-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher A. Lepczyk, Myla F. J. Aronson, Karl L. Evans, Mark A. Goddard, Susannah B. Lerman, J. Scott MacIvor, Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation, BioScience, Volume 67, Issue 9, September 2017, Pages 799–807, https://doi.org/10.1093/biosci/bix079
Close - Share Icon Share
Abstract
As urban areas expand, understanding how ecological processes function in cities has become increasingly important for conserving biodiversity. Urban green spaces are critical habitats to support biodiversity, but we still have a limited understanding of their ecology and how they function to conserve biodiversity at local and landscape scales across multiple taxa. Given this limited view, we discuss five key questions that need to be addressed to advance the ecology of urban green spaces for biodiversity conservation and restoration. Specifically, we discuss the need for research to understand how green space size, connectedness, and type influence the community, population, and life-history dynamics of multiple taxa in cities. A research framework based in landscape and metapopulation ecology will allow for a greater understanding of the ecological function of green spaces and thus allow for planning and management of green spaces to conserve biodiversity and aid in restoration activities.
Urban areas house the majority of the world's population, and there has been a surge in interest in researching urban ecosystems. For many, urban areas are sometimes viewed as concrete jungles, with depauperate fauna and flora dominated by nonnatives and homogenous taxa across regions. Although such views are understandable, in truth, urban areas house a great deal of species both native and nonnative to the surrounding region (Aronson et al. 2014, Ives et al. 2016, Lepczyk et al. 2017). In fact, urban areas can support endemic native species and others of conservation concern both at regional and global scales (Aronson et al. 2014, Ives et al. 2016). These species and the overall diversity in a city rely on the size, quantity, and quality of urban green spaces (Beninde et al. 2015), which are also features vital for human health and well-being (Barton and Pretty 2010). Urban green spaces provide opportunities for citizens to connect with nature, witness ecological processes in action, and potentially become scientifically literate citizens who make informed decisions regarding conservation initiatives and policy.
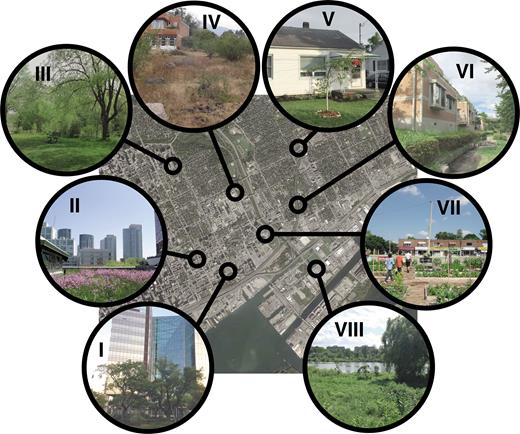
Urban green spaces are often viewed in different lights because ecologists and other stakeholders have contrasting opinions on their role in biodiversity conservation and their value to society. Urban green spaces comprise a range of habitat types that cross a continuum from intact remnant patches of native vegetation, brownfields, gardens, and yards, to essentially terraformed patches of vegetation that may or may not be representative of native community associations (figure 1; Cilliers et al. 2013, Aronson et al. 2017). These diverse green spaces found in cities also represent a gradient of economic and management input. Most urban green spaces represent novel ecosystems (Pickett et al. 2001, Tratalos et al. 2007), because the magnitude and type of selection pressures and resultant assemblages differ from the historical ones present under reduced human influence (Kowarik 2011). This variability affects the species interactions within green spaces and the ecological function of green spaces, as well as how green spaces interact to support biodiversity.
The variety of urban green spaces supports different taxa on the basis of patch size, patch quality, quantity in the landscape, and heterogeneity both within and among green spaces. Urban green spaces include heavily maintained terraformed patches, such as plantings in the city core (I, Pocket park, Incheon, South Korea), green roofs (II, Green Roof at the Mountain Equipment Co-op, Toronto, Canada), bioswales (VI, Private Residence, Hachiōji, Tokyo, Japan), and community gardens (VII, Bloor-Acorn Community Garden, Toronto, Canada); spaces that include both managed and unmanaged vegetation, such as city parks (III, Taylor Massey Creek, Toronto, Canada) and home gardens (V, Private Residence, Guelph, Canada); unmanaged vacant lots and brown fields (IV, Abandoned lot, Morelia, Mexico); and remnant natural areas (VIII, York University, Toronto, Canada). Base map: Toronto, Canada. Urban green spaces throughout the city could be designed to form a network of interconnected spaces to better support biodiversity. Photo Credits: Myla F.J. Aronson (I), J. Scott MacIvor (II–III, V–VIII), Ian MacGregor Fors (IV).
