-
PDF
- Split View
-
Views
-
Cite
Cite
GERHARD GOTTSBERGER, How diverse are Annonaceae with regard to pollination?, Botanical Journal of the Linnean Society, Volume 169, Issue 1, May 2012, Pages 245–261, https://doi.org/10.1111/j.1095-8339.2011.01209.x
Close - Share Icon Share
Abstract
Studies during the last decades on representatives of Annonaceae have revealed their diversification with regard to pollination. Examples are given of species pollinated by beetles (cantharophily), which is the predominant mode of the majority of species worldwide, by small or large beetles (both groups having either diurnal or nocturnal flowers with or without thermogenesis), species pollinated by thrips (Thysanoptera), flies (myiophily and sapromyiophily), cockroaches and even bees (melittophily). Adaptational features of floral structures are compared with the behaviour of flower visitors, emphasizing floral rhythm, flower size, petal thickness, stamen structure and odour production. Anaxagorea, the earliest divergent surviving genus in Annonaceae, exhibits floral characters thought to be basal for the family: tissue-rich thick petals that form a pollination chamber; several Anaxagorea spp. exhibit thermogenesis, emit a fruit-like odour and attract small beetles as pollinators. As cantharophily is plesiomorphic in Anaxagorea and in Annonaceae, characters associated with beetle pollination appear imprinted in members of the whole family. Even non-cantharophilous species retain one or more characteristic features of beetle-pollinated species, such as thick petals, flattened and sclerified connective shields or protogynous dichogamy.
INTRODUCTION
General ideas concerning pollination of flowers of Annonaceae have changed in recent years. The majority of former studies in the Old and New World Tropics showed that flowers of members of the custard apple family were nearly exclusively visited and pollinated by beetles. Cantharophily or beetle pollination therefore was thought to be the overall prevailing pollination system in the family.
Beetle pollination was previously interpreted to be an archaic pollination system in angiosperms. The fact that Annonaceae were shown to be a cantharophilous group, and that other early-divergent angiosperms, such as Magnoliaceae, Eupomatiaceae or Calycanthaceae, have similar pollination modes, therefore fitted well into this conceptual paradigm of antiquity. Studies in the last three decades, however, have modified our understanding of pollination modes in Annonaceae, and early-divergent angiosperms in general. Annonaceae are much more diverse than originally thought, and even the cantharophilous syndrome is quite diverse: the flowers of some species are known to be pollinated by small beetles, whereas others attract relatively large beetles; and there are beetles that visit flowers of certain Annonaceae during the day and others are active during the evening or night. Different beetles have quite different relationships with the flowers they visit. In addition, other species are pollinated by flies or thrips, and a few Annonaceae are even pollinated by cockroaches or bees. Thus, a more differentiated and unexpected picture of pollination modes in Annonaceae has emerged. To what extent is the visiting behaviour of the insects imprinted on the flowers? Are there pollination syndromes and, if so, how are they distinguished from each other? Furthermore, what are the key factors involved in the development and radiation of pollination modes in Annonaceae?
FUNCTIONAL MORPHOLOGY OF ANNONACEAE FLOWERS
The majority of species have hermaphroditic, protogynous flowers and commonly exhibit a trimerous perianth more or less distinctly differentiated into sepals and petals. Sepals are arranged in one whorl and petals are arranged in two whorls; stamens and carpels have a helical arrangement. Valvate and imbricate aestivations are most common. Petals in sexually mature flowers may be reflexed, spreading, erect or connivent. The majority of Annonaceae have many stamens, which are frequently tightly packed and shielded by a broadened apical prolongation of the connective. Furthermore, most Annonaceae are apocarpous, both in flower and fruit, and carpel number varies from one to many within and between genera (van Heusden, 1992). The numerous, centrally located carpels have sessile stigmas of various shapes. In the pistillate phase, stigmas in many species are covered with a sticky secretion; the stigmas usually become detached at the end of the pistillate phase. The sticky mass of stigmas function as a compitum (van Heusden, 1992). With their thick, fleshy petals, flowers of many Annonaceae superficially appear more like fruits; these flowers are tough enough to withstand the rough eating habits of their visitors, which in some cases are large, voracious dynastid scarab beetles. Flowers of scarab-pollinated species always exhibit nocturnal anthesis and remarkable thermogenic flowers. Floral thermogenesis coincides perfectly with the flight patterns of the associated beetles. To appreciate and understand the events in the scarab pollination system, temperature must be correlated with the various functional stages of the flowers.
Examples will be presented of species pollinated by small and large beetles, thrips, flies, cockroaches and bees. Adaptational features of floral structures are compared with the behaviour of the flower visitors, emphasizing floral rhythm, flower size, petal thickness, stamen structure and odour production.
BEETLE POLLINATION
With respect to size, there are two groups of beetles pollinating flowers of Annonaceae. The large majority of cantharophilous species of Annonaceae are pollinated by small beetles (Nitidulidae, Curculionidae, Staphylinidae, Chrysomelidae), with a body length up to 7 mm. A smaller group of species attracts Scarabaeidae (Dynastinae, Rutelinae, Cetoniinae, Trichiinae), which are larger beetles with a body length of 14–20 mm.
POLLINATION BY SMALL BEETLES: THERMOGENIC DIURNAL FLOWERS
Three Anaxagorea A.St.-Hil. studied in Central Amazonia (Webber, 1996) are protogynous with a 2-day flowering rhythm. The flowers enter the pistillate and staminate phases during the day. Anaxagorea brevipes Benth. initiates receptivity (indicated by stigmatic exudates) in the early morning at 06:00 h, A. manausensis Timmerman at approximately 08:00 h and A. phaeocarpa Mart. between 08:00 and 08:30 h. The emission of typical, species-specific fruit-like odours, however, varies in the three species: A. brevipes in the morning, A. manausensis at midday and A. phaeocarpa at 13:30 h. In all three species, floral temperature rises approximately 1.5–5.5 °C above ambient air temperatures, with the highest temperature peaks being either in the pistillate or staminate phases (Webber, 1996). The three species are pollinated by several Colopterus spp. (Nitidulidae), and less commonly by Staphylinidae, which arrive at the flowers after initiation of odour emission in the pistillate phase. On the second day of flowering, during the staminate phase, pollen becomes available. When the petals drop, the pollen-carrying beetles are released and can approach different flowers in the pistillate stage, which at this time are odoriferous and attractive to the beetles.
In another, heterodichogamous species, Anaxagorea prinoides A.St.-Hil. & A.DC. in French Guiana, no heating of the flowers was observed, although emission of a fruit-like scent during anthesis and attraction of Colopterus spp. was similar to the species mentioned above. As in other species, the staminodes, which separate the stamens and the carpels, incline during the staminate stage and curve towards the carpels, in this way preventing pollen being deposited on them (Teichert, Dötterl & Gottsberger, 2011; see also M. Braun & G. Gottsberger, unpubl. data, for A. dolichocarpa, Fig. 1A–C).
