-
PDF
- Split View
-
Views
-
Cite
Cite
Micah G Freedman, Hugh Dingle, Christine A Tabuloc, Joanna C Chiu, Louie H Yang, Myron P Zalucki, Non-migratory monarch butterflies, Danaus plexippus (L.), retain developmental plasticity and a navigational mechanism associated with migration, Biological Journal of the Linnean Society, Volume 123, Issue 2, February 2018, Pages 265–278, https://doi.org/10.1093/biolinnean/blx148
Close - Share Icon Share
Abstract
Monarch butterflies are best known from their migratory North American range, although many resident, year-round breeding populations are established throughout the world. Here, we evaluate two non-exclusive hypotheses for the loss of migration in resident monarch populations: (1) absence of cues that trigger migration; and (2) loss of sensory, neural or physiological systems required for migration. To evaluate the first hypothesis, we exposed resident monarchs from Queensland, Australia to decreasing larval photoperiod and observed reproductive development in resulting females to assess their propensity to show reduced reproductive development, a precursor for long-distance migration. To address the second hypothesis, we measured antennal circadian clock gene expression, a crucial element of the monarch’s ability to orientate directionally, in a resident and a migratory population. We found that Australian resident monarchs show reduced reproductive development in response to decreasing photoperiod, consistent with the ‘loss of cues’ hypothesis. We found no differences in antennal clock gene expression between migratory and resident populations, inconsistent with the ‘loss of mechanism’ hypothesis. Together, these data indicate that even after hundreds of generations of non-migration, monarchs retain two crucial elements of their migratory repertoire: developmental plasticity associated with decreasing photoperiod and antennal circadian rhythms necessary for directional orientation.
INTRODUCTION
Long-distance migration has evolved across the tree of life as an adaptation to temporal and spatial variation in resource availability (Dingle, 2014). Among insects, perhaps the best-known migration is that of the monarch butterfly in North America (Danaus plexippus, Linnaeus, 1758; Urquhart & Urquhart, 1978; Brower, 1995; Gustafsson et al., 2015). To accomplish long-distance migration and subsequent overwintering, monarchs exhibit a correlated syndrome of changes in morphology, physiology and reproductive behaviour (Herman, 1981; Masters, Brower & Malcolm, 1988; Brower, Fink & Walford, 2006). Long-distance migration distinguishes North American monarch populations from long-established non-migratory populations in Central and South America and the Caribbean as well as more recently established non-migratory populations on many Pacific islands.
Monarch migration is preceded by the onset of a physiological state known as reproductive diapause (Herman, 1973; Brower et al., 1977). This diapause is influenced by juvenile hormone titres (Herman, 1981) and entails decreased investment in reproductive development and greater allocation to lipid reserves required for uninterrupted long-distance flight (Beall, 1948; Brown & Chippendale, 1974). Monarchs from eastern North America exhibit true reproductive diapause, whereby migrating and overwintering adults remain reproductively inactive even after prolonged exposure to summer-like conditions that are conducive to reproduction (Herman, Brower & Calvert, 1989). This is in contrast to other monarch populations, which exhibit less pronounced refractory periods and resume reproductive development after relatively short periods of exposure to favourable conditions (James, 1982; Herman et al. 1989). Goehring and Oberhauser (2002) evaluated cues potentially responsible for inducing monarch diapause in eastern North America, including factors such as absolute temperature, fluctuations in temperature, photoperiod, decreases in photoperiod, and age of larval host plant. They found that decreasing photoperiod, older host plant material, and fluctuating temperatures during larval development—all indicative of the onset of North American autumn—were associated with induction of reproductive diapause (Goehring & Oberhauser, 2002).
Migration in monarchs is thought to be highly conserved and dates back at least to the common ancestor of D. plexippus and Danaus erippus (Zhan et al., 2014). Over the past 200 years, monarchs have achieved a nearly global distribution, with at least three independent waves of colonization out of the ancestral North/Central American range (Ackery & Vane Wright, 1984; Zalucki & Clarke, 2004; Pierce et al., 2014; Zhan et al., 2014). Throughout most of their introduced range, monarchs are year-round breeding residents, with the exception of southern Australia, where small-scale seasonal migration occurs (Smithers, 1965; James, 1979, 1993; Dingle, Zalucki & Rochester, 1999). Previous studies have compared resident and migrant populations of monarchs and shown that migrants typically show larger, more elongated forewings, presumably as an adaptation for long-distance flight (Beall & Williams, 1945; Dockx, 2007; Altizer & Davis, 2010; Li, Pierce & de Roode, 2016; Yang et al., 2016). Furthermore, genomic and transcriptomic evidence indicates both fixed differences in haplotype and expression level differences between migrants and residents, despite the recency of the monarch’s introduction in many of these locations (Zhan et al., 2014).
Although it is clear that selection has favoured non-migration and associated phenotypes in recently established monarch populations, the proximate causes of the transition to resident status have yet to be explored fully. One possibility is that residents simply no longer experience the relevant environmental cues that trigger migration, hereby referred to as the ‘loss of cues’ hypothesis. In this scenario, resident monarchs exposed to conditions akin to those that elicit reproductive diapause in eastern North American monarchs may still respond in a similar manner to their migratory ancestors and exhibit phenotypes conducive to long-distance migration.
Another non-mutually exclusive explanation for the loss of migration in residents is that monarchs have lost or suppressed elements of the sensory, neural or physiological systems that link environmental cues with migration, hereby referred to as the ‘loss of mechanism’ hypothesis. For example, the sensory system that enables detection of changing photoperiod, suspected to be related to circadian clock genes expressed in the pars lateralis (Sauman et al., 2005; Zhan et al., 2011), might be altered in resident monarchs. Alternatively, non-migratory monarchs might still be capable of sensing and encoding environmental cues relevant for the onset of migration, but simply do not respond to these cues because of selection against migration out of areas suitable for year-round breeding. One possible target of selection that could inhibit migration is the set of navigational mechanisms that aid in directional orientation (Merlin, Gegear & Reppert, 2009; Guerra, Gegear & Reppert, 2014).
Directional orientation in monarchs involves a time-compensated sun compass, which integrates information from visible and polarized wavelengths with an internal clock to track the changing position of the sun over the course of the day. This clock is expressed in the monarch’s antennae (Froy et al., 2003; Reppert, Zhu & White, 2004; Merlin et al., 2009; Guerra et al., 2012). Populations of reproductive summer butterflies in North America express antennal clocks but do not show the directional characteristics of migration (Zhu et al., 2009), although no studies to date have tested antennal clock gene expression in fully resident monarchs. Thus, the shift from migratory to resident status might be related to altered expression of antennal circadian clock genes and disruption of orientation capabilities. Possible patterns of antennal clock gene expression in residents might include: (1) loss or alteration of clock gene expression/function owing to relaxed selection associated with loss of migration; (2) retention of antennal clock gene function owing to insufficient time for loss of function; (3) retention of clock gene function for use in navigation unrelated to long-distance migration; and (4) retention of clock gene function for uses unrelated to navigation.