As cities expand, urban park managers and ecologists often invest much effort in increasing urban green space through innovative methods and preserving and restoring remnant habitats. The motivations for these actions stem from a perception that all green spaces have biodiversity value. This perception seems intuitive given the strong associations between urban green space and the occurrence of wildlife (Aronson et al. 2014). However, evidence drawn from ecological theory and empirical data suggests that not all green spaces have equal value. In some cases, urban green spaces provide only limited biodiversity benefits, although the evidence base with which to assess the benefits of different forms of urban environmental management is often limited (Beninde et al. 2015). Thus, designing management and restoration plans or advocating for habitat features in urban green spaces often does not make full use of the science that is available, even though that science is itself limited. Identifying the ecological role and conservation value provided by different types of urban green spaces is of particular importance given the continued growth of urban areas, the development of new cities, and the promotion of certain types of green spaces (e.g., community gardens, bioswales, and green roofs; box 1).
Green roofs (figure 2) are an increasingly common form of urban green space in cities and often touted as promoting landscape connectivity. Despite considerable attention paid to the social well-being and economic performance benefits provided by green roofs (Oberndorfer et al. 2007, Clark et al. 2008), there remain considerable gaps in knowledge as to how green roofs can contribute to urban biodiversity conservation (Williams NSG et al. 2014). Numerous taxa have been identified from surveys on green roofs (Brenneisen 2006, MacIvor and Lundholm 2011), but few studies have examined the role of landscape complexity and connectivity of green roofs to other urban green spaces in framing the species diversity observed (Toneitto et al. 2011, Braaker et al. 2014). Because green roofs have only recently been recognized by ecologists as opportunities to contribute to urban wildlife, no studies have yet assessed population dynamics or persistence over more than 3 years (Williams et al. 2014). The size, height, and design (e.g., substrate, planting, irrigation, and maintenance) all contribute to the types and numbers of species frequenting green roofs. However, there remains little evidence of any general trends other than ground-level habitats supporting more variety of taxa (MacIvor and Lundholm 2011).
Green roofs are an increasingly prevalent type of urban green spaces within cities throughout the world, but their ability to support multiple taxa and connect with other green spaces is largely unstudied. Photo Credit: Max R. Piana of a green roof at Cornell University (Ithaca, NY).
For highly mobile insect species such as bees and weevils, the number of green roofs within an area increases their connectedness as a habitat type (Braaker et al. 2014). Still, with increasing building height, any taxa able to reach the top of a building could have difficulty getting down. Moreover, green roofs on taller buildings experience increased exposure to wind and solar radiation, further limiting their value as habitat. For example, bats were shown to use green roofs as foraging areas in London, United Kingdom, preferring low-rise buildings with “biodiverse” plantings (Pearce and Walters 2012). Thus, green roofs that are small and isolated from ground level may result in the creation of urban green space devoid of biodiversity, or worse, as ecological traps attracting local taxa to a difficult environment (MacIvor 2016).
Given the desire of many ecologists and urban managers to enhance the value of urban green spaces for biodiversity conservation, our main goal is to identify key questions that need to be addressed to develop a more robust knowledge base for understanding what factors are important for supporting biodiversity in urban green spaces. We seek to identify not all the outstanding questions but rather those that are among the most important and for which current knowledge regarding the ecological function of urban green spaces for biodiversity is particularly limited. We build on earlier species-specific syntheses (e.g. birds; McKinney 2002, Chace and Walsh 2006), and our questions are focused on those most applicable to urban green spaces planning and design. Although conserving biodiversity is an interdisciplinary problem, our focus here is only the ecological questions, with the socioeconomic and management connections considered in a companion paper (Aronson et al. 2017).