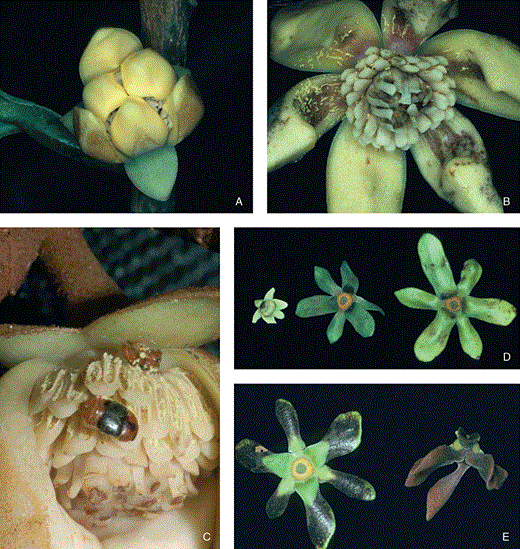
A–C, Anaxagorea dolichocarpa. Length of outer petals c. 14 mm, width c. 8 mm. A, protogynous flower in pistillate stage. Note pollination chamber formed by the inner petals and gaps between petals which permit entrance for pollinators. B, staminate-stage flower with widely open petals. Anthers are dehisced, inner staminodes are lengthened and bent over the stigmatic surface. C, Colopterus sp. ingesting pollen during staminate stage of the flower. D–E, Guatteria foliosa. Length of petals of the largest flower c. 35 mm. D, three open green buds of different sizes. E, left flower developing brown tips indicating initiation of anthesis. Right brown flower is in pistillate stage and has closed its petals over the reproductive organs forming a pollination chamber.
POLLINATION BY SMALL BEETLES: DIURNAL FLOWERS WITHOUT THERMOGENESIS
The vast majority of species of Guatteria Ruiz & Pav. and many species of Duguetia A.St.-Hil. have flowers with relatively small floral chambers. The pendulous flowers of, for example Guatteria neglecta R.E.Fr. (correct name probably G. australis A.St.-Hil.; see Maas et al., 2001) were observed in the Atlantic rainforests in São Paulo State, Brazil. The flowers become dish-shaped after opening (Gottsberger, 1970), but the reproductive organs are exposed long before the flowers are anthetic. After weeks of petal growth, the flowers enter their reproductive stage. The hard and greenish petals then become softer, yellowish and finally brownish. The three inner petals fold over the flower centre and emit a heavy, acetone-like, fruit-like odour.
The anthetic flowers attract fruit-inhabiting and fruit-eating Nitidulidae (Gottsberger, 1970, 1999) by providing floral odours similar to overripe or even decaying fruits. Anthesis begins and ends during the morning hours on two subsequent days; at the same time, attraction and release of the beetles takes place. Small, flat Nitidulidae enter the odoriferous, dark floral or pollination chamber and thus effect pollination of the protogynous flowers. The beetles remain in the flowers until the next morning, which is about the same time at which the flower has entered its pistillate phase the previous morning. Pollen is shed and petals drop. In some other Guatteria spp. of the Amazon forest (e.g. Guatteria foliosa Benth., Fig. 1D, E, and G. megalophylla Diels; see Webber, 1996) small species belonging to Staphylinidae and Chrysomelidae may constitute additional or even dominant flower-visiting and pollinating beetles. The petals of the Guatteria spp. tend to be heavily gnawed or even totally consumed by small curculionid or larger scarabaeid beetles which, however, normally do not have any impact on pollination.
Quite similar processes occur in the cerrado species Duguetia furfuracea (A.St.-Hil.) Saff. (Gottsberger, 1970, 1993), the Brazilian Atlantic forest species D. lanceolata A.St.-Hil. (Silberbauer-Gottsberger, Gottsberger & Webber, 2003) and the Amazon forest species D. stelechantha (Diels) R.E.Fr. (Webber, 1996). All these species are day-active, have small pollination chambers and do not show measurable floral warming.
In the Australian species, Goniothalamus australis Jessup, investigated on the Atherton Tableland in North Queensland (Silberbauer-Gottsberger et al., 2003), the pendulous flowers do not change their shape from the early bud stages to fully functional flowers (Fig. 2A, B), but the thick, fleshy petals continue to grow and enlarge, and the flower colour changes from green to yellow to deep brownish orange–red during their reproductive phases. The flowers are day-active and throughout anthesis the short triangular inner valvate petals remain closed over the centre of the flower. Only the apices of the outer petals retract. Between each of the adjacent bases of the inner petals, there is a small round opening. These three openings are first obstructed by the bases of the outer petals. At the beginning of the pistillate stage, however, the base of the outer petals reflexes and the openings become accessible to small insects.
A–B, Goniothalamus australis. Length of outer petals c. 40 mm. A, yellowish pre-anthetic flower. B, flower in pistillate stage with external petals and one internal petal removed to show the pollination chamber, one nitidulid beetle inside. C–D, Xylopia crinita. Petal length c. 40 mm. C, flower opening during pistillate stage. D, open pistillate stage flower at first evening of anthesis, showing receptive stigmas and the small pollinating beetles (Staphylinidae).
The pistillate stage begins in the early afternoon after the petals turn a deep brownish–red; at this stage the flowers give off a strong odour reminiscent of fermented fruits. The stigmas are white and shiny, the carpels are green and the white stamens are undehisced at this stage. In the later afternoon the odour becomes weaker. During the pistillate stage, nitidulids (Fig. 2B) enter via the small basal openings of the inner petals. On one occasion, we observed up to 25 beetles crowded inside a single flower. On the following day, shortly after 13:00 h, the flowers enter the staminate stage, during which the dried stigmas darken. When the petals drop at approximately 14:00 h, the beetles inside the flower are exposed and leave the flower; when attracted to first-day, pistillate flowers, the pollen-covered beetles pollinate the flowers.
POLLINATION BY SMALL BEETLES: NOCTURNAL FLOWERS WITH THERMOGENESIS
The flowers of Polyalthia korinti (Dunal) Hook.f. & Thomson from Sri Lanka exhibits thermogenesis that initiates before the onset of the pistillate stage (Ratnayake et al., 2006). The internal temperature of the flower reaches approximately 6 °C above ambient levels between 16:00 and 19:00 h. During the staminate phase of the second day, the temperature rises to > 3 °C above ambient air by c. 16:00 h and then gradually diminishes. The elevated temperature coincides with stigmatic receptivity and anther dehiscence during the first and second day of anthesis, respectively.
The most common flower visitors entering the floral chamber in that study were Endaeus spp. (Curculionidae), with up to six individuals inside the chamber. These weevils are crepuscular and nocturnal, arriving at pistillate-stage flowers between 18:00 and 22:00 h, They remain overnight inside the floral chamber and depart in the morning of the following day (between 05:00 and 08:00 h). Another group of Endaeus beetles arrived at the flowers in the staminate phase in the afternoon of the second day and remained until nearly 18:00 h. Their time of leaving the staminate-stage flowers coincided with the arrival of individuals of this beetle in the pistillate-stage ones. Other, but much less common, floral visitors that entered the floral chamber were the nitidulid beetle, Carpophilus plagiatipennis, and two unidentified cockroach species.
Xylopia crinita studied in Central Amazonia around Manaus also has nocturnally active flowers and attracts a small unidentified staphylinid species (Webber, 1996; Küchmeister et al., 1998; Fig. 2C, D). On the first day of anthesis, the unopened buds produce heat and emit a strong spicy, penetrating scent (reminiscent of certain species of Philodendron Schott) from 16:30 h on. At 17:30 h the buds start to gradually open and, when the flowers enter the female stage, the floral scent becomes stronger. The temperature elevation of the flowers accelerates gradually and reaches its maximum of 13.2 °C above ambient air at approximately 19:15 h, the scent is strong and becomes fruity and unpleasant. From 19:15 h the temperature and scent intensity of the flowers diminishes until 22:00 h when the temperature increase stops. Liberation of pollen occurs on the following day, beginning at approximately 15:00 h, and normally finishes at approximately 18:00 h. The small staphylinid beetles start approaching and entering the flowers at approximately 18:00 h on the first day in the pistillate stage and continue to arrive at the flowers until approximately 21:00 h; they remain inside the pollination chamber until 17:30–18:00 h on the following day when the petals and stamens drop. The four to seven beetles attracted by individual flowers apparently eat pollen; when flying away they have pollen grains adhering to their body.
