-
PDF
- Split View
-
Views
-
Cite
Cite
P. Tfelt-Hansen, P. R. Saxena, C. Dahlöf, J. Pascual, M. Láinez, P. Henry, H.-C. Diener, J. Schoenen, M. D. Ferrari, P. J. Goadsby, Ergotamine in the acute treatment of migraine: A review and European consensus, Brain, Volume 123, Issue 1, January 2000, Pages 9–18, https://doi.org/10.1093/brain/123.1.9
Close - Share Icon Share
Abstract
Ergotamine has been used in clinical practice for the acute treatment of migraine for over 50 years, but there has been little agreement on its place in clinical practice. An expert group from Europe reviewed the pre-clinical and clinical data on ergotamine as it relates to the treatment of migraine. From this review, specific suggestions for the patient groups and appropriate use of ergotamine have been agreed. In essence, ergotamine, from a medical perspective, is the drug of choice in a limited number of migraine sufferers who have infrequent or long duration headaches and are likely to comply with dosing restrictions. For most migraine sufferers requiring a specific anti-migraine treatment, a triptan is generally a better option from both an efficacy and side-effect perspective.
Introduction
Ergotamine burst onto the medical scene during the Middle Ages when mass poisoning by ergotamine occurred throughout Europe due to eating bread contaminated with the sclerotia of the mushroom Claviceps purpurea, which is a parasite on rye, wheat, barley and other cultivated grains, climaxing in St Anthony's Fire. Due to its remarkable uterotonic and vasoconstrictor effects, ergotamine was used to precipitate childbirth and to control post-partum haemorrhage, first mentioned clearly by John Stearns in 1808 in a letter published in the Medical Repository of New York (Thoms, 1931). The evolution of the use of ergot derivatives in obstetric practice is covered elsewhere (Moir, 1974). An extract of ergot was used in clinical practice by Eulenberg (1883), and ergotamine itself was first isolated by Stoll (1918) and has been used in the acute treatment of migraine since 1926 (Maier, 1926), with no alternative specific acute anti-migraine treatment for decades. Remarkably, despite widespread use, there is little consensus as to its place in practice. In this review, we attempt to set out information concerning ergotamine and then make conclusions concerning its use based on current evidence. The American Academy of Neurology has published recommendations on ergotamine use (Quality Standards Subcommittee of the American Academy of Neurology, 1995), but here we sought to provide detailed evidence for our position. Most clinicians feel ergotamine has some place in treating acute migraine, and we have attempted a consensus to present the core of its role.
Pharmacology of ergotamine
Receptor binding profile and mode of action
The ergot alkaloids have a complex mode of action that involves interaction with a variety of receptors. Indeed, as shown in Table 1 (Leysen and Gommeren, 1984; Hoyer, 1988; Adham et al., 1993; Hoyer et al., 1994; Glusa and Roos, 1996; Leysen et al., 1996), both ergotamine and dihydroergotamine have affinities for 5-HT (5-hydroxytryptamine), dopamine and noradrenaline receptors. In contrast, sumatriptan and the newer triptans are much more selective, showing high affinity for 5-HT1B and 5-HT1D receptors and a moderate affinity for 5-HT1A and 5-HT1F receptors (Goadsby, 1998).
The α-adrenoceptor-blocking property of ergotamine, first described by Dale (Dale, 1906), is textbook knowledge (Hoffman and Lefkowitz, 1996). However, this property is often overemphasized, since it is observed only with high doses used in some animal experiments and bears no relevance to therapeutic use in humans. In lower therapeutically relevant concentrations, ergotamine acts as an agonist at α-adrenoceptors, 5-HT (particularly 5-HT1B/1D) and dopamine D2 receptors (Müller-Schweinitzer and Weidmann, 1978; Saxena and Cairo-Rawlins, 1979; Müller-Schweinitzer, 1992; De Vries et al., 1998; Villalón et al., 1999). In addition, there is evidence that both ergotamine and dihydroergotamine can activate novel, as yet uncharacterized receptors (De Vries et al., 1998).