To address the knowledge gaps of the ecology of green spaces, we use a landscape ecology framework. Because of the inherent patchiness of cities, which causes urban green spaces to be often small and isolated, island biogeography emerged as an early framework for understanding patterns of urban biodiversity (Davis and Glick 1978, Faeth and Kane 1978). This island-biogeography perspective has largely been replaced with metapopulation theory, in which urban green spaces fall within a landscape patch-matrix framework (Breuste et al. 2008, Wu 2008), because patch size, quality, pattern, and connectedness have been shown to be important for various taxa (Evans et al. 2009, Goddard et al. 2010, Williams and Winfree 2013, Beninde et al. 2015). Although the relative importance of local- versus larger-scale variables in influencing urban biodiversity remains an area of debate and will vary with the scale dependencies of different taxa (Goddard et al. 2010), the weight of evidence suggests that local factors, especially patch size and quality, are paramount (Donnelly and Marzluff 2004, Evans et al. 2009, Lerman and Warren 2011, Shwartz et al. 2013, Williams and Winfree 2013). Nevertheless, the importance of landscape context in determining species richness has been demonstrated for multiple taxa as well (Prevedello and Vieira 2010). As a result, landscape ecology provides the theory and tools of a multiscale spatially explicit perspective that is needed in urban green space ecology. Notably, although a landscape ecology and metapopulation perspective provides a needed framework, fully addressing the questions posed here also requires continued studies on species life history and species responses to both local and landscape factors.
Question 1: How large must an urban green space be for biodiversity conservation?
Urban green spaces are characterized by highly fragmented, small, and isolated patches of green space, as has been exemplified by the United Kingdom, where only 13% of urban tree (or woody vegetation) cover occurs in patches larger than 0.25 hectare (Evans et al. 2009). The positive effect of urban green space area within a city on species richness has been well documented for a range of taxa (Goddard et al. 2010), and it is now well established that the amount of urban green space in cities is an important determinant of biodiversity (Aronson et al. 2014, Beninde et al. 2015). However, much remains unknown about how large individual patch sizes need to be, and evidence suggests that patch size and quality are important factors driving both plant and animal populations in cities (Evans et al. 2009, Shwartz et al. 2013, Williams and Winfree 2013, Matthies et al. 2017). For example, studies on birds have suggested that 10–35 hectares of continuous green space are required to support most urbanized species (Fernández-Juricic and Jokimäki 2001, Chamberlain et al. 2007; i.e., species that today would be considered utilizers and dwellers, sensu Fischer et al. 2015), with forest bird species (avoiders) requiring larger areas (Donnelly and Marzluff 2004). However, most city parks fall considerably below this size range (Jokimäki 1999), and even small urban green spaces can support biodiversity depending on their habitat quality (Holtman et al. 2017, Matthies et al. 2017). Modeling studies predict that adding just a small amount of additional green space (150 square meters) to small neighborhood parks will considerably increase bird species richness (Strohbach et al. 2013). However, there is very little understanding of thresholds in patch size for other animal groups or plants, which makes conserving biodiversity as a whole challenging because different taxa operate at different scales.
Another challenge regarding assessment of the impacts of patch size on biodiversity relates to defining green space patches with reference to their borders with hard surfaces and the composition of the urban matrix. The ability of green space to support biodiversity can be moderated by urban intensity and structure (Matthies et al. 2017, Melliger et al. 2017). For instance, green spaces in the city core may not support the same species numbers as comparable green spaces in a suburban matrix (Carbó-Ramírez and Zuria 2011). Clearly, a better understanding of patch size and of the extent of the overall network of patches of multiple taxa is required to better inform conservation initiatives.
Question 2: How are animal population sizes limited by green spaces during their life cycle?
Animal species often require a variety of habitat types that provide the full spectrum of resource requirements for their life cycle. A variety of green spaces may provide an important component of these requirements. As a result, understanding how green spaces serve as habitat and, in turn, how such habitat influences population size is of particular importance. But we have a limited understanding of how green spaces are used for such important activities as foraging and reproduction. Therefore, although it is clear that many species require access to multiple resources in urban ecosystems, we have a poor understanding of which factors limit population size.
One of the first considerations of how green spaces may limit population size is the degree to which a species requires different habitat types within an urban area and whether these can be met within a single green space or not. For instance, a less mobile species might acquire all required resources within a single patch, whereas others must move across larger areas, such as some bee species that forage for food and nesting resources within and around a fixed nesting site that may include several independent urban green space patches (McFrederick and Lebuhn 2006). Whether or not a species requires a variety of different habitats within or beyond the green spaces, it is critical to identify such factors in terms of how they regulate the urban population size of species, because they may well change spatially and temporally depending on both environmental and anthropogenic conditions.
