-
PDF
- Split View
-
Views
-
Cite
Cite
Elior Moreh, Jeremy M. Jacobs, Jochanan Stessman, Fatigue, Function, and Mortality in Older Adults, The Journals of Gerontology: Series A, Volume 65A, Issue 8, August 2010, Pages 887–895, https://doi.org/10.1093/gerona/glq064
Close - Share Icon Share
Abstract
Although fatigue is common among the elderly people, little is known concerning its relationship with mortality and function over extended periods of time among the very old. This study evaluates the association of fatigue with health, functional status, and mortality from ages 70–88 years.
Mortality data from ages 70–88 years and both health and functional status at age 70, 78, and 85 years were assessed among a representative community-dwelling cohort born 1920–1921 from the Jerusalem Longitudinal Study (1990–2008).
At age 70, 78, and 85, fatigue prevalence was 29%, 53%, and 68%, respectively, with increased prevalence among women. Fatigue was associated with poorer health, function, and psychosocial parameters at all ages and greater likelihood to deteriorate in subsequent self-rated health (SRH), functional status, loneliness, depression, and physical activity level. After adjustment, fatigue at age 70 predicted poor subsequent SRH, difficulty in activities of daily living, reduced levels of physical activity, and poor sleep satisfaction, and at age 78, fatigue predicted subsequent depression. Hazard ratios for mortality among fatigued participants were significant after adjustment for numerous risk factors. The addition of physical activity level and/or depression reduced the significance of the relationship between fatigue and mortality.
Fatigue among the elderly people, up to and including the oldest old, has a significant negative impact on health status, function, and mortality. Pathways of action may be related to the complex relationship of fatigue with depression and levels of physical activity.
“Death seems far less terrible, when you are tired” said Simone de Beauvoir (1).
Indeed, although feeling tired is a common universal experience, nonetheless, fatigue (defined as sense of persistent general tiredness) is becoming increasingly recognized as a specific geriatric entity (2). Both the prevalence and incidence appear to increase with advancing age (2–4), and for the majority, fatigue per se exists independent of any specific diagnostic condition (5). Task-specific measures of tiredness have been examined in clarification of the theoretical assumption that fatigue may be instrumental in the disablement process. In particular, self-reported tiredness while performing daily activities has been examined, and among nondisabled elderly people, it has been found to be a determinant of subsequent utilization of health and social services (6), walking limitations (7), onset of disability (8), and a reduction in both 10- and 15-year survival (9,10). Relatively little research has examined fatigue per se, irrespective of task-specific measures of tiredness. Recent findings from participants in their seventies report that fatigue was associated with poorer functional status both at baseline (11) and at 3-year follow-up (12), as well as increased 10-year mortality (13). The nature of this relationship over longer periods of follow-up and at advancing age remains unknown.
The Jerusalem Longitudinal Cohort Study examined the influence of fatigue among an aging cohort over 18 years of follow-up and addressed the following questions: (a) Is fatigue associated with increased mortality at increasing ages, up to and including the oldest old (>85 years)? and (b) is fatigue at progressively increasing ages associated with subsequent health and functional status?
MATERIALS AND METHODS
Basic Methodology and Sampling
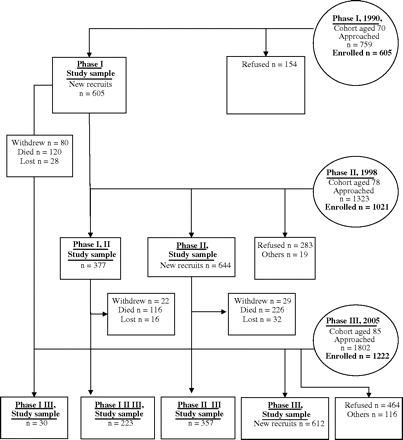
The Jerusalem Longitudinal Cohort Study continues to follow a birth cohort of Jerusalem residents (born June 1920 to May 1921) from age 70 at baseline in 1990 to age 88 at the present time. The study has been described in detail elsewhere (14). In brief, at Phases 1, 2, and 3 (ages 70, 78, and 85, respectively), a total of 605, 1021, and 1222 participants enrolled. The Phase 1 study sample was augmented at Phases 2 and 3, with new participants randomly recruited from the same birth cohort. Each participant, or legal guardian, provided informed consent, and the Hadassah-Hebrew University Medical Center Institutional Review Board approved the study (Figure 1).
The flow of participants in the Jerusalem Longitudinal Cohort Study.
The study sample, which formed about one third of the total birth cohort, was randomly selected from the electoral registry (a complete register of Jerusalem residents born 1920–1921). Participants underwent comprehensive home assessment at ages 70, 78, and 85. At each phase, participants were interviewed twice; an occupational therapist assessed participants for demographic, social, and functional domains, whereas the study physician gathered data concerning self-reported tiredness, medical history, physical examination, and cognitive and psychological tests. The number of participants who underwent both medical and social assessment at each phase, upon whom the present study is based, was 460 (76.0%), 858 (84.0%), and 1162 (95.1%), respectively. The representative nature of the study sample was confirmed by finding similar rates of hospital inpatient morbidity, health service utilization, and mortality between the study sample, participants who refused, and those not approached to enroll (15). Furthermore, no differences in survival rates or in the influence of fatigue on survival were found between participants recruited at different phases in the study and who participated once only, twice, or at all three phases of the study.
Measurements and Data Collection
Fatigue.—
The primary independent study variable of fatigue was operationalized according to the question “Do you feel generally tired?” with available answers being either yes or no.
Functional status.—
We assessed functional status according to self-reported performance on six activities of daily living (ADLs): transferring, dressing, bathing, using the toilet, eating, and continence (16). Participants were questioned on their ability to perform the various ADLs, with possible answers being: (a) able without difficulty, (b) able but with difficulty, (c) only able with assistance from another person, and (d) completely unable or totally reliant on another person. Dependence in ADL was defined as a positive answer for (c) or (d) in at least one of the six ADLs, and difficulty (but not dependence) in ADL was defined as a positive answer for (b) in at least one ADL (17).
Health measures.—
(1) Self-rated health (SRH) was measured according to the question “how do you rate your state of health?” and available answers being good versus poor. (2) Cognitive status was measured using the Mini-Mental State Examination (MMSE) (18) and examined as a continuous variable from 0 to 30. (3) Depression was identified using the Brief Symptom Inventory (19), whereby participants rated from 0 to 4 (0 = none, 4 = greatly) how much they had suffered in the previous month from (a) loneliness, (b) lack of interest, (c) thoughts of ending your life, (d) bad mood, (e) hopelessness concerning the future, and (f) worthlessness. Depression was defined (19) as a total score of ≥6. (4) Chronic back or joint pain participants were asked concerning frequency and site of joint and back pain. Participants reporting pain of more than 1-month duration on an occasional or frequent basis were defined as suffering from chronic pain. (5) Global sleep satisfaction was assessed according to the question “are you satisfied with your sleep in the last month?” (a positive answer being always or generally satisfied vs negative answer—often or never satisfied with their overall sleep, irrespective of hypnotic medication usage) (20). (6) Major diseases (hypertension, ischemic heart disease, diabetes mellitus, and history of neoplasm) were defined according to the International Classification of Diseases, Ninth Edition (21). (7) We also included an assessment of physical activity. Participants were questioned “how often are you physically active?” and answers being (a) less than 4 hours weekly, (b) about 4 hours weekly, (c) vigorous sports at least twice weekly (eg, jogging, swimming), and (d) regular physical activity (eg, walking at least an hour daily). This four-item questionnaire, introduced in 1990 at baseline evaluation, was adapted from the Gothenburg population study of 70 year olds (22) and kept throughout for internal consistency of longitudinal data (23). Physical activity was dichotomized to sedentary (answer a) versus physically active (answers b, c, and d). This cutoff was justified statistically, accounting for distribution and frequency of responses (24).