In this paper, we evaluate two possible explanations (absence of environmental cues and altered antennal clock gene expression) for the shift to resident status in Pacific populations of monarchs (Fig. 1). In the first experiment, we evaluated the loss of cues hypothesis by rearing resident monarchs from Queensland, Australia under either constant or decreasing photoperiod treatments and assessing reproductive development in the adults that emerged. In the second experiment, we evaluated an element of the loss of mechanism hypothesis by comparing diurnal circadian clock gene expression in resident monarchs from the island of Guam with migrants from California, USA. Australian resident monarchs do indeed show reduced reproductive development in response to decreasing photoperiod, consistent with previous studies in migratory monarchs and with the loss of cues hypothesis. We found no differences in antennal clock gene expression between migrants and residents, inconsistent with the loss of mechanism hypothesis and suggesting either retention for use in functions besides migration or insufficient time for loss of function. Together, these data indicate that even after hundreds of generations of resident status, monarchs retain (1) developmental plasticity associated with decreasing photoperiod and (2) a key component of the navigational apparatus necessary for long-distance migration.
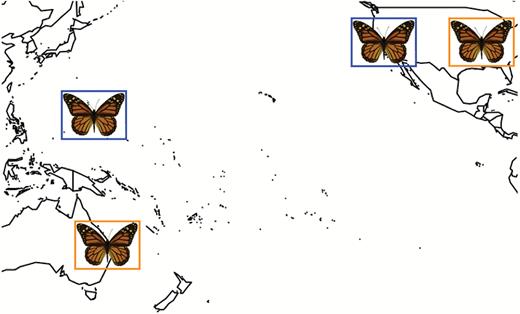
Map of locations of monarch populations described in this study. Orange locations reflect the locations of monarch populations used for assessing diapause responses by Goehring and Oberhauser (2002) (Minnesota, USA) and the present study (Queensland, Australia). Blue locations were used for comparison of antennal circadian clock gene expression between California migrants and Guam residents.
MATERIAL AND METHODS
Reproductive diapause experiment
In our first experiment, we sought to determine whether resident butterflies would respond to an environmental cue associated with induction of reproductive diapause in migratory monarchs. We collected 11 female butterflies from two populations (Pinjarra Hills: 27°32′26.7″S, 152°54′22.7″E; Mount Crosby: 27°31′45.2″S, 152°47′46.2″E) of resident, winter-breeding monarchs in Queensland, Australia between 24 and 28 June 2016. Females were all reproductively active upon collection, and all life-history stages were present on host plants at the time of collection. This continuous breeding is consistent with previous observations from Queensland (Zalucki & Kitching, 1982a), where temperatures rarely fall below developmental zero for monarchs (Zalucki, 1982). Average temperatures at the sites of collection are typically 21 °C: 8 °C in late June, with day lengths of ~11 h (Australian Bureau of Meteorology). These 11 females were enclosed in mesh bags on host plants, and the resulting eggs were used for rearing experiments. All females (and 118 of a total of 122 butterflies collected in Queensland during June and July) used in this experiment were infected with the protozoan parasite Ophryocystis elektroscirrha (hereafter, OE), consistent with high OE infection rates in other continuously breeding populations (Satterfield, Maerz & Altizer, 2015; Satterfield et al., 2016). We examined eggs under ×40 magnification and removed visible OE spores with a paintbrush. Eggs from 11 female lines were used for rearing experiments, and eggs from each line were split evenly between treatments.
All larvae used in the experiment hatched within 24 h of one another and were immediately separated into two Percival growth chambers (GR36-L; Percival Scientific, Inc., Perry, IA, USA) that each included four Phillips F32T8/TL741 32 W fluorescent lights averaging 2470 lumens per light. We chose to focus exclusively on changes in photoperiod as our diapause-inducing cue, because this cue is the most consistent and easily manipulated elicitor of diapause described in the experiments of Goehring and Oberhauser (2002). One growth chamber featured a constant 12 h–12 h light–dark (LD) cycle, with a temperature of 28 °C during the light period and 18 °C during the dark period (constant photoperiod treatment). The other growth chamber featured an LD cycle that started at 14 h–10 h, and decreased by 4 min per day until it reached 12 h–12 h 30 days later (decreasing photoperiod treatment). Temperatures and rate of decreasing photoperiod reflect late August conditions at the northernmost extent of the monarch’s North American range. The decreasing photoperiod growth chamber used a temperature ramp that peaked at 28 °C during the light-to-dark transition and was at its minimum of 18 °C during the dark-to-light transition. The temperature ramp in the decreasing photoperiod treatment ensured that larvae in both growth chambers experienced the same number of developmental degree days in each 24 h window. Degree day calculations are based on Zalucki (1982), which describes a developmental threshold of ~12 °C for all larval instars. Both growth chambers were maintained at 70% humidity. Although our treatments conflate the influence of decreasing photoperiod and total photoperiod (the decreasing photoperiod treatment necessarily featured 43 additional cumulative hours of light), Goehring and Oberhauser (2002) manipulated both factors and suggest that absolute photoperiod is unlikely to be the salient feature controlling diapause.
Larvae were kept in Petri dishes (100 mm × 15 mm) within their respective growth chambers until the second instar. They were then separated into individual 500 mL clear plastic containers with clear plastic lids and fed clipped leaves from the milkweed Gomphocarpus fruticosis (Apocynaceae: Asclepiadoideae) collected in the field. All leaves were washed with a 2% hypochlorite bleach solution and thoroughly rinsed with tap water to kill OE spores. Containers were cleaned and new leaves added every 2–3 days. Individuals pupated in the same containers in which they were reared, and their dates of pupation and eclosion were recorded.
Upon emergence, adults without fully developed wings (N = 12) were discarded, and all other butterflies were placed into glassine envelopes (N = 170 remaining adults). Discarded butterflies came evenly from both larval rearing treatments (N = 7 from decreasing photoperiod treatment, N = 5 from constant photoperiod treatment), and so any subtle selection effects associated with OE infection should be minimal. These adults were further split into two temperature treatments to determine whether conditions experienced immediately post-eclosion influenced reproductive development, according to the results of James (1983). Both treatments included 12 h–12 h LD cycles and 70% relative humidity. One treatment (warm adult treatment) included a 28 °C light phase and an 18 °C dark phase. The other treatment (cool adult treatment) had a 21 °C light phase and a 15 °C dark phase. Adult butterflies in each treatment were fed daily with a 20% honey water solution and were raised until they had accumulated 70 degree days above the reported adult reproductive development threshold of 12 °C, consistent with the findings that females develop mature oocytes after 6 days of summer conditions (Zalucki, 1981; Oberhauser & Hampton, 1995). This entailed 7 days of development for adults in the warm treatment and 11 days of development for adults in the cool treatment. Developmental zero for adults is based on the estimate of 12 °C for Australian monarchs provided by Zalucki (1981). Adults were stored in envelopes whose labels did not indicate their larval rearing treatment in order to minimize potential observer bias (Kardish et al., 2015).
