-
PDF
- Split View
-
Views
-
Cite
Cite
Csilla Ari, Long-term body pigmentation changes on a manta ray (Mobulidae), Biological Journal of the Linnean Society, Volume 114, Issue 2, February 2015, Pages 406–414, https://doi.org/10.1111/bij.12416
Close - Share Icon Share
Abstract
Manta rays (genus Manta) are listed as vulnerable on the IUCN Red List based partly on population studies that use body coloration and ventral spot patterns to identify and count individuals, as a result of such patterns being considered unique and permanent during their lifetime. The present study reports the first evidence of long-term coloration changes of manta rays based on observations of one captive individual. Darkening skin pigmentation was documented on the side of the head, the inner side of the cephalic lobes and the pectoral fin margin of the ventral side, and spot markings appeared on the gill slits within 9 months. These changes were most likely ontogenetic or were connected to maturation. The described specimen's coloration did not fulfill the taxonomic classification criteria of either manta ray species and rather resembled two different manta ray species at the beginning and end of the study. These results show that coloration patterns of manta rays are not as stable as has been assumed previously. Further studies are needed to identify the extent of such changes for accurate identification and classification of manta rays.
Introduction
Manta alfredi (Krefft, 1868) and Manta birostris (Walbaum, 1792) are listed as vulnerable on the IUCN Red List (Marshall et al., 2011a, b) because of the declining populations resulting from the uncontrolled fishing in many countries that harvests their meat, gill rakers and cartilage (Homma et al., 1999; Stevens et al., 2000; Alava et al., 2002; Couturier et al., 2012; Ward-Paige, Davis & Worm, 2013). Monitoring the changes of the population size in response to targeted fishing, by-catch, and the impact of tourism is essential for designing effective conservation actions.
Photo-identification (photo-ID) and visual sight–re-sight techniques using natural distinctive markings are useful for distinguishing and counting individuals of marine species in the wild and represent accepted techniques for population size estimates of whale sharks (Rhincodon typus; Graham and Roberts 2007; Marshall & Pierce, 2012), nurse sharks (Ginglymostoma cirratum; Castro and Rosa 2005), zebra sharks (Stegostoma fasciatum; Dudgeon, Noad & Lanyon, 2008), long finned pilot whales (Globicephala melas; Auger-Méthé and Whitehead 2007), and bottlenose dolphins (Tursiops truncatus; Silva et al., 2009).
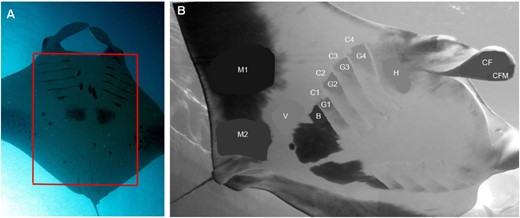
The natural coloration and ventral spot markings of manta rays are considered unique for an individual and permanent during their lifetime, based on population studies on M. alfredi. Individual manta rays have been identified from patterns of ventral markings in recent studies to assess the population size of M. alfredi in Hawaii (Deakos, Baker & Bejder, 2011), Mozambique (Marshall, Dudgeon & Bennett, 2011c), the Maldives (Kitchen-Wheeler, Ari & Edwards, 2012), and Australia (Couturier et al., 2014), or the seasonal occurrence of M. birostris in Brazil (Luiz et al., 2009). To identify individual manta rays, Luiz et al. (2009) and Deakos et al. (2011) used the entire ventral pattern of each individual sighted, whereas Marshall et al. (2011c) defined the standardized region as a rectangle-shaped area extending posterior from the anterior margins of the anterior gill slits, including the pelvic fins. Kitchen-Wheeler et al. (2012) used the ovoid markings between gill slits, as well as markings at the distal ends of gill slits, as an objective differentiator and recorded, as absent or present for each gill slit, markings in the lower abdominal area and pigmentation around the head and posterior–ventral part of fins. The software MANTA MATCHER, used for individual identification, also relies on the spot patterns in the rectangular area on the ventral side, including the spot markings at the end of the gill slits (Town, Marshall & Sethasathien, 2013, Fig. 1A), and the same rectangular area was also used by Couturier et al. 2014.
