselines (Buckingham-Jeffery et al., 2017). The simulations are a potential resource for similar evaluations and can be used by researchers for testing other algorithms in a daily setting.
3.1 Simulated baseline data
The simulations are set to reflect the various syndromes encountered at PHE as well as the reporting patterns. The four services reporting to PHE (i.e. GPOOHSS, GPIHSS, NHS 111 and EDSSS) report data based on the days of the week they operate. GPOOHSS and NHS 111 operate on a 7-day-week basis, with a lower volume of reports during the week and almost double that volume on weekends. GPIHSS operates on a 5-day-week basis (only during weekdays) and portrays two peaks around Mondays and Fridays (the Friday one being smaller). In contrast, for EDSSS the day of the week effects are much smaller.
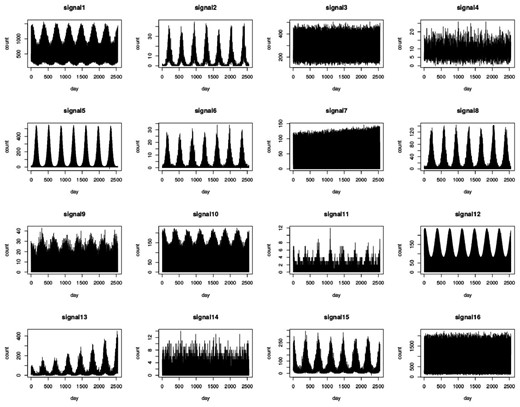
Parameters and criteria used to generate the 16 representative signals
| Signal . | θ . | β . | γ 1 . | γ 2 . | γ 3 . | γ 4 . | . | s . | k 1 . | k 2 . | Trend . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 0 | 0.2 | 0.2 | 0.5 | 0.4 | 2 | 29 | 1 | 2 | 0 |
| 2 | 0.5 | 0 | 1.5 | 1.4 | 0.5 | 0.4 | 1 | −167 | 1 | 2 | 0 |
| 3 | 5.5 | 0 | 0 | 0 | 0.3 | 0.25 | 1 | 1 | 0 | 2 | 0 |
| 4 | 2 | 0 | 0 | 0 | 0.3 | 0.25 | 1 | 1 | 0 | 2 | 0 |
| 5 | 6 | 0 | 0.3 | 2 | 0.3 | 0.5 | 1.5 | −50 | 1 | 2 | 0 |
| 6 | 1 | 0 | 0.1 | 2 | 0.05 | 0.05 | 1 | −50 | 1 | 1 | 0 |
| 7 | 6 | 0.0001 | 0 | 0 | 0.6 | 0.9 | 1.5 | 0 | 0 | 1 | 1 |
| 8 | 3 | 0 | 1.5 | 0.1 | 0.2 | 0.3 | 1 | −150 | 1 | 1 | 0 |
| 9 | 3 | 0 | 0.2 | 0.1 | 0.05 | 0.15 | 1 | −200 | 1 | 1 | 0 |
| 10 | 5 | 0 | 0.2 | 0.1 | 0.05 | 0.1 | 1 | 0 | 1 | 1 | 0 |
| 11 | 0.5 | 0 | 0.4 | 0 | 0.05 | 0.15 | 1 | 0 | 2 | 1 | 0 |
| 12 | 9 | 0 | 0.5 | 0.2 | 0.2 | 0.5 | 1 | 0 | 1 | 1 | 0 |
| 13 | 2 | 0.0005 | 0.8 | 0.8 | 0.8 | 0.4 | 4 | 57 | 1 | 2 | 1 |
| 14 | 0.05 | 0 | 0.01 | 0.01 | 1.8 | 0.1 | 1 | −85 | 4 | 1 | 0 |
| 15 | 3 | 0 | 0.8 | 0.6 | 0.8 | 0.4 | 4 | 29 | 1 | 2 | 0 |
| 16 | 6 | 0 | 0 | 0 | 0.8 | 0.4 | 4 | 1 | 0 | 2 | 0 |
| Signal . | θ . | β . | γ 1 . | γ 2 . | γ 3 . | γ 4 . | . | s . | k 1 . | k 2 . | Trend . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 0 | 0.2 | 0.2 | 0.5 | 0.4 | 2 | 29 | 1 | 2 | 0 |
| 2 | 0.5 | 0 | 1.5 | 1.4 | 0.5 | 0.4 | 1 | −167 | 1 | 2 | 0 |
| 3 | 5.5 | 0 | 0 | 0 | 0.3 | 0.25 | 1 | 1 | 0 | 2 | 0 |
| 4 | 2 | 0 | 0 | 0 | 0.3 | 0.25 | 1 | 1 | 0 | 2 | 0 |
| 5 | 6 | 0 | 0.3 | 2 | 0.3 | 0.5 | 1.5 | −50 | 1 | 2 | 0 |
| 6 | 1 | 0 | 0.1 | 2 | 0.05 | 0.05 | 1 | −50 | 1 | 1 | 0 |
| 7 | 6 | 0.0001 | 0 | 0 | 0.6 | 0.9 | 1.5 | 0 | 0 | 1 | 1 |
| 8 | 3 | 0 | 1.5 | 0.1 | 0.2 | 0.3 | 1 | −150 | 1 | 1 | 0 |
| 9 | 3 | 0 | 0.2 | 0.1 | 0.05 | 0.15 | 1 | −200 | 1 | 1 | 0 |
| 10 | 5 | 0 | 0.2 | 0.1 | 0.05 | 0.1 | 1 | 0 | 1 | 1 | 0 |
| 11 | 0.5 | 0 | 0.4 | 0 | 0.05 | 0.15 | 1 | 0 | 2 | 1 | 0 |