After accumulating 70 degree days, butterflies were dissected and assessed for reproductive development. Female dissections were carried out as described by Oberhauser and Hampton (1995). Oocytes were counted and classified as either yolked (visible yellow coloration) or fully chorionated (ridges along length of oocyte); subsequent analyses use primarily counts of yolked oocytes because few females had fully chorionated oocytes (N = 21 of 80 females). Given that vitellogenesis does not commence until eclosion in monarchs (Pan & Wyatt, 1976), we consider yolked oocyte production an appropriate measure of adult reproductive development. We also assessed male reproductive development by measuring the wet and dry mass of the testes; however, because the signature of adult reproductive development for males is more likely to be the mass of the seminal vesicle, we do not report results for testes. All butterflies were weighed (at the time of dissection, rather than eclosion), and forewings were scanned and measured using the image processing software ImageJ (Schneider, Rasband & Eliceiri, 2012) to assess wing size and shape as described by Altizer and Davis (2010). Finally, adults were assayed for the presence and intensity of OE infection by approximating spore counts on slide mounts and assigning a score based on a relative scale from zero to five that corresponds to log10-transformed spore loads (i.e. a score of zero indicates no infection, and a score of five indicates > 10000 spores per individual; scale adopted from Altizer, Oberhauser & Brower, 2000).
In this study, we refer to the absence of yolked oocytes as reproductive diapause and treat the number of yolked oocytes produced by females as a continuous measure of reproductive development. Reproductive diapause in monarchs is typified by reduced investment in reproductive structures, reallocation of resources into migration-associated physiology, and a pronounced refractory period. Although other authors distinguish between diapause and temporary reproductive dormancy/oligopause (James, 1982; Pocius, 2014), we consider this distinction to be largely semantic and reflective of a continuum of reproductive responses. Given that adult butterflies were exposed to prolonged periods with conditions suitable for reproductive development (7–11 days with temperatures between 15 and 28 °C), we consider the absence of any yolk deposition in these butterflies to indicate a refractory period consistent with diapause.
Data were analysed using linear and generalized linear models in R version 3.4.1 (R Core Development Team, 2017). Briefly, models included the effects of larval treatment (constant vs. decreasing photoperiod) and its interaction with adult treatment (warm vs. cool) and female line, with OE infection status and butterfly sex as covariates. Response variables included whether females were in reproductive diapause (presence or absence of yolked oocytes), number of yolked oocytes, number of mature oocytes, time to eclosion, adult mass and adult forewing area. Models were initially tested with all possible covariates and interactions between larval treatment, adult treatment, female line and sex (when appropriate), and then model comparisons based on Akaike information criterion (AIC) scores were used to determine whether the inclusion of interaction terms was necessary. For the model that used presence or absence of yolked oocytes as a response variable, we used a binomial generalized linear model (GLM) with a logit link function. For the model that used mature oocytes as a response variable, we used a zero-inflated Poisson GLM with a logit link function implemented in the pscl package (Zeileis, Kleiber & Jackman, 2008) because of the high proportion of zeros in our count data; for this model, only larval photoperiod and adult temperature were included as predictors to enable model convergence. Summary statistics were generated using type II ANOVA implemented in the car package (Fox & Weisberg, 2016). For a summary of all models evaluated, see Table 1.
Summary of ANOVAs for each of the response variables tested
| 1. Response: female diapause status (1/0) . | ||||
|---|---|---|---|---|
| Predictor . | Estimate . | SE . | z . | P-value . |
| Larval photoperiod (decreasing) | 0.848 | 0.481 | 2.41 | 0.016* |
| Adult temperature (warm) | 0.423 | 0.352 | 1.34 | 0.18 |
| 2. Response: female reproductive development (yolked oocytes) | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 4558.9 | 7.97 | 0.007** |
| Adult temperature | 1 | 832.8 | 1.46 | 0.233 |
| Female line | 10 | 11573.4 | 2.02 | 0.048* |
| Ophryocystis elektroscirrha status | 1 | 0.4 | 0 | 0.980 |
| Larval photoperiod*adult temperature | 1 | 7110.2 | 12.43 | < 0.001*** |
| Larval photoperiod*female line | 8 | 9936.3 | 2.17 | 0.044** |
| Residuals | 55 | 31466.7 | ||
| 3. Response: female reproductive development (mature oocytes) | ||||
| Predictor | Estimate | SE | z | P-value |
| Larval photoperiod (decreasing) | 1.087 | 0.559 | 1.95 | 0.052 |
| Adult temperature (warm) | −0.709 | 0.585 | −1.29 | 0.196 |
| 4. Response: development time | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 309.7 | 299.49 | < 0.001*** |
| Sex | 1 | 3.7 | 3.56 | 0.062 |
| Female line | 10 | 45.5 | 4.40 | < 0.001*** |
| O. elektroscirrha status | 1 | 1.0 | 0.99 | 0.321 |
| Residuals | 130 | 134.4 | ||
| 5. Response: body mass | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 71236 | 31.02 | < 0.001*** |
| Adult temperature | 1 | 103369 | 45.02 | < 0.001*** |
| Sex | 1 | 36928 | 16.08 | < 0.001*** |
| Female line | 10 | 42411 | 1.85 | 0.059 |
| O. elektroscirrha status | 1 | 0 | 0 | 0.988 |
| Larval photoperiod*adult temperature | 1 | 45041 | 19.62 | < 0.001*** |
| Larval photoperiod*sex | 1 | 9900 | 4.31 | 0.040* |
| Adult temperature*sex | 1 | 13605 | 5.93 | 0.016* |
| Larval photoperiod*adult temperature*sex | 1 | 6564 | 2.86 | 0.093 |
| Residuals | 124 | 284727 | ||
| 6. Response: wing area | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 0.62 | 2.92 | 0.090 |
| Sex | 1 | 4.31 | 20.22 | < 0.001*** |
| Female line | 10 | 6.41 | 3.01 | 0.002** |
