-
PDF
- Split View
-
Views
-
Cite
Cite
Ana Rodriguez-Mateos, Geoffrey Istas, Lisa Boschek, Rodrigo P Feliciano, Charlotte E Mills, Céline Boby, Sergio Gomez-Alonso, Dragan Milenkovic, Christian Heiss, Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights From Randomized Controlled Trials, Metabolomics, and Nutrigenomics, The Journals of Gerontology: Series A, Volume 74, Issue 7, July 2019, Pages 967–976, https://doi.org/10.1093/gerona/glz047
Close - Share Icon Share
Abstract
Potential health benefits of blueberries may be due to vascular effects of anthocyanins that predominantly circulate in blood as phenolic acid metabolites. We investigated which role blueberry anthocyanins and circulating metabolites play in mediating improvements in vascular function and explore potential mechanisms using metabolomics and nutrigenomics. Purified anthocyanins exerted a dose-dependent improvement of endothelial function in healthy humans, as measured by flow-mediated dilation. The effects were similar to those of wild blueberries containing similar amounts of anthocyanins, whereas control drinks containing fiber, minerals, or vitamins had no significant effect. Daily 1-month wild blueberry consumption increased flow-mediated dilation and lowered 24-hour ambulatory systolic blood pressure. Of the 63 anthocyanin plasma metabolites quantified, 14 and 21 correlated with acute and chronic flow-mediated dilation improvements, respectively. Injection of these metabolites improved flow-mediated dilation in mice. Daily wild blueberry consumption led to differential expression (>1.2-fold) of 608 genes and 3 microRNAs, with Mir-181c showing a 13-fold increase in peripheral blood mononuclear cells. Patterns of 13 metabolites were independent predictors of gene expression changes and pathway enrichment analysis revealed significantly modulated biological processes involved in cell adhesion, migration, immune response, and cell differentiation. Our results identify anthocyanin metabolites as major mediators of vascular bioactivities of blueberries and changes of cellular gene programs. Trial registration: NCT025208.
Cardiovascular aging is a dynamic process that goes along with endothelial dysfunction, intimal hyperplasia, and arterial stiffness and may lead to arteriosclerosis and atherosclerosis (1). In fact, the prevalence of coronary, peripheral, and cerebrovascular artery diseases increases with age, is in the order of 25% in individuals older than 75 years, and accounts for the majority of invalidity and mortality in older people (2). Nutritional interventions are promising approaches to slow cardiovascular aging (3,4). The protective effects of these approaches including the Mediterranean diet may be mediated by a high fruit and vegetable intake and novel food bioactives consumed along with them (3,5). A growing body of nutritional science highlights the complex mechanisms and pleiotropic pathways of cardiometabolic effects of different foods to support healthy cardiovascular aging (3). Therefore, there is a growing need to generate robust scientific evidence on the mechanistic and clinical effects of specific foods and in particular the role of bioactive compounds in them that may mediate the effects (6). To be able to understand the mechanisms of action, it is paramount to identify which bioactive compounds in fruits and vegetables are responsible for such beneficial effects and demonstrate causality using accredited end points and understand how such compounds are absorbed, distributed, metabolized, and excreted in healthy humans. To date, the most promising classes of food bioactives present in fruits and vegetables are polyphenols (7,8).
Blueberries are not only rich in polyphenols, in particular anthocyanin (ACN), but also contain other phenolic compounds in smaller amounts such as procyanidins, flavonols, and phenolic acids, as well as being a rich source of fiber, vitamins, and minerals (9). Blueberries have initially been investigated due to their potential beneficial effects on the age-dependent decline in cognitive function (10) but were recently shown to improve cardiovascular function (11). Data from the Nurses’ Health Study II demonstrated that a high intake of blueberries and strawberries and the high intake of ACN associated with it (as calculated based on food frequency questionnaires) was inversely associated with the risk of myocardial infarction (12). Although these data underscore potential real-world relevance that ACN intake with blueberries could lower cardiovascular risk, epidemiological data inherently only provide associative evidence and are further limited by the lack of biomarkers of intake. Causality between ACN intake in berries and cardiovascular benefits in healthy humans has not been established so far. Furthermore, the biological mechanisms of action are not fully understood, and this may be due to the fact that ACN are not present in circulation in relevant amounts but rather as low molecular weight phenolic acid compounds that are the result of chemical and microbial degradation (13,14). However, the role of the circulating metabolites that reach the target organs (cardiovascular system) and can, therefore, only feasibly be regarded as the molecules causing the biological effects is not defined so far (8).
The aims of this work were to investigate which role ACN and their circulating metabolites play in blueberry-related vascular benefits, evaluate potential chronic effects, and explore their mechanisms of action.
Methods
Human Studies
Four studies were conducted in healthy male volunteers (see Supplementary Figures 1–4 for study flow diagrams and study protocols). The human intervention trials (carried out between 2015 and 2017) were approved by the University of Duesseldorf Research Ethics Committee and were conducted according to the guidelines laid down in the Declaration of Helsinki. Human study 4 was registered at ClinicalTrials.gov (NCT02520830).
Human Study 1
In a double-blinded, 5-armed randomized controlled crossover study volunteers (n = 5) received the five treatments in random order on 5 different days separated by 1 week of washout: control drink, control drink with fiber, control drink plus a mix of minerals and vitamins, pure ACN (total ACN content of 160 mg, [Medox, Norway]), or a wild blueberry drink, made of 11 g of freeze-dried wild blueberry powder (Wild Blueberry Association of North America) dissolved in 500 mL low nitrate water (Table 1). The amounts of fiber, minerals, vitamins, and ACN were similar to those present in the wild blueberry drink. The control was matched for flavor and color. Flow-mediated vasodilation (FMD) was measured before (0 hour) and at 1, 2, and 6 hours post-consumption.
