-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher M. Gough, Christoph S. Vogel, Hans Peter Schmid, Peter S. Curtis, Controls on Annual Forest Carbon Storage: Lessons from the Past and Predictions for the Future, BioScience, Volume 58, Issue 7, July 2008, Pages 609–622, https://doi.org/10.1641/B580708
Close - Share Icon Share
Abstract
The temperate forests of North America may play an important role in future carbon (C) sequestration strategies. New, multiyear, ecosystem-scale C cycling studies are providing a process-level understanding of the factors controlling annual forest C storage. Using a combination of ecological and meteorological methods, we quantified the response of annual C storage to historically widespread disturbances, forest succession, and climate variation in a common forest type of the upper Great Lakes region. At our study site in Michigan, repeated clear-cut harvesting and fire disturbance resulted in a lasting decrease in annual forest C storage. However, climate variation exerts a strong control on C storage as well, and future climate change may substantially reduce annual C storage by these forests. Annual C storage varies through ecological succession by rising to a maximum and then slowly declining in old-growth stands. Effective forest C sequestration requires the management of all C pools, including traditionally managed pools such as bole wood and also harvest residues and soils.
Forests are a critical component of the global carbon (C) cycle, storing more than 1 × 1015 metric tons of carbon in biomass, detritus, and soils (Dixon et al. 1994). Forest C storage is an important ecosystem service, locking up C that might otherwise exist in the atmosphere as carbon dioxide (CO2), a potent greenhouse gas. In the northern hemisphere, forests are estimated to sequester up to 7 × 108 metric tons of Cannually (Goodale et al. 2002), or nearly 10% of current global fossil fuel C emissions (IPCC 2007). However, there is a great deal of variation in the capacity of individual forest ecosystems to take up and store C. Annual rates of forest C storage vary across latitudes because of broad gradients in community composition, growing season length, precipitation, temperature, and solar radiation (figure 1a). On average, boreal forests are close to C neutral, whereas those at mid latitudes store approximately four metric tons of C per hectare (ha) per year, but with substantial variation in annual C storage recorded at all latitudes. The difference between maximum and average annual C storage, often exceeding 100%, suggests that forests generally store C at rates below their potential (figure 1). The broad flexibility in forest C storage rates offers opportunities as well as challenges for those considering forests as an important component of strategies to mitigate rising atmospheric CO2.
Average annual carbon (C) storage (a) and maximum annual C storage (b), in metric tons C per hectare per year, across boreal and temperate latitudes in the Northern Hemisphere estimated for 33 forested sites reporting three or more site years of data (see online supplement at http://hdl.handle.net/1811/31687). The difference between maximum and average annual C storage (gray-shaded area) is an indication of flexibility in forest C sequestration rates. Regression functions were selected on the basis of highest goodness of fit.
Multiyear empirical observations of forest C fluxes at the ecosystem scale help provide a quantitative understanding of the biotic and abiotic constraints on C uptake and loss. For example, the broadly distributed terrestrial C cycling research network of the Americas, AmeriFlux, has supported long-term observations of forest C storage to determine how climate, land use, management, disturbance, and forest-stand structure influence rates of C sequestration (http://public.ornl.gov/ameriflux). AmeriFlux is a partner in the worldwide network of C cycling research sites called FLUXNET, which coordinates global syntheses of observations from an extensive array of ecosystems (Baldocchi et al. 2001). Understanding how variation in annual C storage is controlled at the ecosystem scale is central to any approach that employs forest C sequestration to mitigate anthropogenic CO2 emissions (Birdsey et al. 2006).
In this article, we demonstrate how multiyear observations of forest C fluxes provide critical insight into the constraints on annual C storage rates. Using the University of Michigan Biological Station (UMBS) AmeriFlux site (45°35.5′N 84°43′W) as a case example, we examine how climate, disturbance, and forest succession simultaneously influence annual forest C storage. We also describe how foresters and land managers can use knowledge gained from long-term ecosystem-scale studies to better manage forests for C sequestration.
Empirical methods for quantifying annual forest carbon storage
Ecological and meteorological measurements of C fluxes between forests and the atmosphere play complementary roles in quantifying spatial and temporal patterns of ecosystem C storage. Ecological methods offer spatially extensive estimates of individual C pools and fluxes, whereas meteorological methods provide continuous measurements of whole-ecosystem C fluxes, allowing for high temporal resolution of ecosystem C dynamics. Ecological estimates of C storage require the quantification of C gains and losses (i.e., fluxes) for every C-containing pool within a forest. Typically, this approach includes direct or inferred estimates of wood, leaf litter, woody debris, and root mass production or loss; mass losses to herbivory; and respiratory C losses by soil decomposers or soil heterotrophic respiration. The spatially integrated sum of all C gains and losses over a year is the ecological estimate of annual forest C storage. Applied at a similar spatial scale, meteorological methods can measure the C exchange between forest and atmosphere at time intervals of less than one hour (Schmid et al. 2000). This approach provides an integrated measure of net ecosystem C uptake or loss that represents the sum of individual C fluxes occurring within an ecosystem, including those from multiple photosynthetic and respiratory components. The cumulative net exchange of C between the forest and the atmosphere over one year is the meteorological estimate of annual forest C storage. In principle, these independent approaches should yield identical estimates of annual C storage when applied to the same forested area.
Concurrent ecological and meteorological measurements allow independent assessments of ecosystem C storage rates and are important for gauging the accuracy of annual C storage estimates, since multiple sources of uncertainty are associated with both approaches (Schmid et al. 2003, Curtis et al. 2005). Throughout this article, annual C storage is synonymous with net ecosystem production as defined by Chapin and colleagues (2006), and refers to the imbalance between gross primary production and ecosystem respiration. Several publications provide in-depth presentations of ecological and meteorological methods for measuring annual C storage (Clark et al. 2001, Baldocchi et al. 2003). Here, we present a brief introduction to both methods and provide references for those interested in more detailed theory and methodology.
Ecological methods.