Demographic and additional data.—
We examined the following characteristics: gender; financial status was defined according to the following question “do you have financial difficulties?” and available answers were (a) never, (b) rarely, (c) often, and (d) usually. Participants answering (c) or (d) were deemed to be in financial difficulty; education (≥12 years schooling); marital status, loneliness (often or occasionally vs never); body mass index (BMI; kilogram per square meter) was calculated and examined as a continuous variable; smoking history was determined (current smokers vs nonsmokers or ex-smokers); a detailed drug history was taken for hypnotic medications, in view of their potential effects on fatigue; anemia (hemoglobin <12 g/dL for women and <13 g/dL for men). The study physician made diagnoses following medical assessment, system review, and examination.
Outcomes
Mortality.—
Death was the primary outcome obtained from annual review of death certificates issued by the Ministry of Interior from 1990 to 2008. This provided 100% surveillance for participants in Israel, and because a negligible number from this age group (<0.1%) leaves the country, the accuracy of data was considered complete. Mortality data were analyzed among the three phases of participants examined at age 70 (n = 460), age 78 (n = 858), and age 85 (n = 1162).
Deterioration in functional status and health measures.—
Deterioration over time in functional status and health measures was measured among two study populations: (a) participants who participated at both age 70 and age 78 (n = 312), and (b) participants who participated at age 78 and age 85 (n = 545). Only participants who at baseline were free of the poor or disadvantaged category of each single measure under examination were included. Deterioration was defined as the new onset of illness being measured at follow-up among participants who reported being illness free at baseline measurement. Thus, deterioration in (a) loneliness was defined as participants reporting no loneliness at baseline and loneliness at follow-up; (b) deterioration in depression was defined as no depression at baseline and the appearance of depression at follow-up; (c) SRH was defined as participants reporting good SRH at baseline and poor SRH at follow-up; (d) functional decline was defined as independence at baseline and the new onset of either ADL dependence or difficulty at follow-up; (e) deterioration in physical activity was defined as active at baseline and low levels of physical activity at follow-up; (f) sleep satisfaction was defined as good sleep satisfaction at baseline and poor sleep satisfaction at follow-up; (g) onset of the common diseases (hypertension, ischemic heart disease, diabetes mellitus, and anemia) was defined as absence at baseline and their presence at follow-up; (h) MMSE decline was defined as deterioration from >24–30 at baseline to 0 to ≤24 at follow-up; and (i) for BMI, the mean change over follow-up was measured.
Statistical Analyses
Descriptive statistics and the effect of fatigue on subsequent decline in health variables and functional status were performed using chi-squared tests for categorical variables and Wilcoxon test for continuous variables (Tables 1 and 2), and significant associations (p < .05) were further examined using logistic regression in order to account for potential confounders (Table 3). All logistic regression models included gender, education, SRH, ischemic heart disease, diabetes, hypertension, difficulty in ADL, depression, chronic joint or musculoskeletal pain, poor global sleep satisfaction, and fatigue as well as the baseline variable under analysis. Because the definition of depression included a measure of loneliness, the logistic regression models examining loneliness included baseline loneliness and not depression. We employed log rank testing to examine the influence of fatigue (Figure 2) at age 70, 78, and 85 on survival from ages 70–78, 78–85, and 85–88 years, respectively. We used Cox proportional hazards models to calculate hazard ratios (HRs) with 95% confidence interval (CI) for mortality (Table 4). A time-dependent Cox proportional hazards model (25) analyzed the influence of fatigue and risk factors as time-dependent variables throughout the study period on mortality from ages 70–88 years. Participants from all three study phases (ages 70, 78, and 85) were included in the time-dependent analysis, which accounted for participants with one or more measures of fatigue during follow-up. In addition to adjusting for changes in the levels of fatigue, this analysis also adjusted for confounding comorbid factors at the three potential points in time during follow-up. The proportional hazards assumption was met in all models. All Cox proportional hazards models adjusted for gender, education, smoking pack years, hypertension, ischemic heart disease, diabetes mellitus, and history of neoplasm (Table 4, basic model). Additional models also adjusted for physical activity, depression, or both. Kaplan–Meier survival curves (Figure 2) were calculated after adjustment for those variables included in the basic model (Table 4).
Baseline Characteristics According to Fatigue at Age 70, 78, and 85 Years
| Phase 1, Age 70 Years (1990–1991) (n = 460) | Phase 2, Age 78 Years (1997–1998) (n = 858) | Phase 3, Age 85 Years (2005–2006) (n = 1162) | ||||
| Variable | Not Tired, n (%) | Tired, n (%) | Not tired, n (%) | Tired, n (%) | Not tired, n (%) | Tired, n (%) |
| Total | 325 (71) | 135 (29) | 403 (47) | 455 (53) | 373 (32) | 789 (68) |
| Demographic data | ||||||
| Men | 187 (58) | 63 (47)* | 239 (59) | 188 (41)*** | 205 (35) | 320 (41)*** |
| Financial difficulty | 63 (20) | 37 (28) | 41 (11) | 97 (22)*** | 29 (8) | 180 (23)*** |
| Education ≥12 | 169 (51) | 58 (43) | 213 (53) | 175 (38)*** | 197 (53) | 320 (41)*** |
