-
PDF
- Split View
-
Views
-
Cite
Cite
Joanne Feeney, Neil O’Leary, Rachel Moran, Aisling M O’Halloran, John M Nolan, Stephen Beatty, Ian S Young, Rose Anne Kenny, Plasma Lutein and Zeaxanthin Are Associated With Better Cognitive Function Across Multiple Domains in a Large Population-Based Sample of Older Adults: Findings from The Irish Longitudinal Study on Aging, The Journals of Gerontology: Series A, Volume 72, Issue 10, October 2017, Pages 1431–1436, https://doi.org/10.1093/gerona/glw330
Close - Share Icon Share
Abstract
Low blood serum or plasma concentrations of the xanthophyll carotenoids lutein and zeaxanthin have been implicated in poorer cognitive health in older adults. However, equivocal results from smaller studies and clinical trials highlight the need for large population-based studies with comprehensive measures of cognitive function and adjustment for multiple confounders to examine such associations in more depth.
In the current study, we investigated the association between plasma lutein and zeaxanthin and domain-specific cognitive performance in 4,076 community-dwelling adults aged 50 years or older from The Irish Longitudinal Study on Ageing. Mixed-effects models were fitted with adjustment for demographic and socioeconomic factors, health conditions, and health behaviors.
Higher plasma lutein and zeaxanthin were independently associated with better composite scores across the domains of global cognition, memory, and executive function. We also found evidence that higher plasma zeaxanthin, but not lutein, was associated with better processing speed. These associations were consistent across domains.
Further investigation of the prognostic value of carotenoid concentrations, and their changes, on cognition in similar population-based samples longitudinally is warranted.
A large body of evidence suggests that a higher intake of fruit and vegetables is beneficial for health, and higher consumption has been linked to a reduced incidence of cardiovascular disease (1) and several types of cancer (2). In addition to vitamins, fiber, and other related compounds, a group of largely plant-based pigments called carotenoids are implicated in the health benefits arising from a good diet. These lipid soluble compounds are ubiquitous in fruits and vegetables, most notably orange/yellow colored and leafy green vegetables (3). The chemical structure of carotenoids allows them to act as efficient singlet oxygen and peroxyl radical scavengers (4). In addition to their antioxidant properties, it has been suggested that carotenoids may also reduce inflammation by interacting with inflammatory cellular signaling cascades (5). Furthermore, as well as physical health benefits, carotenoids and other dietary antioxidants have been positively associated with brain health and cognitive function, although the results of large population studies and clinical trials have been somewhat equivocal to date (see (6) for a review).
In the last few years, there has been increased interest in the potential role of two carotenoids in particular, lutein and zeaxanthin, in brain health. Lutein and zeaxanthin (total zeaxanthin comprising both zeaxanthin and meso-zeaxanthin) are xanthophyll carotenoids and are the primary constituents of a protective substance in the eye called macular pigment (7). It is well established that these compounds protect the retina from light-induced damage—due to their ability to filter short-wave light (8)—and act to stabilize reactive oxygen species (9). Higher intake of these nutrients, either through diet or supplementation, has been associated with an increase in macular pigment (eg, (10,11)), and there is evidence that they may slow the progression of age-related macular degeneration (12), a leading cause of vision loss among older adults, and improve vision in individuals with the early form of the disease (13). Furthermore, work by our group and others has shown that a higher density of macular pigment is associated with better cognitive function in older adults, across multiple domains (14–16). Importantly, it has been discovered that lutein and zeaxanthin are in the human brain (17,18). Moreover, Johnson and colleagues provided evidence that lutein is the carotenoid which is present in the highest concentration in the brain despite being found at a lower concentration relative to several other carotenoids in the peripheral blood circulation, as assessed using matched brain tissue and serum samples in a group of centenarians (18). Johnson and colleagues also found that a higher concentration of lutein and zeaxanthin in the brain was associated with better cognitive function (18). The factors which influence uptake of these carotenoids into the retina and brain are, as yet, not fully clear, and the question of whether manipulating intake of the xanthophyll carotenoids can improve cognitive function or protect against cognitive decline has not been satisfactorily answered. Results from studies investigating the effects of supplementation or increasing dietary intake of lutein and zeaxanthin on cognition in a healthy elderly population have been contradictory, with some showing a beneficial effect on cognitive performance (19,20), and others finding no effect (21,22). Similarly, previous studies investigating plasma or serum concentrations of lutein and zeaxanthin in relation to cognition have been few (eg, (23,24)). It is noteworthy, however, that in general sample, characteristics and sample sizes have varied widely between and within the various observational and clinical studies, which may in part explain the discrepant findings. Furthermore, the nature and scale of the cognitive testing employed have also been very variable, rendering assessment of domain-specific associations challenging.