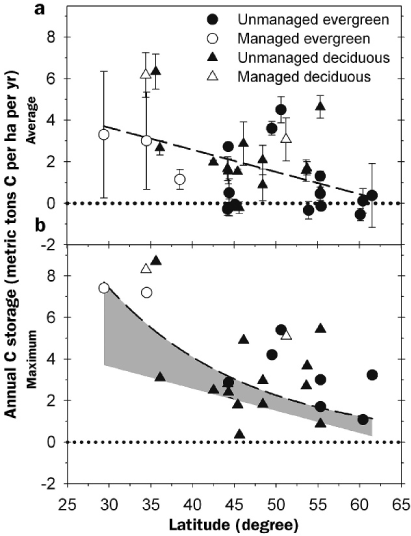
Ecological estimates of C storage rely on direct and indirect measurements of C gains and losses by ecosystem C pools (box 1). Aboveground wood, including stems and branches, is not readily measured directly at the ecosystem scale. Thus, species-specific aboveground wood mass is generally inferred from equations relating tree diameter to wood mass (e.g., Ter-Michaelian and Korzukhinn 1997). Belowground wood, or coarse root mass, can be estimated as a function of tree diameter, or soil cores can be collected and root mass scaled to the whole ecosystem (Gough et al. 2008). Wood production is the incremental change in mass between two measurement periods. Leaf and fine woody debris production is measured directly using litter traps positioned on the forest floor to collect fallen leaves and debris (Gough et al. 2008). Coarse woody debris production often is measured from field surveys of dead wood volume and density (Gough et al. 2007a). Fine root (< 2 millimeters [mm] in diameter) production is measured directly or as the product of standing fine root mass and fine root turnover. This flux is difficult to measure accurately, and thus has been quantified by several different means. Root production can be measured directly using in-growth cores, in which mesh cores containing root-free soil are inserted into the ground and fine root in-growth is quantified. Standing fine rootmass is generally estimated from soil cores. Fine root turnover can be estimated from equations relating turnover to soil nitrogen (N) availability (Raich and Nadelhoffer 1989) or soil temperature (Gough et al. 2008), and from minirhizotron analyses, an approach in which turnover is tracked using digital imagery of soil profiles by inserting a specialized camera into clear plastic soil tubes (Johnson et al. 2001). Annual foliar losses to herbivory can be estimated through the measurement of damage to green leaves, through collection of insect feces below the canopy, and through herbivore feeding trials relating leaf mass loss to feces production (Gough et al. 2008). Dry weight biomass often is converted to C mass by assuming a C fraction of 0.5. However, species- and tissue-specific C fractions can be obtained from publications (Turner et al. 1995, Lamlomand Savidge 2003) or through tissue elemental analysis.
Independent ecological and meteorological methods are used to derive estimates of annual forest carbon (C) storage. Ecological C storage is the sum of annual live (L; wood) and dead (D; leaf, wood, and fine root litter) mass production, and annual herbivory losses (H), less annual heterotrophic respiration (Rh). Ecological estimates of L, D, H, Rh, and annual C storage for the UMBS (University of Michigan Biological Station) forest in 2000 are shown in boxes. Meteorological annual C storage is the difference between annual gross photosynthesis (Pg) and ecosystem respiration (Re) estimated using the eddy-covariance technique in which ecosystem carbon dioxide (CO2) fluxes are correlated with the upward and downward motion of air. In this approach, short-term ecosystem C fluxes (F) are calculated as the average covariance between fluctuations in vertical wind speed (w) and the CO2 mixing ratio (c), plus changes in c integrated across vertical height (h) above the forest floor over time (t). Meteorological annual C storage is the cumulative sum of CO2 exchange over one year between forest and atmosphere. Meteorological estimates of Pg, Re, and annual C storage for UMBS in 2000 are circled. During the day, Pg and Re occur simultaneously and are separated via modeling. In 2000, ecological annual C storage was 13% greater than that estimated from meteorological methods (Curtis et al. 2005, Gough et al. 2008). Agreement between methods generally improves with multiple years of C storage data because there is a lag between photosynthetic C uptake, estimated meteorologically, and growth, estimated ecologically. Mean ecological and meteorological estimates of annual C storage at UMBS converged to within 1% of each other over five years (1999–2003) (Gough et al. 2008).
Carbon loss from soil heterotrophic respiration, part of the decomposition of soil organic matter, must also be accurately estimated. Numerous methods have been developed to estimate this sizable C flux, but a thorough presentation of all methods is beyond the scope of this article (see Hanson et al. [2000] for a review). In brief, respiratory C losses from soil heterotrophs are usually estimated by measuring soil surface CO2 efflux and partitioning this flux into respiration derived from heterotrophic and plant sources (Gough et al. 2008). Partitioning is achieved by (a) separating roots from soil and independently measuring their respiration; (b) using stable isotope analyses of respired CO2 and of bulk roots and soil to determine the fraction of respiration contributed by each respiratory source (Rochette et al. 1999); or (c) amass balance approach in which C losses from soil heterotrophic respiration is assumed to be equivalent to the amount of labile C substrate (i.e., leaf and root litter) produced annually (Gough et al. 2007b). Annual forest C storage is the sum of C growth of all biomass pools less respiration from soil organic matter decomposition.
Meteorological methods.
Meteorological methods for estimating annual forest C storage require continuous, high frequency (10 per second) measurements of three-dimensional wind speed and CO2 concentrations above the forest canopy using a sonic anemometer and infrared gas analyzer, respectively. The eddy-covariance statistical method is used to estimate forest C fluxes from wind and CO2 data (see, e.g., Baldocchi 2003, Schmid et al. 2003). In this approach the vertical transport of air packets, or eddies, covaries with the flux of CO2 into or out of the forest canopy. The net movement of CO2 into the forest indicates that ecosystem photosynthesis is greater than respiration; CO2 transport from the forest to the atmosphere signifies that respiration exceeds photosynthesis. Depending on the height at which instruments are positioned, meteorological methods can measure forest C fluxes integrated from over several hectares to many square kilometers. The area of forest that is measured, or the C flux footprint, also varies with weather conditions because wind speed affects the distance CO2 travels before it is sampled by instruments on the meteorological tower. The year long sum of C fluxes into and out of the forest is the annual forest C storage. Occasionally, instrument failure, heavy rain, or light winds cause gaps in otherwise continuous measurements of wind speed and CO2 concentrations. Filling data gaps requires simulating C fluxes during these periods. Particular caution must be taken when measuring C fluxes over complex terrain because CO2 can drain into and out of these systems and consequently fail to be detected by instruments positioned above the plant canopy (Schmid et al. 2000).
Case study: Forest carbon cycling in northern lower Michigan
Mixed-deciduous forests, such as those at UMBS, represent a dominant ecosystem in the upper Midwest, encompassing 102,000 square kilometers (km2) (USDA Forest Service 2002). Widespread establishment of these forests a century ago has made this region a likely contributor to the terrestrial North American C sink (Birdsey et al. 2006). At UMBS, a diverse array of forest ages and disturbance histories are represented and considerable variation in climate exists from year to year, making this a model landscape for investigating biotic and abiotic controls on whole-ecosystem C cycling. Moreover, the well-studied forested landscape at UMBS encapsulates much of the variation in age and disturbance history present across the region.