Ventral markings used for identification and coloration studies. A, the red rectangle shows the area commonly used for photo-identification studies and the computer software to identify individual manta rays. B, to calculate the difference in darkness of certain body areas, the images were converted to grayscale and the lightness value (L) was measured and compared, where 0 = black and 100 = white, sensuRobbins & Fox (2012). The areas were selected and the average blur function was applied to obtain the mean L value for that area. The measured areas comprised: distal half of cephalic fin's inner side (CF) and, as its control, the black margin on distal half of cephalic fin (CFM); distal edge area below gill slits (G1–G4), control white areas lateral to G areas (C1–C4), head and mouth area below eye and cephalic fin (H), black patch below the last gill slit (B), white ventral side area between the pectoral fin margin and large black patch (V), pectoral fin marginal area around midway (M1), and pectoral fin marginal area above pelvic fin (M2).
The body coloration of manta rays is used for species identification as well, such as the coloration of the head and mouth area, the distribution of ventral spot markings, the extent of the pectoral fin margin on the ventral side, and the presence and shape of shoulder bar markings (Marshall, Compagno & Bennett, 2009). Although the body coloration of manta rays is extensively used for identification and classification purposes, rapid coloration changes of captive manta rays have been described recently that resulted in an intensification of white markings on the dorsal side and head area, which were connected to feeding events and intense social interactions (Ari, 2014).
To be able to use natural markings reliably for individual or species identification, these markings need to be consistent over time. Accurate population size assessments are needed to estimate the vulnerability of all manta ray species and to design effective conservation actions. Therefore, knowledge of the permanence of manta rays' natural body pigmentation is essential.
The present study reports first evidence of long-term pigmentation changes of a captive manta ray, suggesting that pigmentation patterns of manta rays are not as stable over time as previously assumed.
Material and Methods
Studied specimens
The observations recorded during the present study were made in compliance with all ethical standards and were approved by the Kerzner Marine Foundation and the Atlantis Aquarium, Bahamas. Two specimens of captive manta rays were observed extensively in July 2011 for 24 days (first observation period) and also in March 2012 for 16 days (second observation period) at the Atlantis Aquarium, Bahamas. Photographs and video recordings were also collected during these time periods, as well as from March 2013, of the male M. birostris (Manta 1, likely mature, based on body size and clasper morphology) and the female manta ray (Manta 2, stage of maturation uncertain). The morphological characteristics of Manta 2 at the time of the first observation period suggested that the specimen likely belonged to a putative third manta ray species Manta sp. cf. birostris; however, during the next observation period, it could be considered as M. birostris.
Photography, size measurements, and photo analysis
Direct observations were conducted, and photographs and video recordings were made of the manta rays from outside of the tank through the aquarium windows, from the surface, and inside the tank with an underwater still and video camera system (Canon S100 with Fisheye Fix S100 housing for underwater use). Their size was estimated by a snorkeler, who measured the interorbital distance with a measuring tape, and by extrapolating their total width using frontal photographs of the whole body.
Quantification of coloration changes
PHOTOSHOP CS5, version 9.0.2 (Adobe Systems Inc.) was used to quantify the relative darkness of body areas over the years. To calculate the difference in darkness of certain body areas, the images were converted to greyscale and the lightness value (L) was measured and compared in each year, where 0 = black and 100 = white, sensuRobbins & Fox (2012): the areas were selected and an average blur function was applied to obtain the mean L value for that area. Five images per year have been analyzed and pigmentation changes were measured on the mantas' head and ventral side. To eliminate any variation in lighting differences of the photographs potentially caused by depth, clarity of the water, use of flash, and the relative position of the sun, control areas were selected and compared with the areas under investigation. The control areas were selected on the body close to the areas under investigation that were also oriented at the same angle to ensure similar lighting conditions but did not change in darkness noticeably during the study period. The measured body areas are shown in Figure 1B. The difference in the mean L values between the control and studied areas gave the relative difference in tone over the study period.
For statistical analyses, two-way analysis of variance was used with Tukey's post-hoc multiple comparison test. Changes of lightness value of certain body regions over the years are presented as the mean ± SEM. Statistical analysis was performed using GRAPHPAD PRISM, version 6 (GraphPad Software Inc.).