The primary aim of this study was to investigate the independent association between plasma lutein, zeaxanthin, and cognitive function, in a large population-based sample of older adults. A secondary aim was to examine whether the association differed between domains of cognition.
Methods
Participants and Design
Cross-sectional data from the first wave of The Irish Longitudinal Study on Ageing (TILDA) were analyzed. TILDA is a nationally representative prospective cohort study that aims to assess the health, social, and economic characteristics of adults aged 50 years and older in Ireland. TILDA surveyed 8,175 community-dwelling adults aged 50 and older at Wave 1, which was conducted between October 2009 and February 2011. Details of the study design have previously been published (25,26). Briefly, participants were recruited through a two-stage clustered, stratified sampling design by geographic area and by household. The data collected comprised (i) a computer-assisted personal interview (CAPI) carried out in the participant’s own home; (ii) a self-completion questionnaire; and (iii) a health assessment carried out by trained nurses either in a dedicated center or in the respondent’s own home. Ethics approval was obtained by the institutional review board, and all respondents provided signed informed consent prior to participation in the study.
Exclusion Criteria
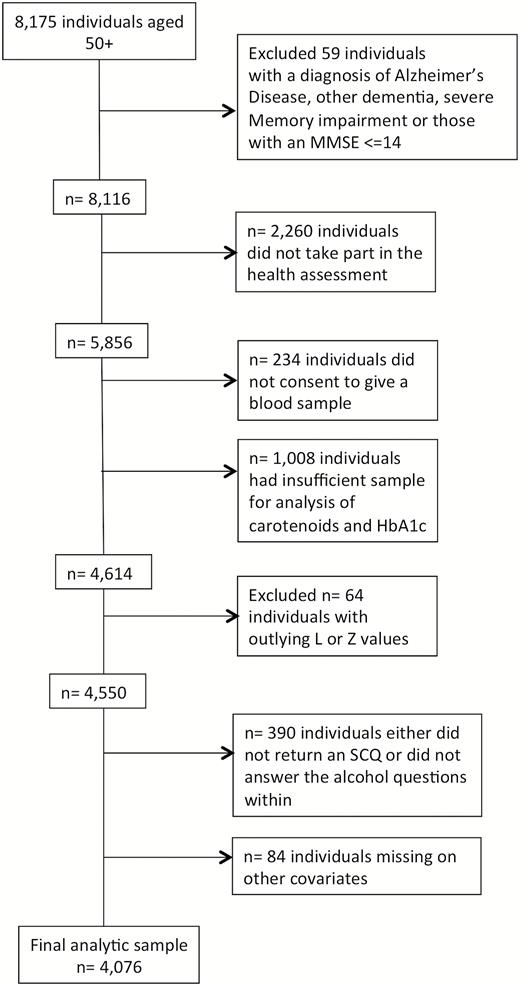
Individual who self-reported a doctor’s diagnosis of Alzheimer’s disease, other dementia, severe memory impairment, or had an Mini-Mental State Examination (MMSE) score of less than 14 (n = 59) were excluded from the analysis. A significant proportion (n = 2,260) of the sample did not undergo a health assessment and thus were necessarily omitted from further analysis. A further 1,242 individuals did not give a sufficient blood sample for the analysis of lutein and zeaxanthin, or did not consent to giving a blood sample. Individuals with outlying lutein or zeaxanthin values (greater or less than 3SD above or below the group mean) were excluded. Additional missingness on covariates left 4,076 individuals with full data for analysis. Figure 1 details the number of individuals excluded or with missing data on the various components of the larger study. A comparison of the analytic sample with those who were excluded from the current analysis due to missing data on one or more variables of interest revealed that the analytic sample was younger, more highly educated, and had higher cognitive scores than those excluded due to missingness (see Supplementary Table 1 for full descriptive analysis).
Flow chart showing the pattern of missing data and the final sample for analysis.
Cognitive Assessment
Cognitive function was assessed using a large battery of neuropsychological tests. Composite scores were derived by combining individual test scores to broadly reflect cognitive performance across four aspects of function: memory, processing speed, executive function, and global cognition. Details on the individual tests are published elsewhere (27). The composite score for global cognition was derived from the scores on the MMSE and Montreal Cognitive Assessment (MoCA) by summing the two scores and then transforming using log([60 − sum score] +3) as suggested by Frewen and colleagues (28). Composite scores for all other domains were created based on an equally unit-weighted approach using standardized scores (z scores). The composite score for memory was derived from the total score from an immediate word recall task (comprising the sum of two recall trials), a delayed word recall task, and a picture recall task. The composite score for processing speed was derived from the time to complete the Colour Trails 1 test, the cognitive reaction time from a choice reaction time task, and mean response time on the Sustained Attention to Response Task. The executive function composite was created from the score on the semantic fluency (animal naming) task, the time to complete Colour Trails 2, and the visual reasoning test from the Cambridge Cognitive Examination. For all composite scores, lower values corresponded with lower performance. All cognitive tests were carried out as part of the health assessment with the exception of the immediate and delayed word recall tasks and the animal naming task, which formed part of the CAPI.