| 12 | 9 | 0 | 0.5 | 0.2 | 0.2 | 0.5 | 1 | 0 | 1 | 1 | 0 |
| 13 | 2 | 0.0005 | 0.8 | 0.8 | 0.8 | 0.4 | 4 | 57 | 1 | 2 | 1 |
| 14 | 0.05 | 0 | 0.01 | 0.01 | 1.8 | 0.1 | 1 | −85 | 4 | 1 | 0 |
| 15 | 3 | 0 | 0.8 | 0.6 | 0.8 | 0.4 | 4 | 29 | 1 | 2 | 0 |
| 16 | 6 | 0 | 0 | 0 | 0.8 | 0.4 | 4 | 1 | 0 | 2 | 0 |
Parameters and criteria used to generate the 16 representative signals
| Signal . | θ . | β . | γ 1 . | γ 2 . | γ 3 . | γ 4 . | . | s . | k 1 . | k 2 . | Trend . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 0 | 0.2 | 0.2 | 0.5 | 0.4 | 2 | 29 | 1 | 2 | 0 |
| 2 | 0.5 | 0 | 1.5 | 1.4 | 0.5 | 0.4 | 1 | −167 | 1 | 2 | 0 |
| 3 | 5.5 | 0 | 0 | 0 | 0.3 | 0.25 | 1 | 1 | 0 | 2 | 0 |
| 4 | 2 | 0 | 0 | 0 | 0.3 | 0.25 | 1 | 1 | 0 | 2 | 0 |
| 5 | 6 | 0 | 0.3 | 2 | 0.3 | 0.5 | 1.5 | −50 | 1 | 2 | 0 |
| 6 | 1 | 0 | 0.1 | 2 | 0.05 | 0.05 | 1 | −50 | 1 | 1 | 0 |
| 7 | 6 | 0.0001 | 0 | 0 | 0.6 | 0.9 | 1.5 | 0 | 0 | 1 | 1 |
| 8 | 3 | 0 | 1.5 | 0.1 | 0.2 | 0.3 | 1 | −150 | 1 | 1 | 0 |
| 9 | 3 | 0 | 0.2 | 0.1 | 0.05 | 0.15 | 1 | −200 | 1 | 1 | 0 |
| 10 | 5 | 0 | 0.2 | 0.1 | 0.05 | 0.1 | 1 | 0 | 1 | 1 | 0 |
| 11 | 0.5 | 0 | 0.4 | 0 | 0.05 | 0.15 | 1 | 0 | 2 | 1 | 0 |
| 12 | 9 | 0 | 0.5 | 0.2 | 0.2 | 0.5 | 1 | 0 | 1 | 1 | 0 |
| 13 | 2 | 0.0005 | 0.8 | 0.8 | 0.8 | 0.4 | 4 | 57 | 1 | 2 | 1 |
| 14 | 0.05 | 0 | 0.01 | 0.01 | 1.8 | 0.1 | 1 | −85 | 4 | 1 | 0 |
| 15 | 3 | 0 | 0.8 | 0.6 | 0.8 | 0.4 | 4 | 29 | 1 | 2 | 0 |
| 16 | 6 | 0 | 0 | 0 | 0.8 | 0.4 | 4 | 1 | 0 | 2 | 0 |
| Signal . | θ . | β . | γ 1 . | γ 2 . | γ 3 . | γ 4 . | . | s . | k 1 . | k 2 . | Trend . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 0 | 0.2 | 0.2 | 0.5 | 0.4 | 2 | 29 | 1 | 2 | 0 |
| 2 | 0.5 | 0 | 1.5 | 1.4 | 0.5 | 0.4 | 1 | −167 | 1 | 2 | 0 |
| 3 | 5.5 | 0 | 0 | 0 | 0.3 | 0.25 | 1 | 1 | 0 | 2 | 0 |
| 4 | 2 | 0 | 0 | 0 | 0.3 | 0.25 | 1 | 1 | 0 | 2 | 0 |
| 5 | 6 | 0 | 0.3 | 2 | 0.3 | 0.5 | 1.5 | −50 | 1 | 2 | 0 |
| 6 | 1 | 0 | 0.1 | 2 | 0.05 | 0.05 | 1 | −50 | 1 | 1 | 0 |
| 7 | 6 | 0.0001 | 0 | 0 | 0.6 | 0.9 | 1.5 | 0 | 0 | 1 | 1 |
| 8 | 3 | 0 | 1.5 | 0.1 | 0.2 | 0.3 | 1 | −150 | 1 | 1 | 0 |
| 9 | 3 | 0 | 0.2 | 0.1 | 0.05 | 0.15 | 1 | −200 | 1 | 1 | 0 |
| 10 | 5 | 0 | 0.2 | 0.1 | 0.05 | 0.1 | 1 | 0 | 1 | 1 | 0 |
| 11 | 0.5 | 0 | 0.4 | 0 | 0.05 | 0.15 | 1 | 0 | 2 | 1 | 0 |
| 12 | 9 | 0 | 0.5 | 0.2 | 0.2 | 0.5 | 1 | 0 | 1 | 1 | 0 |
| 13 | 2 | 0.0005 | 0.8 | 0.8 | 0.8 | 0.4 | 4 | 57 | 1 | 2 | 1 |
| 14 | 0.05 | 0 | 0.01 | 0.01 | 1.8 | 0.1 | 1 | −85 | 4 | 1 | 0 |
| 15 | 3 | 0 | 0.8 | 0.6 | 0.8 | 0.4 | 4 | 29 | 1 | 2 | 0 |
| 16 | 6 | 0 | 0 | 0 | 0.8 | 0.4 | 4 | 1 | 0 | 2 | 0 |
Characteristics of 16 syndromes representative of the 16 simulated data signals
| Signal ID . | Related system . | Related syndrome . | Mean daily count . | Yearly variation . | 5/7 day service . | Trend . |