Results
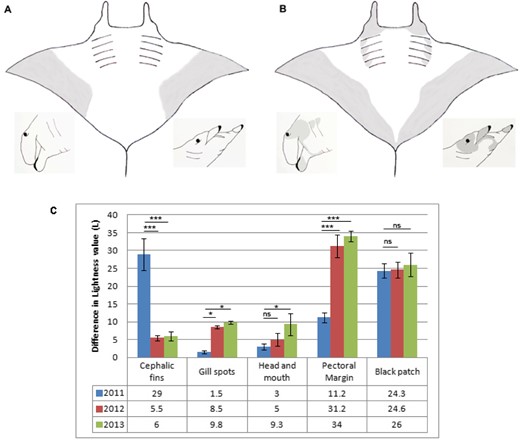
Manta 1 and 2 were estimated to be 3.5 m and 2.6 m disc width (DW), respectively, during the first observation period, whereas their sizes were approximately 4.2 m and 3.4 m DW, respectively, during the second observation period. Manta 1 did not show permanent coloration changes over the study period, either on the dorsal or ventral side, whereas permanent changes in the body pigmentation of Manta 2 were observed. The changes in size and shape of natural coloration and pigmentation markings between the first and last observation periods are presented in a schematic drawing (Fig. 2A, B), whereas Figure 2C shows the difference in lightness value of the described body areas over the study period and the extent of the darkening pigmentation.
The darkening pigmentation of certain body areas is presented. A, B, schematic drawings demonstrate the changes in size, shape, and extent of the pigmentation patterns and natural coloration during the study period (excluding the spot patterns on the lower abdominal area posterior from the last gill slits). The ventral side of the body and lateral side of the head are shown during the first (A) and last (B) observation periods. C, difference in lightness value is presented (as the mean ± SEM) between the body regions under investigation and the control areas over the years. CF areas were compared with CFM, which resulted in the relative darkness of the inner side of cephalic fin; G1–G4 were compared with the darkness of C1–C4 area, which resulted in the relative darkness of the gill spots; H was compared with the C4 area, which resulted in the relative darkness of head and mouth area; M1 and M2 were compared with V, which resulted in the relative darkness of pectoral fin margin; and B was compared with V, which resulted in the relative darkness of the large black patch below the gill slits. (*P < 0.05, **P < 0.005, ***P < 0.0005). For area abbreviations, see Fig. 1. ns, not significant.
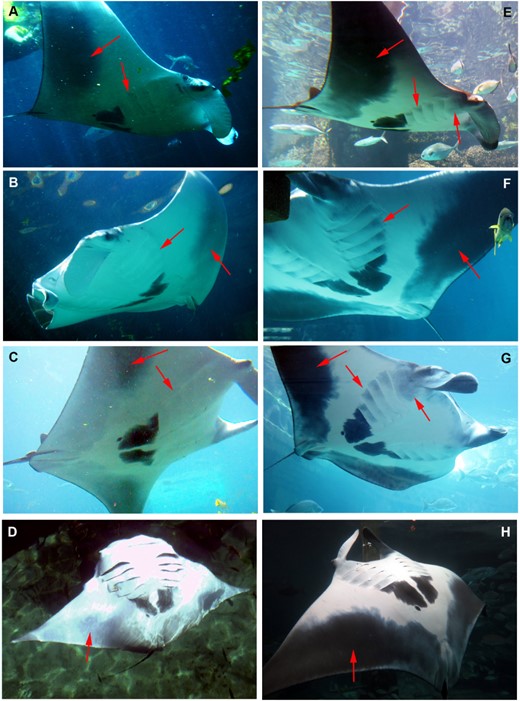
Although Manta 1 and 2 presented rapid coloration changes daily (Ari, 2014) in addition to the long-term changes for Manta 2, the following comparison of body coloration between the observation periods describes the animal when it was in the baseline coloration state. Manta 2 changed its coloration on the side of the head, on the cephalic lobes, and on the ventral surface over a 9-month period (Figs 3, 4). During the first observation period, the inside of the cephalic lobes was completely white with narrow black margins on the outer edge (Fig. 3A, B, C); the side of the head, the mouth region under the cephalic lobes and around the eyes the coloration was mainly white with a small amount of light grey (Fig. 3A, B, C); the pectoral fin margin on the abdominal region was light grey, which extended only until midway and did not reach the pelvic fins (Fig. 4A, B, C, D). During the second observation period, the inside of the cephalic lobes was uniformly dark grey (Fig. 3D, E, F), the side of the head, around the eyes was black (Fig. 3D, E, F), under the cephalic lobes was grey, and the pectoral fin margin on the abdominal region had a strong dark grey coloration that reached all the way to the midline (Fig. 4E, F, G, H). These areas continued darkening over the next year (Fig. 2C). Although the area around the gill slits was completely white without spot markings at the time of the first observation period (Figs 3B, C, 4A, B, C, D), grey patch markings were visible under the distal edge of each gill slit during the second observation period (Fig. 4E, F, G, H), which continued darkening during the next year (Fig. 2C). The most significant difference between years was found in the darkness of cephalic fins and pectoral fin margins (Fig. 2C). The large black patch below the last gill slits did not differ in darkness over the years, whereas the darkness of the gill spots, the head and mouth area significantly increased (Fig. 2C).