Blood Collection and Plasma Carotenoid Analysis
Nonfasting venous blood samples were collected into one 5-mL lithium heparin tube (BD, Becton, Dickinson Limited, Oxford, UK) and two 10-mL ethylene diamine tetra-acetic acid (EDTA; BD, Becton, Dickinson Limited) tubes for long-term storage. Samples were collected during the health assessment. The TILDA protocol for blood sample collection, processing, and storage has been described previously (29). For the measurement of carotenoids, one of the EDTA tubes designated for long-term storage was immediately protected from direct light. This blood sample was centrifuged and 1 mL of EDTA plasma was allocated for carotenoid assessment and stored at −80°C until analysis. Stored blood samples were also analyzed for glycated hemoglobin (HbA1c, used to diagnose diabetes and prediabetes). Separate written and verbal consent from participants was required to obtain and store blood samples. 1 ml of frozen EDTA plasma samples was then transferred to the Macular Pigment Research Group, Waterford Institute of Technology, for analysis. Lutein and total zeaxanthin were analyzed using a reversed phase high-performance liquid chromatography (HPLC) method. Details of the extraction procedures, HPLC analysis, and method validation have been previously described (30,31).
Covariates
Information on demographic characteristics age, sex, education, and living status (living alone, cohabiting with a partner, cohabiting with others) was collected during the CAPI interview. The highest level of education attained was coded into six categories: no formal education or incomplete primary; primary or equivalent; intermediate/junior/group certificate; leaving certificate or equivalent; diploma/certificate; primary degree; or postgraduate degree or higher. Individuals were asked about their smoking habits and were categorized never, past, or current smokers. Physical activity was assessed using the 8-item short form of the International Physical Activity Questionnaire (32). Alcohol intake was assessed by asking individuals to indicate how frequently during the last 6 months they had drunk alcohol (almost every day, 5–6 days a week, 3–4 days a week, 1–2 days a week, once or twice a month, less than once a month, or not at all in the last 6 months/don’t drink alcohol). Participants were also asked during the CAPI whether a doctor had ever told them that they had any chronic condition, which included hypertension, diabetes, glaucoma, cataracts, or age-related macular degeneration. Medication use was recorded during the CAPI and confirmed by cross-checking with medication labels. Medications were classified according to the Anatomical Therapeutic Classification (ATC) (http://www.whocc.no/atc_ddd_index/). Individuals were classified as hypertensive if they reported a doctor’s diagnosis of the condition or were taking an antihypertensive drug (antiadrenergic agents (‘C02’), diuretics (‘C03’), beta blockers (‘C07’), calcium channel blockers (‘C08’), angiotensin-converting enzyme inhibitors/ angiotensin-receptor blockers (‘C09’), and combinations of the above (‘C02’)). They were classified as diabetic if they self-reported a doctor’s diagnosis, if their blood HbA1c was greater than 6.5% (48 mmol/mol) (33), or they were taking a diabetic medication (ATC code ‘A10A’ or ‘A10B’). Use of dietary supplements consisting of minerals, vitamins, or products specifically containing lutein or zeaxanthin was noted and included as a covariate in a sensitivity analyses.
Statistical Analyses
The distribution of all variables of interest was initially examined via tables, histograms, and Q–Q plots. Bivariate tests of differences in lutein and zeaxanthin across categories of the other variables of interest were carried out using t tests, Chi-square tests, analysis of variance, Kruskal–Wallis, or Mann–Whitney U tests as appropriate. A comparison of individuals in the analytic sample versus those with missing data on one or more variables of interest was undertaken. Analysis of the relationship between carotenoids and cognition was carried out using mixed-effects regression modeling. Mixed-effects regression is a more parsimonious alternative to running separate ordinary least squares regression models with each cognitive domain as a separate outcome. It also facilitates joint tests of association between each carotenoid and all cognitive domains and allows direct comparisons of the relationship between lutein or zeaxanthin and cognition, between domains. Two separate mixed-effects hierarchical linear models were fitted for lutein and zeaxanthin, with a nested structure such that scores on the four cognitive domains (Level 1) were nested within each individual (individual as a Level 2 random intercept). Each individual, in turn, was nested within a household (household as a Level 3 random intercept). The following covariates were also included in the model as fixed effects: age and age-squared, sex, education, smoking, physical activity, alcohol use, diabetes, hypertension, and eye disease. An interaction term for education with cognitive domain was also added, in order to allow the potential for the association of the carotenoid with the differing cognitive domains to be stratified by level of education, as education may influence performance on certain tests to a greater degree than others. The error variance was also allowed to vary as a function of cognitive domain to account for the difference in the measurement error variability of the different cognitive tests, specifying an unstructured correlation matrix between domains. Supplement use was added as a covariate in a second model run by a sensitivity analysis.