We used ecological and meteorological approaches to measure annual forest C storage from 1999 to 2005 (Schmid et al. 2003, Gough et al. 2007b, 2008). The secondary successional mixed northern hardwood forest averages 85 years old, but includes stands from 6 to 90 years old. More than half of the standing live tree mass consists of bigtooth aspen (Populus grandidentata Michx.) and trembling aspen (Populus tremuloides Michx.). Other common overstory species include northern red oak (Quercus rubra L.), paper birch (Betula papyrifera Marsh.), American beech (Fagus grandifolia Ehrh.), sugarmaple (Acer saccharum Marsh.), redmaple (Acer rubrum L.), and white pine (Pinus strobes L.). The understory is dominated by bracken fern (Pteridium aquilinum L.) and by red maple, red oak, American beech, and white pine seedlings and saplings. The canopy height is approximately 22 meters. We made ecological C cycling measurements in 60 plots located along transects that radiate from a continuously operating meteorological tower, and in an experimental disturbance chronosequence 1 km away (Gough et al. 2007b). Transects were located 20° apart, from 225° to 15°, toward the prevailing northwest winds and the meteorological tower source footprint. The mean annual temperature (1942–2003) is 5.5 degrees Celsius (°C) and the mean annual precipitation is 817 mm. Old-growth white pine, red pine (Pinus resinosa Ait.), and eastern hemlock (Tsuga canadensis [L.] Carr.) were harvested in the late 19th century, and subsequent cutting and patchy burns were a source of repeated disturbance in the area until the early 20th century.
Where is carbon stored within the forest?
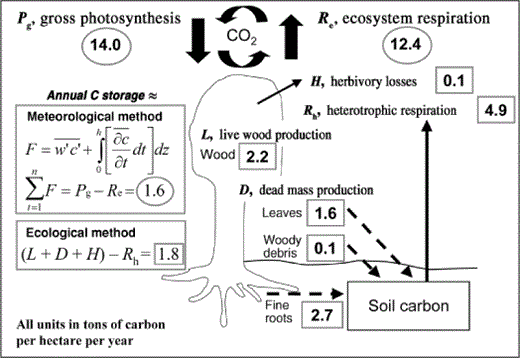
Carbon within the UMBS forest is located primarily in wood mass and soil organic matter, with above- and belowground pools containing 42% and 58%, respectively, of the 180 metric tons C per ha stored in the ecosystem (figure 2a). Soil organic matter was the largest C pool, emphasizing the importance of underground C reservoirs to terrestrial C budgets (Dixon et al. 1994). The amount of C stored in forest soils varies depending on climate and forest type. In a review of the literature, Pregitzer and Euskirchen (2004) reported that soil C stocks in boreal forests averaged approximately twice as much as C stocks in more southern temperate forests. Very high soil C stores of 703 metric tons C per ha were reported for a Canadian boreal black spruce (Piceamariana) forest growing on waterlogged soils, a system in which oxygen-poor environments have inhibited the decomposition of organic matter (Rapalee et al. 1998). The quantity of C stored in forest soils is also a function of species composition (Gower et al. 1997), suggesting that different forest types (e.g., conifer versus deciduous) affect the processes controlling soil C accrual and retention. Stemwood is also a large C store in the UMBS forest, accounting for 95% of all aboveground C. The contribution of this pool to total ecosystem C varies in accordance with forest age, with older stands storing relatively more C in wood (Pregitzer and Euskirchen 2004).
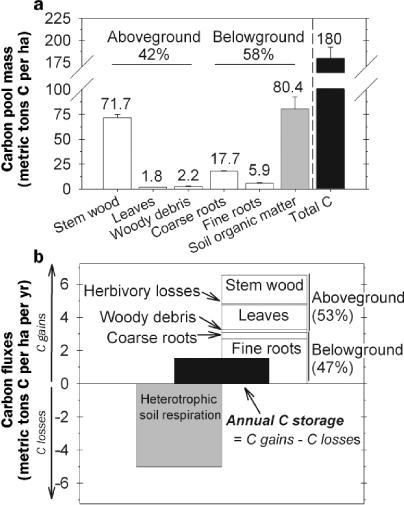
Forest carbon (C) pools (a) and fluxes (a) at the University of Michigan Biological Station (UMBS). The largest storage pool for C is soil organic matter, followed by stem wood (a). Annual C storage in the forest is the small difference between two large fluxes: photosynthetic C gains and respiratory C losses from decomposers or heterotrophs in the soil. The UMBS forest is a moderate C sink, storing an average (1999–2005) of 1.5 metric tons C per hectare per year (b).
How much carbon does the forest store annually?
Annual forest C storage is the small difference between two large opposing fluxes: ecosystem photosynthetic C gains and respiratory C losses. At UMBS, photosynthetic C gains allocated to plant growth averaged 6.5 metric tons C per ha per year (1999–2005), with growth aboveground only slightly exceeding that belowground (figure 2b). More than 40% of total photosynthetic C gain was invested in fine roots; stem wood and leaves also were substantial components of forest growth. The UMBS soil is nutrient poor, which may explain the notably large annual contribution of C to fine root production. Nutrient limitations affect the partitioning of C between above- and belowground biomass components, with plants often investing more C in root growth when soils are poor to enhance nutrient acquisition (Nadelhoffer and Raich 1992). Ecosystem C losses from heterotrophic soil respiration at UMBS average 5 metric tons C per ha per year. The difference between C gains and losses amounts to an annual C storage rate of 1.5metric tons C per ha per year. This rate of annual C sequestration is within the range reported for other aspen-dominated forests (Gough et al. 2008), although it is slightly lower than the general relationship shown in figure 1a would predict for forests at 45°N latitude (figure 1a).
Uncertainty of annual carbon storage estimates
Ecological and meteorological estimates of annual C storage are not free of uncertainty. Here, we report uncertainty as the 95% confidence interval expressed as a percentage of the C flux estimate. Uncertainty in ecological C storage arises in part from spatial variability associated with C fluxes; moreover, many C fluxes are not directly measured but estimated from modeled relationships, also giving rise to uncertainty (Clark et al. 2001, Gough et al. 2008). While the statistical confidence in our ecological C flux estimates was variable (± 11%– 682%), levels of uncertainty were comparable with those reported for other forests (e.g, Clark et al. 2001, Ehman et al. 2002). On average, confidence in stem and coarse root wood mass production (± 11%) was higher than for other pools, in part because allometric equations used to infer mass were developed onsite or in similar forests. Uncertainty in leaf litter production (± 17%) and herbivory (± 8%) was fairly low, reflecting low variability among plots in the case of leaf litter and among methodologies in the case of herbivory. The uncertainty in woody debris production was very high (± 682%), resulting from high variability among coarse debris sampling plots. Methodological constraints and high spatial variation often limit the certainty of woody debris and belowground C fluxes (Clark et al. 2001). Our fine-root litter production and soil heterotrophic respiration uncertainties were modest (± 34% each), resulting from uncertainty in fine root turnover and in empirical models used to estimate continuous soil respiration and partition this flux into autotrophic and heterotrophic components. Aggregated uncertainty in ecological C fluxes (1999–2004) yielded a broad 95%confidence interval for mean annual C storage of −0.75 to 3.75 metric tons C per ha per year.