Pigmentation changes are presented on the head of Manta 2. A, B, C, the pigmentation is presented around the head at the time of the first observation period: the inside of the cephalic lobes was completely white with narrow black margins on the outer edge; the side of the head and the mouth region was white; under the cephalic lobes and around the eyes was mainly white with a small amount of light grey; D, E, F, the pigmentation is presented around the head at the second observation period: the inside of the cephalic lobes turned uniformly grey; the side of the head and around the eyes turned black.
Pigmentation changes are presented on the ventral surface of Manta 2. A, B, C, D, the pigmentation of ventral surface is presented at the time of the first observation period: the pectoral fin margin on the abdominal region was light grey, which extended only until midway, and the gill slits were white without spot markings, E, F, G, H, the pigmentation of the ventral surface is presented at the second observation period: under the cephalic lobes turned grey and the pectoral fin margin on the abdominal region had a strong dark grey, almost black, coloration that reached all the way to the midline. Note the grey spot markings on the gill slits that were not present at the first observation period.
In the Supporting information, Movie S1 shows Manta 2 during the first observation period in 2011 and Movie S2 shows the same animal 9 months later.
Discussion
The present study provides the first description of long-term body pigmentation changes in a manta ray. New markings and darkening pigmentation were documented on a captive, female manta ray over the 2-year observation period.
Some elasmobranchs were reported to show coloration changes as a result of different environmental factors. Scalloped hammerhead sharks (Sphyrna lewini) have been reported to become darker with increasing exposure to solar radiation (Lowe & Goodman-Lowe, 1996), whereas melanism was attributed to cold temperatures in other ectotherm species by Trullas, van Wyk & James (2007). Prota (1992) also reported that the release of melanin-stimulating hormone causes morphological colour changes in sharks. The melanistic changes of the manta ray are unlikely to be caused by sun exposure because the changes occurred on the ventral side (excluding the side of the head); in addition, the other specimen did not show any darkening when exposed to the same ultraviolet radiation. The water temperature and nutritional changes were minimal in captivity and affected both specimens in the same way. The coloration changes could not be associated with healing injury (scarring) because the manta ray in the present study had not suffered injury on the ventral surface, and neither developed skin disease, nor showed any other disorder during the study period. The potential effect of stress on body coloration should also be considered. The animal exhibited rapid coloration changes that could be associated with excitement when introduced into the new environment (Ari, 2014), although these changes did not involve the ventral surface. The animal was observed for more than 3 weeks after capture during the first observation period, during which it started feeding regularly, similarly to the other manta ray, and this might represent a good indication of the animal having become used to the new environment and not being in a stressed state that might cause changes in ventral pigmentation.
It is not uncommon for the pigmentation pattern to change during the juvenile period of an animal (Natusch & Lyons, 2012; Anderson et al., 2013; Martin et al., 2013). Indeed, such a change is known to occur in many species for which the pigmentation pattern is used to identify individuals. Prominent natural colorations or patterns do change during natural growth or at the onset of maturity in white sharks (Carcharodon carcharias; Domeier & Nasby-Lucas, 2006) and in zebra sharks (Stegastoma fasciatum; Dudgeon et al., 2008) and minor changes in the spot patterns were also noted to occur over time in whale sharks (Rhincodon typus; Arzoumanian, Holmberg & Norman, 2005). Melanistic change on the lower caudal lobe of a female white shark was recently described over a 9-month period as evidence of melanism (Robbins & Fox, 2012). Domeier & Nasby-Lucas (2006) also reported melanistic change in the size of gill spots from two white sharks.
The changes observed in the present study were documented on an individual during the early stages of development. Considering previous reports of unchanging patterns of adult M. alfredi in the wild together with the present findings, it is possible that the pigmentation change can be attributed to the maturation process or to ontogenetic changes during early stages of development, or to be only characteristic of M. birostris. It is possible that, once an individual reaches maturity, the ventral coloration does not change or that M. alfredi might not undergo coloration changes similar to those observed in the present study. However, because of a lack of similar observations in the wild, further studies on long-term ventral pattern coloration changes are needed to confirm whether this phenomenon is indeed connected to the maturation process or to ontogenetic changes during early stages of development.
The evolutionary advantage of the white ventral and head coloration of younger individuals might be explained if young animals mainly use shallow habitats, over sandy bottoms with abundant reflecting light (C. Ari, pers. observ.), where lighter body coloration helps them better camouflage from predators. If older animals tend to dive to deeper waters, they most likely achive greater advantage with respect to hiding from predators as a result of darker body colorations.