POLLINATION BY SMALL BEETLES: NOCTURNAL FLOWERS WITHOUT THERMOGENESIS
There are rare cases in which nocturnally active flowers produce no measurable heat. One described case is Polyalthia coffeoides (Thwaites) Hook.f. & Thomson from Sri Lanka. This species is sympatric, and shares the same pollinator, an Endaeus sp. (Nitidulidae), with Polyalthia korinti; but Ratnayake et al. (2006) could not unequivocally detect elevated temperature levels inside the pollination chamber.
POLLINATION BY LARGE BEETLES: DIURNAL FLOWERS WITHOUT THERMOGENESIS
The flowers of Asimina obovata Nash and A. pygmaea Dunal studied in Florida (Norman & Clayton, 1986) are visited by large beetles, specifically Trichiotinus rufobrunneus, T. lunulatus (Trichiinae) and Euphoria sepulchralis (Cetoniinae; both Scarabaeidae) and Typocereus zebra (Cerambycidae). In the pistillate stage beetles feed on special food bodies located in petal tissue, and in the staminate stage on pollen. In A. obovata, the curculionid beetle Notolomus basalis also occurs in large numbers and pollinates flowers (Matthias, 2002). The protogynous receptive phase lasts for 4–6 days, and the staminate phase starts with anther dehiscence in the morning and lasts 1 day.
POLLINATION BY LARGE BEETLES: NOCTURNAL FLOWERS WITH THERMOGENESIS
Several species of Annona, Cymbopetalum Benth., Duguetia and Malmea R.E.Fr. are pollinated by large scarabs (Dynastinae, Scarabaeidae) (e.g. Gottsberger, 1989; Schatz, 1990; Webber, 1996). In the Brazilian cerrado vegetation, near Indianópolis in the State of Minas Gerais, six Annona spp. belonging to several sections grow close together and were studied in regard to their flowering and pollination biology (Gottsberger, 1989). Only two dynastid scarab beetle species, Cyclocephala atricapilla and C. quatuordecimpunctata, are the pollinators of all six Annona spp. Cyclocephala atricapilla is the main or exclusive pollinator of A. coriacea Mart., A. crassiflora Mart., A. dioica A.St.-Hil. and A. monticola Mart., the flowers of which emit a similar, somewhat strong, sharp odour. The flowers of A. tomentosa R.E.Fr. and A. cornifolia A.St.-Hil. have a pleasant fruity odour and are visited nearly exclusively by C. quatuordecimpunctata.
Here we refer to the flowering and pollination of A. coriacea (Gottsberger, 1989), a common and conspicuous tree in the cerrado vegetation. Its flowers are c. 4 cm in diameter and are pendent at the end of twigs. The petals are fleshy. Even during the receptive stages, the reproductive organs at the centre of the flower remain covered by the strongly imbricate inner petals. The hermaphroditic flowers are strongly protogynous, such that there is a distinct temporal separation of pistillate and staminate functional phases of a flower.
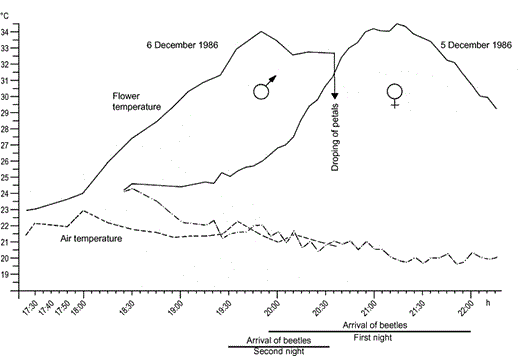
Annona coriacea exhibits a flowering rhythm of approximately 24 h, during which the temperature of the flowers rises during two nights in succession (Fig. 3). On the first day, the flower enters the pistillate phase (Fig. 4A, B) and, during the first evening, the petals and reproductive organs produce heat. The elevated temperature first becomes detectable between 18:30 and 19:00 h and peaks at c. 34 °C between 20:30 and 21:30 h. Thereafter, for the rest of the night, the flowers gradually cool until they finally return to ambient levels the next day. Sometime towards midnight or in the early morning, the stigmas are shed, marking the end of the carpel receptivity. In the afternoon of the second ‘active’ day, at approximately 18:00 h, the temperature of the flowers starts to rise for a second time. This time, the highest temperature is attained before 20:00 h, approximately half an hour or even 1 h earlier than the first night. During this second temperature rise, the dehiscent stamens become detached from the receptacle and, together with the liberated pollen tetrads, fill up the chamber formed by the inner petals (Fig. 4C). At approximately 20:30 h on the second night, the petals drop, marking the end of anthesis.
Rhythm of flower heating in Annona coriacea and visitation by beetles during two subsequent nights. (-) flower temperature; (5 December 1986) and (6 December 1986) air temperature on two subsequent nights.
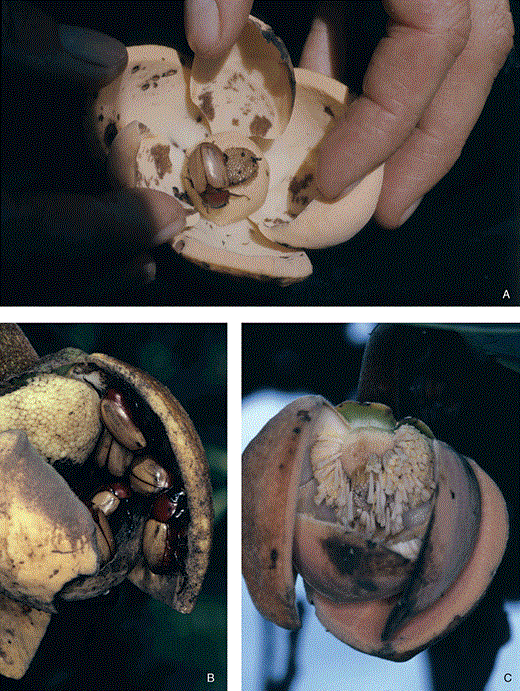
Annona coriacea. Length of outer petals c. 55 mm. A, a flower held open to show Cyclocephala atricapilla in the interior. Note the brown spots at the base of the three inner petals, where the beetle has been feeding on the nutritious tissue. B, post-pistillate- and pre-staminate-stage flower, some petals removed. The flower interior is a meeting place for males and females of C. atricapilla, which gnaw on petals. C, flower in staminate stage, one external and internal petal removed. The stigmatic ‘head’ has dropped and the stamens are detaching from the receptacle.
The strong fragrances of the heated flowers attracts the dynastid scarab beetles. The most frequent and numerous flower visitors throughout the geographical range of A. coriacea is Cyclocephala atricapilla, a robust beetle with a body length of c. 1.7 cm. On the first evening, during the pistillate receptive phase, the beetles begin to arrive shortly before 20:00 h, coinciding with the peak in flower heating. After the peak is reached and the flower has started to cool down, newcomers to the flower diminish and, from c. 22:00 h, no more beetles arrive. Once the beetles have entered the chamber, they start feeding on the three inner petals, where two regions with special nutritional cells are located (Fig. 4A, B). The flowers warm up for a second time on the second night in the staminate stage associated with scent volatilization and attract more beetles. The newcomers enter the floral chamber, joining the beetles already there, and they all become covered with recently liberated sticky pollen tetrads. Pollen now becomes the primary food for the beetles. The beetles are suddenly released from the flower by the abscission of the petals, which occurred about half an hour before other flowers in the pistillate stage attain their temperature peak (Fig. 3). Flight of the pollen-carrying beetles to the ‘hot’, receptive, first-night functional flowers, leads to pollination.