|---|---|---|---|---|---|---|
| 1 | NHS111 | Diarrhoea | >100 | Moderate | 7 | No |
| 2 | ED | Arthropod bites | <10 | Large summer peak | 7 | No |
| 3 | ED | Cardiac | <500 | Small | 7 | No |
| 4 | ED | Cardiac admissions (HCU/ICU) | <10 | Small | 7 | No |
| 5 | GPIHSS | Allergic rhinitis | >100 | Large peak with variable timing | 5 | No |
| 6 | GPIHSS | Heat stroke | <10 | Large peak variable with timing | 5 | No |
| 7 | GPIHSS | Herpes zoster | >100 | Small | 5 | Yes |
| 8 | GPIHSS | Insect bite | 10–100 | Large summer peak | 5 | No |
| 9 | GPIHSS | Pertussis | 10–100 | Moderate | 5 | No |
| 10 | GPIHSS | Pneumonia | >100 | Moderate | 5 | No |
| 11 | GPIHSS | Rubella | <10 | Moderate | 5 | No |
| 12 | GPIHSS | Upper tract respiratory infection | >100 | Moderate | 5 | No |
| 13 | GPOOHSS | Bronchitis | 10–100 | Moderate | 7 | Yes |
| 14 | GPOOHSS | Hepatitis | <10 | Moderate | 7 | No |
| 15 | GPOOHSS | Influenza-like illness | 10–100 | Large peak with variable timing | 7 | No |
| 16 | GPOOHSS | Urinary tract infection | >100 | Small | 7 | No |
| Signal ID . | Related system . | Related syndrome . | Mean daily count . | Yearly variation . | 5/7 day service . | Trend . |
|---|---|---|---|---|---|---|
| 1 | NHS111 | Diarrhoea | >100 | Moderate | 7 | No |
| 2 | ED | Arthropod bites | <10 | Large summer peak | 7 | No |
| 3 | ED | Cardiac | <500 | Small | 7 | No |
| 4 | ED | Cardiac admissions (HCU/ICU) | <10 | Small | 7 | No |
| 5 | GPIHSS | Allergic rhinitis | >100 | Large peak with variable timing | 5 | No |
| 6 | GPIHSS | Heat stroke | <10 | Large peak variable with timing | 5 | No |
| 7 | GPIHSS | Herpes zoster | >100 | Small | 5 | Yes |
| 8 | GPIHSS | Insect bite | 10–100 | Large summer peak | 5 | No |
| 9 | GPIHSS | Pertussis | 10–100 | Moderate | 5 | No |
| 10 | GPIHSS | Pneumonia | >100 | Moderate | 5 | No |
| 11 | GPIHSS | Rubella | <10 | Moderate | 5 | No |
| 12 | GPIHSS | Upper tract respiratory infection | >100 | Moderate | 5 | No |
| 13 | GPOOHSS | Bronchitis | 10–100 | Moderate | 7 | Yes |
| 14 | GPOOHSS | Hepatitis | <10 | Moderate | 7 | No |
| 15 | GPOOHSS | Influenza-like illness | 10–100 | Large peak with variable timing | 7 | No |
| 16 | GPOOHSS | Urinary tract infection | >100 | Small | 7 | No |
Characteristics of 16 syndromes representative of the 16 simulated data signals
| Signal ID . | Related system . | Related syndrome . | Mean daily count . | Yearly variation . | 5/7 day service . | Trend . |
|---|---|---|---|---|---|---|
| 1 | NHS111 | Diarrhoea | >100 | Moderate | 7 | No |
| 2 | ED | Arthropod bites | <10 | Large summer peak | 7 | No |
| 3 | ED | Cardiac | <500 | Small | 7 | No |
| 4 | ED | Cardiac admissions (HCU/ICU) | <10 | Small | 7 | No |
| 5 | GPIHSS | Allergic rhinitis | >100 | Large peak with variable timing | 5 | No |