Composition of Interventions Used in Human Studies (ACN, anthocyanin)
| . | . | Control . | Control . | ACN . | Blueberry . |
|---|---|---|---|---|---|
| . | Control . | (+Fiber) . | (Fiber + minerals + vitamins) . | . | . |
| Dietary fiber (g) | 5 | 5 | 4.4 | ||
| Potassium (mg) | 75 | 68 | |||
| Fructose (g) | 3.5 | 3.5 | 3.5 | 0.05 | 3.6 |
| Total beta carotene (IU) | 50 | 58 | |||
| Vitamin C (mg) | 12.5 | 1.7 | |||
| Calcium (mg) | 20 | 17 | |||
| Iron (mg) | 0.63 | 0.58 | |||
| Vitamin E (IU) | 1.88 | 0.39 | |||
| Vitamin B1 (mg) | 0.18 | 0.03 | |||
| Vitamin B2 (mg) | 0.19 | 0.01 | |||
| Vitamin B6 (mg) | 0.25 | 0.02 | |||
| Phosphorus (mg) | 15.6 | 12.9 | |||
| Magnesium (mg) | 12.5 | 6.5 | |||
| Zinc (mg) | 0.63 | 0.67 | |||
| Manganese (mg) | 2.25 | 2.87 | |||
| Niacin (mg) | 2.5 | 0.61 | |||
| Anthocyanins (mg) | 160 | 150 | |||
| Flavanol monomers (mg) | 3 | ||||
| Flavanol oligomers (mg) | 49 | ||||
| Flavonols (mg) | 31 | ||||
| Chlorogenic acid (mg) | 64 |
| . | . | Control . | Control . | ACN . | Blueberry . |
|---|---|---|---|---|---|
| . | Control . | (+Fiber) . | (Fiber + minerals + vitamins) . | . | . |
| Dietary fiber (g) | 5 | 5 | 4.4 | ||
| Potassium (mg) | 75 | 68 | |||
| Fructose (g) | 3.5 | 3.5 | 3.5 | 0.05 | 3.6 |
| Total beta carotene (IU) | 50 | 58 | |||
| Vitamin C (mg) | 12.5 | 1.7 | |||
| Calcium (mg) | 20 | 17 | |||
| Iron (mg) | 0.63 | 0.58 | |||
| Vitamin E (IU) | 1.88 | 0.39 | |||
| Vitamin B1 (mg) | 0.18 | 0.03 | |||
| Vitamin B2 (mg) | 0.19 | 0.01 | |||
| Vitamin B6 (mg) | 0.25 | 0.02 | |||
| Phosphorus (mg) | 15.6 | 12.9 | |||
| Magnesium (mg) | 12.5 | 6.5 | |||
| Zinc (mg) | 0.63 | 0.67 | |||
| Manganese (mg) | 2.25 | 2.87 | |||
| Niacin (mg) | 2.5 | 0.61 | |||
| Anthocyanins (mg) | 160 | 150 | |||
| Flavanol monomers (mg) | 3 | ||||
| Flavanol oligomers (mg) | 49 | ||||
| Flavonols (mg) | 31 | ||||
| Chlorogenic acid (mg) | 64 |
Composition of Interventions Used in Human Studies (ACN, anthocyanin)
| . | . | Control . | Control . | ACN . | Blueberry . |
|---|---|---|---|---|---|
| . | Control . | (+Fiber) . | (Fiber + minerals + vitamins) . | . | . |
| Dietary fiber (g) | 5 | 5 | 4.4 | ||
| Potassium (mg) | 75 | 68 | |||
| Fructose (g) | 3.5 | 3.5 | 3.5 | 0.05 | 3.6 |
| Total beta carotene (IU) | 50 | 58 | |||
| Vitamin C (mg) | 12.5 | 1.7 | |||
| Calcium (mg) | 20 | 17 | |||
| Iron (mg) | 0.63 | 0.58 | |||
| Vitamin E (IU) | 1.88 | 0.39 | |||
| Vitamin B1 (mg) | 0.18 | 0.03 | |||
| Vitamin B2 (mg) | 0.19 | 0.01 | |||
| Vitamin B6 (mg) | 0.25 | 0.02 | |||
| Phosphorus (mg) | 15.6 | 12.9 | |||
| Magnesium (mg) | 12.5 | 6.5 | |||
| Zinc (mg) | 0.63 | 0.67 | |||
| Manganese (mg) | 2.25 | 2.87 | |||
| Niacin (mg) | 2.5 | 0.61 | |||
| Anthocyanins (mg) | 160 | 150 | |||
| Flavanol monomers (mg) | 3 | ||||
| Flavanol oligomers (mg) | 49 | ||||
| Flavonols (mg) | 31 | ||||
| Chlorogenic acid (mg) | 64 |
| . | . | Control . | Control . | ACN . | Blueberry . |
|---|---|---|---|---|---|
| . | Control . | (+Fiber) . | (Fiber + minerals + vitamins) . | . | . |
| Dietary fiber (g) | 5 | 5 | 4.4 | ||
| Potassium (mg) | 75 | 68 | |||
| Fructose (g) | 3.5 | 3.5 | 3.5 | 0.05 | 3.6 |
| Total beta carotene (IU) | 50 | 58 | |||
| Vitamin C (mg) | 12.5 | 1.7 | |||
| Calcium (mg) | 20 | 17 | |||
| Iron (mg) | 0.63 | 0.58 | |||
| Vitamin E (IU) | 1.88 | 0.39 | |||
| Vitamin B1 (mg) | 0.18 | 0.03 | |||
| Vitamin B2 (mg) | 0.19 | 0.01 | |||
| Vitamin B6 (mg) | 0.25 | 0.02 | |||
| Phosphorus (mg) | 15.6 | 12.9 | |||
| Magnesium (mg) | 12.5 | 6.5 | |||
| Zinc (mg) | 0.63 | 0.67 | |||
| Manganese (mg) | 2.25 | 2.87 | |||
| Niacin (mg) | 2.5 | 0.61 | |||
| Anthocyanins (mg) | 160 | 150 | |||
| Flavanol monomers (mg) | 3 | ||||
| Flavanol oligomers (mg) | 49 | ||||
| Flavonols (mg) | 31 | ||||
| Chlorogenic acid (mg) | 64 |
Human Study 2
To investigate the dose–response of ACN, a randomized, controlled double-blinded crossover trial was conducted (n = 10). FMD was measured at baseline before (0 hour), at 2, and 6 hours post-consumption of ACN capsules (0 [control], 80, 160, 240, 320, or 480 mg ACN) on 6 different days with 1-week washout between study days.
Human Study 3
An uncontrolled, single-armed, pilot study was performed to investigate the time course of the chronic effects of wild blueberry on FMD. Volunteers (n = 5) had 11 g wild blueberry powder, equivalent to 100 g fresh wild blueberries, and containing 150 mg of ACNs dissolved in 500 mL water bi-daily more than 28 days, and FMD was measured at baseline, days 7, 14, 21, and 28.
Human Study 4
An acute-on-chronic 2-arm, parallel, double-blind randomized controlled trial conducted (n = 40, 20 in each arm) comparing effects of wild blueberry drink (11 g wild blueberry powder, bi-daily) with matched control drinks (11 g powder, bi-daily) more than 28 days. The primary outcome was improvement in FMD. Secondary end points were pulse wave velocity and aortic augmentation index, and 24-hour ambulatory blood pressure (BP) was taken in a subpopulation (n = 22). Measurements were taken on day 1 (baseline) and after 28 bi-daily consumption at 0 and 2 hours. Blood samples were drawn at all time points to measure plasma blueberry (poly)phenols and their metabolites. Blood lipids (triglycerides, total cholesterol, high-density and low-density lipoprotein cholesterol), glucose, and routine clinical laboratory parameters were also determined. To explore potential mechanism of action, messenger RNA and microRNA (miRNA) analyses were performed on peripheral blood mononuclear cells (PBMC) isolated from blood samples that were collected after an overnight fasting period from 10 volunteers at the beginning and the end of the 28-day period of blueberry consumption.
Animal Study
To prove the bioactivity of circulating phenolic acid metabolites, a 3-armed double-blinded randomized controlled study was carried out in ten 6-week-old male C57BL/6 mice with a 7-day washout period. The interventions were plasma (poly)phenol metabolite mixes comprising metabolites that correlated with human FMD acutely, chronically (adjusted for human equivalent dose according to the Food and Drug Administration guidelines (15); Table 2B), and vehicle control (0.9% saline solution matched for methanol content at 5.8%). Mice were anesthetized with isoflurane, and FMD measurements performed as published previously (16) before and after blinded administration of 100 μL through intracardiac injection. The analyses of ultrasound images were performed by an operator blinded to allocation of treatments. The metabolites were purchased or synthesized (17), dissolved in methanol, and diluted with saline. Animal procedures were approved by the local authorities (84-02.04.2014.A312) at Düsseldorf University.