Uncertainty in meteorological estimates of annual C storage is a result of filling data gaps, random measurement error, and spatial heterogeneity of the C flux footprint. The filling of data gaps with modeled C fluxes contributed moderately to uncertainty in meteorological annual C storage for our site in 2004 (± 11%). Confidence in annual C storage declines as the number of missing observations increases because of the uncertainty in models used for filling gaps in C flux measurements. Carbon fluxes that were estimated from models rather than measured directly because of inadequate climate conditions or instrument failure averaged 33% at our site, a value that is in the low range for forests (Richardson and Hollinger 2007). We used equations from Richardson and Hollinger (2007) to estimate uncertainty in meteorological annual C storage due to random measurement error. This source of uncertainty, which is slightly higher than that due to the filling of data gaps (± 17%), is caused by instrument and calculation errors (Hollinger and Richardson 2005). Spatial heterogeneity of the C flux footprint may be a substantial source of uncertainty when meteorological methods are applied to patchy landscapes that encompass different plant functional or structural types (Oren et al. 2006). This uncertainty can be quantified using paired meteorological towers by comparing C fluxes from identically equipped towers sampling different areas of vegetation under the same climate conditions. Although we have not quantified uncertainty resulting from spatial variation, we expect that it would be low at our site because of the broad distribution of even-aged, aspen-dominated forest across the meteorological tower footprint (Schmid et al. 2003). Aggregated uncertainties in meteorological annual C storage (1999–2004) produced a 95%confidence interval of 1.2 to 1.8 metric tons C per ha per year.
Although C fluxes are measured with high uncertainty, close long-term agreement between ecological and meteorological estimates of annual C storage at our site provides an essential cross-validation of these independently derived estimates. Both methods yielded a five-year (1999–2003) mean annual C storage estimate of 1.5 metric tons C per ha per year (Gough et al. 2008).
Constraints on annual forest carbon storage
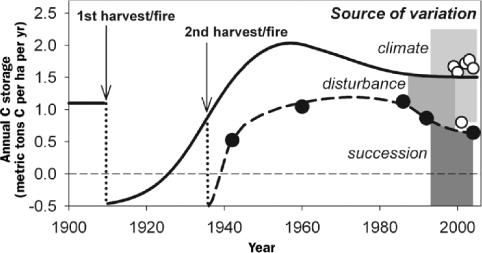
Annual rates of C storage in northern Michigan forests are simultaneously constrained by present climate, past disturbance, and forest successional status (figure 3). Climate exerts a strong influence on the two C metabolic processes that determine annual rates of forest C storage: photosynthesis and respiration. Disturbance by clear-cut harvesting and wildfire during the early 20th century fundamentally reshaped the landscape across the upperMidwest. The region's forests were rapidly transformed from oldgrowth conifer ecosystems to young, mixed deciduous forests, abruptly changing their successional status. We now consider the mechanisms by which climate, disturbance, and successional status affect forest C storage.
Historical reconstruction of annual forest carbon (C) storage at the University of Michigan Biological Station, 1900–2004. Annual forest C storage is simultaneously constrained by forest succession or stand age, past disturbance, and climate. The quantity of variation in annual C storage attributed to these variables is illustrated by vertical gray shading. Forest stands were harvested and burned at different frequencies and times in the early 20th century, changing their successional status. Disturbance frequency also had a direct effect on annual forest C storage, with more frequently disturbed forests having lower annual C storage rates. Climate is the major determinant of current shorter-term, interannual variation in annual C storage. Closed circles are ecological estimates of annual C storage for a disturbance chronosequence that was experimentally harvested and burned, and open circles are meteorological estimates of annual C storage for a nearby control forest (Gough et al. 2007b). Predisturbance annual C storage is from Desai and colleagues (2005) for an old-growth forest in Wisconsin.
Climate.
In temperate forests, the balance between ecosystem photosynthesis and respiration changes seasonally, and is largely a function of climate and leaf phenology (Barr et al. 2004). The UMBS forest is a C source to the atmosphere until the late spring, when leaf expansion begins (figure 4). Conversely, the forest is a net C sink during the growing season, when ecosystem photosynthesis exceeds respiration. This seasonal pattern of net C uptake and loss has been well documented in temperate deciduous forests (Schmid et al. 2000) and in evergreen forests, where winter temperatures inhibit photosynthesis (Kolari et al. 2004).
Cumulative carbon (C) storage by the University of Michigan Biological Station forest, estimated from meteorological methods, 1999–2004. The forest loses C because of respiration in winter, when the deciduous canopy is leafless. Shortly after leaf expansion in the spring, the forest begins to store C because ecosystem photosynthesis is greater than respiration. This upward trajectory of C storage continues until leaf senescence in the autumn. Year-end cumulative C storage equals annual C storage. Annual C storage averaged 1.54metric tons C per hectare per year, but varied by more than 100% (1999–2003 data from Gough et al. [2007a]).
At UMBS, temperature and solar radiation exert strong controls over C storage, but the specific constraints vary seasonally, depending on leaf phenological period. As expected, we observed a strong correlation between ecosystem respiratory C losses and air temperature from October to April, when the canopy was leafless (i.e., during leaf-off) and when photosynthetic C uptake was near zero (figure 5a). Respiratory C losses are correlated with temperature at the tissue, plant, and ecosystem scales (Raich et al. 2002). However, we found that temperature had the opposite effect on ecosystem C losses during May, when leaf expansion occurs and the balance between ecosystem photosynthesis and respiration is in transition. During this phenological period, higher temperatures markedly increased the rate of leaf expansion and hence C uptake through photosynthesis (figure 5b). The onset and rate of canopy development in the spring is correlated with annual C storage rates in other temperate forests (Barr et al. 2004, Baldocchi et al. 2005). At UMBS, there is a 150- day period of full canopy development (i.e., the leaf-on period) that is dominated by C gains. From June through September, photosynthesis was the dominant C metabolic process in the ecosystem, and we found that C gains increased linearly with mean monthly solar radiation (figure 5c). The differences in monthly canopy photosynthesis we observed were due to progressive changes in day length and to variation in cloud cover. Numerous other studies have shown a strong linear or curvilinear relationship between the intensity of solar radiation and the magnitude of forest C uptake (Granier et al. 2002, Law et al. 2002).