| 6 | GPIHSS | Heat stroke | <10 | Large peak variable with timing | 5 | No |
| 7 | GPIHSS | Herpes zoster | >100 | Small | 5 | Yes |
| 8 | GPIHSS | Insect bite | 10–100 | Large summer peak | 5 | No |
| 9 | GPIHSS | Pertussis | 10–100 | Moderate | 5 | No |
| 10 | GPIHSS | Pneumonia | >100 | Moderate | 5 | No |
| 11 | GPIHSS | Rubella | <10 | Moderate | 5 | No |
| 12 | GPIHSS | Upper tract respiratory infection | >100 | Moderate | 5 | No |
| 13 | GPOOHSS | Bronchitis | 10–100 | Moderate | 7 | Yes |
| 14 | GPOOHSS | Hepatitis | <10 | Moderate | 7 | No |
| 15 | GPOOHSS | Influenza-like illness | 10–100 | Large peak with variable timing | 7 | No |
| 16 | GPOOHSS | Urinary tract infection | >100 | Small | 7 | No |
| Signal ID . | Related system . | Related syndrome . | Mean daily count . | Yearly variation . | 5/7 day service . | Trend . |
|---|---|---|---|---|---|---|
| 1 | NHS111 | Diarrhoea | >100 | Moderate | 7 | No |
| 2 | ED | Arthropod bites | <10 | Large summer peak | 7 | No |
| 3 | ED | Cardiac | <500 | Small | 7 | No |
| 4 | ED | Cardiac admissions (HCU/ICU) | <10 | Small | 7 | No |
| 5 | GPIHSS | Allergic rhinitis | >100 | Large peak with variable timing | 5 | No |
| 6 | GPIHSS | Heat stroke | <10 | Large peak variable with timing | 5 | No |
| 7 | GPIHSS | Herpes zoster | >100 | Small | 5 | Yes |
| 8 | GPIHSS | Insect bite | 10–100 | Large summer peak | 5 | No |
| 9 | GPIHSS | Pertussis | 10–100 | Moderate | 5 | No |
| 10 | GPIHSS | Pneumonia | >100 | Moderate | 5 | No |
| 11 | GPIHSS | Rubella | <10 | Moderate | 5 | No |
| 12 | GPIHSS | Upper tract respiratory infection | >100 | Moderate | 5 | No |
| 13 | GPOOHSS | Bronchitis | 10–100 | Moderate | 7 | Yes |
| 14 | GPOOHSS | Hepatitis | <10 | Moderate | 7 | No |
| 15 | GPOOHSS | Influenza-like illness | 10–100 | Large peak with variable timing | 7 | No |
| 16 | GPOOHSS | Urinary tract infection | >100 | Small | 7 | No |
Baseline data, in the absence of outbreaks, is generated using 100 simulations from each of the 16 scenarios; each simulation of size 2548 days (i.e. 7 years consisting of 364 days each or equivalently 52 weeks). Day-of-the-week effects are also reflected within each week. In a 7-day-week system, weekends are set to have around double the volume of reports than weekdays. In a 5-day-week system, weekends are set to zero, whereas weekdays generally consist of 2 peaks, one at the beginning of the week (around Monday) and another later in the week (around Friday). Figure 1 shows the resulting data series and Supplementary Figure S1 shows the first 3 weeks of signals 3 (7-day-week system) and 7 (5-day-week system) to demonstrate the modelled weekly patterns. The outbreaks are added to the most recent 49 weeks (343 days) of the simulated syndromic data.