POLLINATION BY THRIPS
The flowers of the Central Amazonian tree species Bocageopsis multiflora R.E.Fr., investigated around Manaus, were found to be pollinated exclusively by Thysanoptera (Webber & Gottsberger, 1995). Each inflorescence consists of 12–20 flowers, which display all possible orientations on the inflorescence, from erect to pendulous (Fig. 5A). The flowers, which are greenish–cream during anthesis, have three sepals (c. 1.3 mm long), three outer petals (c. 6 mm long) and three inner petals (c. 5 mm long). The androeceum was found to consist of 19–25 stamens, and the apex of the connective is prolonged into a triangular tongue-shaped structure, which is more pronounced in the inner stamens (Fig. 5B, C). There are two to seven carpels per flower.
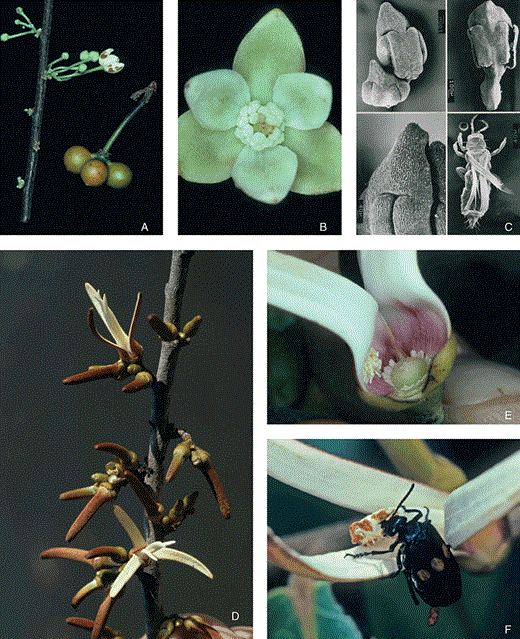
A–C, Bocageopsis multiflora. Outer petals c. 6 mm long. A, inflorescence and fruit. B, widely open flower in the staminate stage. C, (a–c) scanning electron microscope photographs of stamens having a triangular tongue-shaped connective; (d) individual of thrips, the pollinator. D–F, Xylopia aromatica. Petal length c. 30 mm. D, inflorescence with two anthetic flowers. E, flowers in staminate stage with detached stamens. F, Tetraonyx sexguttatus (Meloidae) is a petal-predating beetle but not the pollinator.
Anthesis starts early in the morning when the outer petals begin to unfold. The internal petals only partially open at the apex and thus form a triradiate slit. The receptivity of the stigmas lasts c. 12 h, from the morning until late afternoon of the first day. The staminate stage of a flower begins at c. 05:00 h of the second day and lasts c. 3 h, after which all pollen is liberated and stamens are shed. The inner petals of some but not all flowers open widely before shedding (Fig. 5B). All second-day (staminate) flowers complete anthesis at about the same time that the petals and stamens drop.
The sweetish-rancid floral odour recalls that of the fruits of Genipa americana L. (Rubiaceae). Odour emission is weak during the early morning hours of the first day, intensifies thereafter and becomes weak during the night. During the staminate phase the next morning the odour becomes strong again.
The only insects found visiting the flowers of B. multiflora were Thysanoptera (Fig. 5C), which were observed to approach the pistillate-stage flowers from the morning until the evening and the staminate-stage ones during the morning hours of the second day of anthesis. At the pistillate-stage flowers, these insects settle on the petals and enter the pollination chamber formed by the three internal petals via the opening of the triradiate slit. Three- to five-winged adults each were found inside individual flowers besides a few unwinged nymphs. Their body and legs were covered with pollen grains which they carried on when forced to leave the second-day staminate flowers after the petals and stamens dropped. The simultaneous presence of pistillate-stage and staminate-stage flowers on the same individuals would permit geitonogamous pollination to occur, in addition to xenogamous pollination when the thrips enter the first-day flower of another tree. The pollen/ovule ration of this species (N = 10 flowers) was found to be 1532 (SD 276) (Webber & Gottsberger, 1995), a value characteristic of a breeding system between facultative xenogamy and xenogamy (Cruden, 1977). Another well-documented case of thrips pollination occurs in Popowia pisocarpa Endl. in lowland dipterocarp forest in Sarawak, Malaysia (Momose, Nagamitsu & Inoue, 1998).
MIXED POLLINATION BY THRIPS AND SMALL BEETLES
There are species of Annonaceae that are pollinated by a combination of Thysanoptera and small beetles. The two examples presented are Oxandra euneura Diels (Webber & Gottsberger, 1995), a widespread Amazonian species, investigated c. 700 km south-west of Manaus, and Xylopia aromatica Mart. (Gottsberger, 1970; Webber, 1996) a widespread Neotropical species, investigated in the surroundings of Manaus and at several localities in the Central Brazilian cerrado vegetation.
In O. euneura, each tree has a maximum of three flowers in anthesis at a time. The flower position varies from lateral to erect. The flowers are about the same size as those of Bocageopsis multiflora. The sepals have a length of only 0.5 mm, the three outer and inner petals are 6.5 mm long. The 12–16 stamens in each flower have pronounced and acute tongue-like connective appendages, which in their entirety curve towards the centre of the flower and cover the six to eight carpels.
The protogynous flowers open in the morning at approximately 08:00 h. The outer petals open completely and recurve, but the inner petals open only slightly and remain in a semi-open position for the whole first day. Pollen liberation occurs the following morning between 08:30 and 09:00 h, initiating the staminate phase, during which the inner petals spread apart. The end of anthesis occurs between 11:00 and 11:30 h on the second day; at that time the outer petals fall, and the inner ones and the stamens fall later.
From the first opening of the petals in the pistillate stage on, the flowers emit a sweet perfumed odour reminiscent of that of green Palmolive™ toilet soap. The odour emission ceases by 15:00 h on first day of anthesis, without reappearing in the staminate stage.
Flower-visiting insects include rove beetles (Staphylinidae) and thrips (Thysanoptera). Practically all open flowers contain Staphylinidae (up to eight individuals) and, more rarely, some Thysanoptera. In the staminate-phase flowers, these insects are observed crawling principally on and between the stamens. When the inner petals open on the second morning, the connective appendages still remain curved over the centre of the flower, forming a small chamber where the insects could hide.
Both pistillate- and staminate-stage flowers can occur at the same time on one individual, enabling geitonogamous pollination. At the end of anthesis, the pollen-covered insects fly to anthetic flowers of the same or neighbouring trees. The pollen/ovule ratio (N = 10 flowers) is 3194 (SD 931), which, as in Bocageopsis, is intermediate between facultative xenogamy and xenogamy (Cruden, 1977).
Xylopia aromatica has relatively large flowers with pure white, elongated petals, c. 3 cm long. The flowers are commonly oriented in an upright position (Fig. 5D) and during anthesis give off a strong, sweet, aromatic odour, reminiscent of Convallaria L. flowers. The flowers open early in the morning at c. 06:00 h and remain functionally pistillate until the late afternoon. During this first day of anthesis, the three inner petals are somewhat open, which permits flower-visiting insects to penetrate into the centre of the flower. From 06:40 h of the following day on, during the staminate stage, pollen is liberated, and the phase is ending with the dropping of petals and stamens (Fig. 5E); at this later staminate stage, the inner petals are closed over the centre of the flower. This prevents the insects inside the pollination chamber from leaving the staminate-stage flower before the petals are shed.