Climate constraints on monthly carbon (C) storage by the University of Michigan Biological Station forest, 1999–2004, during three leaf phenological periods: leaf-off (a), leaf expansion (b), and leaf-on (c). Filled circles are data for 2001, when annual C storage reached a six-year low. During the leaf-off period (a), ecosystem respiratory C losses increased with increasing temperature (inset, mean leaf-off temperature in 2001 compared with all other years). Temperature had the opposite effect on respiratory C losses during leaf expansion (b). The forest transitioned more rapidly from a C source to a C sink when mean May temperatures were higher because warmer temperatures accelerated the rate of canopy greening, expressed as the leaf-expansion rate (inset; LAI = leaf area index, squaremeter [m2] leaf area per m2 ground surface area). Mean monthly solar radiation is positively correlated with forest C gains during the leafon (c) period (inset; mean monthly solar radiation in 2001 compared with all other years). Functions were selected based on goodness of fit. P < 0.01 for all regressions.
The collective effects of climate on forest C cycling during the three distinct temperate zone phenological periods of leaf-off, leaf expansion, and leaf-on resulted in variation in C storage rates greater than 100%over six years, although the forest was always a net C sink. Long-term ecosystem C cycling studies conducted within FLUXNET have been instrumental in quantifying the effects of climate on interannual variation in C storage (Goulden et al. 1998, Law et al. 2003, Barr et al. 2004). In 2001, the UMBS forest stored 0.80 metric tons C per ha per year, less than half what was gained in the high year of 2003 and 52% of the six-year average of 1.54metric tons C per ha per year. Reduced C storage in 2001 was due to unusually high respiratory C losses during leaf-off and low photosynthetic C gains during leaf on. Our meteorological data show that winter air temperatures were 1°C warmer, and respiratory C losses from the forest were 10%higher than normal (figure 5a). During the second half of the 2001 growing season, low mean monthly solar radiation resulted in 16%lower photosynthetic C gains compared with the six-year average (figure 5c). Interannual variation in air temperature and radiation has caused very high year-to-year fluctuations in annual C storage of up to 580% in other aspen-dominated forests (Barr et al. 2004). Rates of C storage in boreal and temperate pine forests are similarly constrained by temperature and radiation (Luyssaert et al. 2007).
Disturbance.
The effects of past disturbance are often ignored in studies of forest C cycling, despite a general consensus that historical legacies of land use and disturbance have played major roles in shaping current ecosystem function and forming the modern fragmented landscape (Foster et al. 2003). This is partly because detailed disturbance records from events such as fire, harvesting, wind, flooding, and ice are not readily available for most forests. However, a full understanding of the constraints on forest C sequestration at the landscape scale requires knowledge of prior land use and human activity because nearly all ecosystems have been modified by humans (Foster et al. 2003, Chen et al. 2004, Pregitzer and Euskirchen 2004, Magnani et al. 2007). In North America, many landscapes were shaped by widespread deforestation that occurred through the early 20th century as the nation expanded westward (Birdsey et al. 2006). Ironically, postdisturbance regrowth is responsible in part for the current North American terrestrial C sink (Gaudinski et al. 2000, Birdsey et al. 2006).
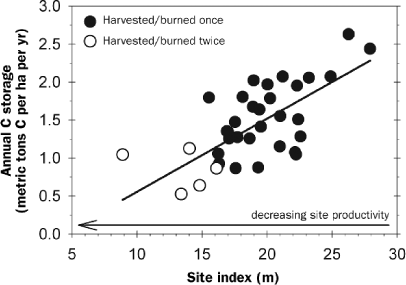
Natural and human-induced disturbances can have long-term, often negative effects on forest C storage capacity. At UMBS, we were able to study the consequences of late 19th- and early 20th-century forest harvest and burning on present-day C storage through the use of a series of disturbance chronosequences (Gough et al. 2007b). We found that experimental forest stands that had been clear-cut and burned twice stored significantly less C than stands disturbed only once (figure 6). Reduced C storage was due to decreased site productivity, expressed as the site index, which is the estimated height of the forest canopy at a reference age of 50 years (Gough et al. 2007b). The combined effects of logging and fire have resulted in reduced soil fertility (Latty et al. 2004) and, in other forests, decreased C sequestration rates (Bergeron and Harvey 1997). Soils, which are often the largest C reservoir in forests (figure 2), may be particularly vulnerable to these disturbances (Latty et al. 2004). The legacy of past land use at UMBS has persisted for more than half a century, demonstrating that poor forest management can have severe and long-lasting effects on C sequestration (Gough et al. 2007b). Fire-adapted ecosystems and infertile sites may be less susceptible to sustained reductions in C storage following fire, but the long-term consequences of fire on productivity are not known for most forest types. Although harvest and fire are becoming less common in the upper Midwest, these disturbances are increasing in the western United States and in tropical regions (Toma et al. 2005, Running 2006). The persistent legacies of poor management practices on forest C storage in northern Michigan should serve as a caution to contemporary forest managers elsewhere.
The effects of clear-cut harvesting and fire on annual carbon (C) storage and site index, a metric of site productivity, at the University of Michigan Biological Station. Repeated disturbance reduced annual C storage by decreasing forest productivity. For the analysis, ecological measurements of annual forest C storage were conducted in 30 stands disturbed once and in 5 stands disturbed twice.
Sustainable management practices can help maintain forest soil fertility and productivity following harvest. For example, even clear-cut harvesting may have little negative effect on soil C and N content when unmerchantable biomass residues, such as leaves and twigs, are left onsite (Johnson and Curtis 2001). Disturbance effects attributed to diminished soil fertility also may be ameliorated by adding limiting nutrients to the ecosystem. Fertilizer is routinely applied to forest plantations to increase wood production. For example, nutrient amendments are applied annually to nearly 5000 km2 of pine plantations in the southeastern United States alone (Fox et al. 2007). However, forest fertilization has C costs associated with its manufacturing and application (Sonne 2006).
Succession.
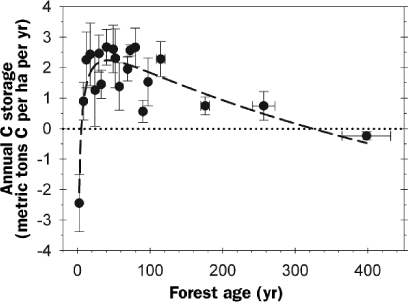
A forest's position along the trajectory of ecological succession is an important determinant of C storage potential (Magnani et al. 2007). In 1969, E. P. Odum hypothesized that forests experience an initial reduction in C storage following initiation because of a low ratio of photosynthesis to respiration, with rates of C storage increasing to a maximum as canopy photosynthesis peaks and slowly declining to near zero thereafter (Odum 1969). This hypothesis was not easily tested in forests at the time because instrumentation and techniques for measuring large-scale C fluxes were not available. Our studies at UMBS support Odum's hypothesis that the shifting balance between ecosystem photosynthesis and respiration over time is a mechanism for this successional pattern of C storage (Gough et al. 2007b). The UMBS forest was a moderate C sink of 0.5 metric tons C per ha per year within six years after experimental clear-cutting and burning (figure 3). In young stands, low annual C storage was due to high annual heterotrophic respiration and low annual photosynthetic C gains. This pattern was reversed in a 50-year-old stand with peak annual growth rates and relatively low C losses from heterotrophic respiration. To examine whether Odum's hypothesis is supported by FLUXNET observations, we compiled above-canopy C flux data and detailed ecological measurements from 33 forested study sites, with results extending three or more site years (see the supplemental material online at http://hdl.handle.net/1811/31687). For the analysis, annual C storage rates were corrected for differences attributable to latitude. This research synthesis shows that, following establishment, forests transition rapidly from C source to C sink, and annual C storage rates decline gradually to near zero in old-growth stands. Although considerable variation in annual C storage exists in intermediate-aged forests, results from modern C-cycling research support Odum's prediction.