Details of Plasma Metabolism From Human (A) and Animal (B) Studies
| . | . | Correlation analysis human study (values are Pearson’s r, all ps < .05) . | . | Estimated concentration mouse plasma after IC injection (mM) . | ||
|---|---|---|---|---|---|---|
| A . | . | 2-h FMD . | 28-d FMD . | B . | 2-h Metabolites . | 28-d Metabolites . |
| Cinnamic acids | Ferulic acid | 0.51 | 0.1 | |||
| Ferulic acid-4-O-sulfate | 0.36 | 0.43 | 1.6 | 1.2 | ||
| Ferulic acid 4-O-ß-D-glucuronide | 0.44 | 2.8 | ||||
| Isoferulic acid 3-O-ß-D-glucuronide | 0.43 | 0.48 | 0.5 | 1.1 | ||
| Dihydroferulic acid | 0.54 | 0.43 | 1.0 | 1.5 | ||
| Dihydroferulic acid 4-O-ß-D-glucuronide | 0.42 | 1.1 | ||||
| Dihydroisoferulic acid 3-O-ß-D-glucuronide | 0.41 | 0.1 | ||||
| Dihydroisoferulic acid 3-O-sulfate | 0.43 | 0.6 | ||||
| Dihydrocaffeic acid 3-O-sulfate | 0.63 | 1.6 | ||||
| p-Coumaric | 0.40 | 0.1 | ||||
| Cinnamic acid | 0.42 | 0.3 | ||||
| Chlorogenic acid | 0.44 | → | 1.0 | |||
| Benzoic acids | Vanillic acid | 0.60 | 0.61 | 6.0 | 10.0 | |
| Homovanillic acid | 0.40 | 0.52 | 0.9 | 1.4 | ||
| Protocatechuic acid | 0.37 | 0.3 | ||||
| Syringic acid | 0.41 | 0.42 | 0.1 | 0.2 | ||
| 4-Hydroxybenzoic acid | 0.37 | 0.4 | ||||
| 2,4-Dihydroxybenzoic acid | 0.64 | 0.4 | ||||
| 4-Methylgallic acid-3-O-sulfate | 0.40 | 0.41 | 0.8 | 0.5 | ||
| Phenols | 4-Methylcatechol-2-O-sulfate | 0.42 | 12.7 | |||
| 1-Methylpyrogallol-O-sulfate | 0.62 | 0.50 | 0.9 | 1.8 | ||
| Hippuric acids | Hippuric acid | 0.57 | 368.9 | |||
| 3-Hydroxyhippuric acid | 0.56 | 10.5 | ||||
| Phenylacetic acids | 3-Hydroxyphenyl acetic acid | 0.46 | 3.0 | |||
| 4-Hydroxyphenyl acetic acid | 0.41 | 0.40 | 3.0 | 5.0 | ||
| Flavonols | Quercetin 3-O-ß-D-glucuronide | 0.38 | 104.5 | |||
| . | . | Correlation analysis human study (values are Pearson’s r, all ps < .05) . | . | Estimated concentration mouse plasma after IC injection (mM) . | ||
|---|---|---|---|---|---|---|
| A . | . | 2-h FMD . | 28-d FMD . | B . | 2-h Metabolites . | 28-d Metabolites . |
| Cinnamic acids | Ferulic acid | 0.51 | 0.1 | |||
| Ferulic acid-4-O-sulfate | 0.36 | 0.43 | 1.6 | 1.2 | ||
| Ferulic acid 4-O-ß-D-glucuronide | 0.44 | 2.8 | ||||
| Isoferulic acid 3-O-ß-D-glucuronide | 0.43 | 0.48 | 0.5 | 1.1 | ||
| Dihydroferulic acid | 0.54 | 0.43 | 1.0 | 1.5 | ||
| Dihydroferulic acid 4-O-ß-D-glucuronide | 0.42 | 1.1 | ||||
| Dihydroisoferulic acid 3-O-ß-D-glucuronide | 0.41 | 0.1 | ||||
| Dihydroisoferulic acid 3-O-sulfate | 0.43 | 0.6 | ||||
| Dihydrocaffeic acid 3-O-sulfate | 0.63 | 1.6 | ||||
| p-Coumaric | 0.40 | 0.1 | ||||
| Cinnamic acid | 0.42 | 0.3 | ||||
| Chlorogenic acid | 0.44 | → | 1.0 | |||
| Benzoic acids | Vanillic acid | 0.60 | 0.61 | 6.0 | 10.0 | |
| Homovanillic acid | 0.40 | 0.52 | 0.9 | 1.4 | ||
| Protocatechuic acid | 0.37 | 0.3 | ||||
| Syringic acid | 0.41 | 0.42 | 0.1 | 0.2 | ||
| 4-Hydroxybenzoic acid | 0.37 | 0.4 | ||||
| 2,4-Dihydroxybenzoic acid | 0.64 | 0.4 | ||||
| 4-Methylgallic acid-3-O-sulfate | 0.40 | 0.41 | 0.8 | 0.5 | ||
| Phenols | 4-Methylcatechol-2-O-sulfate | 0.42 | 12.7 | |||
| 1-Methylpyrogallol-O-sulfate | 0.62 | 0.50 | 0.9 | 1.8 | ||
| Hippuric acids | Hippuric acid | 0.57 | 368.9 | |||
| 3-Hydroxyhippuric acid | 0.56 | 10.5 | ||||
| Phenylacetic acids | 3-Hydroxyphenyl acetic acid | 0.46 | 3.0 | |||
| 4-Hydroxyphenyl acetic acid | 0.41 | 0.40 | 3.0 | 5.0 | ||
| Flavonols | Quercetin 3-O-ß-D-glucuronide | 0.38 | 104.5 | |||
Notes: A Univariate correlation between human plasma metabolites (Study 4) and change in flow-mediated dilation (FMD) at 2 h (2-h FMD), “acute effect”) and after 28 d (28-d FMD, “chronic effect”) of blueberry consumption in humans (values are Pearson’s r and all ps < .05) and B composition of metabolite mixes that were injected intracardially (IC) in 100 µL vehicle into mice to demonstrate bioactivity of 2 h and 28 d metabolite profiles. Values represent estimated instantaneous concentrations (µM) in mouse plasma.