At the cerrado site, X. aromatica is visited by thrips and small beetles of the genus Cillaeus (Nitidulidae) (Gottsberger, 1970), whereas in the Amazon region this species is principally visited by thrips, and more occasionally by nitidulid and some staphylinid beetles (Webber, 1996). The insects that leave the flowers after the end of the staminate stage, when the petals abscise, are covered by pollen grains. They then fly to newly opened flowers in the pistillate stage, enabling pollination. Large beetles (e.g. Tetraonyx sexguttatus, Meloidae; Fig. 5F) feed on the petals but hardly ever destroy the reproductive organs, which are protected by the partially closed inner petals.
Tests reveal that X. aromatica is self-compatible. The pollen/ovule rate is 610, which would indicate that, following the classification by Cruden (1977), the species is facultatively xenogamous (Webber, 1996).
POLLINATION BY FLIES
Fly pollination is well documented among Asian/Australian species of Pseuduvaria Miq. For example, the dioecious, cauliflorous species, P. froggattii (F.Muell.) Jessup, was observed at Cape Tribulation in North Queensland (Silberbauer-Gottsberger et al., 2003). The flowers (c. 1.5 cm in diameter) are grouped in inflorescences of 20 or more. The petals of the pendulous flowers are cream-coloured abaxially and deep wine-red adaxially. After flower opening, the inner petals form a half-closed roundish mitriform structure. The outer petals spread apart revealing large roundish openings framed by the narrow bases of the semi-closed inner petals (Fig. 6A, B). Flower opening is diurnal and asynchronous among different flowers. After a few hours of being open, flowers begin to function, pistillate flowers have tumescent stigmas, indicating receptivity, or anthers open and expose their pollen in staminate flowers. At the beginning of this reproductive phase, the interior of the flowers turns purple. Two large, oval, dark-purple glands situated marginally along the inner petals of both flower types (Fig. 6B) begin to secrete nectar through small superficial openings from an underlying nectariferous tissue, and concomitantly both pistillate and staminate flowers start emitting of an unpleasant scent for the human nose, reminiscent of old dishwater and vomit.
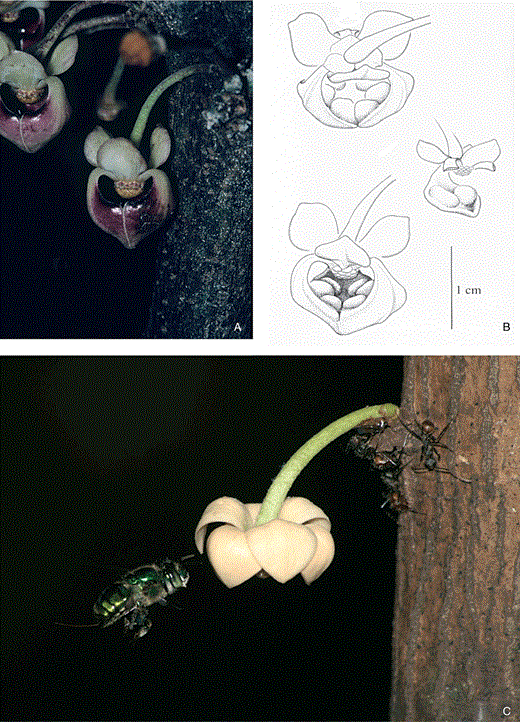
A–B, Pseuduvaria froggattii. Flower c. 18 mm long. A, inflorescence with open, mitriform flowers. B, drawings to show the mitriform flower and the large glands situated at the border of the inner petals. C, Unonopsis stipitata flower (external petal c. 12 mm long) being approached by one of its pollinating euglossine bees, Euglossa imperialis (photo credit Holger Teichert).
Both pistillate and staminate anthetic flowers of P. froggattii are highly attractive to certain flies, probably lured by the strong stench and licked the nectar. They move around in the floral cavity and touch the reproductive organs of both flower types, being potential effective pollinators. Although the type of scent is indicative of sapromyiophily, non-dung flies, only interested in nectar, also appear to be attracted to flowers of Pseuduvaria.
The African, monoecious Uvariopsis bakeriana (Hutch. & Dalziel) Robyns & Ghesq. has a diffuse dung-fly pollination syndrome (sapromyiophily) (Gottsberger, Meinke & Porembski, 2011). Their flowers have reddish fleshy petals and look like a small version of the fruiting body of certain stinkhorn fungi (Phallaceae), although without emitting the stench of those fungi. Visual cues of the flowers seem to be more strongly developed than olfactory ones. Pistillate and staminate flowers attract a diffuse population of dung flies and non-dung flies.
POLLINATION BY COCKROACHES
Uvaria elmeri Merr., a tall woody climber in the understory of the forest, was analysed by Nagamitsu & Inoue (1997) in Sarawak, Malaysia. The flowers are protogynous and bloom for c. 50 h. The creamy–white or brown petals remain open and spread during anthesis, exposing the reproductive organs and emitting a smell of decaying wood or mushrooms. The main and most regular flower visitors and supposed principal pollinators are cockroaches (genus Hemithyrsocera), which approach pistillate and staminate flowers, feeding on stigmatic exudates at the former and pollen at the latter.
POLLINATION BY BEES
This phenomenon was described for Uvaria concava Teijsm. & Binn. in North Queensland (Silberbauer-Gottsberger et al., 2003). Its large, hermaphroditic, bright-red flowers emit a slight odour in the receptive stage, frequently starting in the morning and being correlated with the formation of resinous stigmatic exudates. In the early morning of the third day after flower opening, when the stigmas still appear receptive, the anthers release their pollen. Thus, there is an overlap of pistillate and staminate stages. Small pollen-collecting Meliponinae bees have been observed to visit the transitional pistillate-staminate third-day flowers. It appears, however, that the bees also collect resinous stigmatic exudates, thus being potential pollinators of pistillate (non-staminate)-phase flowers.
A spectacular type of bee pollination in Annonaceae was described by Carvalho & Webber (2000) and Silberbauer-Gottsberger et al. (2003). It is the case of perfume-collecting by male euglossine bees in Unonopsis guatterioides A.DC. ex R.E.Fr. studied in Central Amazonia around Manaus. Recently, the same phenomenon has been studied in U. stipitata Diels in the Nouragues Natural Reserve approximately 100 km south-west of Cayenne in French Guyana (Teichert et al., 2009).
Unonopsis stipitata has hermaphrodite flowers with diurnal protogynous anthesis lasting for 2 days. The flowers emit a spearmint-like scent during the first and the second day of anthesis. During the first day, the flowers are in the pistillate stage and during the second day in the late morning/early afternoon there is an overlap of 1–2 h of pistillate and staminate floral stages. Principally on the second day of anthesis, during the overlapping pistillate/staminate stages of flowering, the flowers are intensively visited by males of the euglossine bees Euglossa imperialis (Fig. 6C) and Eulaema bombiformis. The bees approach the flowers, which keep their petals spread and open during the entire anthesis, and after landing they hang upside down on the petals of the inner whorl. Perfume (odour in liquid form) is produced on the inner side of the inner petals. With their foretarsal organs the male bees brush these scent-emitting osmophores and afterwards, in flight, they transfer the collected fragrances to the tibial capsule, which functions as a scent recipient. The male euglossine bees are efficient pollinators and carry pollen and transfer it to the stigmas.
ADAPTIVE CHARACTERS OF FLOWERS AND PHYLOGENETIC CONSIDERATIONS
As shown above, Annonaceae have diverse modes of insect pollination, with beetle-, thrips-, cockroach-, fly- and even bee-pollinated flowers (Table 1). According to analytical approaches (e.g. Scharaschkin & Doyle, 2006), the earliest diverging genus in the family is Anaxagorea. On the one hand, Anaxagorea has several features, for example stamen morphology, non-peltate stamen connectives, inner staminodes and leaf architecture, considered ancestral in Annonaceae and shared with other Magnoliales (Scharaschkin & Doyle, 2006). On the other hand, Anaxagorea also has many floral morphological and functional characters that are present in other genera and species of Annonaceae, pollinated by a variety of insects including beetles, thrips, flies and bees.