3.2 Simulated outbreaks and public holiday effects
The simulation models described in this paper enable the control of baselines for different scenarios, and therefore outbreaks and unusual increases of different shapes can be added to the baselines. We consider two types of outbreaks: ‘spiked outbreaks’ which last around 3 weeks on average and ‘seasonal outbreaks’ which have a duration of about 8 weeks on average. We also consider the effects of public holidays (called bank holidays in the UK), which usually last 1 or 2 days, because of their impact on syndromic baselines. All outbreaks and public holiday effects take into account the day-of-the-week pattern.
3.2.1 Seasonal outbreaks
Examples of syndromes with seasonal outbreaks are syndromes designed to detect seasonal influenza and allergic rhinitis (i.e. hay fever). Although, baseline data already take into account seasonality, ‘seasonal outbreaks’ differ from the usual seasonality in the sense that the size and timing of their peak is more variable. They are added to a similar window of weeks within each year of simulated data. Based on Noufaily et al. (2013), outbreak sizes are simulated using a Poisson distribution with mean equal to m times the standard deviation of the baseline count of the day at which the outbreak started and then distributed randomly in time according to a lognormal distribution with mean 0 and standard deviation 0.5. Outbreaks are then re-weighted based on the day of the week they fall on, since outbreaks tend to be influenced by the weekly patterns. In the 5-day-week system, Monday (Tuesday) outbreaks are over-weighted by a factor of 1.5 (1.1). Outbreak days falling on the remaining weekdays are kept the same. In the 7-day-week systems, weekend outbreaks are over-weighted by a factor of 2, whereas weekday outbreaks are kept the same. ‘Seasonal outbreaks’ are only added to signals 5 (m = 1680), 6 (m = 1050) and 15 (m = 3150) as shown in Supplementary Figure S2.
3.2.2 Spiked outbreaks
‘Spiked outbreaks’ are added to the most recent 49 weeks of our 16 simulated signals. They are generated in a similar fashion to ‘seasonal outbreaks’, however they are of shorter duration. Day-of-the-week re-weighting is also considered. ‘Seasonal outbreaks’ were added first to signals 5, 6 and 15, then ‘spiked outbreaks’ were added to all signals. ‘Spiked outbreaks’ of different sizes—very small, small, medium and large using respective m values of 2, 3, 5 and 10—have been considered and this study will involve repeating the analysis of the 16 signals 4 times, each time using a different ‘spiked outbreak’ size. For the purpose of demonstration, Supplementary Figure S3 shows baseline data for signals 1, 2, 9 and 12 with examples of medium (m = 5) ‘spiked outbreaks’.
3.2.3 Public holidays
Public holiday effects are added to the simulations following the addition of ‘seasonal outbreaks’ and ‘spiked outbreaks’. We chose the public holiday dates to be on similar days as the United Kingdom ‘bank holidays’ (Supplementary Data). In the 5-day-system, the public holiday count was set to zero and the weekday after the public holiday was multiplied by 1.5; in the 7-day-system, the public holiday count was doubled.
4 Evaluation measures
A number of additional measures, such as receiver operator characteristic curves, have been used elsewhere to assess the performance of algorithms. However, here we focused on measures that can be easily explained to the users and policy makers who will be choosing which algorithms to implement. Hence, we have POD to measure the probability of detecting specific outbreaks but also specificity and sensitivity measures that tell users how accurate daily alarms will be during and outside outbreaks. PPV is useful when outbreaks are very rare because even if specificity is high, an alarm is more likely to be false than true. For example, a system monitoring two outbreak types (one from a common disease, and one from a rare disease) may have the same specificity for both of them; however, the PPV for the common disease would be higher than that of the rare disease. It is noted that in our study PPV is not very informative because we specified outbreak occurrence to be exactly one outbreak for each 343 day simulation. Thus, PPV does not give the user any more useful information than does specificity.
