-
PDF
- Split View
-
Views
-
Cite
Cite
Grace A Freymiller, Malachi D Whitford, Timothy E Higham, Rulon W Clark, Escape dynamics of free-ranging desert kangaroo rats (Rodentia: Heteromyidae) evading rattlesnake strikes, Biological Journal of the Linnean Society, Volume 127, Issue 1, May 2019, Pages 164–172, https://doi.org/10.1093/biolinnean/blz027
Close - Share Icon Share
Abstract
Many animals exhibit morphological specializations driven by the extreme selective pressure of predation, and understanding how such specializations shape escape behaviours can elucidate the evolutionary context of these morphologies. We examined the kinematics of the evasive leaps of desert kangaroo rats (Dipodomys deserti) during strikes from sidewinder rattlesnakes (Crotalus cerastes) to understand the potential importance of predator evasion in shaping bipedalism in desert rodents. We found that kangaroo rats escaping from snake strikes relied on rapid response times to initiate effective evasions. During jumps, their enlarged hindlimbs propelled vertical leaps that were multiple body lengths into the air, and these leaps were often accompanied by mid-air kicks and other manoeuvres that deterred snakes. Although we found high levels of variability in kinematic factors, all kangaroo rats that successfully evaded attacks escaped in a path away from the snake and thus did not have random/protean escape trajectories. In general, our findings support the idea that bipedalism, which has evolved independently in several desert rodent lineages, might be favoured because it allows for rapid and powerful vertical leaps that are crucial for avoiding ambush predators, such as vipers and owls.
INTRODUCTION
Physical performance inextricably links morphology and fitness. Performance is generally defined as the ability to execute an ecologically relevant act (Arnold, 1983) and is often used to characterize the locomotor abilities of animals as they either flee from a threat or pursue prey. Locomotor performance is often examined in the laboratory to control external factors; this context allows for detailed analyses of a few potentially influential variables, but comes at the cost of reduced ecological realism of the results (Irschick & Garland, 2001; Domenici et al., 2011a, b). For example, sprinting is used in a variety of contexts, and sprint speed varies depending on whether the animal is fleeing from predators, chasing prey or simply moving from one location to another (Irschick & Losos, 1998). Laboratory-based measurements of performance also frequently focus on ‘maximal performance’, and animals in their natural environments may only occasionally (or never) perform at maximal levels (Hertz et al., 1988; Husak, 2006; Combes et al., 2012; Wilson et al., 2018). Trade-offs that are commonplace in nature may be lacking in controlled environments, such as high energetic costs of maximal performance (Taylor et al., 1980) or speed–manoeuvrability trade-offs in complex habitats (Alexander, 1982; Wynn et al., 2015), either of which may prevent animals from performing at maximal capacity in the wild. Alternatively, animals in natural environments may exhibit enhanced performance if they are motivated by factors that do not exist in the laboratory (Moore et al., 2017b). Thus, although it is clear that quantification of some aspects of performance requires controlled laboratory settings, measurement of performance in free-ranging animals can complement laboratory studies by providing a better understanding of the ecological relevance of the morphology and physiology underlying performance.
In situ measures of performance and kinematics (i.e. quantitative measure of motion) are particularly important for predator–prey interactions given the high costs for prey associated with predation and the motivation to perform maximally. The strong selective pressure to detect or avoid predators has produced some of the fastest sensory responses and motor actions in nature. These mechanisms often result from changes to morphology (Dayton et al., 2005) and fine-tuning of physiological systems (Jacobs, 1995; Domenici & Blake, 1997). Therefore, the aspects of an organism’s morphology that enhance performance are intimately linked to survival and fitness. For example, crucian carp (Carassius carassius Linnaeus) exhibit intraspecific variation in body shape, where the deep-bodied phenotype (elongated dorsoventrally) improves escape performance compared with the shallow-bodied phenotype (Domenici et al., 2008). Instances such as this, where organisms are morphologically specialized for predator evasion, provide model systems to explore how performance links morphology to fitness.