| O. elektroscirrha status | 1 | 0.00 | 0.01 | 0.910 |
| Residuals | 129 | 27.52 | ||
| 1. Response: female diapause status (1/0) . | ||||
|---|---|---|---|---|
| Predictor . | Estimate . | SE . | z . | P-value . |
| Larval photoperiod (decreasing) | 0.848 | 0.481 | 2.41 | 0.016* |
| Adult temperature (warm) | 0.423 | 0.352 | 1.34 | 0.18 |
| 2. Response: female reproductive development (yolked oocytes) | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 4558.9 | 7.97 | 0.007** |
| Adult temperature | 1 | 832.8 | 1.46 | 0.233 |
| Female line | 10 | 11573.4 | 2.02 | 0.048* |
| Ophryocystis elektroscirrha status | 1 | 0.4 | 0 | 0.980 |
| Larval photoperiod*adult temperature | 1 | 7110.2 | 12.43 | < 0.001*** |
| Larval photoperiod*female line | 8 | 9936.3 | 2.17 | 0.044** |
| Residuals | 55 | 31466.7 | ||
| 3. Response: female reproductive development (mature oocytes) | ||||
| Predictor | Estimate | SE | z | P-value |
| Larval photoperiod (decreasing) | 1.087 | 0.559 | 1.95 | 0.052 |
| Adult temperature (warm) | −0.709 | 0.585 | −1.29 | 0.196 |
| 4. Response: development time | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 309.7 | 299.49 | < 0.001*** |
| Sex | 1 | 3.7 | 3.56 | 0.062 |
| Female line | 10 | 45.5 | 4.40 | < 0.001*** |
| O. elektroscirrha status | 1 | 1.0 | 0.99 | 0.321 |
| Residuals | 130 | 134.4 | ||
| 5. Response: body mass | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 71236 | 31.02 | < 0.001*** |
| Adult temperature | 1 | 103369 | 45.02 | < 0.001*** |
| Sex | 1 | 36928 | 16.08 | < 0.001*** |
| Female line | 10 | 42411 | 1.85 | 0.059 |
| O. elektroscirrha status | 1 | 0 | 0 | 0.988 |
| Larval photoperiod*adult temperature | 1 | 45041 | 19.62 | < 0.001*** |
| Larval photoperiod*sex | 1 | 9900 | 4.31 | 0.040* |
| Adult temperature*sex | 1 | 13605 | 5.93 | 0.016* |
| Larval photoperiod*adult temperature*sex | 1 | 6564 | 2.86 | 0.093 |
| Residuals | 124 | 284727 | ||
| 6. Response: wing area | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 0.62 | 2.92 | 0.090 |
| Sex | 1 | 4.31 | 20.22 | < 0.001*** |
| Female line | 10 | 6.41 | 3.01 | 0.002** |
| O. elektroscirrha status | 1 | 0.00 | 0.01 | 0.910 |
| Residuals | 129 | 27.52 | ||
For response variables 1 and 3, estimates are based on comparison with reference states (constant larval photoperiod and cool adult treatment). All predictors with P < 0.1 are shown in bold, with asterisks denoting levels of significance (*P < 0.05, **P < 0.01, ***P < 0.001).
Summary of ANOVAs for each of the response variables tested
| 1. Response: female diapause status (1/0) . | ||||
|---|---|---|---|---|
| Predictor . | Estimate . | SE . | z . | P-value . |
| Larval photoperiod (decreasing) | 0.848 | 0.481 | 2.41 | 0.016* |
| Adult temperature (warm) | 0.423 | 0.352 | 1.34 | 0.18 |
| 2. Response: female reproductive development (yolked oocytes) | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 4558.9 | 7.97 | 0.007** |
| Adult temperature | 1 | 832.8 | 1.46 | 0.233 |
| Female line | 10 | 11573.4 | 2.02 | 0.048* |
| Ophryocystis elektroscirrha status | 1 | 0.4 | 0 | 0.980 |
| Larval photoperiod*adult temperature | 1 | 7110.2 | 12.43 | < 0.001*** |
| Larval photoperiod*female line | 8 | 9936.3 | 2.17 | 0.044** |
| Residuals | 55 | 31466.7 | ||
| 3. Response: female reproductive development (mature oocytes) | ||||
| Predictor | Estimate | SE | z | P-value |
| Larval photoperiod (decreasing) | 1.087 | 0.559 | 1.95 | 0.052 |
| Adult temperature (warm) | −0.709 | 0.585 | −1.29 | 0.196 |
| 4. Response: development time | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 309.7 | 299.49 | < 0.001*** |
| Sex | 1 | 3.7 | 3.56 | 0.062 |
| Female line | 10 | 45.5 | 4.40 | < 0.001*** |
| O. elektroscirrha status | 1 | 1.0 | 0.99 | 0.321 |
| Residuals | 130 | 134.4 | ||
| 5. Response: body mass | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 71236 | 31.02 | < 0.001*** |
| Adult temperature | 1 | 103369 | 45.02 | < 0.001*** |
| Sex | 1 | 36928 | 16.08 | < 0.001*** |
| Female line | 10 | 42411 | 1.85 | 0.059 |
| O. elektroscirrha status | 1 | 0 | 0 | 0.988 |
| Larval photoperiod*adult temperature | 1 | 45041 | 19.62 | < 0.001*** |
| Larval photoperiod*sex | 1 | 9900 | 4.31 | 0.040* |
| Adult temperature*sex | 1 | 13605 | 5.93 | 0.016* |
| Larval photoperiod*adult temperature*sex | 1 | 6564 | 2.86 | 0.093 |
| Residuals | 124 | 284727 | ||
| 6. Response: wing area | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 0.62 | 2.92 | 0.090 |
| Sex | 1 | 4.31 | 20.22 | < 0.001*** |
| Female line | 10 | 6.41 | 3.01 | 0.002** |
| O. elektroscirrha status | 1 | 0.00 | 0.01 | 0.910 |
| Residuals | 129 | 27.52 | ||
| 1. Response: female diapause status (1/0) . | ||||
|---|---|---|---|---|
| Predictor . | Estimate . | SE . | z . | P-value . |
| Larval photoperiod (decreasing) | 0.848 | 0.481 | 2.41 | 0.016* |
| Adult temperature (warm) | 0.423 | 0.352 | 1.34 | 0.18 |
| 2. Response: female reproductive development (yolked oocytes) | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 4558.9 | 7.97 | 0.007** |
| Adult temperature | 1 | 832.8 | 1.46 | 0.233 |
| Female line | 10 | 11573.4 | 2.02 | 0.048* |
| Ophryocystis elektroscirrha status | 1 | 0.4 | 0 | 0.980 |
| Larval photoperiod*adult temperature | 1 | 7110.2 | 12.43 | < 0.001*** |
| Larval photoperiod*female line | 8 | 9936.3 | 2.17 | 0.044** |
| Residuals | 55 | 31466.7 | ||
| 3. Response: female reproductive development (mature oocytes) | ||||
| Predictor | Estimate | SE | z | P-value |
| Larval photoperiod (decreasing) | 1.087 | 0.559 | 1.95 | 0.052 |
| Adult temperature (warm) | −0.709 | 0.585 | −1.29 | 0.196 |
| 4. Response: development time | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 309.7 | 299.49 | < 0.001*** |
| Sex | 1 | 3.7 | 3.56 | 0.062 |
| Female line | 10 | 45.5 | 4.40 | < 0.001*** |
| O. elektroscirrha status | 1 | 1.0 | 0.99 | 0.321 |
| Residuals | 130 | 134.4 | ||
| 5. Response: body mass | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 71236 | 31.02 | < 0.001*** |
| Adult temperature | 1 | 103369 | 45.02 | < 0.001*** |
| Sex | 1 | 36928 | 16.08 | < 0.001*** |
| Female line | 10 | 42411 | 1.85 | 0.059 |