5 Simulation study results
As well as giving overall performance we report on differences between the 16 scenarios of synthetic syndromes. RAMMIE without trend, RAMMIE with trend, Farrington Flexible, EARS-C1, EARS-C2, EARS-C3 and EARS-NB were implemented to the most recent 49 weeks of each of the 100 simulations from each of the 16 signals (i.e. 49 weeks × 16 signals × 100 simulations 78 400 simulated time series) and the evaluation measures defined in Section 4 were computed across all four sizes of ‘spiked outbreaks’ (notice that PPV is discussed at the end of this section). As previously mentioned, both RAMMIE versions were run on daily counts whereas Farrington Flexible and the EARS variants were run on 7-day moving totals. Results for RAMMIE with or without trend are very similar in cases where a trend does not exist; however, RAMMIE without trend produces a much lower specificity in some cases where data has an increasing trend, such as signal 13. Given that RAMMIE with trend produces a much higher specificity and similar sensitivity and timeliness (for signals with trend) as well as comparable results for signals without trend, we only include the results corresponding to RAMMIE with trend (referred to as RAMMIE) in the analysis below.
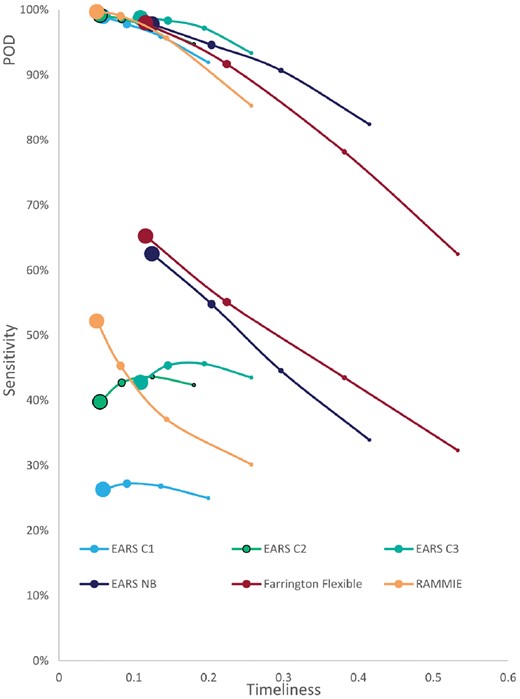
We first investigate how performance is affected by outbreak size. Figure 2 shows the detection capability of each algorithm for different outbreak sizes. It displays both POD and sensitivity versus timeliness (note that a lower score for timeliness indicates better results). Specificity was not included in this figure because it varies only very slightly with outbreak size (for each of the algorithms, a difference of less than 0.003, on average, between the different outbreak sizes). The average specificity across all 16 signals and all outbreak sizes for each of the algorithms is: 0.981 for Farrington Flexible; 0.953 for RAMMIE; 0.922 for EARS-C1; 0.834 for EARS-C2; 0.812 for EARS-C3; 0.969 for EARS-NB. The figure shows that, all algorithms were very likely to detect the larger outbreaks, but POD was considerably lower for the smallest outbreaks, particularly for the Farrington Flexible method. Farrington Flexible detection capability is the most affected by outbreak size, though generally algorithm ranking is not affected by outbreak size and as the latter increases, POD, sensitivity and timeliness improve. Farrington Flexible and EARS-NB have a much higher sensitivity than RAMMIE but lower POD, most likely due to the smoothing methods used in adjusting from weekly to daily surveillance. In particular, Farrington Flexible has the highest sensitivity but lowest POD, though its POD is similar to the other algorithms with outbreaks of size 10. EARS-C1 and EARS-C2 were the most timely, although RAMMIE has similar timeliness except for the smallest outbreaks. (See Supplementary Fig. S4 for further demonstration on how detection is influenced by outbreak size).