Kangaroo rats (Dipodomys spp.) are an abundant and widespread radiation of bipedal rodents common throughout arid environments of North America, and they exhibit several specialized adaptations for avoiding predatory attacks. First, they have enlarged auditory bullae that allow them to hear low-frequency sounds often produced by the sudden attack of an ambush predator (e.g. rattlesnake striking or owl swooping), which is key in predator evasion (Webster, 1962; Webster & Webster, 1971). Second, their enlarged hindlimb muscles and thick tendons are important for withstanding high acceleration during the rapid and forceful jumps used to evade predator attacks (Biewener & Blickhan, 1988). Bipedality evolved four times independently within Rodentia (kangaroo rats, jerboas, springhares and jumping mice), presumably as an adaptation for vertical leaping and predator evasion (McGowan & Collins, 2018). Although there are some recent studies of how jerboa locomotion and predator evasion are influenced and driven by this unique morphology (Moore et al., 2017a, b) and a series of studies exploring the sensory basis for predator avoidance in kangaroo rats (Webster, 1962; Webster & Webster, 1971), no field-based studies have quantitatively analysed the biomechanics of the forceful evasive jumps made by bipedal rodents leaping away from ambush predators.
The goals of this study were to examine how kangaroo rats avoid the rapid strike of rattlesnakes during natural encounters and quantify several aspects of these evasions to gain a better understanding of how the specialized morphology of the kangaroo rat may underlie its extraordinary performance during predator evasion. Using three-dimensional high-speed videos, we analysed several key kinematic details of the evasive jumps used by free-ranging desert kangaroo rats (Dipodomys deserti Stephens) that successfully escaped rattlesnake (Crotalus cerastes Hallowell) strikes in order to determine how these leaps vary in natural conditions and provide new insights into predator evasion by bipedal rodents. We predicted that desert kangaroo rats would exhibit greater evasive jumping abilities compared with other small mammals. We also predicted that they would display truly protean (i.e. random) escape trajectories by occasionally escaping towards/over the snake because: (1) other rodents have been described as having protean escape paths (Domenici et al., 2011b); and (2) escape trajectories should, in theory, be highly variable during rapid predator–prey interactions, such as those between rattlesnakes and their prey (Domenici et al., 2011a). This study is an important next step towards understanding why bipedalism evolved convergently in desert rodents, because it elucidates how this morphology aids in avoiding ambush predators and provides information necessary to quantify the fitness consequences of high levels of performance.
MATERIAL AND METHODS
Study site
Our study took place on the southwestern side of the Barry M. Goldwater Range in Yuma, AZ, USA (32°22′13.508″N, 114°22′23.783″W), which is managed by the United States Marine Corps. The site is bisected by a dirt road, separating the site into two distinct habitats: wind-blown sand dunes to the west and creosote scrub (Larrea tridentate Coville) to the east (Malusa & Sundt, 2015). Data were collected from mid-May to early August in 2016. All interactions were recorded between sunset and sunrise, because both rattlesnakes and kangaroo rats are nocturnal at this time of year.
Study animals
All procedures were approved by the San Diego State University Institutional Animal Care and Use Committee (APF 16-08-014C). We first located sidewinder rattlesnakes by following the distinct tracks they leave in the sand. Adults were captured and surgically implanted with temperature-sensitive radio transmitters following the methods of Reinert & Cundall (1982). While the snakes were anaesthetized for surgery, we measured mass to the nearest gram, sex, snout–vent length, tail length, head length and width, and the width of each rattle segment, all to the nearest millimetre. Once normal activities were resumed, snakes were released at the site of capture.
Kangaroo rats were trapped using Sherman live traps baited with black oil sunflower seed placed adjacent to D. deserti burrow systems. We marked kangaroo rats with fingerling ear tags (National Band and Tag #1005-1) for long-term identification and a unique dye mark using Nyanzol fur dye for short-term identification. During the marking process, we recorded sex, mass to the nearest gram, and snout–anus length, tail length and hindfoot length, all to the nearest millimetre. All individuals were processed in the field and released immediately at the site of capture.