Details of Plasma Metabolism From Human (A) and Animal (B) Studies
| . | . | Correlation analysis human study (values are Pearson’s r, all ps < .05) . | . | Estimated concentration mouse plasma after IC injection (mM) . | ||
|---|---|---|---|---|---|---|
| A . | . | 2-h FMD . | 28-d FMD . | B . | 2-h Metabolites . | 28-d Metabolites . |
| Cinnamic acids | Ferulic acid | 0.51 | 0.1 | |||
| Ferulic acid-4-O-sulfate | 0.36 | 0.43 | 1.6 | 1.2 | ||
| Ferulic acid 4-O-ß-D-glucuronide | 0.44 | 2.8 | ||||
| Isoferulic acid 3-O-ß-D-glucuronide | 0.43 | 0.48 | 0.5 | 1.1 | ||
| Dihydroferulic acid | 0.54 | 0.43 | 1.0 | 1.5 | ||
| Dihydroferulic acid 4-O-ß-D-glucuronide | 0.42 | 1.1 | ||||
| Dihydroisoferulic acid 3-O-ß-D-glucuronide | 0.41 | 0.1 | ||||
| Dihydroisoferulic acid 3-O-sulfate | 0.43 | 0.6 | ||||
| Dihydrocaffeic acid 3-O-sulfate | 0.63 | 1.6 | ||||
| p-Coumaric | 0.40 | 0.1 | ||||
| Cinnamic acid | 0.42 | 0.3 | ||||
| Chlorogenic acid | 0.44 | → | 1.0 | |||
| Benzoic acids | Vanillic acid | 0.60 | 0.61 | 6.0 | 10.0 | |
| Homovanillic acid | 0.40 | 0.52 | 0.9 | 1.4 | ||
| Protocatechuic acid | 0.37 | 0.3 | ||||
| Syringic acid | 0.41 | 0.42 | 0.1 | 0.2 | ||
| 4-Hydroxybenzoic acid | 0.37 | 0.4 | ||||
| 2,4-Dihydroxybenzoic acid | 0.64 | 0.4 | ||||
| 4-Methylgallic acid-3-O-sulfate | 0.40 | 0.41 | 0.8 | 0.5 | ||
| Phenols | 4-Methylcatechol-2-O-sulfate | 0.42 | 12.7 | |||
| 1-Methylpyrogallol-O-sulfate | 0.62 | 0.50 | 0.9 | 1.8 | ||
| Hippuric acids | Hippuric acid | 0.57 | 368.9 | |||
| 3-Hydroxyhippuric acid | 0.56 | 10.5 | ||||
| Phenylacetic acids | 3-Hydroxyphenyl acetic acid | 0.46 | 3.0 | |||
| 4-Hydroxyphenyl acetic acid | 0.41 | 0.40 | 3.0 | 5.0 | ||
| Flavonols | Quercetin 3-O-ß-D-glucuronide | 0.38 | 104.5 | |||
| . | . | Correlation analysis human study (values are Pearson’s r, all ps < .05) . | . | Estimated concentration mouse plasma after IC injection (mM) . | ||
|---|---|---|---|---|---|---|
| A . | . | 2-h FMD . | 28-d FMD . | B . | 2-h Metabolites . | 28-d Metabolites . |
| Cinnamic acids | Ferulic acid | 0.51 | 0.1 | |||
| Ferulic acid-4-O-sulfate | 0.36 | 0.43 | 1.6 | 1.2 | ||
| Ferulic acid 4-O-ß-D-glucuronide | 0.44 | 2.8 | ||||
| Isoferulic acid 3-O-ß-D-glucuronide | 0.43 | 0.48 | 0.5 | 1.1 | ||
| Dihydroferulic acid | 0.54 | 0.43 | 1.0 | 1.5 | ||
| Dihydroferulic acid 4-O-ß-D-glucuronide | 0.42 | 1.1 | ||||
| Dihydroisoferulic acid 3-O-ß-D-glucuronide | 0.41 | 0.1 | ||||
| Dihydroisoferulic acid 3-O-sulfate | 0.43 | 0.6 | ||||
| Dihydrocaffeic acid 3-O-sulfate | 0.63 | 1.6 | ||||
| p-Coumaric | 0.40 | 0.1 | ||||
| Cinnamic acid | 0.42 | 0.3 | ||||
| Chlorogenic acid | 0.44 | → | 1.0 | |||
| Benzoic acids | Vanillic acid | 0.60 | 0.61 | 6.0 | 10.0 | |
| Homovanillic acid | 0.40 | 0.52 | 0.9 | 1.4 | ||
| Protocatechuic acid | 0.37 | 0.3 | ||||
| Syringic acid | 0.41 | 0.42 | 0.1 | 0.2 | ||
| 4-Hydroxybenzoic acid | 0.37 | 0.4 | ||||
| 2,4-Dihydroxybenzoic acid | 0.64 | 0.4 | ||||
| 4-Methylgallic acid-3-O-sulfate | 0.40 | 0.41 | 0.8 | 0.5 | ||
| Phenols | 4-Methylcatechol-2-O-sulfate | 0.42 | 12.7 | |||
| 1-Methylpyrogallol-O-sulfate | 0.62 | 0.50 | 0.9 | 1.8 | ||
| Hippuric acids | Hippuric acid | 0.57 | 368.9 | |||
| 3-Hydroxyhippuric acid | 0.56 | 10.5 | ||||
| Phenylacetic acids | 3-Hydroxyphenyl acetic acid | 0.46 | 3.0 | |||
| 4-Hydroxyphenyl acetic acid | 0.41 | 0.40 | 3.0 | 5.0 | ||
| Flavonols | Quercetin 3-O-ß-D-glucuronide | 0.38 | 104.5 | |||
Notes: A Univariate correlation between human plasma metabolites (Study 4) and change in flow-mediated dilation (FMD) at 2 h (2-h FMD), “acute effect”) and after 28 d (28-d FMD, “chronic effect”) of blueberry consumption in humans (values are Pearson’s r and all ps < .05) and B composition of metabolite mixes that were injected intracardially (IC) in 100 µL vehicle into mice to demonstrate bioactivity of 2 h and 28 d metabolite profiles. Values represent estimated instantaneous concentrations (µM) in mouse plasma.
Vascular Measurements
All vascular measures were performed by a trained researcher in a temperature-controlled room after a period of 15 minutes rest as detailed later. FMD of the brachial artery was measured as described previously (18) using a 12-MHz transducer (Vivid I; GE Healthcare, Berlin, Germany) with automatic edge-detection software (Brachial Analyzer; Medical Imaging Applications, Iowa City, IA). A single trained operator performed the analysis of all images within a single study. Office BP (mean of second and third measurements) was taken using an automated clinical digital sphygmomanometer (DynaMap, Tampa, FL). 24-hour ambulatory BP measurements were performed on days 1 and 28, using a Tonoport V monitor (GE Healthcare). Pulse wave velocity and aortic augmentation index were measured by applanation tonometry using the SphygmoCor (SMART Medical, Gloucestershire, UK) system determined from measurements taken at the carotid and femoral artery as described previously (4).
Biochemical Analysis
The blood samples collected in ethylenediaminetetraacetic acid/heparin tubes were spun (1,700g; 15 minutes; 4°C) immediately after collection. Samples for (poly)phenol analysis were spiked with 2% formic acid. All samples were aliquoted and frozen at –80°C until analysis. Screening clinical parameters including total, high-density lipoprotein and high-density lipoprotein cholesterol, triglycerides (enzymatic photometric assay; Roche Diagnostics), HbA1c, glucose (hexokinase assay), and whole-blood count (flow cytometry; Sysmex) were measured using standard techniques by the Institute for Clinical Chemistry, University Hospital Düsseldorf, Germany.
Nutrient and (Poly)phenol Analysis of Wild Blueberry Interventions
Nutrient analysis was performed by Medallion Labs (Minneapolis, MN) using standard procedures. (Poly)phenol analysis of blueberry interventions was performed as described previously (9).
UPLC-Q-TOF MS Analysis of Plasma (Poly)phenols
Plasma and urinary analysis of (poly)phenol metabolites was performed using microelution solid phase extraction coupled with ultra-high-performance liquid chromatography quadrupole-time-of-flight mass spectrometry (UPLC-Q-TOF MS) and authentic standards for quantification as described previously (19).