Analyses were carried out using Stata 12.0 and statistical significance was set at p less than .05.
Results
Sample Characteristics
Mean (SD) lutein in the analytic sample was 0.20 (0.10) µmol/L, range = 0.01 to 0.59. Mean (SD) of zeaxanthin was 0.053 (0.036) µmol/L, range = 0.003 to 0.284. Concentrations of lutein and zeaxanthin were related to nearly all demographic and health variables investigated (see Supplementary Table 2 for full descriptive analysis).
Mean scores on each of the individual cognitive variables by quartile of lutein and zeaxanthin are displayed in Table 1. A consistent pattern in these unadjusted associations can be observed whereby lower cognitive performance was associated with lower lutein and zeaxanthin, which was also evident when both carotenoids were examined as continuous variables (data not shown).
Individual Item Cognitive Scores by Quartile of Lutein and Zeaxanthin (N = 4,074)
| . | Lutein (quartiles) . | . | Zeaxanthin (quartiles) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) . | . | Mean (SD) . | . | |||||||
| . | 1st . | 2nd . | 3rd . | 4th . | p . | 1st . | 2nd . | 3rd . | 4th . | p . |
| Global | ||||||||||
| MMSE | 28.4 (1.9) | 28.7 (1.7) | 28.6 (1.7) | 28.9 (1.4) | <.001 | 28.2 (2.0) | 28.6 (1.6) | 28.8 (1.6) | 28.9 (1.4) | <.001 |
| MoCA | 24.7 (3.4) | 25.2 (3.2) | 25.3 (3.3) | 25.8 (2.9) | <.001 | 24.4 (3.6) | 25.1 (3.1) | 25.6 (3.1) | 25.8 (2.9) | <.001 |
| Memory | ||||||||||
| Immediate recall | 13.4 (3.0) | 13.7 (2.9) | 13.9 (2.9) | 14.2 (2.8) | <.001 | 13.1 (3.0) | 13.7 (2.9) | 14.2 (2.8) | 14.2 (2.9) | <.001 |
| Delayed recall | 5.9 (2.2) | 6.3 (2.2) | 6.3 (2.2) | 6.6 (2.2) | <.001 | 5.8 (2.2) | 6.2 (2.2) | 6.4 (2.2) | 6.6 (2.1) | <.001 |
| Picture recall | 3.2 (1.1) | 3.3 (1.1) | 3.3 (1.1) | 3.3 (1.1) | .19 | 3.2 (1.1) | 3.2 (1.1) | 3.3 (1.0) | 3.3 (1.0) | .029 |
| Processing speed | ||||||||||
| CRT RT (ms) | 50.5 (10.2) | 50.1 (9.8) | 49.1 (9.3) | 49.2 (9.7) | .002 | 51.1 (10.7) | 50.1 (9.6) | 49.4 (9.4) | 48.4 (9.3) | <.001 |
| SART RT (ms) | 38.2 (10.3) | 38.1 (10.0) | 37.8 (9.8) | 37.6 (9.5) | .48 | 38.3 (10.1) | 38.3 (10.0) | 37.9 (9.8) | 37.1 (9.5) | .012 |
| Colour Trail 1 time (s) | 55.7 (21.8) | 53.0 (20.4) | 53.3 (21.4) | 52.0 (21.1) | .001 | 57.8 (23.0) | 54.3 (20.8) | 52.5 (20.6) | 49.4 (19.4) | <.001 |
| Executive function | ||||||||||
| Visual reasoning | 3.0 (1.3) | 3.1 (1.3) | 3.2 (1.3) | 3.3 (1.3) | <.001 | 2.9 (1.3) | 3.1 (1.4) | 3.2 (1.3) | 3.4 (1.3) | <.001 |
| Semantic fluency | 20.6 (6.7) | 21.8 (6.9) | 21.7 (6.9) | 22.6 (6.8) | <.001 | 20.7 (6.7) | 21.2 (6.9) | 22.4 (6.7) | 22.5 (7.0) | <.001 |
| Colour Trail 2 time (s) | 110.2 (36.9) | 105.0 (33.3) | 106.8 (36.2) | 103.0 (33.4) | <.001 | 114.2 (37.4) | 108.3 (35.4) | 104.2 (33.9) | 98.3 (31.4) | <.001 |
| . | Lutein (quartiles) . | . | Zeaxanthin (quartiles) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) . | . | Mean (SD) . | . | |||||||