Filming interactions
We used a modified version of the methods used by Whitford et al. (2017) and Higham et al. (2017) to record natural interactions between free-ranging sidewinders and desert kangaroo rats. Rattlesnakes with transmitters were tracked at least once nightly via radio telemetry. When a telemetered snake was found hunting on the surface, we moved recording equipment to the snake’s location. Two tethered high-speed cameras (Edgertronic, model SC1) recording at 500 Hz and four to six infrared lights were positioned ~3 m away from the ambushing snake. The cameras were connected to laptop computers via 30.5 m Ethernet cables, which allowed the observers to remain a minimum of 20 m away from the snake. Observers watched the live video feed on the laptops until either an interaction with a kangaroo rat occurred or the snake abandoned ambush. Immediately after snake strikes, observers triggered cameras to save the preceding 10 s of footage. We encouraged the aboveground movements of kangaroo rats at our site by sprinkling small amounts of black oil sunflower seed (5–10 g), thereby increasing kangaroo rat foraging activity in the area. Although placing seed increases the foraging movements of kangaroo rats, we do not believe this impacts their ability to perceive and escape snake strikes. In our previous studies of natural encounters between rattlesnakes and kangaroo rats (Clark et al., 2016; Higham et al., 2017; Whitford et al., 2017), we found that kangaroo rats respond to strikes in a similar manner regardless of the presence of supplemental seed in the vicinity. Furthermore, our experimental study of kangaroo rat escape performance (Freymiller et al., 2017) found that head position (i.e. down/foraging or up/alert) does not alter kangaroo rat escape kinematics. Owing to the high density of desert kangaroo rats at this field site, most of the kangaroo rats that interacted with snakes were not marked despite intensive trapping efforts. However, unmarked kangaroo rats are highly likely to be unique individuals, because our recording locations were typically hundreds of metres apart, and desert kangaroo rat home ranges are ~120 m wide (Langley, 1994). After recording of interactions, we calibrated the video frames with a large object of known dimensions (metal rods screwed in place and fixed to a 30 cm × 25 cm metal plate) placed in the space where the strike occurred.
Video and statistical analyses
All videos were calibrated and digitized in MATLAB (R2016b) using the software DltDataviewer, v.5 (Hedrick, 2008). In order to measure the velocity of the kangaroo rats, we digitized a point on the back of the head in between the ears, then applied a quintic spline to the raw data using the package ‘pspline’ in RStudio (v.0.99.473). We used a generalized cross-validation smoothing parameter (Walker, 1998) to avoid introducing bias with hand-selected smoothing parameters and to ensure that our results were reproducible. We then took the first derivative of the splined data to obtain velocity. Owing to the high levels of noise in the data and the amplification of noise with each derivative, our field data were not suitable for calculating acceleration values.
We calculated take-off angle as the angle between a point on the foot immediately before toe-off, the back of the head 60 ms after toe-off, and a point on the ground. We chose to use the back of the head instead of the centre of mass because this is a more easily distinguishable landmark on the body, and we chose 60 ms after toe-off instead of the highest point of the jump because kangaroo rats often jumped off screen by the height of the jump. The ground point was placed such that the body of the kangaroo rat was between the snake and the ground point; therefore, jumps away from the snake would have angles < 90°. We then used the coordinates for those points to calculate the three-dimensional jump angle.
We recorded reaction time as the amount of time between the first movement of the strike by the snake and the first movement of the evasion by the kangaroo rat. We also recorded the amount of time the kangaroo rat remained airborne by measuring the amount of time between toe-off and touchdown. Ground contact time was measured as the time between the first visible reaction by the kangaroo rat and toe-off. Given that kangaroo rats often jumped out of the camera view during the highest point of the evasive leap, we calculated jump height using the time spent in air with the following equation:
Lastly, we quantified escape trajectory as the angle between the path of the evasion by the kangaroo rat and the strike trajectory in the horizontal plane (i.e. only two dimensions). To do this, we created two vectors: one between the snake’s head and the kangaroo rat’s head in the frame of first kangaroo rat reaction (strike trajectory), and one between the kangaroo rat’s head in the frame of the first reaction and the kangaroo rat’s head from either the frame of landing or, if it landed off screen, the last frame in which the kangaroo rat’s head was visible (kangaroo rat’s evasion). We tested escape trajectory randomness across a 360° circle using a Rao’s spacing test of uniformity (Pewsey et al., 2013). All values are reported as the mean ± SE.
RESULTS
We recorded 32 strikes: 15 ‘hits’ (rattlesnakes contacting and biting kangaroo rats) and 17 ‘misses’ (snakes did not physically contact kangaroo rats). Given that the snakes often made contact with the kangaroo rats before toe-off and this contact influenced the movements of the kangaroo rats, we excluded hits from our analyses. We also removed six misses from quantitative analyses for the following reasons. One miss was removed because the cameras moved slightly between the strike and calibration, and therefore we could not make accurate measurements. Two misses were removed because the evasive manoeuvres by the kangaroo rats were so extreme that the body could not be digitized properly. Two additional misses were removed because the kangaroo rats were not in strike range and did not perform evasive manoeuvres (i.e. snakes struck prematurely and reached maximal extension well short of kangaroo rats). Lastly, one miss was removed because the kangaroo rat immediately jumped off screen, preventing analysis. Thus, we retained 11 misses in our analyses.