Gene Expression Analyses
PBMC isolation
PBMCs were isolated from whole blood using BD Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). Blood samples were collected after an overnight fasting period from 10 volunteers at the beginning and the end of the 28-day period of consumption of 11 g of freeze-dried wild blueberry powder dissolved in 500 mL low nitrate water twice a day (Human study 4). Eight-milliliter blood collected into BD Vacutainer tubes were immediately centrifuged at room temperature in a horizontal rotor for 20 minutes at 1,500g. The cell layer was collected and washed twice with sterile phosphate-buffered saline with centrifugation at 300g for 10 minutes between each washing step. The obtained pellet of PBMCs was immediately frozen at –80°C and kept at this temperature until use.
Total RNA extraction
The PBMCs were lysed using lysing buffer solution from the RNeasy Micro Kit (Qiagen, Hilden, Germany). Total RNA extraction has been performed using RNeasy Micro Kit as recommended by the manufacturer. RNA quality and quantity were checked by 1% agarose gel electrophoresis and by the determination of the absorbencies at 260 and 280 nm on NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE). The total RNA was stored at –80°C until used.
Microarray analysis
Total RNA (50 ng per sample) for 20 RNA samples (10 from the volunteers at the beginning and 10 at the end of the 28-day study period) was amplified and fluorescently labeled to produce Cy5 or Cy3 complementary RNA using the Low Input Quick Amp Labeling two color Kit (Agilent, Santa Clara, CA) in the presence of spike-in two colors control as recommended by the manufacturer. After purification, 825 ng of labeled complementary RNA was hybridized onto G4845A Human GE 4×44K v2 Microarray (Agilent) according to the manufacturer’s instructions. The G4845A Human GE 4×44K v2 Microarray contains 27958 Entrez Gene RNAs sequences. After hybridization, microarrays were scanned with Agilent G2505 scanner (Agilent) and data were extracted with Feature Extraction software (Agilent) using linear and Lowess normalization. GeneSpring GX10 software (Agilent) was used to quantify the signal and background intensity for each feature and to substantially normalize the data by the 75th percentile method. Statistical analyses were performed using GeneSpring GX10 software to identify differentially expressed genes using Student’s t test and the probability values were adjusted false discovery rate to eliminate false positives. Genes with false discovery rate corrected p value is less than .05 and with a fold change more than 1.2 were referred to as differentially expressed genes (608). A gene list was chosen based on strict criteria to perform a multivariate analysis on plasma (poly)phenol concentrations. At first, the coefficient of variation of fold change expression was calculated between individuals where genes with less than 20% variation were designated resulting in a list of 152 genes. A fold change cutoff of at least 1.3 was chosen resulting in 20 remaining genes (Figure 3A). Next, a Pearson correlation followed by stepwise multivariate linear regression analysis of metabolite concentrations against the top 20 selected genes was performed. The analysis was executed 20 times, inserting every time one gene against the 63 metabolites.
miRNA expression analysis
The impact of blueberry consumption on the expression of miRNAs was analyzed using Human miRNA Microarray (V3) 8×15K (Agilent). miRNAs were labeled using miRNA labeling and hybridization kit from Agilent technologies as recommended by the manufacturer. Briefly, 100 ng of each total RNA sample was treated with calf intestinal phosphatase for 30 minutes at 37°C before denaturing the samples using pure dimethyl sulfoxide at 100°C for 5 minutes and rapid transfer in an ice water bath to prevent RNA reannealing. RNA samples were labeled with pCp-Cy3 using T4 RNA ligase by incubation at 16°C for 2 hours. After purification with Micro Bio-Spin columns, labeled samples were hybridized to Agilent human miRNA microarrays. Hybridizations were performed for 24 hours at 55°C after which the microarrays were washed in GE Wash Buffer 1 (Agilent) and GE Wash Buffer 2 (Agilent) for 5 minutes. Following washing step, the microarrays were scanned with Aligent Microarray Scanner. The scanned images were analyzed using Feature Extraction Software (Agilent). GeneSpring GX10 software (Agilent) was used to quantify the signal and background intensity for each feature and to substantially normalize the data by the 75th percentile method. The statistical significance was the corrected ratios of hybridization signal intensity between blueberry-exposed samples and control samples. miRNAs selected by these criteria are referred to as the “differentially expressed miRNAs.”
Biological interpretation
To extract maximum biological information of differentially expressed genes, together with gene ontology (biological processes) and gene networks, genes were also classified according to their role(s) in cellular or metabolic pathways using the Kyoto Encyclopedia of Genes and Genomes (http://www.genome.jp/kegg/pathway.html) and Metacore (https://portal.genego.com) databases. Gene network interactions were based on data mining tools where a score was given to every gene occurring in the same research abstracts. Potential transcription factors involved in the regulation of differentially expressed genes were searched using network algorithms for transcription factors development in Metacore software. Potential target genes of miRNA were identified using miRWalk database (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/).
Power Calculation and Statistical Analyses
FMD was defined as the primary outcome. On the basis of previous intervention studies with blueberries, we expected a change in FMD by 1%–2% (11). The intra- and interindividual variabilities for FMD measurements established in our laboratory are 0.9% (standard deviation of difference between repeated FMD measurements in n = 20 middle-aged healthy subjects, unpublished data) and 1% (SD within a group of healthy old subjects) (20).
Study 1–3
Assuming an SD of difference between repeated FMD measurements of 0.9%, intra-individual measurements in 5 and 10 experimental subjects would provide sufficient power to detect an absolute change in FMD of 1.5% and 0.9% (two-sided a of 5%, power = 0.80).
Study 4
Assuming an SD of change in FMD of 1%, 20 experimental and 20 control subjects would provide sufficient power to detect an absolute change in FMD of 0.9% (two-sided a of 5%, power = 0.80). Changes in FMD values were compared to control by one-way repeated measurements analysis of variance (Study 1–2) and are presented as mean values and 95% confidence intervals (CI). In Study 3, FMD values were compared to baseline by one-way repeated measurements analysis of variance. In Study 4, changes in FMD values at 2 hours, 1 month, and 1 month/2 hours relative to baseline values at day 1/0 hour were compared to control by one-way repeated measurements analysis of variance (Study 1–2) and are presented as mean values and 95% confidence intervals. Sample size calculations for the mouse study were based on our previously described method (16) to detect significant FMD changes with a power of 0.8 and error probability of 0.05. Changes in % FMD in between control and acute/chronic mixes were performed using one-way repeated measurements analysis of variance with Dunnett’s post hoc test. Correlations are presented as Pearson’s r for normal distribution and as Spearman for non-normal distribution. Analyses were performed in Prism 6 and 7 and SPSS, version 20 (IBM). See earlier sections for microarray analyses.
Results
The characteristics of the healthy volunteers (mean age 33 ± 6 years [Study 4]) are shown in Supplementary Table 1.
ACN Are Major Contributors of Endothelial Function Improvement After Blueberry Consumption
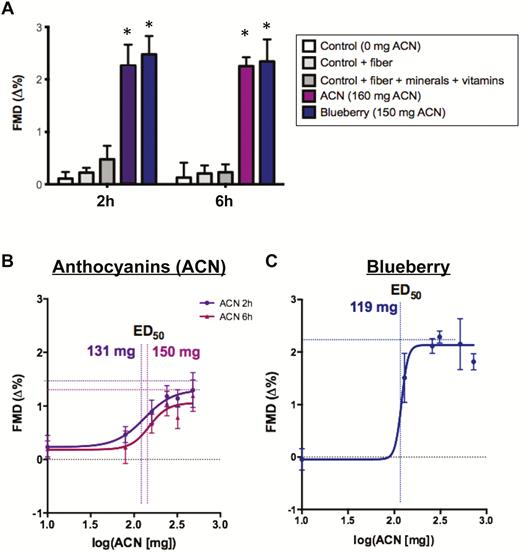
We first evaluated the potential role of ACN as major bioactives in blueberries to increase endothelial function. In a randomized crossover for controlled study (Study 1; Supplementary Figure 1), we compared the effects of fiber, minerals and vitamins, and ACN with the effects of blueberries containing the compounds in similar amounts on FMD. Our present results demonstrated that the vehicle control drink, the control with fiber, or the control with minerals and vitamins in similar amounts as present in 100 g of fresh blueberries (Table 1) had no significant effect on FMD at 2 and 6 hours post-consumption (Figure 1A). More importantly, 160 mg of pure ACN was sufficient to increase FMD in a similar magnitude as blueberries containing 150 mg ACN did.