Average (across the 16 signals) sensitivity (lower end of plot) and POD (upper end of plot) versus timeliness for evaluating the impact of ‘spiked outbreak’ size on detection capabilities obtained from applying RAMMIE, Farrington Flexible, EARS-C1, EARS-C2, EARS-C3 and EARS-NB to the most recent 49 weeks of each of the 100 simulations of the 16 signals. Marker size is proportional to outbreak size (i.e. largest point refers to large outbreaks; second largest refers to medium outbreaks; third largest refers to small outbreaks; smallest point refers to very small outbreaks)
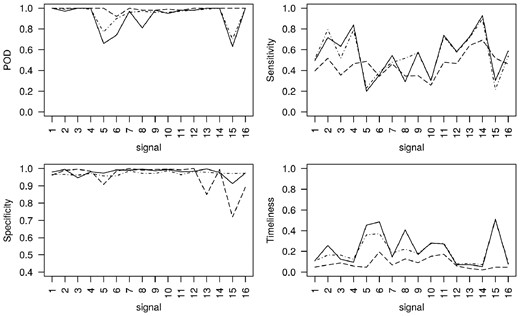
Second, we investigate the algorithms’ performance with the particular characteristics depicted by each of the 16 simulated signals. Figure 3 displays the algorithms’ 4 performance measures for each of the 16 signals, when ‘spiked outbreaks’ of medium size (m = 5) were added to the most recent 49 weeks. Figures corresponding to very small, small and large outbreaks can be found in the Supplementary Data. EARS-C1, EARS-C2 and EARS-C3 have much lower sensitivity and specificity than the other algorithms making them not very useful in our setting, therefore Figure 3 reports on the results corresponding to just RAMMIE, Farrington Flexible and EARS-NB. The figure shows that Farrington Flexible has the highest specificity on average followed by EARS-NB then RAMMIE. RAMMIE specificity is similar to Farrington Flexible, however it has particularly low values for signals 5, 13 and 15, all of which have high volume and seasonality. Signal 13 has a trend and signals 5 and 15 have the added ‘seasonal outbreaks’, which could explain the low specificity. RAMMIE produces particularly low specificity for signal 15 and so the false alarms might be due to the fact that RAMMIE detects ‘seasonal outbreak’ better (which in reality could be the seasonal influenza outbreak). RAMMIE sensitivity is the least variable across signals. Farrington Flexible produces low POD and is the least timely particularly for signals 5, 6, 8 and 15 which have high seasonality and added seasonal outbreaks (for signals 5, 6 and 15). EARS-NB gives a similar picture but with a higher POD and lower timeliness on average. The timeliness for simulations where an outbreak is not detected is set to 1, which explains why signals with particularly low POD are also the least timely. RAMMIE produces, on average, the highest (lowest) and most consistent POD and timeliness across all signals.
POD, sensitivity, specificity and timeliness for each of the simulated signals, with added medium ‘spiked outbreaks’, obtained from applying RAMMIE (dashed lines), Farrington Flexible (solid lines) and EARS-NB (dot dash lines) to the most recent 49 weeks of each of the 100 simulations from each signal
Every algorithm scored highly in terms of PPV. Farrington Flexible had the highest overall PPV (99.73%) followed by EARS-NB (99.56%), RAMMIE (99.04%), EARS-C1 (99.03%), EARS-C2 (98.00%) and EARS-C3 (97.70%). The slightly lower values for EARS-C methods reflects their lower scores for specificity.
6 Discussion
During the testing of algorithm performance across a range of scenarios, we found that EARS-C1, EARS-C2 and EARS-C3 have considerably lower specificity than the other algorithm variants tested. Amongst the other algorithms, Farrington Flexible has the highest sensitivity and specificity, whereas RAMMIE has the highest POD and is the most timely.
Farrington Flexible and EARS-NB smooth the data by taking moving totals and so dilute the signal, whereas RAMMIE is designed for daily surveillance which allows it to detect more outbreaks, typically 2–3 days earlier. However, RAMMIE is less consistent in generating alarms during spiked outbreak days and produces the lowest sensitivity. Due to smoothing of the day-of-the-week effects, once Farrington Flexible and EARS-NB detect an outbreak, they are more likely to generate alarms consistently during the remaining spike outbreak days.
Although there are differences in the performance measures across the syndromes, the differences mostly affect all the algorithms in similar ways, apart from some signals with high seasonality or added seasonal outbreaks. The Flexible Farrington method has the biggest variation in detection capabilities across the different signals. Our results show that the performance of RAMMIE can be improved by adjusting it for long-term trends.
6.1 Implications for public health authorities
In this paper, we provide an assessment of algorithm detection capability to help decision makers and researchers performing daily surveillance decide which algorithm would be more efficient for their needs, and which aspects of detection are more important i.e. POD, sensitivity, specificity, PPV or timeliness. POD is the most important measure if the priority of the surveillance system is to ensure that all outbreaks are detected. Whilst our sensitivity measure is important if a clear consistent signal is required, i.e. an alarm every day during an outbreak, specificity is important if the user needs to ensure that there are no false alarms. PPV provides a measure of how likely an alarm is to be true, which is particularly important when outbreaks do not occur often. Finally, timeliness may be the most important measure if the focus of the surveillance activity is on providing early warning and other public health systems exist which can reliably detect outbreaks. For each of these measures the user may want to set a minimum threshold required by the algorithms and/or prioritize which measures are most important for their surveillance needs. Furthermore, their requirements may vary depending on the public health hazard they are trying to detect.