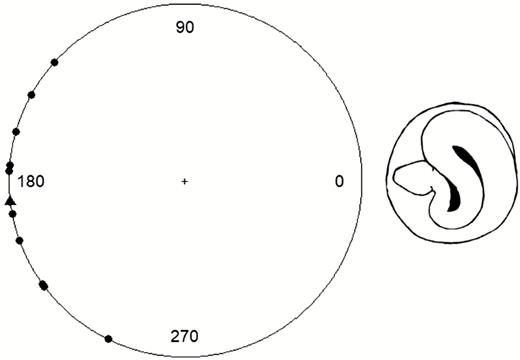
Kangaroo rats avoiding snake strikes exhibited a remarkable ability to move their bodies rapidly out of the initial strike trajectories. Reaction times were highly variable and ranged from 38 to 150 ms, with an average of 81 ± 8.7 ms. Mean maximal velocity was 3.5 ± 0.2 m s−1 (range: 2.7–4.4 m s−1) and always occurred within 10 ms of toe-off but was not consistently achieved either before or after toe-off (before toe-off in 45%, after toe-off in 55%). Ground contact time ranged from 28 to 46 ms (average: 37.8 ± 2.1 ms). The leaps of kangaroo rats were typically near-vertical and propelled them high into the air to evade the strikes. Successful kangaroo rat evasions had take-off angles ranging from 56 to 97°, with an average of 80 ± 4°. Successful evaders also jumped an average of 0.39 ± 0.05 m in the air (range: 0.16–0.82 m) and spent 0.55 ± 0.04 s airborne (range: 0.36–0.82 s). Lastly, kangaroo rats always jumped in a path away from the snake (Fig. 1). Escape trajectory angles varied from 138 to 244°, with an average of 187°. Given that no kangaroo rats ever evaded towards/over the snake in the horizontal plane, the escape trajectory was not random (i.e. not uniformly spread) across 360° (U = 221.3, P < 0.001).
Circle plot showing the escape trajectory angles for all 11 kangaroo rats that successfully evaded rattlesnake strikes. Strikes come from 0°, and the escape trajectory angle (in degrees) for each individual kangaroo rat is plotted along the circumference. No individual ever jumped over/towards the snake, which would be expected if escape trajectories were truly protean/random. Triangle shows mean escape trajectory angle.
Qualitatively, another important aspect of the evasive jump was the high degree of manoeuvrability displayed by most kangaroo rats, consisting of kicks, flips, twists, body contortions and other rapid mid-air movements. Torquing of the body in the air appeared to rely on movements of their long tails (Table 1). We were unable to quantify these aspects of the evasions, however, because many of the points on the kangaroo rats’ bodies would frequently go in and out of frame, preventing more detailed three-dimensional motion analysis.
Video examples of kangaroo rat evasions
| Description . | Video link . |
|---|---|
| Kangaroo rat uses tail to torque body during jump | https://youtu.be/aV8_iv6SXqc |
| Kangaroo rat uses hindlimbs to kick away snake mid-air during leaps | https://youtu.be/HTjX8YilcJg |
| Kangaroo rats using mid-air kicks during antagonistic interactions with conspecifics | https://youtu.be/pPqk3PrFH6E |
| Description . | Video link . |
|---|---|
| Kangaroo rat uses tail to torque body during jump | https://youtu.be/aV8_iv6SXqc |
| Kangaroo rat uses hindlimbs to kick away snake mid-air during leaps | https://youtu.be/HTjX8YilcJg |
| Kangaroo rats using mid-air kicks during antagonistic interactions with conspecifics | https://youtu.be/pPqk3PrFH6E |
All videos were filmed at 500 Hz using dual Edgertronic SC-1 cameras.