Pollination in Annonaceae: Presented are species or genera and the respective authors for the mentioned cases; timing of anthesis (diurnal or nocturnal); presence (+) or absence (–) of flower self-heating; respective pollinators and their size class (size). Two size classes of insects are distinguished: small pollinators, with a body length up to 7 mm and large ones, with a body length between 14 and 20 mm
| Plant taxa . | Anthesis . | Thermogenesis . | Pollinators . | Size . | Authors . |
|---|---|---|---|---|---|
| Pollination by beetles (cantharophily) | |||||
| Anaxagorea prinoides | Diurnal | − | Nitidulidae (Colopterus spp.) | Small | Teichert et al., 2011 |
| Several Anaxagorea spp. | Diurnal | + | Nitidulidae (Colopterus spp.) | Small | Webber, 1996 |
| Guatteria spp., e.g. G. foliosa | Diurnal | − | Nitidulidae (partly Colopterus spp.), Chrysomelidae, Curculionidae | Small | Gottsberger, 1970, 1993, 1999; Webber, 1996; Silberbauer-Gottsberger et al., 2003 |
| Duguetia spp., e.g. D. furfuracea | |||||
| Goniothalamus australis | |||||
| Polyalthia korinti | Nocturnal | + | Curculionidae (Endaeus sp.) | Small | Webber, 1996; Küchmeister et al., 1998; Ratnayake et al., 2006 |
| Xylopia crinita | Nitidulidae (Carpophilus plagiatipennis) | ||||
| Staphylinidae | |||||
| Polyalthia coffeoides | Nocturnal | − | Nitidulidae | Small | Ratnayake et al., 2006 |
| Asimina obovata | Diurnal | − | Scarabaeidae, Trichiinae: Trichiotinus spp., Cetoniinae: Euphoria sepulchralis, | Large | Norman & Clayton, 1986; Matthias, 2002 |
| A. pygmaea | Cerambycidae (Typocereus zebra) | ||||
| Curculionidae (Notalomus basalis) | |||||
| Annona, Cymbopetalum, Duguetia and Malmea spp. | Nocturnal | + | Scarabaeidae, Dynastinae: Cyclocephala spp. | Large | Gottsberger, 1989; Schatz, 1990; Webber, 1996 |
| Pollination by Thysanoptera (thrips) | |||||
| Bocageopsis multiflora | Diurnal | − | Thysanoptera | Small | Webber & Gottsberger, 1995; Momose et al., 1998 |
| Popowia pisocarpa | |||||
| Mixed pollination by thrips and beetles | |||||
| Oxandra euneura | Diurnal | − | Thysanoptera and beetles (Staphylinidae) | Small | Gottsberger, 1970; Webber & Gottsberger, 1995; Webber, 1996 |
| Xylopia aromatica | Thysanoptera and beetles (Nitidulidae, Cillaeus sp., Staphylinidae) | ||||
| Pollination by dung flies (sapromyiophily) | |||||
| Pseuduvaria froggattii | Diurnal | − | Dung flies | Small | Silberbauer-Gottsberger et al., 2003; Gottsberger et al., 2011 |
| Uvariopsis bakeriana | |||||
| Pollination by cockroaches | |||||
| Uvaria elmeri | Nocturnal | − | Cockroaches (Hemithyrsocera sp.) | ± Large | Nagamitsu & Inoue, 1997 |
| Pollination by bees (melittophily) | |||||
| Uvaria concava | Diurnal | Meliponinae | Small | Silberbauer-Gottsberger et al., 2003 | |
| Unonopsis guatterioides | Diurnal | − | Euglossinae (Eulaema bombiformis and Euglossa imperialis) | Large | Carvalho & Webber, 2000; Teichert et al., 2009 |
| U. stipitata | |||||
| Plant taxa . | Anthesis . | Thermogenesis . | Pollinators . | Size . | Authors . |
|---|---|---|---|---|---|
| Pollination by beetles (cantharophily) | |||||
| Anaxagorea prinoides | Diurnal | − | Nitidulidae (Colopterus spp.) | Small | Teichert et al., 2011 |
| Several Anaxagorea spp. | Diurnal | + | Nitidulidae (Colopterus spp.) | Small | Webber, 1996 |
| Guatteria spp., e.g. G. foliosa | Diurnal | − | Nitidulidae (partly Colopterus spp.), Chrysomelidae, Curculionidae | Small | Gottsberger, 1970, 1993, 1999; Webber, 1996; Silberbauer-Gottsberger et al., 2003 |
| Duguetia spp., e.g. D. furfuracea | |||||
| Goniothalamus australis | |||||
| Polyalthia korinti | Nocturnal | + | Curculionidae (Endaeus sp.) | Small | Webber, 1996; Küchmeister et al., 1998; Ratnayake et al., 2006 |
| Xylopia crinita | Nitidulidae (Carpophilus plagiatipennis) | ||||
| Staphylinidae | |||||
| Polyalthia coffeoides | Nocturnal | − | Nitidulidae | Small | Ratnayake et al., 2006 |
| Asimina obovata | Diurnal | − | Scarabaeidae, Trichiinae: Trichiotinus spp., Cetoniinae: Euphoria sepulchralis, | Large | Norman & Clayton, 1986; Matthias, 2002 |
| A. pygmaea | Cerambycidae (Typocereus zebra) | ||||
| Curculionidae (Notalomus basalis) | |||||
| Annona, Cymbopetalum, Duguetia and Malmea spp. | Nocturnal | + | Scarabaeidae, Dynastinae: Cyclocephala spp. | Large | Gottsberger, 1989; Schatz, 1990; Webber, 1996 |
| Pollination by Thysanoptera (thrips) | |||||
| Bocageopsis multiflora | Diurnal | − | Thysanoptera | Small | Webber & Gottsberger, 1995; Momose et al., 1998 |
| Popowia pisocarpa | |||||
| Mixed pollination by thrips and beetles | |||||
| Oxandra euneura | Diurnal | − | Thysanoptera and beetles (Staphylinidae) | Small | Gottsberger, 1970; Webber & Gottsberger, 1995; Webber, 1996 |
| Xylopia aromatica | Thysanoptera and beetles (Nitidulidae, Cillaeus sp., Staphylinidae) | ||||
| Pollination by dung flies (sapromyiophily) | |||||
| Pseuduvaria froggattii | Diurnal | − | Dung flies | Small | Silberbauer-Gottsberger et al., 2003; Gottsberger et al., 2011 |
| Uvariopsis bakeriana | |||||
| Pollination by cockroaches | |||||
| Uvaria elmeri | Nocturnal | − | Cockroaches (Hemithyrsocera sp.) | ± Large | Nagamitsu & Inoue, 1997 |
| Pollination by bees (melittophily) | |||||
| Uvaria concava | Diurnal | Meliponinae | Small | Silberbauer-Gottsberger et al., 2003 | |
| Unonopsis guatterioides | Diurnal | − | Euglossinae (Eulaema bombiformis and Euglossa imperialis) | Large | Carvalho & Webber, 2000; Teichert et al., 2009 |
| U. stipitata | |||||
Pollination in Annonaceae: Presented are species or genera and the respective authors for the mentioned cases; timing of anthesis (diurnal or nocturnal); presence (+) or absence (–) of flower self-heating; respective pollinators and their size class (size). Two size classes of insects are distinguished: small pollinators, with a body length up to 7 mm and large ones, with a body length between 14 and 20 mm