Importance of anthocyanin (ACN) in blueberry-mediated acute improvements in vascular function. Comparison of ACN effect on flow-mediated dilation (FMD) with (A) other components of blueberry at 2 and 6 h after ingestion (see Table 1 for composition of interventions), (B) dose–response of ACN at 2 and 6 h (0 mg control arbitrarily set to 1), and (C) dose–response of blueberry at 2 h (adapted from ref. (119 *p < .05 versus 0 mg ACN control.
Pure ACN Dose-dependently Increase Endothelial Function
To further evaluate the causal role of ACN in the mediation of vascular effects of blueberries, we performed dose–response experiments with pure ACN (Study 2; Supplementary Figure 2). Our data demonstrate a dose-dependent increase in FMD at 2 and 6 hours after consumption of pure ACN (0–480 mg) (Figure 1B). The amount to achieve half-maximal FMD improvements (ED50) was 131 mg (95% CI = 59 to 290 mg) and 150 mg (95% CI = 78,290 mg) ACN at 2 and 6 hours, respectively. The top of the sigmoid curve fit indicated maximal FMD increases of 1.3% (95% CI = 0.8% to 1.9%) and 1.1% (95% CI = 0.6% to 1.5%). This was similar to our previously published dose–response study with blueberries (11). The current analysis of dose–response data from the previously published study (11) is shown in Figure 1C. It showed that the amount of ACN in blueberries to achieve half-maximal FMD improvements was 120 mg and did not significantly differ from pure ACN. The top of the sigmoid curve fit indicated a maximal FMD increases after blueberries of 2.1% (95% CI = 1.8% to 2.5%) which is significantly larger than achieved with ACN when consumed alone.
Daily Blueberry Consumption Leads to Sustained Effects on Endothelial Function and BP
We then evaluated (a) which ACN metabolites circulate in blood and may, therefore, qualify as bioactives causing the vascular functional effects and (b) whether acute effects translate into sustained effects with a potential to impact vascular health.
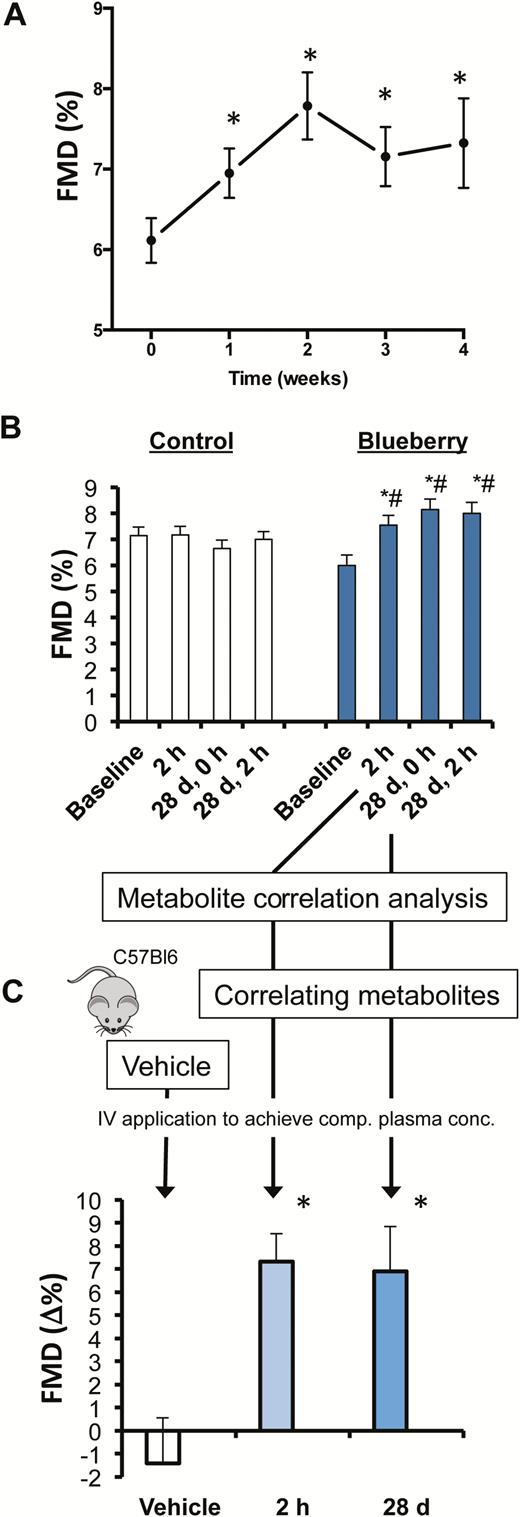
In a pilot open-label study (Study 3; Supplementary Figure 3) to evaluate the time course of chronic effects, we administered wild blueberries containing 150 mg ACN bi-daily (300 mg/day) more than 1 month and measured FMD every week in the morning after overnight fasting. FMD significantly increased already after 1 week, increased further after 2 weeks, and plateaued thereafter (Figure 2A). This suggests that at least 2 weeks of daily blueberry consumption are necessary to achieve a sustained improvement in endothelial function that persists after overnight fasting.
Circulating anthocyanin metabolites improve vascular function. (A) Pilot study to demonstrate time course of flow-mediated dilation (FMD) during daily consumption of blueberries. (B) FMD values at baseline after acute, chronic, and acute on chronic ingestion of control (white bars) and blueberry (blue bars). In these subjects, a targeted metabolomics analysis of plasma metabolites was performed. We identified metabolites that correlated with the 2 h and day 28 changes (*see Table 2A for correlation analysis) and composed a chemically pure mix of the identified metabolites that we injected intracardially into mice. (C) FMD in mice at before and after 15 min injection of mixtures of anthocyanin metabolite profiles that correlated with of acute (2 h) and chronic (28 d) FMD improvements in human study (*see Table 2B for composition).
We then performed a 1-month randomized controlled intervention study (Study 4; Supplementary Figure 4) with additional secondary end points, including 24-hour BP measurements, aortic augmentation index, pulse wave velocity, and blood lipids, and detailed metabolomics analyses (Supplementary Table 2). The very first (acute) consumption of wild blueberry containing 150 mg of ACN significantly increased FMD by 1.5% (95% CI = 0.6% to 2.3%) at 2 hour post-ingestion compared to control (Figure 2B). After 28 days of bi-daily consumption, FMD was significantly increased after overnight fasting in the wild blueberry group compared to control by 2.3% (95% CI = 1.4% to 3.2%). Interestingly, no further improvement in FMD was observed when blueberries were acutely consumed on day 28 (acute on chronic; 0.3% [95% CI = –1.3% to 0.6%]) suggesting a saturation of effect indicating that acute and chronic effects may be mediated via similar pathways.