| O. elektroscirrha status | 1 | 0 | 0 | 0.988 |
| Larval photoperiod*adult temperature | 1 | 45041 | 19.62 | < 0.001*** |
| Larval photoperiod*sex | 1 | 9900 | 4.31 | 0.040* |
| Adult temperature*sex | 1 | 13605 | 5.93 | 0.016* |
| Larval photoperiod*adult temperature*sex | 1 | 6564 | 2.86 | 0.093 |
| Residuals | 124 | 284727 | ||
| 6. Response: wing area | ||||
| Predictor | d.f. | Sum of squares | F | P-value |
| Larval photoperiod | 1 | 0.62 | 2.92 | 0.090 |
| Sex | 1 | 4.31 | 20.22 | < 0.001*** |
| Female line | 10 | 6.41 | 3.01 | 0.002** |
| O. elektroscirrha status | 1 | 0.00 | 0.01 | 0.910 |
| Residuals | 129 | 27.52 | ||
For response variables 1 and 3, estimates are based on comparison with reference states (constant larval photoperiod and cool adult treatment). All predictors with P < 0.1 are shown in bold, with asterisks denoting levels of significance (*P < 0.05, **P < 0.01, ***P < 0.001).
Circadian clock gene expression experiment
To determine whether resident and migratory monarchs possess functional antennal circadian clocks, we measured expression of key clock genes in residents from Guam and compared expression levels with migrants from California. Butterflies captured on Guam (N = 12 females) were returned to laboratories in Davis, CA, USA in July 2015. Their offspring were reared in growth chambers in conditions similar to the July Guam environment (LD 14 h–10 h, 28 and 27.5 °C) and within 5–8 days of adult eclosion were processed for detection of diurnal differences in clock gene expression in antennae and the head. Using reverse transcription of total RNA to cDNA followed by quantitative real-time polymerase chain reaction (qPCR), we analysed per, tim and cry2 steady-state mRNA levels as a function of two circadian time points (zeitgeber times), ZT5 (day) and ZT14 (night). These times were chosen because in migratory monarchs the circadian expression of these genes was at or near low points 5–6 h after light onset (ZT5–6) and at or near high points 2–3 h after the onset of darkness (ZT14–15; Merlin et al., 2009). Identical analyses were performed on the offspring of California migrants reared in the same conditions.
At ZT5 and ZT14, butterflies were killed and immediately frozen on dry ice. The antennae and heads were separated from the bodies and stored at −80 °C until RNA extraction. The antennae were homogenized as follows: two stainless-steel 5 mm beads (Qiagen, Valencia, CA, USA) were placed in a round-bottomed tube containing three or four pairs of antennae per pooled sample and 1 mL of TRI-reagent (Sigma, St Louis, MO, USA). The sample was shaken three times at 50 Hz for 45 s using a TissueLyser (Qiagen). The heads from the same individuals were frozen in liquid nitrogen and manually ground using a mortar and pestle. Three times TRI-reagent was added to the homogenized head tissue, and total RNA extraction was performed as described by Hamby et al. (2013). Extracted RNA was treated with a Turbo DNA-free kit (Life Technologies, Grand Island, NY, USA).
The RNA was quantified and its quality assessed using the Experion Bioanalyzer (Bio-Rad, Hercules, CA, USA). Total RNA (1.5 µg) was used to synthesize cDNA using the Thermoscript RT-PCR System (Life Technologies). Dilutions (1:2) of cDNA samples were used in qPCR. Gene-specific primers were designed to amplify monarch period (per), cryptochrome2 (cry2) and timeless (tim) with amplicon size of ~150 bp and optimized at an annealing temperature of 62 °C. Internal control primers to amplify rpl32 were optimized at the same annealing temperature for relative quantification. Primer sequences are provided in the Supporting Information (Table S1). The qPCR reactions were performed using SsoAdvanced SYBR Green Supermix (Bio-Rad) in a CFX 96 Touch Real-Time PCR Detection thermal cycler (Bio-Rad). The cycling parameters were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and an extension step at 62 °C for 30 s. The reaction was proceeded with a melt curve analysis ranging from 65 to 95 °C, with temperature increases of 0.5 °C every 5 s. Data were analysed as outlined by Hamby et al. (2013) using the ΔΔCt method. At least three biological replicates were performed for each combination of population and ZT, with each biological replicate consisting of three technical replicates for qPCR. We analysed data in R using ANOVA that included expression levels nested within technical replicate, with antennae and heads evaluated separately. Here, an effect of ZT time implies differences in expression levels between ZT5 and ZT14, and an interaction between [population*ZT time] implies differential diurnal expression patterns between populations.
RESULTS
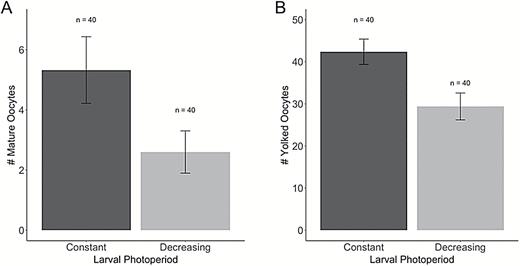
Female butterflies reared in the decreasing photoperiod treatment were significantly more likely to exhibit reproductive diapause (z = 2.41, P = 0.016) and produced significantly fewer yolked oocytes (F1,55 = 7.97, P = 0.007) and marginally fewer mature oocytes (z = 1.95, P = 0.052) than females reared in a constant photoperiod treatment (Table 1 and Fig. 2). Of the 16 females that produced no yolked oocytes, 12 of 40 were from the decreasing photoperiod treatment, compared with 4 of 40 from the constant photoperiod treatment. Among the 64 females with reproductive development, yolked oocyte production was significantly higher in the constant (42.4 ± 4.2) compared with the decreasing photoperiod treatment (29.4 ± 3.2; Fig. 2); the same pattern was observed for mature oocytes, with more produced by females from the constant (5.3 ± 1.5) compared with the decreasing photoperiod treatment (2.6 ± 1.1; Fig. 2). The decreasing photoperiod treatment was associated with a significantly longer development period (323.3 ± 11.1 degree days from egg to eclosion) compared with the constant photoperiod treatment (289.1 ± 13.9 degree days; F1,130 = 304.12, P < 0.001; Table 1). The resultant butterflies from the decreasing photoperiod treatment had significantly higher body masses (F1,124 = 31.02, P < 0.001; Table 1 and Fig. 3A) and marginally larger forewings (F1,129 = 2.92, P = 0.090; Table 1 and Fig. 3) than those reared under constant photoperiod.