| Plant taxa . | Anthesis . | Thermogenesis . | Pollinators . | Size . | Authors . |
|---|---|---|---|---|---|
| Pollination by beetles (cantharophily) | |||||
| Anaxagorea prinoides | Diurnal | − | Nitidulidae (Colopterus spp.) | Small | Teichert et al., 2011 |
| Several Anaxagorea spp. | Diurnal | + | Nitidulidae (Colopterus spp.) | Small | Webber, 1996 |
| Guatteria spp., e.g. G. foliosa | Diurnal | − | Nitidulidae (partly Colopterus spp.), Chrysomelidae, Curculionidae | Small | Gottsberger, 1970, 1993, 1999; Webber, 1996; Silberbauer-Gottsberger et al., 2003 |
| Duguetia spp., e.g. D. furfuracea | |||||
| Goniothalamus australis | |||||
| Polyalthia korinti | Nocturnal | + | Curculionidae (Endaeus sp.) | Small | Webber, 1996; Küchmeister et al., 1998; Ratnayake et al., 2006 |
| Xylopia crinita | Nitidulidae (Carpophilus plagiatipennis) | ||||
| Staphylinidae | |||||
| Polyalthia coffeoides | Nocturnal | − | Nitidulidae | Small | Ratnayake et al., 2006 |
| Asimina obovata | Diurnal | − | Scarabaeidae, Trichiinae: Trichiotinus spp., Cetoniinae: Euphoria sepulchralis, | Large | Norman & Clayton, 1986; Matthias, 2002 |
| A. pygmaea | Cerambycidae (Typocereus zebra) | ||||
| Curculionidae (Notalomus basalis) | |||||
| Annona, Cymbopetalum, Duguetia and Malmea spp. | Nocturnal | + | Scarabaeidae, Dynastinae: Cyclocephala spp. | Large | Gottsberger, 1989; Schatz, 1990; Webber, 1996 |
| Pollination by Thysanoptera (thrips) | |||||
| Bocageopsis multiflora | Diurnal | − | Thysanoptera | Small | Webber & Gottsberger, 1995; Momose et al., 1998 |
| Popowia pisocarpa | |||||
| Mixed pollination by thrips and beetles | |||||
| Oxandra euneura | Diurnal | − | Thysanoptera and beetles (Staphylinidae) | Small | Gottsberger, 1970; Webber & Gottsberger, 1995; Webber, 1996 |
| Xylopia aromatica | Thysanoptera and beetles (Nitidulidae, Cillaeus sp., Staphylinidae) | ||||
| Pollination by dung flies (sapromyiophily) | |||||
| Pseuduvaria froggattii | Diurnal | − | Dung flies | Small | Silberbauer-Gottsberger et al., 2003; Gottsberger et al., 2011 |
| Uvariopsis bakeriana | |||||
| Pollination by cockroaches | |||||
| Uvaria elmeri | Nocturnal | − | Cockroaches (Hemithyrsocera sp.) | ± Large | Nagamitsu & Inoue, 1997 |
| Pollination by bees (melittophily) | |||||
| Uvaria concava | Diurnal | Meliponinae | Small | Silberbauer-Gottsberger et al., 2003 | |
| Unonopsis guatterioides | Diurnal | − | Euglossinae (Eulaema bombiformis and Euglossa imperialis) | Large | Carvalho & Webber, 2000; Teichert et al., 2009 |
| U. stipitata | |||||
| Plant taxa . | Anthesis . | Thermogenesis . | Pollinators . | Size . | Authors . |
|---|---|---|---|---|---|
| Pollination by beetles (cantharophily) | |||||
| Anaxagorea prinoides | Diurnal | − | Nitidulidae (Colopterus spp.) | Small | Teichert et al., 2011 |
| Several Anaxagorea spp. | Diurnal | + | Nitidulidae (Colopterus spp.) | Small | Webber, 1996 |
| Guatteria spp., e.g. G. foliosa | Diurnal | − | Nitidulidae (partly Colopterus spp.), Chrysomelidae, Curculionidae | Small | Gottsberger, 1970, 1993, 1999; Webber, 1996; Silberbauer-Gottsberger et al., 2003 |
| Duguetia spp., e.g. D. furfuracea | |||||
| Goniothalamus australis | |||||
| Polyalthia korinti | Nocturnal | + | Curculionidae (Endaeus sp.) | Small | Webber, 1996; Küchmeister et al., 1998; Ratnayake et al., 2006 |
| Xylopia crinita | Nitidulidae (Carpophilus plagiatipennis) | ||||
| Staphylinidae | |||||
| Polyalthia coffeoides | Nocturnal | − | Nitidulidae | Small | Ratnayake et al., 2006 |
| Asimina obovata | Diurnal | − | Scarabaeidae, Trichiinae: Trichiotinus spp., Cetoniinae: Euphoria sepulchralis, | Large | Norman & Clayton, 1986; Matthias, 2002 |
| A. pygmaea | Cerambycidae (Typocereus zebra) | ||||
| Curculionidae (Notalomus basalis) | |||||
| Annona, Cymbopetalum, Duguetia and Malmea spp. | Nocturnal | + | Scarabaeidae, Dynastinae: Cyclocephala spp. | Large | Gottsberger, 1989; Schatz, 1990; Webber, 1996 |
| Pollination by Thysanoptera (thrips) | |||||
| Bocageopsis multiflora | Diurnal | − | Thysanoptera | Small | Webber & Gottsberger, 1995; Momose et al., 1998 |
| Popowia pisocarpa | |||||
| Mixed pollination by thrips and beetles | |||||
| Oxandra euneura | Diurnal | − | Thysanoptera and beetles (Staphylinidae) | Small | Gottsberger, 1970; Webber & Gottsberger, 1995; Webber, 1996 |
| Xylopia aromatica | Thysanoptera and beetles (Nitidulidae, Cillaeus sp., Staphylinidae) | ||||
| Pollination by dung flies (sapromyiophily) | |||||
| Pseuduvaria froggattii | Diurnal | − | Dung flies | Small | Silberbauer-Gottsberger et al., 2003; Gottsberger et al., 2011 |
| Uvariopsis bakeriana | |||||
| Pollination by cockroaches | |||||
| Uvaria elmeri | Nocturnal | − | Cockroaches (Hemithyrsocera sp.) | ± Large | Nagamitsu & Inoue, 1997 |
| Pollination by bees (melittophily) | |||||
| Uvaria concava | Diurnal | Meliponinae | Small | Silberbauer-Gottsberger et al., 2003 | |
| Unonopsis guatterioides | Diurnal | − | Euglossinae (Eulaema bombiformis and Euglossa imperialis) | Large | Carvalho & Webber, 2000; Teichert et al., 2009 |
| U. stipitata | |||||
Besides the ancestral characteristics mentioned above, flowers of Anaxagorea spp. are also characterized by developing remarkably thick petals. These thick and tissue-rich petals are curved over the centre of the flower and form a kind of pollination chamber, a semi-closed structure that only gives the specific pollinators access to the floral centre and reproductive organs. The tissue on the inner side of the petals produces heat, a respiratory process (thermogenesis). This warming of the flowers during anthesis intensifies the floral odours and, at the same time, provides the pollinators inside the pollination chamber with a warm environment. Flower visitors of Anaxagorea spp. studied in Amazonia include nitidulid beetles of the genus Colopterus, and, more occasionally, staphylinid beetles that are attracted by fruity floral odours intensified by thermogenesis.