In species where natural coloration changes are observed during maturation, juveniles are excluded from the photo-ID catalogue and/or the analyses (Huffard et al., 2008). As such, as long as the pigmentation pattern of the adult manta rays is stable over time and researchers can differentiate juveniles from adults, pigmentation changes in juveniles should not hinder the use of photo-ID in most population estimate analyses. No studies on long-term re-sightings and permanence of ventral markings of M. birostris have been published so far. Indeed, it is possible that the pigmentation of the described areas (gill slit, ventral pectoral fin margin, cephalic fin areas) further increases over time; therefore, the degree of melanism might be correlated with age to a certain degree in M. birostris. In addition, the much lower quality underwater photographs from decades ago might mean that it is not possible to detect the subtle changes reported in the present study.
The body coloration of manta rays is also used for taxonomic identification (Marshall et al., 2009); therefore, the results of the present study suggest that certain areas of their body might not be suitable for such a purpose. It is possible that the different coloration states represent different developmental stages rather than separate species. At the beginning of the study, Manta 2 could be identified as the putative third species based on its coloration (Marshall et al., 2009), although, from the second observation period, it could be identified as M. birostris, except that the distinctive shoulder bars were missing. Most likely, greater variety exists in coloration, pigmentation, and shapes of areas with pigmentation, between species and between individuals, than indicated by the present taxonomic classification.
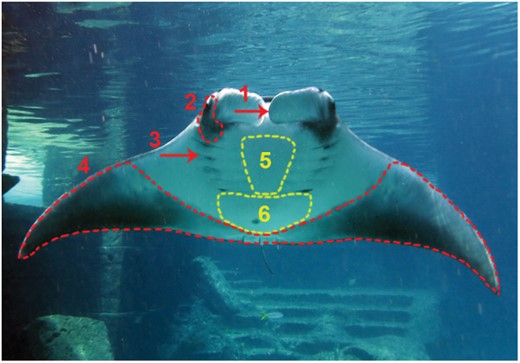
Based on the results of the present study, caution is warranted when considering the coloration of gill slit area, the ventral pectoral fin margin area, and the head (including the eyes, cephalic fins and mouth area) for identification or classification purposes (Fig. 5). Changes in the shape, darkness, and size of spot markings in the lower abdominal area, posterior from the gill slits, were not observed during the study period, making this area the most reliable for using in photo-ID studies, especially if computer software is applied to determine matching markings of resighted individuals (Fig. 5). Even if most of the changes did not affect the markings generally used for individual photo-ID studies, the recent discovery of rapid coloration changes and the described long-term pigmentation changes make it essential to confirm the stability of the natural markings, especially for M. birostris, which can further improve both techniques and the software used for identification in the future.
Body regions of a manta ray (Manta 1) are presented that changed pigmentation and those that did not change during the study period: Red labelling shows areas where coloration changed over 9 months; 1: inner side of cephalic fins; 2: side of head, around eyes, under cephalic lobes; 3: gill slit markings, 4; ventral margin of pectoral fins. Yellow labelling shows the areas where the markings did not change during the study period; 5: ventral inter-branchial region; 6: markings in lower abdominal area posterior from the last gill slits, excluding the pectoral fin margin.
Although the reported pigmentation changes were observed on one specimen, these changes raise new questions about the biology and physiology of manta rays, and are sufficient to highlight the need to properly assess the reliability of the current identification methods. It is uncertain whether the documented pigmentation changes are characteristic of this specimen, the species or the genus Manta, although this physiological trait of coloration change would be expected to be consistent within a genus, or at least within a species. Because recent emerging evidence on several large bodied, marine species suggests that natural markings, spot patterns, and body coloration are not as permanent as previously assumed, more studies will be needed, specifically on the permanence of natural coloration of each manta ray species, as well as on the mechanism and extent of these pigmentation changes.
Acknowledgements
The Save Our Seas Foundation is thanked for providing full funding for the present study. I also thank Michelle Liu, Dave Wert, and the aquarium staff of Atlantis Aquarium, Bahamas, for their support and for providing the opportunity to study the manta rays at their exhibit. Dr Dominic D'Agostino and Dr Huntington Potter are thanked for their continuous and irreplaceable support during the research period. DAN Europe provided essential support. Dr Dominic D'Agostino, Dr Christopher Rogers, and anonymous reviewers further improved the manuscript with their thoughtful comments.