In addition to size and proximity, it is vital to understand how the specific habitat features present within green spaces support individuals during different life stages, highlighting the need for model validations and experimental work in cities. For example, most urban pollinator studies have concluded that rich floral resources exist in urban green spaces and are linked to diverse bee communities (Lowenstein et al. 2014). Although research exists on the factors limiting populations for many migratory species, such as the combination of loss of wintering habitat and the destruction of larval plants for monarch butterflies (Vidal et al. 2014), understanding which life stages limit urban populations is far from complete.
Question 3: How does heterogeneity within and across green spaces affect plant and animal assemblages?
On one hand, because many species require access to different habitats either simultaneously or in different seasons or stages of their life cycle, a positive relationship between urban habitat heterogeneity and diverse assemblages can occur. On the other hand, habitat heterogeneity can enable a diverse assemblage of habitat specialists to coexist, but only if the patch size of each individual habitat is sufficiently large to support viable populations of habitat specialists. Consequently, the beneficial impact of habitat heterogeneity on assemblage diversity is likely to be a nonlinear function of total patch size. However, empirical assessments of such relationships are lacking and needed.
To maximize ecological functioning in urban landscapes, we need to consider how the networks of different green-space types interact with each other at multiple spatial scales (Borgström et al. 2006, Colding 2007). At the landscape scale, the heterogeneity of green spaces within urban areas increases plant species diversity (Kowarik 2011), and the presence of diverse resources across green space types explains the presence of diverse animal communities (e.g., pollinators; Baldock et al. 2015). At the local scale, habitat heterogeneity within green spaces increases the species richness of multiple taxa (Nielsen et al. 2014), whereas vegetation structure and complexity enhance the diversity of urban forest bird communities (Kang et al. 2015). However, more research is required regarding how species richness, population size, and viability respond to heterogeneity across green spaces. In reality, most species, especially the more specialized ones that are rare in urban environments, cannot use all the habitats present within a single block of green space.
Question 4: How connected should green spaces be to support biodiversity?
Wildlife corridors are now a prominent feature of urban planning and appear to be a useful tool for enhancing biodiversity in urban green spaces (Vergnes et al. 2013). However, the question still remains whether or not corridors provide functional landscape connectivity and, ultimately, improve population viability (Douglas and Sadler 2011). The patchy nature of urban green spaces makes them ideally suited for understanding population dynamics from a metapopulation perspective (e.g., Bastin and Thomas 1999, Dornier et al. 2011). A growing body of evidence has shown that landscape connectivity enhances biodiversity in fragmented urban habitats (e.g., Shanahan et al. 2011). Landscape genetic techniques have confirmed that connectivity can increase gene flow between urban green spaces (Munshi-South 2012, Saarikivi et al. 2013) and that fragmentation reduces genetic connectivity between isolated urban habitat patches (Delaney et al. 2010, Jha and Kremen 2013). Thus, networks of urban green spaces may provide corridors through the urban matrix, and when plentiful and within close proximity to each other, they have the potential to lessen the risk of sink habitats in urban areas. In fact, models of networks suggest that even small patches within a city have the potential to connect populations of highly mobile and small animals (e.g., butterflies) with source habitat in the periurban area (Rudd et al. 2002, Snep et al. 2006). However, empirical studies within urban green spaces that validate these models are lacking.
One key unknown regarding how to enhance connectivity is the relative merits of corridors, which form continuous connections between habitats, versus the role of stepping stones that may enhance connectivity using less land than corridors use. Recent evidence suggests that corridors may be more effective than stepping-stone habitats for multiple taxa (Beninde et al. 2015), but these results were based on only two cities. Proponents of maintaining and adding small green spaces to the urban landscape state that they help to maintain the connectivity of isolated populations. Network analyses have confirmed this potential (e.g., Rudd et al. 2002), but the magnitude of small fragments’ contribution to connectivity will depend on the characteristics of the patch and focal biota. Furthermore, stepping-stone habitats offer an opportunity to increase connectivity if they offer additional pathways (path redundancy) through the matrix, which are important for mobile organisms such as birds and insects.
Corridors may enhance plant and animal biodiversity, as has been shown in forest ecosystems (Tewksbury et al. 2002). Narrow linear patches of green space can enhance connectivity; for example, the 40-meter-wide Long Island Motor Parkway increases gene flow between white-footed mouse (Peromyscus leucopus) populations in New York City, although other apparently similar corridors do not (Munshi-South 2012). Translocation experiments demonstrate that habitat type and the number of gaps influences the ability of forest birds to cross gaps in urban areas, but there is considerable variation within and between species to dispersal barriers (Tremblay and St. Clair 2011).