Video examples of kangaroo rat evasions
| Description . | Video link . |
|---|---|
| Kangaroo rat uses tail to torque body during jump | https://youtu.be/aV8_iv6SXqc |
| Kangaroo rat uses hindlimbs to kick away snake mid-air during leaps | https://youtu.be/HTjX8YilcJg |
| Kangaroo rats using mid-air kicks during antagonistic interactions with conspecifics | https://youtu.be/pPqk3PrFH6E |
| Description . | Video link . |
|---|---|
| Kangaroo rat uses tail to torque body during jump | https://youtu.be/aV8_iv6SXqc |
| Kangaroo rat uses hindlimbs to kick away snake mid-air during leaps | https://youtu.be/HTjX8YilcJg |
| Kangaroo rats using mid-air kicks during antagonistic interactions with conspecifics | https://youtu.be/pPqk3PrFH6E |
All videos were filmed at 500 Hz using dual Edgertronic SC-1 cameras.
DISCUSSION
Kangaroo rats that successfully evaded snake strikes exhibited incredible performance, jumping more than six body lengths vertically into the air with an average maximal velocity of more than 27 body lengths s−1 and reacting 3.5 times faster than the average human response time to visual stimuli (Marshall et al., 1998). Despite the obvious importance of evasive jumping as a predator-avoidance mechanism, there have been surprisingly few studies of the biomechanics of such manoeuvres. Past studies that quantify kangaroo rat and jerboa locomotion have focused mainly on ‘richochetal’ locomotion (i.e. a series of hops used to move between locations in the environment), rather than the ‘single-shot’ explosive escape jumps used to avoid surprise attacks (but see Biewener & Blickhan, 1988; Moore et al., 2017b).
Although the present study provides the first detailed quantitative analysis of the vertical evasive manoeuvres made by a bipedal rodent during natural predatory attacks, we are aware of several studies in which basic kinematics of escape jumps have been measured in other rodent species. The quadrupedal jumping mouse (Zapus trinotatus Rhoads), which exhibits morphological specializations for jumping, is capable of attaining take-off velocities ranging from 1.2 to 3.5 m s−1 (Harty & Roberts, 2010). Owing to the high power outputs calculated in the study, these authors found support for the use of elastic energy during jumping. Studies of bipedal rodents [lesser Egyptian jerboas (Moore et al., 2017b), banner-tailed kangaroo rats (Biewener & Blickhan, 1988) and desert kangaroo rats (Schwaner et al., 2018)], however, have not found evidence for power amplification via elastic energy storage; these species rely solely on power production by their enlarged hindlimb muscles during jumps. Because of this, bipedal rodents are capable of faster jumps with higher force production and accelerations. Our team (Higham et al., 2017) estimated the maximal velocity of Dipodomys merriami Mearns using the same methodology as used here and found a velocity range of 1.5–4.5 m s−1, comparable to the values in the present study of 2.7–4.4 m s−1. These comparisons indicate that, although all five species have been shaped by natural selection into specialized jumpers, bipedal rodents show specializations that favour high force and acceleration for faster, more controlled jumps, whereas the quadrupedal jumping mice favour power amplification via elastic mechanisms to compensate for their relatively smaller hindlimb muscle mass.
The remarkable reaction time of kangaroo rats appears to be the crux of their evasion strategy. In Whitford et al. (2019), we compared various performance variables of both snakes and kangaroo rats that potentially influence the outcome of these interactions and found that kangaroo rat reaction time was the main determinant of whether or not a strike would make contact. Reaction times of kangaroo rats that evaded strikes (i.e. the jumps we analyse here) were typically faster than those of kangaroo rats that were bitten, regardless of the distance between the kangaroo rat and the snake. Thus, the effectiveness of a kangaroo rat escape manoeuvre is largely determined by their ability to initiate a response as rapidly as possible. Interestingly, our estimates of kangaroo rat reactions times to rattlesnake strikes are markedly slower than those we measured in a previous study examining kangaroo rat evasions to an uncoiling metal spring designed to simulate a snake strike (Freymiller et al., 2017). Given that kangaroo rats are likely to rely predominantly on acoustic cues to evade predators (Webster, 1962), we assume that the noise associated with our simulated strike was different from the noise made by a real rattlesnake strike, owing either to mechanical noise associated with the triggering mechanism or to a difference in the bow wave of air moved towards the kangaroo rat during the forward motion of the device.