| Married | 228 (71) | 88 (66)* | 256 (64) | 255 (56) | 180 (49) | 316 (40)** |
| Affective data | ||||||
| Loneliness | 101 (32) | 63 (47)** | 106 (27) | 220 (51)*** | 92 (25) | 406 (53)*** |
| Depression | 29 (10) | 38 (31)*** | 30 (9) | 100 (34)*** | 51 (14) | 356 (47)*** |
| Poor self-rated health | 62 (19) | 69 (51)*** | 81 (20) | 269 (59)*** | 33 (9) | 367 (48)*** |
| Functional status | ||||||
| ADL dependent | 13 (4) | 12 (9)* | 18 (5) | 69 (16)*** | 66 (18) | 360 (47)*** |
| ADL difficulty | 28 (9) | 45 (34)*** | 36 (9) | 171 (39)*** | 117 (32) | 443 (57)*** |
| Heath measures | ||||||
| Low physical activity | 138 (43) | 74 (55)* | 40 (11) | 138 (32)*** | 79 (22) | 328 (42)*** |
| Smoking (currently) | 40 (12) | 19 (14) | 18 (5) | 29 (7) | 20 (5) | 35 (5) |
| Back or joint pain | 171 (52) | 106 (79)*** | 269 (66) | 382 (84)*** | 74 (20) | 280 (35)*** |
| Poor sleep satisfaction | 61 (19) | 54 (40)*** | 53 (13) | 159 (36)*** | 69 (19) | 264 (35)*** |
| Use of sleeping pills | 62 (20) | 32 (25) | 81 (21) | 154 (35)*** | 100 (27) | 291 (38)** |
| Hypertension | 108 (33) | 70 (52)*** | 196 (48) | 267 (59)** | 247 (66) | 593 (75)** |
| Ischemic heart disease | 71 (22) | 49 (36)** | 106 (26) | 184 (40)*** | 116 (31) | 321 (41)** |
| Diabetes mellitus | 43 (13) | 30 (22)* | 71 (18) | 87 (19) | 68 (18) | 177 (22) |
| History of neoplasm | 10 (3) | 7 (5) | 23 (6) | 28 (6) | 32 (9) | 64 (8) |
| Anemia | 19 (6) | 14 (10) | 28 (13) | 55 (20)* | 60 (25) | 163 (32)* |
| MMSE ± SD | 29.1 ± 1.9 | 28.7 ± 2.4 | 28.8 ± 1.9 | 28.4 ± 2.1 | 27.1 ± 4.0 | 25.1 ± 5.4*** |
| MMSE ≤24 (%) | 5 (2.3) | 5 (6.3) | 8 (3) | 12 (5) | 51 (14) | 227 (30)*** |
| Body mass index ± SD | 27.1 ± 4.0 | 27.1 ± 3.9 | 27.2 ± 4.2 | 28.4 ± 4.8 | 26.9 ± 4.4 | 27.5 ± 4.6 |
| Phase 1, Age 70 Years (1990–1991) (n = 460) | Phase 2, Age 78 Years (1997–1998) (n = 858) | Phase 3, Age 85 Years (2005–2006) (n = 1162) | ||||
| Variable | Not Tired, n (%) | Tired, n (%) | Not tired, n (%) | Tired, n (%) | Not tired, n (%) | Tired, n (%) |
| Total | 325 (71) | 135 (29) | 403 (47) | 455 (53) | 373 (32) | 789 (68) |
| Demographic data | ||||||
| Men | 187 (58) | 63 (47)* | 239 (59) | 188 (41)*** | 205 (35) | 320 (41)*** |
| Financial difficulty | 63 (20) | 37 (28) | 41 (11) | 97 (22)*** | 29 (8) | 180 (23)*** |
| Education ≥12 | 169 (51) | 58 (43) | 213 (53) | 175 (38)*** | 197 (53) | 320 (41)*** |
| Married | 228 (71) | 88 (66)* | 256 (64) | 255 (56) | 180 (49) | 316 (40)** |
| Affective data | ||||||
| Loneliness | 101 (32) | 63 (47)** | 106 (27) | 220 (51)*** | 92 (25) | 406 (53)*** |
| Depression | 29 (10) | 38 (31)*** | 30 (9) | 100 (34)*** | 51 (14) | 356 (47)*** |
| Poor self-rated health | 62 (19) | 69 (51)*** | 81 (20) | 269 (59)*** | 33 (9) | 367 (48)*** |
| Functional status | ||||||
| ADL dependent | 13 (4) | 12 (9)* | 18 (5) | 69 (16)*** | 66 (18) | 360 (47)*** |
| ADL difficulty | 28 (9) | 45 (34)*** | 36 (9) | 171 (39)*** | 117 (32) | 443 (57)*** |
| Heath measures | ||||||
| Low physical activity | 138 (43) | 74 (55)* | 40 (11) | 138 (32)*** | 79 (22) | 328 (42)*** |
| Smoking (currently) | 40 (12) | 19 (14) | 18 (5) | 29 (7) | 20 (5) | 35 (5) |
| Back or joint pain | 171 (52) | 106 (79)*** | 269 (66) | 382 (84)*** | 74 (20) | 280 (35)*** |
| Poor sleep satisfaction | 61 (19) | 54 (40)*** | 53 (13) | 159 (36)*** | 69 (19) | 264 (35)*** |
| Use of sleeping pills | 62 (20) | 32 (25) | 81 (21) | 154 (35)*** | 100 (27) | 291 (38)** |
| Hypertension | 108 (33) | 70 (52)*** | 196 (48) | 267 (59)** | 247 (66) | 593 (75)** |
| Ischemic heart disease | 71 (22) | 49 (36)** | 106 (26) | 184 (40)*** | 116 (31) | 321 (41)** |
| Diabetes mellitus | 43 (13) | 30 (22)* | 71 (18) | 87 (19) | 68 (18) | 177 (22) |
| History of neoplasm | 10 (3) | 7 (5) | 23 (6) | 28 (6) | 32 (9) | 64 (8) |
| Anemia | 19 (6) | 14 (10) | 28 (13) | 55 (20)* | 60 (25) | 163 (32)* |
| MMSE ± SD | 29.1 ± 1.9 | 28.7 ± 2.4 | 28.8 ± 1.9 | 28.4 ± 2.1 | 27.1 ± 4.0 | 25.1 ± 5.4*** |
| MMSE ≤24 (%) | 5 (2.3) | 5 (6.3) | 8 (3) | 12 (5) | 51 (14) | 227 (30)*** |
| Body mass index ± SD | 27.1 ± 4.0 | 27.1 ± 3.9 | 27.2 ± 4.2 | 28.4 ± 4.8 | 26.9 ± 4.4 | 27.5 ± 4.6 |
Notes: ADL = activities of daily living; MMSE = Mini-Mental State Examination.
*p ≤ .05, **p ≤ .005, ***p ≤ .0001.
Baseline Characteristics According to Fatigue at Age 70, 78, and 85 Years
| Phase 1, Age 70 Years (1990–1991) (n = 460) | Phase 2, Age 78 Years (1997–1998) (n = 858) | Phase 3, Age 85 Years (2005–2006) (n = 1162) | ||||
| Variable | Not Tired, n (%) | Tired, n (%) | Not tired, n (%) | Tired, n (%) | Not tired, n (%) | Tired, n (%) |
| Total | 325 (71) | 135 (29) | 403 (47) | 455 (53) | 373 (32) | 789 (68) |
| Demographic data | ||||||
| Men | 187 (58) | 63 (47)* | 239 (59) | 188 (41)*** | 205 (35) | 320 (41)*** |
| Financial difficulty | 63 (20) | 37 (28) | 41 (11) | 97 (22)*** | 29 (8) | 180 (23)*** |
| Education ≥12 | 169 (51) | 58 (43) | 213 (53) | 175 (38)*** | 197 (53) | 320 (41)*** |
| Married | 228 (71) | 88 (66)* | 256 (64) | 255 (56) | 180 (49) | 316 (40)** |
| Affective data | ||||||
| Loneliness | 101 (32) | 63 (47)** | 106 (27) | 220 (51)*** | 92 (25) | 406 (53)*** |
| Depression | 29 (10) | 38 (31)*** | 30 (9) | 100 (34)*** | 51 (14) | 356 (47)*** |
| Poor self-rated health | 62 (19) | 69 (51)*** | 81 (20) | 269 (59)*** | 33 (9) | 367 (48)*** |
| Functional status | ||||||
| ADL dependent | 13 (4) | 12 (9)* | 18 (5) | 69 (16)*** | 66 (18) | 360 (47)*** |
| ADL difficulty | 28 (9) | 45 (34)*** | 36 (9) | 171 (39)*** | 117 (32) | 443 (57)*** |
| Heath measures | ||||||
| Low physical activity | 138 (43) | 74 (55)* | 40 (11) | 138 (32)*** | 79 (22) | 328 (42)*** |
| Smoking (currently) | 40 (12) | 19 (14) | 18 (5) | 29 (7) | 20 (5) | 35 (5) |
| Back or joint pain | 171 (52) | 106 (79)*** | 269 (66) | 382 (84)*** | 74 (20) | 280 (35)*** |