It is important to note that the magnitude and timing of changes in annual C storage through ecological succession vary considerably among ecosystems, and our analysis of annual forest C storage over time (shown in figure 7) illustrates a generalized response. For example, some forests have been shown to be strong C sources to the atmosphere up to four decades following stand-replacing disturbance (Law et al. 2003, Campbell et al. 2004). Despite confirmation of Odum's hypothesis, our understanding of how annual forest C storage changes over time is based on relatively few studies. Our assessment of annual C storage in old-growth forests (mean age = 400 years) includes only two coniferous forest types in the western United States (Law et al. 2003, Campbell et al. 2004). Regional appraisals of terrestrial C storage require quantitative knowledge of how annual C storage changes through ecological succession because most landscapes encompass forest stands of varying age (Chen et al. 2004).
The general pattern of annual forest C storage through ecological succession constructed from pooled data for 33 forested sites comprising a total of 184 site years (see the online supplement at http://hdl.handle.net/1811/31687). Ecosystem-scale studies of annual forest C storage have supported E. P. Odum's hypothesis (Odum 1969), which predicted that ecosystems would transition from C source to sink following establishment as respiration from decomposers (or heterotrophs) declines and photosynthesis increases with expansion of the forest canopy. Odum predicted a gradual decline in annual C storage as the photosynthetic capacity of the forest declines upon maturity. Annual C storage rates for each forested site and site year were corrected for differences due to latitude (figure 1a) and pooled into 5-year (< 100 years) and 75-year (≥ 100 years) increments for the analysis. Bars illustrate one standard error.
Global change and forest carbon storage
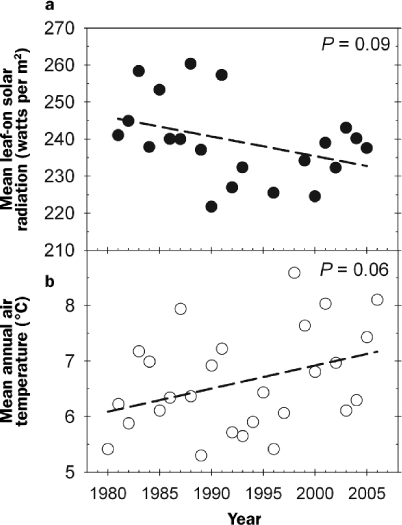
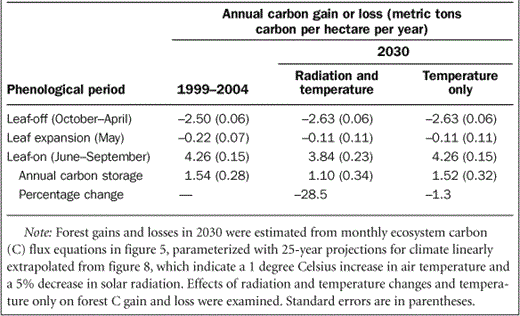
How will changes in climate and disturbance regimes together with continued ecological succession constrain future forest C storage in the upper Midwest? Climate records at UMBS over the past 25 years indicate rising mean annual air temperatures and decreasing solar radiation in the growing season—trends that, should they continue, will negatively affect this forest's rate of C storage (figure 8, table 1). Air temperatures rose by an average of approximately 1°C over 25 years while growing-season solar radiation decreased by 5%, with high interannual variation in both climate parameters. On the basis of our understanding of C cycling in northern forests, climate change at the current rate would reduce the existing forest's mean annual C storage by 28%in 25 years, from1.5 to 1.1metric tons C per ha per year, primarily through reduced solar radiation and a resulting 10% decrease in photosynthetic C uptake. However, if solar radiation stabilized at current levels, annual C storage losses would be negligible, amounting to a 1%decrease relative to current rates. Although there would be higher respiration during leaf-off, the rate of canopy greening would increase by more than 50%in response to higher May temperatures. This more rapid transition from forest C source to sink during the spring would offset the higher wintertime C losses. Our extrapolated estimate of +1°C over 25 years is within the range predicted by the Intergovernmental Panel on Climate Change (IPCC 2007), but changes in solar radiation over the next quarter century are much less certain (Wild et al. 2005).
Mean growing season solar radiation (a) and mean annual air temperatures (b) at the University of Michigan Biological Station, 1980–2005. On average, mean growing season net radiation decreased by 5%and mean annual air temperature increased by 1.1 degree Celsius over 25 years.
Recent (1999–2004 mean) and projected (2030) rates of carbon gain and loss for the UMBS forest during three phenological periods.
Whole-ecosystem approaches are required to gain a comprehensive understanding of the relationships between climate change and total forest C storage, especially if forests are managed for C sequestration. Most studies have examined how climate change affects one component of forest C storage, such as wood growth. Boisvenue and Running (2006) found that wood growth rates increased globally in a majority of forests (n = 49) over the past 55 years, possibly because of warmer air temperatures and rising CO2. In general, there is a positive relationship between mean annual temperature and annual C storage rates, with higher temperatures extending the leaf-on period and consequently increasing annual photosynthetic C gains (Baldocchi et al. 2005). However, rising air temperatures also increase ecosystem respiratory C losses, often exponentially (Lawet al. 2002, Curtis et al. 2005). Thus, rates of C loss from forests may be accelerated with more extreme warming. In addition to changes in air temperature and solar radiation, global changes that include rising atmospheric CO2 and ozone, increasing atmospheric N deposition, and changes in precipitation may affect annual forest C storage both as primary drivers and in interaction with each other (Boisvenue and Running 2006, Hyvonen et al. 2007, Magnani et al. 2007). Experimental approaches that examine the effects of multiple forms of climate change on whole ecosystem C storage, combined with long-term monitoring programs, are essential to understand how forest C-cycling processes realistically will respond to a changing environment. In addition, process models are essential tools for synthesizing empirical C cycling data and examining the multiple, interacting effects of global change.