| . | 1st . | 2nd . | 3rd . | 4th . | p . | 1st . | 2nd . | 3rd . | 4th . | p . |
| Global | ||||||||||
| MMSE | 28.4 (1.9) | 28.7 (1.7) | 28.6 (1.7) | 28.9 (1.4) | <.001 | 28.2 (2.0) | 28.6 (1.6) | 28.8 (1.6) | 28.9 (1.4) | <.001 |
| MoCA | 24.7 (3.4) | 25.2 (3.2) | 25.3 (3.3) | 25.8 (2.9) | <.001 | 24.4 (3.6) | 25.1 (3.1) | 25.6 (3.1) | 25.8 (2.9) | <.001 |
| Memory | ||||||||||
| Immediate recall | 13.4 (3.0) | 13.7 (2.9) | 13.9 (2.9) | 14.2 (2.8) | <.001 | 13.1 (3.0) | 13.7 (2.9) | 14.2 (2.8) | 14.2 (2.9) | <.001 |
| Delayed recall | 5.9 (2.2) | 6.3 (2.2) | 6.3 (2.2) | 6.6 (2.2) | <.001 | 5.8 (2.2) | 6.2 (2.2) | 6.4 (2.2) | 6.6 (2.1) | <.001 |
| Picture recall | 3.2 (1.1) | 3.3 (1.1) | 3.3 (1.1) | 3.3 (1.1) | .19 | 3.2 (1.1) | 3.2 (1.1) | 3.3 (1.0) | 3.3 (1.0) | .029 |
| Processing speed | ||||||||||
| CRT RT (ms) | 50.5 (10.2) | 50.1 (9.8) | 49.1 (9.3) | 49.2 (9.7) | .002 | 51.1 (10.7) | 50.1 (9.6) | 49.4 (9.4) | 48.4 (9.3) | <.001 |
| SART RT (ms) | 38.2 (10.3) | 38.1 (10.0) | 37.8 (9.8) | 37.6 (9.5) | .48 | 38.3 (10.1) | 38.3 (10.0) | 37.9 (9.8) | 37.1 (9.5) | .012 |
| Colour Trail 1 time (s) | 55.7 (21.8) | 53.0 (20.4) | 53.3 (21.4) | 52.0 (21.1) | .001 | 57.8 (23.0) | 54.3 (20.8) | 52.5 (20.6) | 49.4 (19.4) | <.001 |
| Executive function | ||||||||||
| Visual reasoning | 3.0 (1.3) | 3.1 (1.3) | 3.2 (1.3) | 3.3 (1.3) | <.001 | 2.9 (1.3) | 3.1 (1.4) | 3.2 (1.3) | 3.4 (1.3) | <.001 |
| Semantic fluency | 20.6 (6.7) | 21.8 (6.9) | 21.7 (6.9) | 22.6 (6.8) | <.001 | 20.7 (6.7) | 21.2 (6.9) | 22.4 (6.7) | 22.5 (7.0) | <.001 |
| Colour Trail 2 time (s) | 110.2 (36.9) | 105.0 (33.3) | 106.8 (36.2) | 103.0 (33.4) | <.001 | 114.2 (37.4) | 108.3 (35.4) | 104.2 (33.9) | 98.3 (31.4) | <.001 |
Note: CRT = choice reaction time; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; SART = Sustained Attention to Response Task.
Individual Item Cognitive Scores by Quartile of Lutein and Zeaxanthin (N = 4,074)
| . | Lutein (quartiles) . | . | Zeaxanthin (quartiles) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) . | . | Mean (SD) . | . | |||||||
| . | 1st . | 2nd . | 3rd . | 4th . | p . | 1st . | 2nd . | 3rd . | 4th . | p . |
| Global | ||||||||||
| MMSE | 28.4 (1.9) | 28.7 (1.7) | 28.6 (1.7) | 28.9 (1.4) | <.001 | 28.2 (2.0) | 28.6 (1.6) | 28.8 (1.6) | 28.9 (1.4) | <.001 |
| MoCA | 24.7 (3.4) | 25.2 (3.2) | 25.3 (3.3) | 25.8 (2.9) | <.001 | 24.4 (3.6) | 25.1 (3.1) | 25.6 (3.1) | 25.8 (2.9) | <.001 |
| Memory | ||||||||||
| Immediate recall | 13.4 (3.0) | 13.7 (2.9) | 13.9 (2.9) | 14.2 (2.8) | <.001 | 13.1 (3.0) | 13.7 (2.9) | 14.2 (2.8) | 14.2 (2.9) | <.001 |
| Delayed recall | 5.9 (2.2) | 6.3 (2.2) | 6.3 (2.2) | 6.6 (2.2) | <.001 | 5.8 (2.2) | 6.2 (2.2) | 6.4 (2.2) | 6.6 (2.1) | <.001 |
| Picture recall | 3.2 (1.1) | 3.3 (1.1) | 3.3 (1.1) | 3.3 (1.1) | .19 | 3.2 (1.1) | 3.2 (1.1) | 3.3 (1.0) | 3.3 (1.0) | .029 |
| Processing speed | ||||||||||