Females reared under decreasing photoperiod conditions (LD 14:10 > LD 12:12) produced marginally fewer mature (A) and signficantly fewer yolked oocytes (B) when compared with females reared under constant photoperiod conditions (LD 12:12). Error bars represent ±1 mean standard error.
Larvae reared under decreasing photoperiod conditions [light–dark (LD) 14 h–10 h > LD 12 h–12 h] had significantly higher body mass as adults (A) and marginally larger forewings (B) than larvae reared under constant photoperiod (LD 12 h–12 h).
Conditions experienced post-eclosion did not significantly affect the reproductive development of females, and reproductive development was, in fact, slightly greater in the cool adult treatment (F1,55 = 1.46, P = 0.233). Larval and adult treatments interacted significantly to predict female reproductive development (F1,55 = 12.43, P < 0.001), with highest yolked oocyte production in the treatment that combined constant larval photoperiod and cool adult temperature. Post-eclosion conditions significantly affected the body mass of adults, with adults that experienced warm conditions weighing significantly less than those in the cool temperature treatment (F1,124 = 45.02, P < 0.001). Approximately half of the assayed butterflies (86 of 170) were infected with OE, although OE infection status did not significantly impact reproductive development, development time, body mass or wing morphology in adult butterflies (Table 1; Supporting Information, Fig. S1).
We found significant family-level effects for female reproductive development (F1,55 = 2.02, P = 0.048), development time (F1,130 = 4.40, P < 0.001) and forewing area (F1,129 = 6.41, P = 0.002; Table 1). Maternal lines differed in their response to decreasing photoperiod, as indicated by a significant interaction effect between maternal line and larval treatment (F1,55 = 2.17, P = 0.044; Supporting Information, Fig. S2). Maternal line was only a marginal predictor for adult body mass (F1,124 = 1.85, P = 0.059; Table 1).
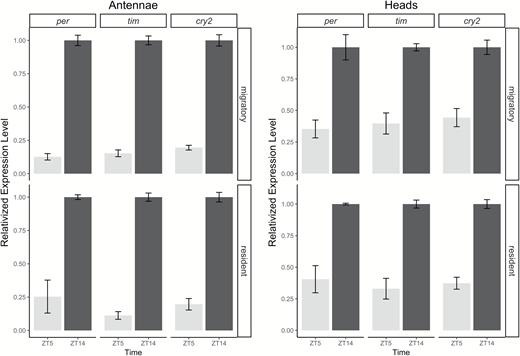
Migrants from California and residents from Guam displayed identical diurnal patterns of clock gene expression in both heads and antennae (Fig. 4). Clock gene expression in heads was significantly greater at ZT14 than at ZT5 (F1,96 = 76.28, P < 0.001), but there were no expression differences between resident and migratory populations (F1,96 = 0.31, P = 0.58). Likewise, antennal clock gene expression was significantly greater at ZT14 than at ZT5 (F1,96 = 122.76, P < 0.001), but this effect did not differ based on the source population (F1,96 = 0.02, P = 0.90).
Expression analysis of clock genes in antennae (left panel) and heads (right panel) of migratory (California) and non-migratory (Guam) monarch butterflies. Messenger RNA expression levels of per, tim and cry2 were assayed in heads and antennae of migratory (top row) and non-migratory (bottom row) butterflies collected at zeitgeber time ZT5 (light bars) and ZT14 (dark bars). Steady-state mRNA levels were normalized to non-cycling rpl32 levels and expressed as a fraction of peak expression level (peak = 1). Each biological replicate consists of pooled antennae from three or four individuals of the same sex, and at least three biological replicates were performed for the combination of population and ZT. Sexes were combined because they did not differ. Error bars represent ±1 SEM.
DISCUSSION
In this paper, we show that resident Australian monarchs exhibit reduced reproductive development when exposed to environmental conditions known to stimulate migration in North American monarchs. Furthermore, larval exposure to decreasing photoperiod is associated with a suite of correlated responses, including a longer development period, greater adult mass and slightly larger forewings, a pattern that has not been shown in any population of monarch butterfly. These responses varied between maternal lines, suggesting heritable genetic variation for diapause responses. Finally, resident butterflies from Guam exhibit identical patterns of antennal circadian clock expression to migratory monarchs from California. This suggests that resident butterflies retain a necessary but not sufficient component of their time-compensated sun compass. We discuss possible functions of this sun compass in resident monarchs.
We found that resident Australian monarchs respond to a decreasing photoperiod treatment during larval development, in accordance with the loss of cues hypothesis for non-migration. The fact that female monarchs reared under decreasing photoperiod were both more likely to show no reproductive development (i.e. no yolked oocytes), and that females in this treatment produced significantly fewer yolked oocytes, provides strong evidence that monarch butterflies, regardless of source population and migratory status, respond to photoperiod cues during larval development. Our results from the diapause experiment are consistent with Goehring and Oberhauser (2002) in showing that decreasing photoperiod elicits reduced reproductive development. Observing this same result in a non-migratory population suggests that plastic responses to seasonal changes are a feature of all monarch populations and that the transition to residency may not be irreversible. These results are consistent with the deep evolutionary origins of migration within Danaine butterflies. Migration is thought to be the ancestral condition for monarchs and is likely to be rooted in genetic variation that has been maintained for millions of years (Zhan et al., 2014). Thus, even after hundreds or thousands of generations of non-migration, ancestral variation associated with migration may be maintained and expressed upon exposure to relevant conditions.