| Poor sleep satisfaction | 61 (19) | 54 (40)*** | 53 (13) | 159 (36)*** | 69 (19) | 264 (35)*** |
| Use of sleeping pills | 62 (20) | 32 (25) | 81 (21) | 154 (35)*** | 100 (27) | 291 (38)** |
| Hypertension | 108 (33) | 70 (52)*** | 196 (48) | 267 (59)** | 247 (66) | 593 (75)** |
| Ischemic heart disease | 71 (22) | 49 (36)** | 106 (26) | 184 (40)*** | 116 (31) | 321 (41)** |
| Diabetes mellitus | 43 (13) | 30 (22)* | 71 (18) | 87 (19) | 68 (18) | 177 (22) |
| History of neoplasm | 10 (3) | 7 (5) | 23 (6) | 28 (6) | 32 (9) | 64 (8) |
| Anemia | 19 (6) | 14 (10) | 28 (13) | 55 (20)* | 60 (25) | 163 (32)* |
| MMSE ± SD | 29.1 ± 1.9 | 28.7 ± 2.4 | 28.8 ± 1.9 | 28.4 ± 2.1 | 27.1 ± 4.0 | 25.1 ± 5.4*** |
| MMSE ≤24 (%) | 5 (2.3) | 5 (6.3) | 8 (3) | 12 (5) | 51 (14) | 227 (30)*** |
| Body mass index ± SD | 27.1 ± 4.0 | 27.1 ± 3.9 | 27.2 ± 4.2 | 28.4 ± 4.8 | 26.9 ± 4.4 | 27.5 ± 4.6 |
| Phase 1, Age 70 Years (1990–1991) (n = 460) | Phase 2, Age 78 Years (1997–1998) (n = 858) | Phase 3, Age 85 Years (2005–2006) (n = 1162) | ||||
| Variable | Not Tired, n (%) | Tired, n (%) | Not tired, n (%) | Tired, n (%) | Not tired, n (%) | Tired, n (%) |
| Total | 325 (71) | 135 (29) | 403 (47) | 455 (53) | 373 (32) | 789 (68) |
| Demographic data | ||||||
| Men | 187 (58) | 63 (47)* | 239 (59) | 188 (41)*** | 205 (35) | 320 (41)*** |
| Financial difficulty | 63 (20) | 37 (28) | 41 (11) | 97 (22)*** | 29 (8) | 180 (23)*** |
| Education ≥12 | 169 (51) | 58 (43) | 213 (53) | 175 (38)*** | 197 (53) | 320 (41)*** |
| Married | 228 (71) | 88 (66)* | 256 (64) | 255 (56) | 180 (49) | 316 (40)** |
| Affective data | ||||||
| Loneliness | 101 (32) | 63 (47)** | 106 (27) | 220 (51)*** | 92 (25) | 406 (53)*** |
| Depression | 29 (10) | 38 (31)*** | 30 (9) | 100 (34)*** | 51 (14) | 356 (47)*** |
| Poor self-rated health | 62 (19) | 69 (51)*** | 81 (20) | 269 (59)*** | 33 (9) | 367 (48)*** |
| Functional status | ||||||
| ADL dependent | 13 (4) | 12 (9)* | 18 (5) | 69 (16)*** | 66 (18) | 360 (47)*** |
| ADL difficulty | 28 (9) | 45 (34)*** | 36 (9) | 171 (39)*** | 117 (32) | 443 (57)*** |
| Heath measures | ||||||
| Low physical activity | 138 (43) | 74 (55)* | 40 (11) | 138 (32)*** | 79 (22) | 328 (42)*** |
| Smoking (currently) | 40 (12) | 19 (14) | 18 (5) | 29 (7) | 20 (5) | 35 (5) |
| Back or joint pain | 171 (52) | 106 (79)*** | 269 (66) | 382 (84)*** | 74 (20) | 280 (35)*** |
| Poor sleep satisfaction | 61 (19) | 54 (40)*** | 53 (13) | 159 (36)*** | 69 (19) | 264 (35)*** |
| Use of sleeping pills | 62 (20) | 32 (25) | 81 (21) | 154 (35)*** | 100 (27) | 291 (38)** |
| Hypertension | 108 (33) | 70 (52)*** | 196 (48) | 267 (59)** | 247 (66) | 593 (75)** |
| Ischemic heart disease | 71 (22) | 49 (36)** | 106 (26) | 184 (40)*** | 116 (31) | 321 (41)** |
| Diabetes mellitus | 43 (13) | 30 (22)* | 71 (18) | 87 (19) | 68 (18) | 177 (22) |
| History of neoplasm | 10 (3) | 7 (5) | 23 (6) | 28 (6) | 32 (9) | 64 (8) |
| Anemia | 19 (6) | 14 (10) | 28 (13) | 55 (20)* | 60 (25) | 163 (32)* |
| MMSE ± SD | 29.1 ± 1.9 | 28.7 ± 2.4 | 28.8 ± 1.9 | 28.4 ± 2.1 | 27.1 ± 4.0 | 25.1 ± 5.4*** |
| MMSE ≤24 (%) | 5 (2.3) | 5 (6.3) | 8 (3) | 12 (5) | 51 (14) | 227 (30)*** |
| Body mass index ± SD | 27.1 ± 4.0 | 27.1 ± 3.9 | 27.2 ± 4.2 | 28.4 ± 4.8 | 26.9 ± 4.4 | 27.5 ± 4.6 |
Notes: ADL = activities of daily living; MMSE = Mini-Mental State Examination.
*p ≤ .05, **p ≤ .005, ***p ≤ .0001.
Decline in Variables According to Fatigue at Age 70 and 78 Years
| Follow-up Age 70–78 (1990–1998) | Follow-up Age 78–85 (1998–2005) | |||
| Deterioration at Follow-up Among Participants Free of Poor or Disadvantaged Category at Baseline | Number of Participants With Deterioration at Age 78/Number of Participants With No Fatigue at Age 70, n (%) | Number of Participants With Deterioration at Age 78/Number of Participants With Fatigue at Age 70, n (%) | Number of Participants With Deterioration at Age 85/Number of Participants With No Fatigue at Age 78, n (%) | Number of Participants With Deterioration at Age 85/Number of Participants With Fatigue at Age 78, n (%) |
| Loneliness | 17/140 (12) | 12/38 (32)** | 38/193 (20) | 57/141 (40)*** |
| Depression | 13/162 (8) | 3/40 (8) | 31/206(15) | 46/138 (33)*** |
| Poor self-rated health | 49/190 (26) | 19/45 (42)* | 36/225(16) | 25/127 (20) |
| ADL dependence | 12/199 (6) | 12/71 (17)** | 64/263 (24) | 81/222(36)** |
| ADL difficulty | 27/192 (14) | 24/57 (42)*** | 93/250 (37) | 67/169 (39) |
| Low physical activity | 8/136 (6) | 8/37 (22)** | 58/227 (25) | 65/184 (36)* |
| Poor sleep satisfaction | 20/173 (12) | 16/48 (33)** | 44/240 (18) | 40/164 (24) |
| Hypertension | 49/150 (32) | 14/35 (40) | 59/140 (42) | 52/108 (48) |
| Ischemic heart disease | 46/177 (26) | 12/53 (23) | 31/198 (16) | 30/150 (20) |
| Diabetes mellitus | 13/196 (6) | 4/62 (6) | 18/221 (8) | 17/213(8) |
| Anemia | 19/120 (16) | 8/46 (17) | 30/121 (25) | 31/117 (26) |
| Mini-Mental State Examination ≤24 | 1/102 (1) | 2/21 (10) | 20/175 (11) | 24/144 (17) |
| Body mass index, mean change (±SD) | 0.15 (±2.31) | 0.62 (±3.01) | −0.5 (±2.55) | −0.63 (±2.71) |
| Follow-up Age 70–78 (1990–1998) | Follow-up Age 78–85 (1998–2005) | |||
| Deterioration at Follow-up Among Participants Free of Poor or Disadvantaged Category at Baseline | Number of Participants With Deterioration at Age 78/Number of Participants With No Fatigue at Age 70, n (%) | Number of Participants With Deterioration at Age 78/Number of Participants With Fatigue at Age 70, n (%) | Number of Participants With Deterioration at Age 85/Number of Participants With No Fatigue at Age 78, n (%) | Number of Participants With Deterioration at Age 85/Number of Participants With Fatigue at Age 78, n (%) |
| Loneliness | 17/140 (12) | 12/38 (32)** | 38/193 (20) | 57/141 (40)*** |
| Depression | 13/162 (8) | 3/40 (8) | 31/206(15) | 46/138 (33)*** |