The improvement in FMD was accompanied by a lowering of 24-hour systolic BP (–5.6 mmHg [95% CI =–0.2 to –11.1 mmHg)]. Changes in 24-hour diastolic BP were not significantly different from control (–5.5 mmHg [95% CI = –13.0 to 1.9 mmHg)]. No changes were seen with respect to pulse wave velocity, aortic augmentation index, or blood lipids in our present study.
Acute and Chronic Blueberry Effects Are Linked to Circulating ACN Metabolome
To identify potential mediators of vascular effects, we performed a metabolomics analysis of circulating ACN metabolites. A total of 63 phenolic metabolites were quantified in plasma taken from subjects after blueberry at 2 hour after first dose and after 1 month of bi-daily consumption after an overnight fasting. Most of the metabolites were conjugated and nonconjugated phenolic acid derivatives, with only three of them being flavonoid derivatives (see Supplementary Table 2; data published elsewhere (21)). To link the circulating metabolites with vascular effects, we performed univariate correlation analyses with the increases in FMD at 2 hours and after 28-day consumption of blueberry and all individual plasma metabolites (Table 2A). Fourteen phenolic metabolites significantly correlated with the acute effects and 21 with the chronic responses, with 9 of them correlating with both acute and chronic responses.
ACN Metabolites Lead to Endothelial Function Improvements When Injected Into a Translational Experimental Model
To demonstrate the biological activity of the circulating ACN metabolites that correlated with the FMD responses in the human study (Study 4), we facilitated a translational animal model that allows the measurement of FMD in living mice (16). We assessed FMD in crossover study in 10 anesthetized mice before and at 15 minutes after injection of mixtures of the ACN metabolites that were significantly correlated with acute and chronic blueberries effects in Study 4 or vehicle (Table 2B). Both metabolite mixes led to significant increases in FMD over vehicle (“acute” metabolites: 8.7% [95% CI = 3% to 15%]; “chronic” metabolites: 8.3% [95% CI = 2% to 14%]; Figure 2C).
Gene and miRNA Expression Changes Linked With Chronic Consumption of Blueberries
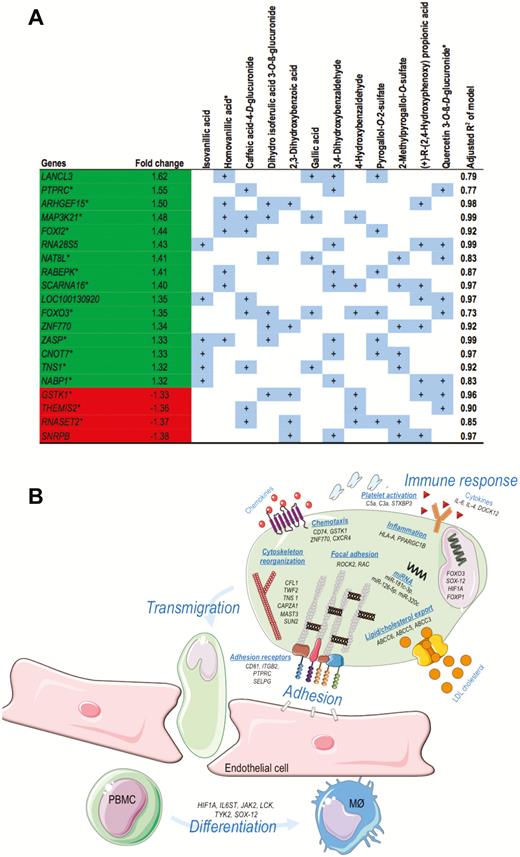
To gain insight into the nutrigenomic effect of chronic wild blueberry consumption, we performed exploratory gene expression analyses on PBMCs from a subgroup (n = 10) of the subjects participating in Study 4 comparing samples taken at baseline before and after having received blueberries more than 28 days. The analyses showed that 608 genes were significantly differentially expressed (Supplementary Figure 5), with 357 genes upregulated and 251 identified as downregulated with fold changes varying from –1.58 to 1.61 (see Figure 3A for top 20). A homogenous fold change expression across all 10 individuals was observed with a minor variation in volunteer 1 for a subset of the genes (Supplementary Figure 5). To evaluate the biological significance of the nutrigenomic data, a functional annotation of the 608 genes according to biological processes (gene ontology) was performed. Gene network analysis was conducted to investigate gene–gene interactions. Among the 35 significant gene networks identified, 11 are known to be involved in the regulation of chemotaxis and inflammation/immune response, 9 in cell adhesion and cytoskeleton organization, and few networks regulating signal transduction, apoptosis, or development. Together with gene network analyses, pathway enrichment analyses using Kyoto Encyclopedia of Genes and Genomes and Metacore databases showed that among the most overrepresented pathways identified are those involved in the regulation of cell adhesion, cell migration, inflammation, and cell differentiation processes (Figure 3A; Supplementary Figure 6).
(A) Summary of individual stepwise regression analyses identifying circulating metabolites as significant independent predictors of gene expression changes of the top 20 differentially expressed genes after 1 mo of blueberry consumption (n = 10). * Designates that also significant correlations existed with changes in flow-mediated dilation. (B) Schematic illustration of biological processes and molecular functions occurring in peripheral blood mononuclear cells (PBMC) on blueberry intake. A subset of genes from the 608 significant differentially expressed list was chosen for this figure as they are involved in previously established cell processes, which are highlighted in blue.
Together with the impact of blueberry polyphenol compounds on the expression of genes, we also analyzed their impact on expression of small non-coding RNA, miRNA, involved in the posttranscriptional regulation of gene expression. Our nutrigenomic study identified three differentially expressed miRNA in PBMCs of the volunteers consuming blueberries for 1 month: miR-181c-3p*, miR-126-5p*, and miR-30c-5p (Supplementary Table 3). The most striking finding was the 13-fold increase in expression of miR-181c. With the aim to retrieve potential biological effects of modulation of the three miRNAs, we identified target genes of these miRNAs using the miRWalk database and performed network and pathway analyses. Our comparison of differentially expressed genes with potential target genes of differentially expressed miRNAs revealed 69 genes in common. This observation suggests that 11% differentially expressed genes could be regulated at posttranscriptional level by the miRNAs. Bioinformatic analyses to identify pathways and networks in which target genes are involved in have shown that among the most overrepresented ones are those involved in the regulation of focal adhesion, chemotaxis, cytoskeletal reorganization, cytokine–cytokine interactions, cellular development but also lipid absorption, accumulation, and excretion (Figure 3B, Supplementary Figure 6).
Metabolites Predict Expression Changes in the Top 20 Selected Genes
One-third (n = 21) of the quantified 63 metabolites showed significant correlation with improvements in FMD. To explore whether circulating blueberry derived phenolic metabolites might be responsible for the changes in gene activity in PBMCs, a correlation followed by a stepwise multivariate analysis was performed on a selection of top 20 genes with interindividual variability less than 20%. From these top 20 genes, 15 are involved in processes of inflammation or have a functional link to cardiovascular disease development. Twelve metabolites were identified as significant independent predictors for changes in expression of the 20 genes (Figure 3A). Notably, the R2 of the individual multivariate linear regression models including sets of metabolites being significant independent predictors of gene changes were 0.73–0.99, suggesting that 73%–99% of the variability in gene expression changes were explained by the metabolites. Two of the 12 metabolite independent predictors of gene expression changes (namely quercetin 3-O-ß-D-glucuronide and homovanillic acid) positively correlated with improved human vascular function and were also part of the polyphenol mix that significantly increased FMD in mice.