| CRT RT (ms) | 50.5 (10.2) | 50.1 (9.8) | 49.1 (9.3) | 49.2 (9.7) | .002 | 51.1 (10.7) | 50.1 (9.6) | 49.4 (9.4) | 48.4 (9.3) | <.001 |
| SART RT (ms) | 38.2 (10.3) | 38.1 (10.0) | 37.8 (9.8) | 37.6 (9.5) | .48 | 38.3 (10.1) | 38.3 (10.0) | 37.9 (9.8) | 37.1 (9.5) | .012 |
| Colour Trail 1 time (s) | 55.7 (21.8) | 53.0 (20.4) | 53.3 (21.4) | 52.0 (21.1) | .001 | 57.8 (23.0) | 54.3 (20.8) | 52.5 (20.6) | 49.4 (19.4) | <.001 |
| Executive function | ||||||||||
| Visual reasoning | 3.0 (1.3) | 3.1 (1.3) | 3.2 (1.3) | 3.3 (1.3) | <.001 | 2.9 (1.3) | 3.1 (1.4) | 3.2 (1.3) | 3.4 (1.3) | <.001 |
| Semantic fluency | 20.6 (6.7) | 21.8 (6.9) | 21.7 (6.9) | 22.6 (6.8) | <.001 | 20.7 (6.7) | 21.2 (6.9) | 22.4 (6.7) | 22.5 (7.0) | <.001 |
| Colour Trail 2 time (s) | 110.2 (36.9) | 105.0 (33.3) | 106.8 (36.2) | 103.0 (33.4) | <.001 | 114.2 (37.4) | 108.3 (35.4) | 104.2 (33.9) | 98.3 (31.4) | <.001 |
| . | Lutein (quartiles) . | . | Zeaxanthin (quartiles) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) . | . | Mean (SD) . | . | |||||||
| . | 1st . | 2nd . | 3rd . | 4th . | p . | 1st . | 2nd . | 3rd . | 4th . | p . |
| Global | ||||||||||
| MMSE | 28.4 (1.9) | 28.7 (1.7) | 28.6 (1.7) | 28.9 (1.4) | <.001 | 28.2 (2.0) | 28.6 (1.6) | 28.8 (1.6) | 28.9 (1.4) | <.001 |
| MoCA | 24.7 (3.4) | 25.2 (3.2) | 25.3 (3.3) | 25.8 (2.9) | <.001 | 24.4 (3.6) | 25.1 (3.1) | 25.6 (3.1) | 25.8 (2.9) | <.001 |
| Memory | ||||||||||
| Immediate recall | 13.4 (3.0) | 13.7 (2.9) | 13.9 (2.9) | 14.2 (2.8) | <.001 | 13.1 (3.0) | 13.7 (2.9) | 14.2 (2.8) | 14.2 (2.9) | <.001 |
| Delayed recall | 5.9 (2.2) | 6.3 (2.2) | 6.3 (2.2) | 6.6 (2.2) | <.001 | 5.8 (2.2) | 6.2 (2.2) | 6.4 (2.2) | 6.6 (2.1) | <.001 |
| Picture recall | 3.2 (1.1) | 3.3 (1.1) | 3.3 (1.1) | 3.3 (1.1) | .19 | 3.2 (1.1) | 3.2 (1.1) | 3.3 (1.0) | 3.3 (1.0) | .029 |
| Processing speed | ||||||||||
| CRT RT (ms) | 50.5 (10.2) | 50.1 (9.8) | 49.1 (9.3) | 49.2 (9.7) | .002 | 51.1 (10.7) | 50.1 (9.6) | 49.4 (9.4) | 48.4 (9.3) | <.001 |
| SART RT (ms) | 38.2 (10.3) | 38.1 (10.0) | 37.8 (9.8) | 37.6 (9.5) | .48 | 38.3 (10.1) | 38.3 (10.0) | 37.9 (9.8) | 37.1 (9.5) | .012 |
| Colour Trail 1 time (s) | 55.7 (21.8) | 53.0 (20.4) | 53.3 (21.4) | 52.0 (21.1) | .001 | 57.8 (23.0) | 54.3 (20.8) | 52.5 (20.6) | 49.4 (19.4) | <.001 |
| Executive function | ||||||||||
| Visual reasoning | 3.0 (1.3) | 3.1 (1.3) | 3.2 (1.3) | 3.3 (1.3) | <.001 | 2.9 (1.3) | 3.1 (1.4) | 3.2 (1.3) | 3.4 (1.3) | <.001 |
| Semantic fluency | 20.6 (6.7) | 21.8 (6.9) | 21.7 (6.9) | 22.6 (6.8) | <.001 | 20.7 (6.7) | 21.2 (6.9) | 22.4 (6.7) | 22.5 (7.0) | <.001 |
| Colour Trail 2 time (s) | 110.2 (36.9) | 105.0 (33.3) | 106.8 (36.2) | 103.0 (33.4) | <.001 | 114.2 (37.4) | 108.3 (35.4) | 104.2 (33.9) | 98.3 (31.4) | <.001 |
Note: CRT = choice reaction time; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; SART = Sustained Attention to Response Task.