| Poor self-rated health | 49/190 (26) | 19/45 (42)* | 36/225(16) | 25/127 (20) |
| ADL dependence | 12/199 (6) | 12/71 (17)** | 64/263 (24) | 81/222(36)** |
| ADL difficulty | 27/192 (14) | 24/57 (42)*** | 93/250 (37) | 67/169 (39) |
| Low physical activity | 8/136 (6) | 8/37 (22)** | 58/227 (25) | 65/184 (36)* |
| Poor sleep satisfaction | 20/173 (12) | 16/48 (33)** | 44/240 (18) | 40/164 (24) |
| Hypertension | 49/150 (32) | 14/35 (40) | 59/140 (42) | 52/108 (48) |
| Ischemic heart disease | 46/177 (26) | 12/53 (23) | 31/198 (16) | 30/150 (20) |
| Diabetes mellitus | 13/196 (6) | 4/62 (6) | 18/221 (8) | 17/213(8) |
| Anemia | 19/120 (16) | 8/46 (17) | 30/121 (25) | 31/117 (26) |
| Mini-Mental State Examination ≤24 | 1/102 (1) | 2/21 (10) | 20/175 (11) | 24/144 (17) |
| Body mass index, mean change (±SD) | 0.15 (±2.31) | 0.62 (±3.01) | −0.5 (±2.55) | −0.63 (±2.71) |
Notes: For all numbers shown, the numerator represents the number of participants who were free of the poor or disadvantaged category at baseline, who deteriorated in the variable being examined during the follow-up period (either from ages 70–78 years or from ages 78–85 years), according to fatigue status at baseline. The denominator represents the number of participants who were free of the poor or disadvantaged category at baseline according to fatigue status, thus grouped to participants with no fatigue or participants with fatigue. Thus, at age 70, 140 participants were neither lonely nor fatigued. By age 78, 17/140 (12%) had become lonely. In contrast, among the 38 not lonely yet fatigued participant at age 70, 12/38 (22%) had become lonely at follow-up at age 78. ADL = activities of daily living.
*p ≤ .05, **p ≤ .005, ***p ≤ .0001.
Decline in Variables According to Fatigue at Age 70 and 78 Years
| Follow-up Age 70–78 (1990–1998) | Follow-up Age 78–85 (1998–2005) | |||
| Deterioration at Follow-up Among Participants Free of Poor or Disadvantaged Category at Baseline | Number of Participants With Deterioration at Age 78/Number of Participants With No Fatigue at Age 70, n (%) | Number of Participants With Deterioration at Age 78/Number of Participants With Fatigue at Age 70, n (%) | Number of Participants With Deterioration at Age 85/Number of Participants With No Fatigue at Age 78, n (%) | Number of Participants With Deterioration at Age 85/Number of Participants With Fatigue at Age 78, n (%) |
| Loneliness | 17/140 (12) | 12/38 (32)** | 38/193 (20) | 57/141 (40)*** |
| Depression | 13/162 (8) | 3/40 (8) | 31/206(15) | 46/138 (33)*** |
| Poor self-rated health | 49/190 (26) | 19/45 (42)* | 36/225(16) | 25/127 (20) |
| ADL dependence | 12/199 (6) | 12/71 (17)** | 64/263 (24) | 81/222(36)** |
| ADL difficulty | 27/192 (14) | 24/57 (42)*** | 93/250 (37) | 67/169 (39) |
| Low physical activity | 8/136 (6) | 8/37 (22)** | 58/227 (25) | 65/184 (36)* |
| Poor sleep satisfaction | 20/173 (12) | 16/48 (33)** | 44/240 (18) | 40/164 (24) |
| Hypertension | 49/150 (32) | 14/35 (40) | 59/140 (42) | 52/108 (48) |
| Ischemic heart disease | 46/177 (26) | 12/53 (23) | 31/198 (16) | 30/150 (20) |
| Diabetes mellitus | 13/196 (6) | 4/62 (6) | 18/221 (8) | 17/213(8) |
| Anemia | 19/120 (16) | 8/46 (17) | 30/121 (25) | 31/117 (26) |
| Mini-Mental State Examination ≤24 | 1/102 (1) | 2/21 (10) | 20/175 (11) | 24/144 (17) |
| Body mass index, mean change (±SD) | 0.15 (±2.31) | 0.62 (±3.01) | −0.5 (±2.55) | −0.63 (±2.71) |
| Follow-up Age 70–78 (1990–1998) | Follow-up Age 78–85 (1998–2005) | |||
| Deterioration at Follow-up Among Participants Free of Poor or Disadvantaged Category at Baseline | Number of Participants With Deterioration at Age 78/Number of Participants With No Fatigue at Age 70, n (%) | Number of Participants With Deterioration at Age 78/Number of Participants With Fatigue at Age 70, n (%) | Number of Participants With Deterioration at Age 85/Number of Participants With No Fatigue at Age 78, n (%) | Number of Participants With Deterioration at Age 85/Number of Participants With Fatigue at Age 78, n (%) |
| Loneliness | 17/140 (12) | 12/38 (32)** | 38/193 (20) | 57/141 (40)*** |
| Depression | 13/162 (8) | 3/40 (8) | 31/206(15) | 46/138 (33)*** |
| Poor self-rated health | 49/190 (26) | 19/45 (42)* | 36/225(16) | 25/127 (20) |
| ADL dependence | 12/199 (6) | 12/71 (17)** | 64/263 (24) | 81/222(36)** |
| ADL difficulty | 27/192 (14) | 24/57 (42)*** | 93/250 (37) | 67/169 (39) |
| Low physical activity | 8/136 (6) | 8/37 (22)** | 58/227 (25) | 65/184 (36)* |
| Poor sleep satisfaction | 20/173 (12) | 16/48 (33)** | 44/240 (18) | 40/164 (24) |
| Hypertension | 49/150 (32) | 14/35 (40) | 59/140 (42) | 52/108 (48) |
| Ischemic heart disease | 46/177 (26) | 12/53 (23) | 31/198 (16) | 30/150 (20) |
| Diabetes mellitus | 13/196 (6) | 4/62 (6) | 18/221 (8) | 17/213(8) |
| Anemia | 19/120 (16) | 8/46 (17) | 30/121 (25) | 31/117 (26) |
| Mini-Mental State Examination ≤24 | 1/102 (1) | 2/21 (10) | 20/175 (11) | 24/144 (17) |
| Body mass index, mean change (±SD) | 0.15 (±2.31) | 0.62 (±3.01) | −0.5 (±2.55) | −0.63 (±2.71) |
Notes: For all numbers shown, the numerator represents the number of participants who were free of the poor or disadvantaged category at baseline, who deteriorated in the variable being examined during the follow-up period (either from ages 70–78 years or from ages 78–85 years), according to fatigue status at baseline. The denominator represents the number of participants who were free of the poor or disadvantaged category at baseline according to fatigue status, thus grouped to participants with no fatigue or participants with fatigue. Thus, at age 70, 140 participants were neither lonely nor fatigued. By age 78, 17/140 (12%) had become lonely. In contrast, among the 38 not lonely yet fatigued participant at age 70, 12/38 (22%) had become lonely at follow-up at age 78. ADL = activities of daily living.