Directly measuring species dispersal in ecological networks (e.g., using mark–recapture methods) remains a challenge (Jacobson and Peres-Neto 2010), but radio tracking and translocation studies are now emerging that quantify species movement in urban landscapes (Gaughan and Destefano 2005, Tremblay and St. Clair 2011, Caryl et al. 2013). It is increasingly apparent that the permeability of urban landscapes is taxon dependent, and more research is required into how different types of urban green space influence dispersal across a wide range of taxa. Moreover, to assess accurately whether habitat connectivity improves population viability in urban green spaces, we need more experimental studies that collect data on demographic parameters (Beier and Noss 1998).
Question 5: When are green spaces more likely to act as ecological traps or population sinks?
An ecological trap exists when an animal selects low-quality habitat over other available higher-quality habitat such that their resulting reproduction and survival rates are unable to sustain a population (Donovan and Thompson 2001). In other words, an ecological trap is a sink habitat that is preferred rather than avoided (Battin 2004), which is why they are sometimes called an attractive sink (Delibes et al. 2001). Within urban ecosystems and human-dominated landscapes, ecological traps arise when anthropogenic change means that previously adaptive cues used in habitat-selection results in individuals selecting habitats in which fitness is reduced relative to alternative habitat-selection decisions. Modeling ecological traps in an evolutionary genetic framework indicates that they are more likely to lead to population extinction when traps arise through degradation of existing habitat (Fletcher et al. 2012). Such degradation, rather than the creation of entirely new habitats, frequently occurs in urban areas. Novel features that dominate urban areas, such as buildings, roads, and light pollution, create ecological traps for a wide variety of taxa (Robertson et al. 2013). Ecological traps are thus likely to be contributors to the local extirpation of urban populations of fauna and flora, but evidence concerning how and when urban green space generates ecological traps—and whether or not they are just simply sinks—is limited and contrasting. One notable example of this concerns the role of urban habitats in generating ecological traps for breeding songbirds by increasing nest predation rates (Bonnington et al. 2015).
Similar to ecological traps, sink habitats are those that have negative population growth (i.e., λ < 1) but differ in that they are avoided by animals until all higher-quality habitats have been filled first (Pulliam 1988, Battin 2004). Claims that traps and sinks occur in urban areas across a wide range of taxonomic groups strongly contradict the perception that all urban green spaces have conservation value. Rather, urban green spaces may have a suite of conservation values, which can include both traps and sinks. Although population growth is negative within traps and sinks, they still have some reproductive output and could be the only place in an urban area that less-fit individuals are able to breed, thereby providing conservation value. However, distinguishing whether specific types of urban green spaces are being chosen (trap) or avoided (sink) is of critical importance for determining their conservation value. Ultimately, the probability of such traps and sinks occurring depends on the focal species’ dispersal mechanism, its population density, its life history, and qualities of the urban green space, including spatial configuration.
Urban source–sink dynamics are perhaps most frequently discussed with regard to birds. Only a minority of avian species have population densities in urban areas that are higher than or equivalent to those in less-developed regions (Evans et al. 2011), although this does not by itself provide evidence for an urban-sink population. Some species also have poorer reproductive success in highly urbanized areas (Mennechez and Clergeau 2006, Chamberlain et al. 2009), and in some cases, their populations are sustained by dispersal from distant nonurban regions (Withey and Marzluff 2005). These data strongly suggest that urban green spaces could act as sinks, although data on survival rates are rarely available to facilitate a robust assessment (Stracey and Robinson 2012, Shipley et al. 2013). Turning to other taxa, storm-water basins designed to reduce the impacts of urban runoff almost invariably contain fish and thus act as a sink for numerous amphibian species in their larval stages (McCarthy and Lathrop 2011). Likewise, artificial light in urban areas can act as ecological traps for urban moth species (Bates et al. 2014). There is clearly evidence that urban green spaces can generate ecological traps and sinks, but well-documented empirical examples are surprisingly rare—although see Crooks and Soulé’s (1999) work on mesopredator release. Insufficient evidence exists to enable firm conclusions to be made regarding when traps and sinks arise, although they seem more likely to do so in degraded and highly fragmented patches surrounded by an intensely urbanized matrix. Furthermore, attempts to increase the attractiveness of such sites to wildlife may draw individuals to relatively low-quality habitats, thus creating ecological traps. Indeed, ecological restoration appears to be one of the three most frequent causes of ecological traps (Robertson et al. 2013). More work is needed to understand the mechanisms that lead to ecological traps and sinks and the interventions necessary to make these green spaces more biodiversity friendly.