Mixed-Effects Regression of Composite Cognitive Scores on Lutein and Zeaxanthin
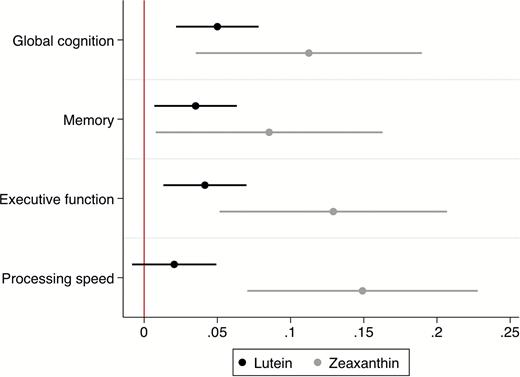
Figure 2 displays the fully adjusted mean estimated differences from the mixed-effects model for each of the four cognition composite scores for a 0.1-µmol/L increase in (a) lutein and (b) zeaxanthin. A significant, positive, independent association was observed between lutein and global cognition (β = 0.048, 95% confidence interval [CI] 0.021 to 0.075, p < .001), memory (β = 0.033, 95% CI 0.004 to 0.061, p = .026), and executive function (β = 0.040, 95% CI 0.013 to 0.068, p = .004). The relationship between lutein and processing speed was not statistically significant (β = 0.018, 95% CI −0.012 to 0.047, p = .245). Similar to the pattern observed with lutein, there was a significant association between zeaxanthin and global cognition (β = 0.112, 95% CI 0.039 to 0.185, p = .003), memory (β = 0.081, 95% CI 0.004 to 0.157, p = .039), and executive function (β = 0.120, 95% CI 0.043 to 0.197, p = .002). However, the association between zeaxanthin and processing speed was also significant (β = 0.130, 95% CI 0.052 to 0.208, p = .001). Post hoc pairwise comparisons did not reveal any significant difference between domains in the relationship of either carotenoid to cognitive performance. Further adjustment for supplement use did not alter the size of the coefficients. Conducting a sensitivity analysis by removing individuals with cognitive scores in the bottom 10% of the sample also did not markedly attenuate the observed effects, although the association between both carotenoids and the memory composite score was rendered nonsignificant (p values > .05)
Adjusted mean increase (with 95% confidence intervals) in the standardized cognitive score for each domain per 0.1-µmol/L increase in lutein and zeaxanthin. Models were adjusted for age, sex, education, smoking, alcohol use, exercise, body mass index, diabetes, hypertension, cataracts, glaucoma, age-related macular degeneration, and supplement use.
To compare the magnitude of the associations with the effect of age on cognition, we compared the coefficients associated with a 0.1-µmol/L increase in lutein and zeaxanthin with the association between age and cognition in a separate model adjusted for education and gender. The estimated age coefficient in the latter model was −0.034 per year compared with a coefficient for lutein in our fully adjusted model of 0.048 per 0.1 µmol/L, and zeaxanthin of 0.112 per 0.1 µmol/L. Therefore, the expected difference in cognition associated with an increase from the 25th to the 75th percentile in terms of lutein in our sample (a difference of 0.119 µmol/L) would be approximately equivalent to the difference in global cognition between a 62-year-old and a 64-year-old matched for gender and educational attainment. An increase from the 25th to the 75th percentile in zeaxanthin (a difference of 0.038 µmol/L) would correspond approximately to the expected difference between a 62- and 63-year-old matched for gender and educational status.
Discussion
In the present study, we investigated the cross-sectional association of plasma concentrations of lutein and zeaxanthin with domain- specific cognitive function among 4,076 individuals aged 50 and older from a nationally representative aging cohort. We found an independent positive association between plasma concentrations of both lutein and zeaxanthin and performance on three of the four domains assessed—global, memory, and executive function, with a significant association also evident between zeaxanthin and the processing speed composite score. Comparisons of the size of the association of cognition to lutein and zeaxanthin between domains did not yield any significant differences, indicating that having a higher concentration of lutein or zeaxanthin did not affect any one area of cognitive performance to a significantly greater extent than any other.