Discussion
Although blueberries contain many potentially “healthy” bioactive molecules including vitamins, fiber, and minerals, our present results demonstrate that ACN are major bioactive compounds in blueberry that can account for the increases in endothelial function after blueberry consumption. Our data demonstrate for the first time that purified ACN cause dose-dependent improvements in endothelial function. The comparison of pure ACN dose–responses experiments with the previously obtained results with ACN-rich blueberries (11) supports that the majority of blueberry effects can be explained by ACN but also indicate that the beneficial effects of blueberries are still larger than achieved with the consumption of ACN alone. This may be due to other blueberry (poly)phenols likely chlorogenic acids, which have been previously shown to be capable of inducing favorable effects on endothelial function (21). The amounts of vitamins, fibers, and minerals but also the amount of flavanols (3 mg of flavanol monomers) (22) present in blueberries were too low to exert significant effects. However, we cannot discard possible synergistic and/or antagonistic effects of components when consumed as a whole food, rather than individual compounds, as well as matrix effects affecting the liberation or absorption of ACN from blueberries, which have been described previously to play a role in the context of flavanols (23).
We also report for the first time in healthy adults that chronic blueberry consumption leads to a significant sustained improvement in endothelial function and lowering of 24-hour systolic BP. A few studies in at-risk populations, however, have demonstrated a reduction in BP after blueberry consumption (24). The potential clinical relevance of the findings is underscored by the fact that the lowering of BP in the magnitude observed in our study of 5 mmHg is similar to what is commonly observed in clinical studies with BP lowering medication (eg, ACE inhibitors) in patients (25). Taken together, our data demonstrate that blueberries not only acutely and transiently improve endothelial function (11) but also induce sustained improvement in endothelial function and systolic BP after repetitive consumption for 1 month.
The molecular mechanisms of action of blueberries have still not been fully characterized, and this may be due to the fact that only recently significant advances in the understanding of the absorption and metabolism of ACN and blueberry polyphenols were made indicating that, for instance, ACN primarily circulate as phenolic metabolites (11,14). However, most clinical studies with cardiovascular outcomes have not reported plasma or urine levels of circulating polyphenol metabolites in the participants. In a targeted metabolomics approach, we identified here in healthy humans a panel of circulating metabolites that correlated with vascular FMD responses and demonstrated in a translational model that these metabolites are indeed bioactive and can improve FMD after injection. Importantly, these metabolites do not represent metabolites specific for ACN and are common metabolites of other common dietary (poly)phenols (8). It may be argued that it is a limitation of the present work that we did not quantify parent ACN and their phase II metabolites. However, we believe that such results would likely not affect the outcomes of our present work as the majority of ACN are transformed into low molecular weight phenolic metabolites, which we quantified in this work. Intact ACN are present in very low concentrations in plasma and typically represent less than 1% of the ACN metabolome, therefore, do not significantly contribute to the pool of circulating ACN metabolites (11,14).
Future work is needed to dissect if it is one or several of the metabolites or combinations of metabolites that mediate the effects and if the individual metabolites act via the same or different potentially synergistic mechanism(s). The investigation of structure–function relationships with these candidate metabolites may help to identify the molecular target structure and investigate potential class effects. Many (poly)phenols share similar phase II and gut microbiome derived metabolites, and similar metabolites have been observed to correlate with FMD after consumption of coffee (21) and cranberries (26). Furthermore, whether these effects slow or even reverses components of cardiovascular aging itself and can increase health span or longevity remains to be determined. Future randomized controlled trials in larger populations including older subjects with relevant clinical end points will answer this question.
Identifying molecular targets of polyphenol has proven to be a real challenge due to the complex mechanisms and pleiotropic pathways of cardiometabolic effects of different foods. We, here, used an exploratory nutrigenomic approach aiming at identifying gene networks in circulating mononuclear cells that were modulated by blueberry consumption in healthy humans and explored which gene and expression changes correlate with circulating phenolic metabolites and vascular responses. It is a limitation of this approach that gene expression changes were investigated in blood cells and not endothelial cells. However, immune responses, mediated by both circulating and resident leukocytes (27), play pathophysiological roles in the development and progression of cardiovascular disease (CVD), including neutrophil recruitment, coronary atherosclerotic plaque development and stability, heart failure, and endothelial dysfunction (28,29). A few studies have proposed molecular mechanisms of action of other (poly)phenols in vitro and in vivo using nutrigenomic approaches (30). These studies suggest that dietary (poly)phenols exert anti-inflammatory properties by binding to molecular targets in human cells making them attractive candidates for dietary CVD prevention strategies. Our present data corroborate these overall observations and add to the current body of knowledge by supplying in vivo data on nutrigenomic effects of blueberries and link these with ACN-metabolites in healthy humans. Among the significant gene networks identified from genes whose expression was affected by blueberry, one-third is involved in the regulation of immune response and inflammation. This observation suggests that blueberry consumption can modulate inflammatory cellular processes of PBMCs that represent cellular targets of vascular function maintenance. The fact that we also identified circulating ACN metabolites in plasma as strong predictors of expression changes in the most overexpressed genes with the majority being involved in inflammation suggest that these metabolites may play a mechanistic role in the mediation of effects. Only two metabolites correlated both with vascular function improvements and gene expression changes indicating that the mechanism by which ACN metabolites modulate vascular function and PBMC expression changes may differ and the interaction is likely complex. We also observed significant changes in the expression of three miRNAs. The little data available for miR-30c-5p and miR-181c-3p suggest that they could play a role in the cancer development but a possible link with cardiovascular disease remains to be determined. miR-126-5p was described as being involved in enhancing the inflammatory responses of monocytes (31) or showing increased expression in patients with carotid artery disease (32) and acute pancreatitis (33). Taken together, our nutrigenomic data showed that blueberry consumption can modulate the expression of genes and miRNA toward an anti-inflammatory and CVD protective profile, revealing new molecular targets that may be underlying the health properties of berries.
In conclusion, our results demonstrate a key role of ACN metabolites in the mediation of biological activities of blueberries that could contribute to healthy cardiovascular aging. We provide further scientific evidence that in healthy humans chronic blueberry consumption leads to sustained cardiovascular benefits, which are linked with circulating ACN metabolites and the modulation of cellular gene programs towards an anti-inflammatory and CVD protective profile. Future studies will help to further characterize the mechanisms of action of individual metabolites, establish general structure–function relationships, and identify relevant interactions. As the identified metabolites are common for a range of food bioactive classes, this knowledge represents an important building block necessary for the development of evidence-based dietary recommendations for food bioactives in primary prevention.
Funding
This work was supported by the Medical Research Committee of the University of Düsseldorf (9772574) and by an unrestricted grant from the Wild Blueberry Association of North America. C.H. is supported by the Deutsche Forschungsgemeinschaft (SFB1116 TP A07). We also acknowledge a Susanne Bunnenberg grant to Düsseldorf Heart Centre.
Conflict of Interest
ARM and CH have received unrestricted research grants from the Wild Blueberry Association of North America. The other authors declare no conflicts of interests.
Acknowledgements
We thank Prof. Dr. Helmut Sies for reviewing the manuscript and important critical discussions on the topic.