The finding that Australian females respond to decreasing photoperiod during larval development is in contrast to the findings of James (1983), who suggested that conditions experienced immediately post-eclosion and not during larval development influence reproductive status in Australian monarchs. However, James (1983) did not formally evaluate the influence of larval rearing conditions and instead made this assertion based on observations of overwintering cluster formation and reproductive development. We also note that the conditions experienced by adults in our experiment did not significantly affect reproductive development. This may be attributable to the relatively high temperatures (light 21 °C and dark 15 °C) used in the cool adult treatment, which is warmer than the conditions evaluated by James (1983) and consistent with winter temperatures in Queensland, where monarchs breed year round.
Monarchs reared under decreasing photoperiod had significantly higher body mass and somewhat larger forewing area than monarchs reared under constant photoperiod. Although we did not specifically measure lipid content in adult butterflies, higher lean body mass is generally consistent with greater lipid reserves, a characteristic reported for migratory monarchs and monarchs in reproductive diapause (Alonso-Mejia et al., 1997; Brower et al., 2006). Previous studies have not shown any link between larval rearing conditions and monarch wing morphology, but larger forewings are thought to be conducive to soaring/gliding and the long-distance movements associated with migration (Doccx, 2007; Altizer & Davis, 2010; Yang et al., 2016). Wing area scaled isometrically with body size and independently of larval photoperiod (Supporting Information, Fig. S3). Plasticity in monarch wing morphology as a function of larval rearing conditions should be investigated further, as this could help to explain some of the observed morphological differences between migratory and non-migratory monarch populations (e.g. Altizer & Davis, 2010).
Given that decreasing/short photoperiod and cool temperatures have been associated with induction of reproductive diapause in monarchs, it is interesting to consider why all wild-caught adult females used in this experiment were reproductively competent at the time of capture, despite the short day lengths (LD 11 h–13 h) and cool temperatures (light 21 °C and dark 8 °C) that they were experiencing. The most likely explanation is that these butterflies are themselves responsive to seasonal changes, but that the year-round availability of their milkweed host plants overrides developmental plasticity associated with seasonality. Previous work has shown that exposure to milkweed can stimulate reproductive development in female monarchs (Goehring & Oberhauser, 2004), and recent research has highlighted that milkweed availability along the monarch’s southerly migration route in eastern North America can elicit breeding in adults that were previously in diapause (Batalden & Oberhauser, 2015). Thus, even though monarchs within Queensland may develop and emerge in preparation for adverse conditions, cues associated with milkweed availability may supersede other seasonal cues. Another, less likely, explanation is that there is a threshold for decreasing photoperiod required to elicit reproductive diapause. The latitudes sampled here have relatively modest seasonal changes in photoperiod, with maximal daily day length decreasing by only 1.5 min per day, compared with the 4 min per day imposed in our experiment.
Our data from the photoperiod manipulation experiment show that maternal lines differ in the magnitude of their response to decreasing photoperiod. We found significant family-level effects for female reproductive development, development time and wing area. Perhaps most interestingly, we found a significant interaction between maternal line and larval photoperiod treatment for female reproductive development, suggesting heritable variation among family lines in the response to decreasing photoperiod. Heritable variation for diapause responses has been recorded for numerous species, including milkweed bugs (Dingle, Brown & Hegmann, 1977), ground crickets (Mousseau & Roff, 1989) and pitcher plant mosquitoes (Bradshaw & Holzapfel, 2001). As we used wild-caught females that might have mated on multiple occasions (Oberhauser, 1988), we do not provide estimates of the narrow-sense heritability of the diapause response, but this is a promising area for future study. We also note that maternal effects could influence diapause responses (Mousseau & Dingle, 1991). However, because females used for oviposition in this study were all naturally reproductively active and were experiencing similar environmental conditions at the time of collection, the contribution of maternal effects within this experiment should be similar between female lines.
A possible explanation for the maintenance of photoperiodic responses in the resident Australian populations described here is gene flow with putatively migratory populations in southern Australia. Although this might be a possibility, population genetic and historical data suggest that Australian monarchs are themselves descended from other non-migratory populations that colonized the Pacific in a stepping-stone manner (Clarke & Zalucki, 2004; Zalucki & Clarke, 2004; Pierce et al., 2014; Zhan et al., 2014). Zhan et al. (2014) sampled six Pacific island groups and found that all (including Australia) share derived haplotypes with resident populations from Central and South America, suggesting recurrent selection on ancestral variation associated with resident status. Thus, even if there is gene flow within Australia, this scenario still requires that the genetic variation underlying developmental plasticity and migratory behaviour persisted during the monarch’s dispersal across the Pacific.
Although not a primary focus of our study, we were surprised to find that infection by the protozoan parasite OE did not strongly affect the phenotype of adult butterflies in our experiment. Specifically, OE infection load was not significantly associated with adult body mass or wing size, in contrast to studies showing deleterious effects of OE infection in eastern North American monarchs (Altizer & Oberhauser, 1999; Bradley & Altizer, 2005; Altizer et al., 2015). We did find modestly stronger impacts of OE infection status for male compared with female monarchs (Supporting Information, Fig. S2), consistent with Altizer and Oberhauser (1999), although the interaction between infection status and sex was not significant. One possible explanation for the apparent lack of association between parasite infection load and adult phenotypes is the evolution of increased tolerance to OE in non-migratory populations. Whereas OE-monarch interactions are thought to be shaped by transmission–virulence trade-offs in migratory monarch populations (De Roode, Yates & Altizer, 2008), selection might instead favour the evolution of resistance or tolerance mechanisms in non-migratory populations, where monarchs feed recurrently on milkweed patches and OE infection rates are high (Satterfield, Maerz & Altizer, 2015). Such a scenario has been demonstrated in Hawaii; Hawaiian OE is more virulent than OE strains from other monarch populations, yet Hawaiian monarch hosts exhibit only modest reductions in fitness when exposed to OE (Sternberg et al., 2013). Given that we observed extremely high OE infection rates in wild-caught Australian monarchs (> 95%), we tentatively suggest that Australian populations have also evolved mechanisms of tolerance that mitigate the fitness effects of OE infection.
Our second study addressed the loss of mechanisms hypothesis and evaluated whether resident and migratory monarchs exhibit differential expression of clock genes involved in directional orientation and migration. We found that resident populations from Guam exhibited identical patterns of antennal clock gene expression to those seen in migratory California individuals. This indicates that even derived non-migratory monarch populations retain the antennal clocks necessary for directional orientation in migrants. We also found identical clock gene expression patterns between residents and migrants in heads. Although this experiment addressed only a subset of the loss of mechanism hypothesis, the results allow us to rule out the loss of antennal clock gene expression as an explanation for the cessation of migration.