Escape trajectory
The optimal escape path for evading a rattlesnake strike is predicted to be a relatively vertical path that is perpendicular to the oncoming strike (Freymiller et al., 2017; Higham et al., 2017), which was seen in the present study. Truly protean escape behaviours should result in a random mixture of escape trajectories, including occasional escapes towards predators (Domenici et al., 2011a, b). Although ostensibly riskier than escaping away from a predator, this risk would be mitigated when dealing with ‘single-strike’ predators that cannot launch a second attack quickly; additionally, moving towards a predator gives the predator less time to make major adjustments to the attack path, especially if it is moving quickly and must overcome a higher moment of inertia to manoeuvre (Shifferman & Eilam, 2004). As such, we expected that kangaroo rats escaping rattlesnakes (a classic example of a rapid, single-strike predator) would have occasional escapes towards the position of the snake. However, we found that in the horizontal plane, kangaroo rat jumps were almost always directed away from the snake and thus were not random. Kangaroo rats do appear to use random, zig-zagging trajectories in the horizontal plane when being chased (Djawdan & Garland, 1988), suggesting that bipedal rodents might use different escape trajectories depending on the hunting mode of the predator. The strike path of a viper is inherently limited by the body length of the snake, and we have never observed rattlesnakes to strike more than once when attacking prey in natural conditions (Clark, 2006; Clark et al., 2012, 2016; Putman et al., 2016; Whitford et al., 2017; Whitford et al., 2019), whereas cursorial pursuits often take place over longer distances. Previous studies have also noted that kangaroo rats use rapid vertical leaps to escape owls, another common single-strike predator (Webster, 1962). Thus, movement into the vertical plane is likely to be more important when escaping a single-strike, sit-and-wait ambush predator, whereas unpredictability in the horizontal plane might be more important for escaping pursuit predators.
Role of bipedalism in predator evasion
Several studies have found evidence that bipedal rodents are better at predator evasion when compared with their quadrupedal counterparts (Kotler, 1985; Longland & Price, 1991). Conversely, another study found no difference in predation rate between bipedal and quadrupedal rodents (Kotler et al., 1988). Although very limited data exist for comparison, a series of past studies from our group have recorded various species of quadrupedal small mammals jumping away from rattlesnake strikes, including ground squirrels (Otospermophilus beecheyi Richardson), gray squirrels (Sciurus carolinensis Gmelin), chipmunks (Tamias striatus Linnaeus), woodrats (Neotoma lepida Thomas), field mice (Peromyscus sp.) and voles (Microtus sp.) (Clark, 2006; Clark et al., 2012; Putman et al., 2016); qualitatively, the evasive jumps of kangaroo rats are unique among these species. Kangaroo rats exhibit more forceful jumps that propel individuals much further and faster from snakes, and we have not observed other species exhibit the mid-air kicks and turns used by kangaroo rats (for details of kicking snakes away, see Whitford et al., 2019). Additionally, using an experimental approach, Freymiller et al. (2017) found that desert kangaroo rats far outperformed California ground squirrels in the speed and force of their evasive jumps. Thus, we hypothesize that bipedalism and enlarged, powerful muscles provide kangaroo rats with the following unique abilities: (1) to produce rapid, vertical evasions; (2) to narrowly evade capture via extreme levels of manoeuvrability; and (3) to reduce the likelihood of envenomation by kicking away snakes attempting to embed their fangs.
Vipers present a significant risk in desert environments across the globe because they can occur in high abundances (see Nowak et al., 2008) and specialize in killing and consuming small mammals with rapid, envenomating strikes. In order to evade these predators successfully, kangaroo rats used complex, intricate and variable manoeuvres. We found a high degree of variability between leaps in all the factors we measured. Some individuals, for example, jumped almost 90° into the air, whereas others jumped at more acute angles away from the body of the snake. Although we focused on different forms of evasion, studies of jerboa bounding away from pursuit predators also found a high degree of variability in evasions (Moore et al., 2017a). Both kangaroo rats and jerboas execute predominantly muscle-powered leaps; by not having to load tendons for power amplification, these bipedal rodents are capable of performing more rapid and complex manoeuvres (Biewener & Blickhan, 1988; Moore et al., 2017b). When taken together, this evidence supports the idea that powerful and variable jumping to evade predators makes bipedal rodents more difficult to capture and is a key reason why this unique morphology has evolved independently multiple times in desert rodents.