To our knowledge, this is the first study to investigate plasma lutein and zeaxanthin in relation to cognition, with between-domain comparisons, in a large population-based sample of older adults. The results are in concordance with smaller studies of the association between plasma/serum and brain xanthophyll carotenoids and cognition (19,21,24). Furthermore, the current findings further support previous work by our group showing that macular pigment was associated with better cognition in the same cohort of older adults (15). Establishing the factors governing uptake of lutein and zeaxanthin from the peripheral circulation into the central nervous system is crucial in order to better interpret the relationship between blood, retinal, and brain concentrations of lutein and zeaxanthin and to better compare and interpret findings from these disparate regions. While plasma concentrations of zeaxanthin were much lower than lutein in the current study (as has been reported in previous investigations), lutein concentrations also have a larger scale than zeaxanthin concentrations (the mean and standard deviation of lutein was approximately four and three times, respectively, that of zeaxanthin), and thus a larger effect on cognition in terms of age can be observed with an increase in lutein by the interquartile range in our cohort. Nonetheless, given that the coefficients associated with zeaxanthin for all cognitive composite scores were at least twice to four times that for lutein, a marked increase in absolute concentrations of plasma zeaxanthin would be associated with a greater improvement in cognition scores.
Although it is premature to draw inferences from these results as to the absolute or relative importance of the carotenoids for cognitive function, there is a good biological basis for hypothesizing that these compounds may be neuroprotective, owing to their antioxidant and putative anti-inflammatory cell signaling properties. Inflammatory markers have been implicated in increasing risk of dementia (34). Markers of lipid peroxidation are increased in individuals with mild cognitive impairment (35), and it has been hypothesized that oxidative stress in tandem with a reduction in neuronal survival and dendrite growth in the aging hippocampus plays an important role in impaired cognitive function in senescence (36). A substantial body of evidence has shown that nutrition influences the aging process (for a review see (37)). In particular, numerous studies have suggested that diet plays a role in cognitive aging and age-related disease (eg, (38,39)). Recently, several distinct nutrient patterns, including one centered on high carotenoid intake, have been associated with imaging biomarkers of Alzheimer’s disease in the brains of cognitively normal individuals (40).
The current study has several strengths including the large population-based sample of older adults, a comprehensive cognitive test battery facilitating between-domain comparisons, and detailed information on covariates; however, several limitations must be acknowledged. In general, the final analytic sample was observably healthier and had better cognition than those who did not take part in all the requisite components of the study. The cross-sectional nature of the study is also a considerable limitation when attempting to infer causality. It is plausible that reverse causality exists, whereby individuals with better cognitive function are those who are more likely to eat well and better look after their health, thus reflecting in higher concentrations of carotenoids. However, overt dementia cases and individuals with very low MMSE scores were excluded from the study at the initial recruitment and analysis stages, respectively. In addition, after rerunning our statistical models and further excluding individuals in the bottom 10th percentile for cognition, the association between lutein, zeaxanthin, and cognition persisted. While we did adjust for health behaviors such as smoking, alcohol intake, and physical activity, as well as chronic health conditions in our analysis, it is possible that measurement error in these variables has allowed some residual confounding. There is also the potential for unobserved confounding—for example, it was not feasible for participants to fast prior to blood sampling and we were unable to gather data on plasma levels of other dietary-derived compounds such as omega-3 fatty acids which may possibly also influence circulating carotenoid levels and cognitive function. However, we carried out a further sensitivity analysis including use of dietary supplements containing lutein, zeaxanthin, fatty acids, vitamins, or minerals as an additional covariate showing our findings to be robust to the further adjustment for this health behavior. We believe that the potential for unobserved lifestyle or behavior confounding is reduced due to the adjustment for the aforementioned factors, along with other individual characteristics such as age, gender, educational attainment, and living arrangements.
Conclusions
The results from this large study of community-dwelling older adults show that plasma lutein and zeaxanthin are independently associated with cognitive function across multiple domains. Longitudinal investigations in similarly large population cohorts are needed to establish the prognostic significance of this relationship.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by The Atlantic Philanthropies, The Irish Government, Irish Life plc, The Centre for Ageing Research and Development in Ireland (CARDI), which became the Ageing Research and Development Division within the Institute of Public Health in Ireland (IPH) in September 2015 (authors J.F. and A.M.O.H), Waterford Institute of Technology and Bayer Ireland (R.M.), and the European Research Council (J.M.N.)
Conflict of Interest
S.B. and J.M.N. are Directors of Nutrasight Consultancy Ltd, where they do consultancy work for companies with an interest in supplements for eye care. All other authors report no potential conflicts of interest.
References
Author notes
Decision Editor: Stephen Kritchevsky, PhD