*p ≤ .05, **p ≤ .005, ***p ≤ .0001.
The Predictive Value (OR) of Fatigue at Ages 70 and 78 on Decline in Health Outcome Variables at Ages 78 and 85
| Age 70 | Age 78 | |||||||
| Decline in Response Variable | Unadjusted OR (95% CI) | Adjusted OR | 95% CI | p | Unadjusted OR (95% CI) | Adjusted OR | 95% CI | p |
| Poor self-rated health | 2.9 (1.6–5.1) | 2.12 | 1.05–4.3 | .03 | 1.79 (1.2–2.8) | 1 | 0.6–1.8 | .9 |
| ADL dependence | * | * | * | * | 1.8 (1.2–2.6) | 0.9 | 0.5–1.6 | .8 |
| ADL difficulty | 4 (2.1–7.4) | 2.9 | 1.4–6.1 | .004 | 1.2 (0.8–1.7) | 0.7 | 0.4–1.2 | .2 |
| Depression | 1.1 (0.4–2.9) | 0.65 | 0.2–2.1 | .4 | 2.8 (1.7–4.5) | 2 | 1.2–3.5 | .01 |
| Loneliness† | 2.8 (1.6–4.7) | 1.8 | 0.8–3.7 | .1 | 2.6 (1.8–3.7) | 4.7 | 3.0–7.6 | .001 |
| Low physical activity | 4.7 (2.5–8.7) | 5.1 | 1.9–13.4 | .0009 | 1.84 (1.25–2.7) | 1.3 | 0.8–2.2 | .3 |
| Poor sleep satisfaction | 2.7 (1.5–5) | 2.1 | 1–4.5 | .048 | 1.4 (0.9–2.1) | 1.2 | 0.7–2.1 | .5 |
| Age 70 | Age 78 | |||||||
| Decline in Response Variable | Unadjusted OR (95% CI) | Adjusted OR | 95% CI | p | Unadjusted OR (95% CI) | Adjusted OR | 95% CI | p |
| Poor self-rated health | 2.9 (1.6–5.1) | 2.12 | 1.05–4.3 | .03 | 1.79 (1.2–2.8) | 1 | 0.6–1.8 | .9 |
| ADL dependence | * | * | * | * | 1.8 (1.2–2.6) | 0.9 | 0.5–1.6 | .8 |
| ADL difficulty | 4 (2.1–7.4) | 2.9 | 1.4–6.1 | .004 | 1.2 (0.8–1.7) | 0.7 | 0.4–1.2 | .2 |
| Depression | 1.1 (0.4–2.9) | 0.65 | 0.2–2.1 | .4 | 2.8 (1.7–4.5) | 2 | 1.2–3.5 | .01 |
| Loneliness† | 2.8 (1.6–4.7) | 1.8 | 0.8–3.7 | .1 | 2.6 (1.8–3.7) | 4.7 | 3.0–7.6 | .001 |
| Low physical activity | 4.7 (2.5–8.7) | 5.1 | 1.9–13.4 | .0009 | 1.84 (1.25–2.7) | 1.3 | 0.8–2.2 | .3 |
| Poor sleep satisfaction | 2.7 (1.5–5) | 2.1 | 1–4.5 | .048 | 1.4 (0.9–2.1) | 1.2 | 0.7–2.1 | .5 |
Notes: All models, except for the loneliness model, include at baseline gender, education, self-rated health, ischemic heart disease, diabetes, hypertension, ADL difficulty, depression, back or joint pain, poor sleep satisfaction, and fatigue. ADL = activities of daily living; CI = confidence interval; OR = odds ratio.
The validity of the model fit is questionable due to low numbers of incident ADL dependence from ages 70–78 years.
The model for loneliness includes all the above except for depression at baseline. Instead, loneliness at baseline is included.
The Predictive Value (OR) of Fatigue at Ages 70 and 78 on Decline in Health Outcome Variables at Ages 78 and 85
| Age 70 | Age 78 | |||||||
| Decline in Response Variable | Unadjusted OR (95% CI) | Adjusted OR | 95% CI | p | Unadjusted OR (95% CI) | Adjusted OR | 95% CI | p |
| Poor self-rated health | 2.9 (1.6–5.1) | 2.12 | 1.05–4.3 | .03 | 1.79 (1.2–2.8) | 1 | 0.6–1.8 | .9 |
| ADL dependence | * | * | * | * | 1.8 (1.2–2.6) | 0.9 | 0.5–1.6 | .8 |
| ADL difficulty | 4 (2.1–7.4) | 2.9 | 1.4–6.1 | .004 | 1.2 (0.8–1.7) | 0.7 | 0.4–1.2 | .2 |
| Depression | 1.1 (0.4–2.9) | 0.65 | 0.2–2.1 | .4 | 2.8 (1.7–4.5) | 2 | 1.2–3.5 | .01 |
| Loneliness† | 2.8 (1.6–4.7) | 1.8 | 0.8–3.7 | .1 | 2.6 (1.8–3.7) | 4.7 | 3.0–7.6 | .001 |
| Low physical activity | 4.7 (2.5–8.7) | 5.1 | 1.9–13.4 | .0009 | 1.84 (1.25–2.7) | 1.3 | 0.8–2.2 | .3 |
| Poor sleep satisfaction | 2.7 (1.5–5) | 2.1 | 1–4.5 | .048 | 1.4 (0.9–2.1) | 1.2 | 0.7–2.1 | .5 |
| Age 70 | Age 78 | |||||||
| Decline in Response Variable | Unadjusted OR (95% CI) | Adjusted OR | 95% CI | p | Unadjusted OR (95% CI) | Adjusted OR | 95% CI | p |
| Poor self-rated health | 2.9 (1.6–5.1) | 2.12 | 1.05–4.3 | .03 | 1.79 (1.2–2.8) | 1 | 0.6–1.8 | .9 |
| ADL dependence | * | * | * | * | 1.8 (1.2–2.6) | 0.9 | 0.5–1.6 | .8 |
| ADL difficulty | 4 (2.1–7.4) | 2.9 | 1.4–6.1 | .004 | 1.2 (0.8–1.7) | 0.7 | 0.4–1.2 | .2 |
| Depression | 1.1 (0.4–2.9) | 0.65 | 0.2–2.1 | .4 | 2.8 (1.7–4.5) | 2 | 1.2–3.5 | .01 |
| Loneliness† | 2.8 (1.6–4.7) | 1.8 | 0.8–3.7 | .1 | 2.6 (1.8–3.7) | 4.7 | 3.0–7.6 | .001 |
| Low physical activity | 4.7 (2.5–8.7) | 5.1 | 1.9–13.4 | .0009 | 1.84 (1.25–2.7) | 1.3 | 0.8–2.2 | .3 |
| Poor sleep satisfaction | 2.7 (1.5–5) | 2.1 | 1–4.5 | .048 | 1.4 (0.9–2.1) | 1.2 | 0.7–2.1 | .5 |
Notes: All models, except for the loneliness model, include at baseline gender, education, self-rated health, ischemic heart disease, diabetes, hypertension, ADL difficulty, depression, back or joint pain, poor sleep satisfaction, and fatigue. ADL = activities of daily living; CI = confidence interval; OR = odds ratio.