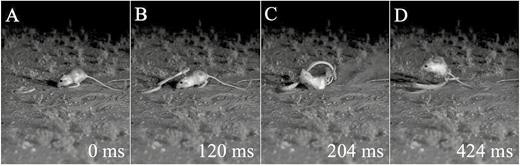
Although our video set-up was not sufficient for accurately quantifying manoeuvrability during these evasions, we observed many kangaroo rats narrowly evade the strikes by quickly moving different parts of their bodies out of the strike path. For example, one kangaroo rat rotated its body upside down, kicked the snake’s head away from its body, then righted itself before bounding away (Fig. 2). All our evasion sequences exhibited unique elements, underscoring the high degree of manoeuvrability and acrobatics used to avoid snake strikes. Analyses of these complex manoeuvres will be undertaken in the future with a larger number of synchronized cameras to allow for a more intricate examination of the remarkable manoeuvrability of these rodents. From the qualitative observations, we noted that much of this manoeuvrability may also stem from their specialized morphology. Given that they were still at risk from being hit after initiating the jump (see Whitford et al., 2019), individuals would frequently rotate and twist their bodies in mid-air, potentially with the aid of their long tail (Table 1). Lastly, we observed the kangaroo rats using their large hindlimbs physically to kick the snakes away from their bodies and prevent envenomation (Table 1). It is also worth noting that bipedal jumping and kicking may be crucial for other facets of kangaroo rat life history (Bartholomew & Caswell, 1951; Eisenberg, 1963; Kenagy, 1976); anecdotal recordings of intraspecific interactions that we recorded opportunistically show that jumping and kicking play central roles in antagonistic encounters (Table 1).
Panel of stills showing the extreme manoeuvrability of desert kangaroo rats during evasions from snakes. A, snake initiates strike. B, kangaroo rat begins reaction. C, kangaroo rat flips upside down and kicks snake away. D, kangaroo rat rights self before landing. Time (in milliseconds) is shown in lower right corner of each image, with 0 ms being the moment of strike initiation. Video footage of this interaction is viewable at https://youtu.be/svz9MPebQRw.
Conclusion
This study is the first, to our knowledge, to describe the kinematics of evasive leaps by bipedal rodents avoiding real attacks from predators. In order to evade rattlesnakes successfully, kangaroo rats combine highly enhanced auditory senses with morphological specializations for jumping and kicking, resulting in remarkable levels of physical performance in natural escape manoeuvres. Laboratory-based studies of bipedal rodents have elucidated the relative importance of various muscles and tendons for jumping, and they provide a framework for understanding why bipedalism has evolved several times in desert rodents. Taking these studies into the field (an ‘ecomechanical’ approach) further underscores the importance of predator evasion in the evolution of bipedalism in small desert mammals.
ACKNOWLEDGEMENTS
We would like to thank Jessica Ryan, Drew Steele, Katherine Phillips and Colin Goodman for their assistance and dedication during data collection. We would also like to thank the Marine Corps Air Station, Yuma and Abigail Rosenberg for providing us with access to the Barry M. Goldwater Range; we appreciate the opportunity to work in habitat that has been maintained and preserved by the United States Marine Corps. We thank two anonymous reviewers for their valuable feedback on earlier versions of this manuscript. Lastly, we thank San Diego State University, the Animal Behavior Society and the American Society of Mammalogists for financial support to carry out this work.
REFERENCES
Domenici P, Blagburn JM, Bacon JP. 2011a. Animal escapology I: theoretical issues and emerging trends in escape trajectories. Journal of Experimental Biology 214: 2463–2473.
Domenici P, Blagburn JM, Bacon JP. 2011b. Animal escapology II: escape trajectory case studies. Journal of Experimental biology 214: 2474–2494.
Hertz PE, Huey RB, Garland T, Jr. 1988. Time budgets, thermoregulation, and maximal locomotor performance: are reptiles Olympians or boy scouts? Integrative and Comparative Biology 28: 927–938.
Irschick DJ, Garland T, Jr. 2001. Integrating function and ecology in studies of adaptation: investigations of locomotor capacity as a model system. Annual Review of Ecology and Systematics 32: 367–396.
Whitford MD, Freymiller GA, Higham TE, Clark RW. 2019. Determinants of predation success: how to survive an attack from a rattlesnake. Functional Ecology. doi:10.1111/1365-2435.13318
Wilson AM, Hubel TY, Wilshin SD, Lowe JC, Lorenc M, Dewhirst OP, Bartlam-Brooks HLA, Diack R, Bennitt E, Golabek KA, Woledge RC, McNutt JW, Curtin NA, West TG. 2018. Biomechanics of predator-prey arms race in lion, zebra, cheetah, and impala. Nature 554: 183–188.