Future forest C storage also will depend on ecological changes that result from on going forest disturbance and succession. While severe disturbance is common in fire-prone and intensively managed ecosystems, many maturing forests of the upper Midwest and eastern United States are likely to undergo a less severe and subtler successional transition that includes the emergence of a more species-diverse and structurally complex forest, but does not involve complete canopy replacement (Frelich and Reich 1995). Although the successional dynamics likely to occur in these forests are well understood, the C cycling processes in the emerging ecosystem are not. At UMBS and throughout the surrounding region, many forests that were established following disturbance at the beginning of the 20th century are undergoing a major successional transition in which aspen, the dominant canopy species, is senescing and being replaced by other deciduous and evergreen species (USDA Forest Service 2002). This subtler transition to a heterogeneous secondary forest is likely to be much more common because fire suppression and less aggressive forest harvesting have greatly reduced catastrophic stand replacement (Caspersen et al. 2000).However, fire frequency in the upper Midwest is predicted to increase by 20% to 40% over the next 50 years because of climate change, so future fire regimes may be different from those today. Understanding how ecological succession and future disturbance regimes will affect the permanence of C stored in forest reservoirs is essential when managing ecosystems for long-term C sequestration. This is especially true in unmanaged low-productivity forests of the upper Midwest because these ecosystems have the potential to serve as long-term C reservoirs.
Putting knowledge to work: Translating ecosystem-scale research into management for carbon sequestration
Managing forests for C sequestration is consistently supported by international scientists and policymakers as a strategy for mitigating anthropogenic CO2 emissions (IPCC2007). Although a robust market for C trading as an approach to stabilize greenhouse gas emissions has not yet emerged in the United States, several major industrial forestry corporations have voluntarily enlisted in pilot greenhouse gas emission reduction and trading programs, including the Chicago Climate Exchange (www.chicagoclimatex.com). Thus, this approach could be employed immediately. Multiyear assessments of annual C storage, such as those conducted within FLUXNET, are helping to evaluate the potential for different ecosystems to sequester C, and they are providing estimates that eventually could be used in C accounting efforts. Here, we conclude by briefly discussing challenges associated with the management of forests for C sequestration, and we describe how ecosystem studies of forest C cycling may be used to inform forest and land managers.
The high variation in C storage at all latitudes (figure 1) suggests that forests often store Cat rates well below their potential and thus could be responsive to management for enhanced C sequestration. However, forests at lower latitudes may offer greater potential since longer growing seasons support higher average C storage rates (figure 1a), and because larger differences between average and maximum C storage suggest that there is greater flexibility to improve C sequestration rates through management (figure 1b). Global remote sensing and modeling studies suggest that abiotic enhancements have amore pronounced effect on plant growth at lower latitudes (Nemani et al. 2003). Distinct regional differences in timber management intensity also will influence how forests are managed for C sequestration. As forest management intensifies and becomes more concentrated in the southeastern and northwestern United States, low-productivity forests that are not managed for timber, such as some forests in the upper Midwest, may serve as long-term terrestrial C reservoirs. Alternatively, industrial timberlands may be managed both for forest products and C sequestration to offset anthropogenic CO2 emissions (Birdsey et al. 2006). In forests where wood will be harvested, management for long-term C sequestration must center on augmenting belowground C pools and increasing the residence time of C stored in harvest residues and soils. Contemporary forest management practices emphasize sustainability of wood production and site quality (Frelich and Reich 1995, Houghton et al. 1999), but generally disregard other pools that contribute to forest C sequestration.
A quantitative understanding of how forest management practices may simultaneously enhance wood production and C sequestration is lacking for most forest types because few whole-ecosystem C storage studies have been conducted in managed forests. In our survey of 33 sites reporting annual C storage for three or more site years, only 5 were managed forests (see http://hdl.handle.net/1811/31687). Nonetheless, some general recommendations for enhancing soil and residue C pools can be made on the basis of these few studies that focus explicitly on C storage in managed ecosystems. For example, many of the techniques established in agricultural systems to conserve organic matter apply to C conservation and enhancement efforts in forest soils. These include (a) cropping intensification to enhance below ground C allocation, (b) conservation tillage to reduce erosion and minimize the disruption of soil aggregates containing C, (c) applying organic amendments to soils to increase soil C content, and (d) replanting following harvesting to minimize the transition time from C source to C sink (Post et al. 2004).
Many of these agricultural practices are already used in common silvicultural applications. Cropping intensification (e.g., fertilization, pest management) is widely employed in intensively managed forests, and the benefits of this practice on above- and belowground growth are well documented (Maier and Kress 2000). Logging residues can be left onsite, providing organic amendments that increase soil C (Johnson and Curtis 2001). Common silvicultural practices such as forest thinning can enhance total wood yield over a rotation and augment soil C pools (Selig et al. 2008). Other silvicultural practices may require modification to enhance forest soil C storage. For example, tillage prior to planting may increase forest soil C emissions (Gough et al. 2005) and lower the soil C pool. Forest canopies are commonly manipulated to maintain an age structure that is within the window of peak wood production, but the effects of these practices on other C pools have not been quantified for most forested ecosystems. Very little is known about the effects of repeated, short rotation harvesting on soil C storage, despite an increase in intensive plantation management worldwide (FAO 2005). Clearly, experimental studies in managed forests are required to provide ecosystem-specific management guidelines for C sequestration without compromising wood production. Additionally, it is essential to quantify the C costs of management and to consider how such practices may compromise other ecosystem goods and services (Sonne 2006).
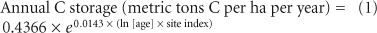
In addition to simple ecosystem-specific empirical models, more sophisticated forest growth and yield models and process-based models developed for both wood and C sequestration management are now available. For example, the USDA Forest Service's Forest Vegetation Simulator (FVS) predicts forest stand-level changes in plant and woody debris C stocks over time and in response to common management practices (www.fs.fed.us/fmsc/fvs/). The model is parameterized using readily obtained forest inventory data. One major limitation of the current FVS model is its inability to predict changes in soil C stocks. Continued development of easily parameterized models is essential for low-cost assessments of annual forest C storage (Birdsey et al. 2006). Multiyear, spatially extensive measurements of C storage at FLUXNET research sites such as UMBS are helping to refine and improve the precision of these predictive models. Ultimately, models developed and tested using ecosystem C cycling data will inform forest managers how best to achieve maximum rates of annual C storage across an array of ecosystems varying in disturbance history, successional status, and climate.