Implications for conservation, management, and restoration
The five questions posed here all have direct links to conservation, management, and restoration. Answering these questions can aid in how urban green spaces are managed, planned, and designed. Furthermore, by drilling down into each question we can seek more nuanced answers. For example, how does habitat quality of a green space vary depending on the composition of native, nonnative, and invasive species? Given that nonnative species can play important roles in green spaces, distinguishing their roles relative to native and invasive species can be important.
Although the five questions relate to biodiversity, it is important to keep in mind the various definitions of biodiversity and how they are used in a given context. For instance, in many biodiversity assessments, only overall species richness is considered. However, considering only species richness can lead to areas with a large number of nonnative and invasive species achieving a greater biodiversity value than an equally representative area devoid of such species. Therefore, what type of biodiversity and how it is being measured must be considered when addressing them—and, ultimately, when those assessments are then applied.
As we have discussed elsewhere (Aronson et al. 2017), conserving, designing, and managing urban green spaces require balancing human perceptions, needs, and use with ecological requirements for preserving and enhancing biodiversity. Thus, within a full socioecological-systems perspective, it is important to consider both the ecological questions presented here and what they mean for society concomitantly. Furthermore, there are many additional sociologically based questions that dovetail with our ecological questions that are in need of addressing, such as how biodiversity benefits human health and well-being and what the relationships are between biodiversity and ecosystem services.
Conclusions
The principles of landscape ecology are a central tenet of the urban green infrastructure movement to establish networks of interconnected urban green spaces. Urban green infrastructure is rising on the agenda for policymakers at national (POST 2013) and continental scales (European Commission 2013), despite the fact that the effectiveness of green infrastructure projects is rarely evaluated (Felson et al. 2013). Furthermore, the geographic bias in the Northern Hemisphere and the taxonomic bias primarily on birds and some mammals limit the successful application of ecological research to urban green space planning. We suggest that research on the ecology of urban green spaces should focus in particular on the response of multiple taxa to landscape and local-scale factors. In addition, globally comparative analyses are necessary to aid in conservation, restoration, and urban planning.
As we decide how best to expand growing cities, redevelop those experiencing population redistributions (e.g., gentrification), and rethink those declining in size, ecologists are increasingly urging urban planners to gauge the impacts of development and vacant land conversion on biodiversity at the city or regional scales (Sushinsky et al. 2013, Gardiner et al. 2014). The relationship between urban form and ecological processes is not straightforward (Alberti 2005), and the consensus from spatial simulation models is that there is often no single optimal solution when examining how different taxa respond to alternative urban landscape configuration scenarios (Tannier et al. 2012). Here, we have identified five key questions for a research framework in urban green space ecology that need to be considered as we move forward. By no means are these the only questions in need of attention, given that green spaces are critical and many other questions need to be addressed. However, we hope that these questions foster a broader discussion of the value of urban green spaces and ecology as a whole, as well as to provide managers with more useful information.
Acknowledgments
We thank the participants and speakers of the 26th International Congress for Conservation Biology symposium, entitled “The Role of Urban Green Spaces in Maintaining Biodiversity and Ecosystem,” for shaping the ideas that led to the development of the main questions presented here. In addition, we would like to thank Roarke Donnelly, the two anonymous reviewers, and the handling editor, who all provided valuable critiques on the draft manuscript. This work was supported in part by the National Science Foundation (NSF; Research Coordination Networks, Division of Environmental Biology [DEB] no. 1354676 and no. 1355151; NSF Science, Engineering, and Education for Sustainability, DEB no. 1215859). KLE was funded by grant NE/J015369/1 from the Biodiversity and Ecosystem Service Sustainability (BESS) programme. BESS is a six year program (2011– 2017) funded by the UK Natural Environment Research Council (NERC) and the Biotechnology and Biological Research Council (BBSRC) as part of the UK’s Living with Environmental Change (LWEC) programme.