There are a number of possible explanations for retention of antennal circadian clocks in resident monarchs. First, residents may still use antennal clock gene expression for navigational purposes unrelated to long-distance migration. For example, directional orientation could still be adaptive for locating widely distributed patches of milkweed host plant. Zalucki and Kitching (1982b) showed that monarchs typically fly in straight lines when found outside milkweed patches, and optimal foraging theory dictates that linear movements are adaptive for searching during between-patch movements (Zalucki, 1983; Viswanathan et al., 1999). Second, retention of antennal clock expression might be related to functions entirely unrelated to navigation. For example, antennal clocks in other insects have been shown to coordinate sensitivity of olfactory and gustatory receptors (Rund et al., 2013). Antennal clocks in monarchs might function in a similar manner and be retained in residents for regulation of receptor sensitivity related to detection of host plants or pheromones. Finally, antennal clocks might no longer serve any useful function but have not been lost owing to insufficient time for selection or drift to eliminate their expression. However, given the likely role of monarch antennal clocks in the aforementioned activities, we consider this last possibility unlikely. Again, the deep evolutionary origin of migration within monarchs may help to explain why migration-associated features, such as antennal clocks, are retained even in long-established residents (Zhan et al., 2014).
The findings that resident monarchs retain a reponse to photoperiodic cues and a crucial element of their navigational sun compass raises an interesting question: are non-migratory monarchs capable of resuming long-distance migration? Although resident populations have shorter and rounder wings than migrants (Altizer & Davis, 2010) and fixed and expression-level differences in collagen expression related to wing development (Zhan et al., 2014), these differences do not preclude the resumption of migration. One clue to this question comes from the southern parts of the monarch’s Australian range. Australian monarchs are themselves derived from non-migratory populations from other Pacific islands (Pierce et al., 2014; Zhan et al., 2014), and strong circumstantial evidence suggests that they may be directly descended from a resident population on New Caledonia (Clarke & Zalucki, 2004). Still, southern Australian monarchs exhibit seasonal migration akin to that seen in western North America, with long-distance flights of up to 380 km (James, 1983) and overwintering clusters of hundreds to thousands of butterflies in New South Wales and Victoria (Smithers, 1965; James, 1979); similar overwintering colonies have been reported in New Zealand (Wise, 1980). Further research should attempt to rear permanent resident populations in conditions conducive to diapause and migration to see if these butterflies directionally orient in flight simulators (e.g. Mouritsen & Frost, 2002).
It is interesting to consider why natural selection has not acted more strongly against migration-associated traits in resident monarchs. One hypothesis is that there has not been enough time for selection to erode these traits fully, and that populations from locations such as Ecuador, where monarchs may have become established as residents longer ago, would show more pronounced loss of migratory capabilities. Another possibility is that in transitions to resident status, monarchs may be exhibiting pre-existing developmental plasticity that is already expressed in North American migrants as an adaptation for conditions experienced during summer breeding. In this scenario, selection in resident populations would act only against genotypes associated with the strongest diapause responses that are induced by even modest seasonal changes in their introduced range. Other resident monarchs may retain pre-adaptations necessary for migration (e.g. diapause induction responses, directional orientation using antennal clocks) even after hundreds or thousands of generations of non-migration, either because these genotypes are never expressed and are therefore shielded from selection (Ghalambor et al., 2007) or because these traits have additional functions unrelated to migration.
We have shown that monarch butterflies established as permanent residents in the Pacific retain two necessary elements of their migratory repertoire: the ability to respond to diapause-inducing cues and the antennal clocks needed for directional orientation. Recent research has begun to highlight the genetic and transcriptional differences between resident and migrant monarch populations and provide hypotheses as to the origins of monarch migration (Zhan et al., 2014; Pfeiler et al., 2017). Especially in light of ongoing population declines in migratory overwintering North American monarchs (Brower et al., 2012), understanding the causes and consequences of shifts to resident status is an important part of understanding monarch butterfly biology.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Table S1. Primers for gene expression analysis.
Figure S1.Ophryocystis elektroscirrha (OE) infection status was not significantly associated with any of the measured response variables, including body mass (A) and forewing area (B). The impacts of OE infection appear to be stronger in males than in females, although the interaction between infection status and sex was not significant for either measure. OE infection status reflects log10 spore loads.
Figure S2. Maternal lines varied significantly in the strength of their response to decreasing photoperiod. Of 11 maternal lines tested, eight showed greater development under constant larval photoperiod [light–dark (LD) 12 h–12 h], one showed greater development under decreasing larval photoperiod (LD 14 h−10 h > LD 12 h–12 h), and two could not be assessed because they were tested in only one condition. Error bars represent ± 1 SEM.
Figure S3. Wing area and body mass scale isometrically (slope = 0.29 ± 0.05 g cm−2; isometry = 0.33); the slope of this relationship does not depend on larval photoperiod treatment.
ACKNOWLEDGEMENTS
M.G.F., H.D. and M.P.Z. designed and performed the reproductive diapause experiment with Australian monarchs. H.D., M.G.F., J.C.C., C.A.T. and L.H.Y. designed and performed the antennal clock experiment. M.G.F. performed all statistical analyses. We thank Ali Kerr, Dan Fagin, Haldre Rogers, Aubrey Moore and Ross Miller for assistance with monarch collections from Guam. This manuscript was greatly improved by the suggestions of four anonymous reviewers. Experiments in Australia were funded by an NSF EAPSI fellowship (OISE-1614052) and NSF Graduate Research Fellowship to M.G.F.; the study using Guam butterflies was funded by a Dickson Emeritus Grant from UC Davis to H.D. Studies performed in the laboratory of J.C.C. were funded by the NSF (IOS-1456297). Additional support was provided by an NSF CAREER grant (DEB-1253101) to L.H.Y.



![Larvae reared under decreasing photoperiod conditions [light–dark (LD) 14 h–10 h > LD 12 h–12 h] had significantly higher body mass as adults (A) and marginally larger forewings (B) than larvae reared under constant photoperiod (LD 12 h–12 h).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/biolinnean/123/2/10.1093_biolinnean_blx148/1/m_blx14803.jpeg?Expires=1716316951&Signature=KKuLAjvcW8w7UF19ZiKCFtw-usnYpP9snMsDuNpA5ynL6KYdEQbzRUsWhNzoaCf6epcare6mFTQbvaAVMrGehGpgRKzJCq9KCwPsN7CE5eWJtfTioXsDr~2OTCIpts4xOWOyE7XZp14r~jdRRl3B0bimmWOc1kd0J60TjLG73Rzm3FGnoVAGgAseGPANMHnCxlXkB2aWn~NSPSRuliO0o4o0yRAEayr1YOWt01xDadqHcmQWT3g6tECZreVE0jxf1ZQfud4cKa0vX8nLUzgZvblliPNF9YnV~8NqetYFswPxdVq35fricCyyYNQNmEwPvz3DpgM~Pqs12948tzr7rg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)