Easily parameterized ecosystem-specific models for predicting annual forest C storage are essential to carbon (C) accounting. Site index, a metric of forest productivity, and stand age are two predictors of annual forest C storage in deciduous forests of northern lower Michigan (equation 1) and are routinely measured by foresters. Equation 1 can be parameterized to make regional predictions of forest C sequestration using USDA Forest Inventory and Analysis (FIA) data, which are available for all 50 states (USDA 2002). We estimated annual forest C storage by 2400 square kilometers of deciduous forests in the four northern counties of lower Michigan (Charlevoix, Emmet, Cheboygan, and Presque Isle) by parameterizing equation 1 with forest site index and age distribution data from the FIA database (n = 567 plots; http://ncrs2.fs.fed.us/4801/fiadb). In this heavily forested region, deciduous forests (41% of the total land area) store an average of 1.32 ± 2.25 metric tons C per hectare per year, or a total of 320,000 metric tons of C per year. The US Energy Information Administration (EIA 2007) estimates that people living in this area emit 520,000 metric tons of C per year. Thus, these forests currently sequester 62% of the region's anthropogenic C emissions, or the equivalent annual C emissions from 225,000 cars (EPA 2007).
Conclusions
Recent ecosystem-scale forest C cycling studies have tested several fundamental hypotheses about the controls on annual C storage. We now have a quantitative understanding of how climate, past disturbance, and ecological succession constrain annual C storage for several forest types. Our results show that at UMBS, solar radiation and air temperature are important regulators of short-term forest C uptake and loss, respectively, causing interannual variation in annual C storage that exceeds 100%. Harvest and fire disturbance in northern Michigan during the early 20th century continues to exert a negative effect on annual C storage, indicating that the legacy of disturbance in the region is severe and long lasting. We have also shown that annual C storage changes over ecological succession, as hypothesized by ecologists decades ago.
Whole-ecosystem C storage studies provide quantitative information that can be applied to C sequestration management. Ecosystem-specific models for predicting annual C storage will be essential if forests are to be managed for C sequestration. Many questions remain unanswered about how whole-ecosystem C storage responds to contemporary forest management practices and to treatments that may enhance C sequestration. Moreover, we know very little about how annual C storage will respond to future changes in climate, new disturbance regimes, and altered forest composition and structure. Ecosystem-scale forest C cycling studies have provided many answers about how past and present conditions constrain current annual C storage. As portfolios for offsetting greenhouse gas emissions are likely to include forest C sequestration, ecosystem-scale studies must now anticipate how natural and anthropogenic forces will diminish or enhance annual C storage in forests of the future.
Acknowledgments
This research was supported by the US Department of Energy's Office of Science (Biological and Environmental Research) through the Midwestern Regional Center of the National Institute for Global Environmental Change under cooperative agreement no. DE-FC03-90ER610100; the Midwestern Regional Center of the National Institute for Climatic Change Research at Michigan Technological University, under award no. DE-FC02-06ER64158; and the US Department of Agriculture's Forest Service award no. 06-JV-11242300-145. We acknowledge the University of Michigan Biological Station for facilities and material support, and the National Science Foundation's Research Experience for Undergraduates program (award no. NSF-DBI 0453328) and Integrative Graduate Education and Research Traineeship program (award no.DGE 0504552) for student training and support. We appreciate field and technical assistance from Bob Vande Kopple.
References cited
Author notes
Christopher M. Gough (e-mail: gough.21@osu.edu; cmgough@vcu.edu) was with the Department of Evolution, Ecology, and Organismal Biology at Ohio State University in Columbus when this article was being prepared; he is now with the Department of Biology at Virginia Commonwealth University in Richmond.
Christoph S. Vogel is with the University of Michigan Biological Station in Pellston.
Hans Peter Schmid is with Atmospheric Environmental Research, Institute of Meteorology and Climate Research, Research Center Karlsruhe, in Kreuzeckbahnstr, Germany.
Peter S. Curtis is in the Department of Evolution, Ecology, and Organismal Biology at Ohio State.



![Cumulative carbon (C) storage by the University of Michigan Biological Station forest, estimated from meteorological methods, 1999–2004. The forest loses C because of respiration in winter, when the deciduous canopy is leafless. Shortly after leaf expansion in the spring, the forest begins to store C because ecosystem photosynthesis is greater than respiration. This upward trajectory of C storage continues until leaf senescence in the autumn. Year-end cumulative C storage equals annual C storage. Annual C storage averaged 1.54metric tons C per hectare per year, but varied by more than 100% (1999–2003 data from Gough et al. [2007a]).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/bioscience/58/7/10.1641_B580708/5/m_i0006-3568-58-7-609-f04.gif?Expires=1716403394&Signature=SP37XbaNlYMhhK7mBW-3zxdSL3maRO5LBccaSAapnNmrM2x-NJk4rhldePdMjFaIy1yrVNqNCyudHUIWXwbQGWj1MyWDyeUS66xtdN~bxS0NqjGh3QoZPj8lK9RcosR6aTuKSNZp1mGGqBMPQpAA7zqn8BthXO4wbTYwT1B4UymTULms9XDWk-2oDU6TDSlro6IR3X-c3a~8tXbmbyIJMi~PS91UOlyYkjo5JFgFZAEN~jYmIhGCVB6MEFuoiO4IBq5Gboq512YYqXNdOGUAAVEu0bcKzIDH150q6oAhKO6ImHzc57Edh8CpYjFlTOV47TWPKmMXz1JoRaxDPI3evA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Climate constraints on monthly carbon (C) storage by the University of Michigan Biological Station forest, 1999–2004, during three leaf phenological periods: leaf-off (a), leaf expansion (b), and leaf-on (c). Filled circles are data for 2001, when annual C storage reached a six-year low. During the leaf-off period (a), ecosystem respiratory C losses increased with increasing temperature (inset, mean leaf-off temperature in 2001 compared with all other years). Temperature had the opposite effect on respiratory C losses during leaf expansion (b). The forest transitioned more rapidly from a C source to a C sink when mean May temperatures were higher because warmer temperatures accelerated the rate of canopy greening, expressed as the leaf-expansion rate (inset; LAI = leaf area index, squaremeter [m2] leaf area per m2 ground surface area). Mean monthly solar radiation is positively correlated with forest C gains during the leafon (c) period (inset; mean monthly solar radiation in 2001 compared with all other years). Functions were selected based on goodness of fit. P < 0.01 for all regressions.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/bioscience/58/7/10.1641_B580708/5/m_i0006-3568-58-7-609-f05.gif?Expires=1716403394&Signature=aA9-g4u5UgMS0sF7-DJ16ejEO1gJaxUJd4g9vK~8wQ49PSJ9FnOWTHdUeuFL2ilcEDxCbe8BjEaI~6eK~dfk1GP16XZQLrL5B2dwe8~RaokBWGPSoi08YsKAiJU~yS7IkqhXomqzs12XfB7lIwn1TiGOmzfkIH5qqQFDbsFqL6C2J119doBdqacdnn5HS-iSDZOAsAdseSZoVFnqttvypN0XNaoJXzLWU6wapHZwZVbOceXgwIKoBMgelpH1rezsd38KLXgXX6vW75dHHz5U-f-~Eyf6VNGZjKsjIC~j6fNgPl~wdpw~-1iI1tVC3wlHv83mvpwplTuRek3Yq9uHyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)