Thick petals are not only a characteristic feature of Anaxagorea flowers, but are a prominent character of a large number of species in Annonaceae. Strongly constructed, thick petal tissue is functionally important for at least two reasons. Excess tissue provides food for the attracted beetles, and thick petals are also functional in protecting the flowers against non-pollinating beetles. In addition to attracting beetles as potential pollinators, cantharophilous Annonaceae often also attract non-pollinating beetles, which gnaw the petals from the outside and even use the thick petal tissue as an ovipositing site. Thus, thick petals are an efficient anti-predator structure, and this character reappears in many species of this pantropical family; this certainly is a legacy from the basal cantharophilous representatives of this group, such as the progenitors of Anaxagorea or any extinct proto-Annonaceae (Takahashi et al., 2008). Thick petals combined with large flowers are most prominently developed in species that attract large, voracious dynastid scarab beetles, such as those described above for Annona spp. In these species, thick petals are an essential condition, without which the flowers would not be able to host and maintain the pollinating beetle population, much less the non-pollinating predators.
Thick petal tissue not only acts as a mechanical device in Annonaceae to protect against the aggressive behaviour of flower-visiting beetles, but allows cantharophilous flowers to fulfil another important function, i.e. heat production. Anaxagorea and many other diurnal and especially nocturnal cantharophilous species of Annonaceae have thermogenic flowers, in which they heat up most intensively in the pistillate stage and less intensively pronounced in the staminate stage. Heating of flowers during certain hours when their pollinating beetles start their diurnal or nocturnal activities, coupled with concomitant intensification of scent emissions, is an efficient method for transforming more occasional visits of beetles into a precise event. For thermogenesis to occur, flowers have to accumulate large amounts of starch and lipids in their petals, additionally resulting in profuse and thick petal tissue. Floral thermogenesis not only causes more efficient and stronger volatilization of odour constituents, but also provides a warm and stable environment. The beetles are better able to carry out their activities such as eating, digesting, mating and preparing to fly off at the end of the staminate stage (Seymour, White & Gibernau, 2003, 2009; Seymour, Silberbauer-Gottsberger & Gottsberger, 2010).
Another remarkable functional character of the large majority of Annonaceae, especially beetle-pollinated ones, is a character that exists in Anaxagorea, the floral or ‘pollination’ chamber, formed by petals that are closed over the flower centre during anthesis. In these floral chambers, specific odours are produced (often intensified by thermogenesis) that entice the beetles to enter these dark chambers. Inside, the beetles are sheltered from daylight and predators (e.g. beetle-eating toucans and other birds) and find an often warm environment, plenty of food (perianth tissue and pollen) and members of the opposite sex, ready for mating. Chamber size, amount of food available and petal thickness are correlated with size, number and voraciousness of the associated beetles (Gottsberger & Silberbauer-Gottsberger, 2006).
The ‘basal’ form of stamens with elongated, tongue-shaped connectives, as observed in Anaxagorea, also occurs in a few other genera, notably pollinated by non-destructive insects such as thrips; for example, in Bocageopsis, Oxandra, Trigynaea Schltdl. or in some Xylopia spp. Flowers associated with thrips and non-destructive beetle groups have more commonly maintained the stamen form with elongate connectives (Webber & Gottsberger, 1995); see also Deeringothamnus rugelii Small and D. pulchellus Small in Florida, which appear to be pollinated by tumbling beetles, Mordellidae, and thrips (Norman, 2003). The disc-like sclerified connective shield of the majority of Annonaceae is apparently a secondary and modified structure, in which the densely arranged stamens with their hard connective shields and the copious resinous exudates sealing the receptive stigmas are principally anti-predator structures.
Thus, Anaxagorea has several plesiomorphic floral characters (e.g. inner staminodes and tongue-shaped, non-sclerified connectives) linking Annonaceae and other families of Magnoliales. In addition, Anaxagorea exhibits plesiomorphic functional floral characters found in the family: its tissue-rich thick petals form a pollination chamber, flowers of several species exhibit thermogenesis, emit a fruit-like odour and attract beetles as pollinators. As Anaxagorea and Annonaceae are basically a beetle-pollinated group, characters associated with beetle pollination appear imprinted in members of the entire family.
The majority of the species of Annonaceae that are beetle-pollinated form a pollination chamber, and most of these produce fruity or otherwise strong and enticing odours. Several of the beetle-pollinated species are more efficient and produce strong volatile floral odours enhanced by heat. Species pollinated by dynastid scarab beetles in the Neotropics have obligate nocturnal anthesis (as these beetles are nearly all nocturnal), and heating and odour emission is accentuated in their flowers. This scarab pollination system in Annonaceae is certainly evolutionarily derived.
Thrips-pollinated species also have a pollination chamber. Excess tissue in the form of thick petals is, however, less prominently developed in the thrips-pollinated species and the flowers are usually smaller than in cantharophilous species. The few species studied in this pollination mode, have retained the ‘basal’ form of stamens of Annonaceae, as found in Anaxagorea.
In the cockroach-pollinated Uvaria elmeri the flowers are fully open. Also fly- and bee-pollinated Annonaceae can obviously function only with open flowers and freely accessible reproductive organs. Fly-pollinated species are surprisingly different in their appearance from other Annonaceae and have a half-closed mitriform or otherwise open corolla. In Pseuduvaria, unpleasant scent emissions lure dung flies, and secretions of nectar from the inner petals provide them and other, non-dung flies with liquid resources. The flowers of Uvariopsis bakeriana visually look like the fruiting bodies of small stinkhorn fungi, but do not produce the strong stench of such fungi and thus attract flies more occasionally.
In the few known bee-pollinated species, the flowers have open petals and also provide bees with easy and unhindered access to the centre of the flower. In Uvaria concava, small meliponine bees collect pollen and the flowers have strong overlapping pistillate/staminate flower phases; such overlapping of the two phases does not usually occur in beetle-pollinated species; these are usually dichogamous. Neotropical Unonopsis spp. are even more curious in that they have developed a pollination system in which male euglossine bees collect perfume and store it for further use in their tibial capsule. Such sophisticated systems are only otherwise known in orchids and a few representatives of some other monocot and eudicot families (Carvalho & Webber, 2000; Teichert et al., 2011).
Annonaceae have developed a surprising number of different, sophisticated and apparently highly specialized pollination syndromes (see Table 1 and also Saunders, 2010). The majority of species in this family have retained beetle pollination, but different lines of cantharophily evolved: pollination by small beetles vs. pollination by large beetles, diurnally vs. nocturnally active flowers and some flowers with thermogenesis. Other lines of specialization are evident in the few species pollinated by thrips, cockroaches, flies and bees, which also show characters in accordance with the behaviour of the respective pollinating insects and the necessary access of these insects to the reproductive organs of the flowers. Notwithstanding, these non-cantharophilous species still retain one or more of the specialized cantharophilous features, for example thick petals, flattened and sclerified connective shields or protogynous dichogamy.
ACKNOWLEDGEMENTS
This work was supported by the German Research Council (DFG), the German Federal Ministry for Education and Research (BMBF) and the Brazilian Research Council (CNPq). The author also thanks the authorities of his former university in Botucatu (UNESP, Campus de Botucatu) for support during the years of his employment (1968–1981). The cooperation of Silvia R. Machado, Universidade Estadual Paulista, Campus de Botucatu, Maria Jesus N. Rodal, Universidade Federal Rural de Pernambuco, Recife, and Antonio C. Webber, Universidade do Amazonas, Manaus, is greatly appreciated. Thanks also go to Cristina Mattos, Edy de Lello Montenegro, Carmen Regina Marcati, Luzia Gonçalves, José Angelo (Zico) Gonçalves, Oswaldo Rodrigues and Clemente José Campos for assistance in Botucatu and surroundings. Thanks go to Ilse Silberbauer-Gottsberger and Graciela Hintze for technical help with the manuscript and the illustrations. Holger Teichert provided the photograph of Unonopsis stipitata and Richard M. K. Saunders and two anonymous reviewers kindly read and improved the manuscript.