The validity of the model fit is questionable due to low numbers of incident ADL dependence from ages 70–78 years.
The model for loneliness includes all the above except for depression at baseline. Instead, loneliness at baseline is included.
Mortality From Any Cause According to Age of Baseline Fatigue
| Hazard Ratio (95% confidence interval) | ||||
| Age at baseline fatigue, y | 70 | 78 | 85 | 70–85* |
| Follow-up period, y | 70–78 | 78–85 | 85–88 | 70–88 |
| Adjusted for sex | 1.87 (1.26–2.78) | 1.6 (1.3–2) | 2.1 (1.53–2.8) | 1.64 (1.25–2.14) |
| Basic model† | 1.52 (1.01–2.28) | 1.6 (1.2–2) | 2.08 (1.35–3.22) | 1.52 (1.14–2.03) |
| Basic model + physical activity | 1.35 (0.89–2.05) | 1.5 (1.1–1.9) | 1.68 (1.08–2.62) | 1.32 (0.97–1.79) |
| Basic model + depression | 1.38 (0.86–2.20) | 1.2 (0.9–1.7) | 1.80 (1.09–2.97) | 1.43 (1.02–2.00) |
| Basic model + physical activity + depression | 1.29 (0.79–2.11) | 1.2 (0.9–1.7) | 1.55 (0.93–2.59) | 1.31 (0.92–1.86) |
| Hazard Ratio (95% confidence interval) | ||||
| Age at baseline fatigue, y | 70 | 78 | 85 | 70–85* |
| Follow-up period, y | 70–78 | 78–85 | 85–88 | 70–88 |
| Adjusted for sex | 1.87 (1.26–2.78) | 1.6 (1.3–2) | 2.1 (1.53–2.8) | 1.64 (1.25–2.14) |
| Basic model† | 1.52 (1.01–2.28) | 1.6 (1.2–2) | 2.08 (1.35–3.22) | 1.52 (1.14–2.03) |
| Basic model + physical activity | 1.35 (0.89–2.05) | 1.5 (1.1–1.9) | 1.68 (1.08–2.62) | 1.32 (0.97–1.79) |
| Basic model + depression | 1.38 (0.86–2.20) | 1.2 (0.9–1.7) | 1.80 (1.09–2.97) | 1.43 (1.02–2.00) |
| Basic model + physical activity + depression | 1.29 (0.79–2.11) | 1.2 (0.9–1.7) | 1.55 (0.93–2.59) | 1.31 (0.92–1.86) |
Notes: *Time-dependent Cox proportional hazards models using time-dependent variables. All participants from the entire study included in this model. According to number of times participants participated, they had one, two, or three measurements for fatigue.
The Cox proportional hazards basic model adjusted for gender, education, smoking, history of neoplasm, diabetes mellitus, ischemic heart disease, and hypertension in addition to fatigue measured at baseline.
Mortality From Any Cause According to Age of Baseline Fatigue
| Hazard Ratio (95% confidence interval) | ||||
| Age at baseline fatigue, y | 70 | 78 | 85 | 70–85* |
| Follow-up period, y | 70–78 | 78–85 | 85–88 | 70–88 |
| Adjusted for sex | 1.87 (1.26–2.78) | 1.6 (1.3–2) | 2.1 (1.53–2.8) | 1.64 (1.25–2.14) |
| Basic model† | 1.52 (1.01–2.28) | 1.6 (1.2–2) | 2.08 (1.35–3.22) | 1.52 (1.14–2.03) |
| Basic model + physical activity | 1.35 (0.89–2.05) | 1.5 (1.1–1.9) | 1.68 (1.08–2.62) | 1.32 (0.97–1.79) |
| Basic model + depression | 1.38 (0.86–2.20) | 1.2 (0.9–1.7) | 1.80 (1.09–2.97) | 1.43 (1.02–2.00) |
| Basic model + physical activity + depression | 1.29 (0.79–2.11) | 1.2 (0.9–1.7) | 1.55 (0.93–2.59) | 1.31 (0.92–1.86) |
| Hazard Ratio (95% confidence interval) | ||||
| Age at baseline fatigue, y | 70 | 78 | 85 | 70–85* |
| Follow-up period, y | 70–78 | 78–85 | 85–88 | 70–88 |
| Adjusted for sex | 1.87 (1.26–2.78) | 1.6 (1.3–2) | 2.1 (1.53–2.8) | 1.64 (1.25–2.14) |
| Basic model† | 1.52 (1.01–2.28) | 1.6 (1.2–2) | 2.08 (1.35–3.22) | 1.52 (1.14–2.03) |
| Basic model + physical activity | 1.35 (0.89–2.05) | 1.5 (1.1–1.9) | 1.68 (1.08–2.62) | 1.32 (0.97–1.79) |
| Basic model + depression | 1.38 (0.86–2.20) | 1.2 (0.9–1.7) | 1.80 (1.09–2.97) | 1.43 (1.02–2.00) |
| Basic model + physical activity + depression | 1.29 (0.79–2.11) | 1.2 (0.9–1.7) | 1.55 (0.93–2.59) | 1.31 (0.92–1.86) |
Notes: *Time-dependent Cox proportional hazards models using time-dependent variables. All participants from the entire study included in this model. According to number of times participants participated, they had one, two, or three measurements for fatigue.
The Cox proportional hazards basic model adjusted for gender, education, smoking, history of neoplasm, diabetes mellitus, ischemic heart disease, and hypertension in addition to fatigue measured at baseline.
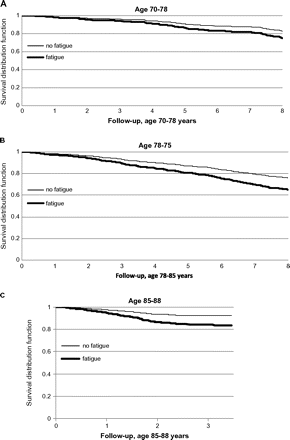
Adjusted Kaplan–Meier survival curves according to fatigue. (A) Cumulative survival according to fatigue from ages 70–78 years (p = .045). (B) Cumulative survival according to fatigue from ages 78–85 years (p < .001). (C) Cumulative survival according to fatigue from ages 85–88 years (p = .001). Adjusted according to Cox proportional hazards basic model for gender, education, smoking, history of neoplasm, diabetes mellitus, ischemic heart disease, and hypertension.
Data storage and analysis was performed using SAS 9.1e package (SAS Institute Inc., Cary, NC). All p values were two tailed, and p < .05 was considered significant.
RESULTS
Fatigue was a frequent complaint throughout the entire study period being reported by 29%, 53%, and 68% of participants at ages 70, 78, and 85, respectively, with prevalence increased among women. As seen in Table 1, cross-sectional associations were observed between fatigue and a range of psychosocial, functional, and physical parameters. In particular, fatigue at ages 70, 78, and 85 was consistently associated with loneliness, depression, SRH, ADL (both dependence and difficulty), low physical activity, chronic back or joint pain, and poor sleep satisfaction. Similarly, hypertension and ischemic heart disease were associated with fatigue throughout follow-up, whereas diabetes was not.
Separate comparison according to gender did not change the findings, except for BMI at age 78 and for dependence in ADL, which were significantly more common in fatigued women.
Participants with fatigue were significantly more likely to deteriorate during follow-up in a number of different health and functional parameters, as shown in Table 2.
Fatigued participants at both ages were more likely to complain of subsequent loneliness, poor SRH, and reduced likelihood of engaging in regular physical activity. In contrast, fatigued participants did not have an increased likelihood for subsequent onset of diabetes, hypertension, or ischemic heart disease.