In effect, there is no one algorithm that is better across all detection measures. However, due to their lower specificity, the EARS-C1, EARS-C2 and EARS-C3 variants may not be as suited for a multi-purpose daily surveillance system. Farrington Flexible had the highest sensitivity and specificity so may be preferred if the priority is for daily alarms that are as accurate as possible. However, RAMMIE was more timely and had a slightly higher POD so it may be more useful where early warning is important or the top priority is that at least one alarm occurs during an outbreak. Alternatively, EARS-NB may be preferred as a compromise because its sensitivity was better than RAMMIE and its POD slightly better than Flexible Farrington.
Specifically, we provide PHE with an evaluation of RAMMIE that will help them improve their service by modifying RAMMIE or replacing it with another algorithm. We recommend adjusting RAMMIE to allow for any long-term trends in the underlying syndromic data. We also provide a range of developed simulations that researchers can use in testing other algorithms for use in a daily setting elsewhere (We aim in the future to provide public access to these simulations).
One of our research aims was to discover which algorithm worked best in particular situations, (e.g. which algorithm is best for small numbers or which is best for 5-day-week systems, or which is best for different outbreak sizes or types). However, we show that the ranking of algorithms is not affected by these different situations. Therefore, whichever algorithm is preferred by users should be used for all types of signals.
6.2 Limitations
The timeliness penalty of 1 for failing to detect an outbreak is set quite high. Consequently, when an algorithm has a low POD score it will also have very poor timeliness, e.g. Farrington Flexible for signals with seasonal outbreaks. An alternative approach, given that the simulated outbreaks are nearly symmetric could be to impose a penalty of 0.5, because it is highly unlikely that an outbreak will first be detected after it has peaked. It would also be possible to not impose any penalty, although that could result in algorithms that can only detect big outbreaks with an initial sharp rise in cases being scored as more timely than algorithms with a much better POD.
6.3 Future work
In further research, we aim to explore different decision rules for evaluating the algorithms. We can use the results from this study to see how different priorities for timeliness, sensitivity, specificity or POD would affect the decision of which algorithm we should use. For instance, we can ask decision makers to specify a set of preferred requirements for algorithms (e.g. specificity > 98%) and their priorities, (e.g. whether timeliness is a priority over alarming every day). Then we can apply these decision rules to our study results to determine which algorithm performed best against the criteria set by the decision makers. This approach would also allow them to set different priorities for different public health events (e.g. a short spike in vomiting cases caused by a norovirus outbreak or a longer-term gradual rise in scarlet fever incidence).
We attribute some of the differences in performance between algorithms to whether or not they were designed for daily or weekly surveillance. Future work could create new versions of the Farrington Flexible and EARS algorithms which are specifically adapted to model day-of-the-week effects inherent in the daily surveillance data.
This research focuses on the detection of spiked outbreaks. The seasonal outbreak detection is part of the bigger question of what are we trying to model versus what we are trying to detect. This is an issue that can be addressed in future work. Also, further research on RAMMIE day-of-the-week detection efficiency (e.g. weekends versus weekdays; public holidays versus non-public holidays; detection on different days of the week) can be undertaken.
7 Conclusion
We have created simulated data representing the wide range of data structures seen in a multi-purpose daily surveillance system. We have used this data to compare three algorithms already in use and made these modelled data structures available for the evaluation of other new algorithms. We have shown that the decision as to which algorithm to use should depend on which detection characteristics are most important to the user and not the characteristics of the data signal being monitored. In particular, the Farrington Flexible method has the highest sensitivity and specificity, whereas RAMMIE has the highest POD and is the most timely.
Acknowledgements
Simulations are based on data from Public Health England’s registries for syndromic surveillance. We acknowledge support from: NHS 111 and NHS Digital for their assistance and support in the NHS 111 system; OOH providers submitting data to the GPOOHSS and Advanced; ED clinicians and NHS Trust staff supporting EDSSS, the Royal College of Emergency Medicine and technical support provided by EMIS Health and L2S2 Ltd; TPP and participating SystmOne practices and University of Nottingham, ClinRisk, EMIS and EMIS practices submitting data to the QSurveillance database.
Funding
Iain R Lake and Noel McCarthy were funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections at University of Liverpool in partnership with Public Health England (PHE), in collaboration with University of East Anglia, University of Oxford and the Quadram Institute (grant HPRU-2012-10038). Roger A Morbey, Felipe J Colón-González, Alex J Elliot, Gillian E Smith and Iain R Lake were funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Emergency Preparedness and Response (grant HPRU-2012-10141). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Conflict of Interest: none declared.
References
R Development Core Team (