The value of fatigue in the prediction of subsequent health and functional status was further examined in multiple logistic regression analyses (Table 3). Fatigue at age 70 was found to remain significantly associated at age 78 with poor SRH, difficulty in performing ADL, reduced levels of physical activity, and poor global sleep satisfaction. After adjusting for the same covariates, fatigue at age 78 was significantly associated with the onset of depression and loneliness at age 85.
Fatigue was associated with decreased survival throughout the entire follow-up. The survival rate among fatigued vs nonfatigued participants was 70% vs 81% (p = .0085) from ages 70–78 years, 65% vs 74% (p < .0012) from ages 78–85 years, and 81% vs 91% (p < .0001) from ages 85–88 years. Thus, the magnitude of survival difference between fatigued vs nonfatigued participants at ages 70, 78, and 85 for subsequent survival was 11% (age 70–78), 9% (age 78–85), and 10% (age 85–88). After adjusting for known mortality risk factors, fatigue at age 70, 78, and 85 was associated with increased mortality (Table 4, Figure 2). The addition of physical activity to the model reduced the significance of the association at age 70, and the addition of depression to the model reduced the significance at both age 70 and age 78. After the addition of both depression and physical activity to the model, fatigue was no longer predictive of increased mortality. Similarly, when treated as a time-dependent variable, fatigue was associated with increased mortality in the basic model. Addition of either depression or physical activity to the time-dependent model reduced the significance of the relationship between fatigue and mortality.
We examined the interaction term of fatigue with age (ie, ages 70, 78, and 85) in the basic mortality model from ages 70–88 years. The interaction term at age 70 served as the reference (HR = 1). The HR for the interaction terms at age 78 was 0.9 (95% CI = 0.64–1.38; p = .76), and at age 85, the HR was 1.47 (95% CI = 0.77–2.79; p = .23). Fatigue remained independently associated with mortality (HR = 1.60, 95% CI = 1.22–2.05).
DISCUSSION
The results of this 18-year longitudinal study of an age-homogenous cohort studied from ages 70–88 years support the hypothesis that fatigue across advancing age is associated with increased mortality and greater likelihood of subsequent decline in health and levels of physical activity, functional status, and depression.
These findings both corroborate and extend those of Hardy and Studenski (13), which found that fatigue predicted increased 10-year mortality risk in an age-heterogeneous cohort of community-dwelling elderly people. Like Vestergaard and coworkers (11), Hardy and Studenski (12) also described cross-sectional associations between fatigue and functional limitations, which remained over 3 years of follow-up. While reproducing similar cross-sectional findings at ages 70, 78 and 85, our findings also emphasize the value of fatigue in predicting subsequent deterioration in health and functional status. We also have shown that the negative influence of fatigue on mortality remains of similar magnitude irrespective of advancing age to include even the oldest old. This was a robust finding emerging from both unadjusted and adjusted survival curves and from the proportional hazards models, which included a time-dependent model spanning the entire study period. Furthermore, the inclusion of the interaction term of fatigue and age did not detract from the significant independent association between fatigue per se and increased mortality.
Generalized fatigue is a separate more generic entity from “tiredness in daily activities,” which has been studied by Avlund and coworkers (6–9). The finding that tiredness in daily activities may be a subjective measure for the identification of frailty (10) may also apply to fatigue, which is implicated in theoretical understanding and definitions of frailty (26,27).
The complex coexistence, and potential interaction, of fatigue, comorbidity, psychosocial and functional decline, as well as risk factors such as reduced levels of physical activity, raises the issues of reverse causality when interpreting observational studies. Among younger populations, tiredness has been found to be associated with a number of modifiable risk factors, including obesity, poor diet, and reduced physical activity (28), and among older adults, tiredness in daily activities was shown to be associated with deteriorating level of physical activity over 4.5 years of follow-up (29). The importance of not only continuing but also initiating physical activity among the oldest old has been described in our cohort (23), and the present finding that fatigue predicts subsequent decline in physical activity may suggest possible pathways in the mechanism whereby fatigue feeds into a cycle of frailty, functional decline, and reduced survival. Including physical activity in the mortality models reduced the significance of the relationship between fatigue and mortality. Similarly, including an interaction variable (fatigue and physical activity) did not alter the findings (data not shown). Thus, our findings suggest that fatigue may indeed lead to a reduction in physical activity levels, the negative effect of which may overshadow any residual effects of fatigue per se.
An additional point deserving clarification is the relationship between fatigue and depression. Fatigue may be a proxy for depression, reflecting either an early or subclinical symptom. Depression was three times more frequent among fatigued participants at all ages, and fatigued nondepressed participants at age 78 were twice as likely to develop subsequent depression. However, adding depression to the proportional hazard models reduced the significance of the influence of fatigue on mortality, and the introduction of an interaction variable (fatigue and depression) did not alter the findings (data not shown). The weakening of the effect of fatigue on outcomes following adjustment for depression has been reported elsewhere (11) and reinforces the complex interplay between depressive symptoms and fatigue. It is interesting to notice that “feeling tired” does not figure among the symptoms checklist of many common depression scales—Brief Symptom Inventory (18), Beck Depression Inventory (30), or the Geriatric Depression Scale (31). The Center for Epidemiological Studies-Depression scale (32) includes two questions on tiredness, which have themselves been used as the basis for research into fatigue (11).
It remains to be clarified if early recognition of fatigue may enable the initiation of an appropriate intervention aimed at addressing both the causes and the potential consequences of fatigue. For instance, improving sleep quality by environmental or pharmaceutical means, controlling pain, searching for underlying depression, and providing nutritional support, together with the encouragement of physical activity, could help to break the vicious cycle involving fatigue, decreased activity, and depression, themselves inducing further increased fatigue.
The study limitations include a healthy survivor bias, a product of diminishing sample size during follow-up due to death and dropout. However, the study sample was augmented at age 78 and 85 with randomly chosen recruits from the same birth cohort, which served to redress the sample's representative nature. Nonetheless, the sample size was relatively small, in particular among the analysis of deteriorating measures of health and function. An additional concern is the validity and sensitivity of the single question used to detect fatigue. However, despite a number of instruments in existence measuring various dimensions of fatigue, consensus is lacking as to any single measure and different researchers use different dimensions according to the context of their work (11,33). Finally, it should be noted that the data acquisition was based upon self-reported data at the time of assessment. While the study physician confirmed medical diagnosis, nonetheless, the self-reported nature of the data might have served as a source of inaccuracy.
In conclusion, our study provides evidence that fatigue among older people, up to and including the oldest old, is a harbinger of declining health and functional trajectories, culminating in increased mortality. The intimate association of fatigue, depression, and physical activity suggest potential lines of research, aimed at understanding the transition from perceived fatigue to declining performance, and developing strategies for diminishing its effects on longevity and quality of life.
FUNDING
This work was supported by funds from the Ministry of Labor and Social Affairs of the State of Israel, ESHEL—the Association for the Planning and Development of Services for the Aged in Israel, The National Insurance Institute, and various private charitable donors. The funding sources had no role in the design and conduct of the study, analysis, or interpretation of the data or preparation or final approval of the manuscript.
CONFLICT OF INTEREST
There were no conflicts of interest involved in the undertaking of this study for any of the authors.
We thank Ms Ella Ein-Mor, Ms Aliza Hammerman-Rozenberg, and Ms Irene Kornilenko for statistical work and Ms Etty Arbel for organizational contribution.
References
Author notes
These authors contributed equally and share joint first authorship.
Decision Editor: Luigi Ferrucci, MD, PhD