Effects on blood vessels
The most important and conspicuous pharmacological effect of ergot alkaloids is undeniably the vasoconstrictor action (Müller-Schweinitzer and Weidmann, 1978; Müller-Schweinitzer, 1992). Extensive studies in animals show that this vasoconstrictor effect is particularly marked within the carotid vascular bed and the selectivity extends to the arteriovenous anastomotic part; blood flow to a number of tissues, including that to the brain, is little affected (Johnston and Saxena, 1978; De Vries et al., 1998). Similar vasoconstrictor effects on cephalic arteriovenous anastomoses are also observed with sumatriptan as well as with other triptans (Saxena and Ferrari, 1996).
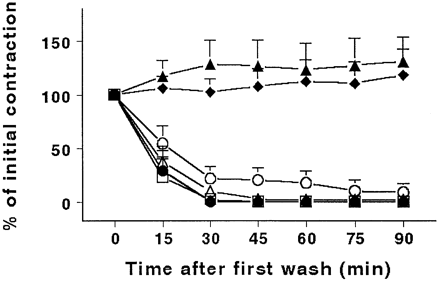
In humans, ergotamine can constrict several isolated blood vessels, including the pulmonary (Cortijo et al., 1997), cerebral (Müller-Schweinitzer, 1992), temporal (Østergaard et al., 1981) and coronary (MaassenVanDenBrink et al., 1998) arteries. The drug seems to be more active on large arteries (conducting vessels) than on arterioles (resistance vessels). Basal cerebral (Andersen et al., 1987; Dixon et al., 1997) or myocardial (Gnecchi-Ruscone et al., 1998) blood flow may not change, although ergotamine does affect coronary vasodilator reserve (Gnecchi-Ruscone et al., 1998). Arterial blood pressure is moderately increased in therapeutic doses (Bulow et al., 1986; Dixon et al., 1997). An important feature of ergotamine and dihydroergotamine, as illustrated in Fig. 1 (MaassenVanDenBrink et al., 1998), is that their effects in isolated human coronary arteries are resistant to repeated wash. This appears to be due mainly to slow diffusion from the receptor biophase and, therefore, their effects last far longer than can be expected from plasma concentrations (Bulow et al., 1986; Tfelt-Hansen and Johnson, 1993).
Other effects
Ergotamine and dihydroergotamine have been reported to inhibit dural plasma extravasation after stimulation of the trigeminal ganglion in rats (Buzzi and Moskowitz, 1991; Buzzi et al., 1991). In addition, as has been demonstrated for dihydroergotamine (Goadsby and Edvinsson, 1993; Hoskin et al., 1996), ergotamine derivatives may block the trigeminovascular pathway centrally. Ergotamine also has a prominent uterotonic action (Graves, 1996).
Pharmacokinetics of ergotamine
Oral absorption of ergotamine is 60–70%, and the concurrent administration of caffeine improves both the rate and extent of absorption. Due to high first-pass metabolism, ergotamine has a very low bioavailability from oral administration. There is considerable subject variability with respect to bioavailability and lack of consistency in the clinical response over multiple attacks. Compared with intravenous bioavailability (100%), oral bioavailability of ergotamine is <1% (Sanders et al., 1983; Ibraheem et al., 1983), rectal bioavailability is 1–3% and intramuscular bioavailability is 47% (Tfelt-Hansen and Johnson, 1993). Ergotamine is metabolized in the liver by largely undefined pathways; 90% of the metabolites are excreted in the bile and the elimination half-life is 2 h (Tfelt-Hansen and Johnson, 1993). An interaction with erythromycin may dramatically increase the oral bioavailability of ergotamine (Francis et al., 1984), and ergotism is a reported complication of co-administration with clarithromycin (Horowitz et al., 1996) and ritonavir (Liaudet et al., 1999). Since the same cytochrome P450 enzyme metabolizes a number of other drugs, including bromocriptine, dexamethasone, ethinyloestradiol, ketoconazole, nifedipine, omeprazole and verapamil (Christians et al., 1996), this interaction may extend to these drugs as well.
Ergotamine formulations
Most formulations of ergotamine are not very useful due to an inappropriate amount of ergotamine or compounding with other drugs, such as caffeine, chlorcyclizine or meprobamate. Ergotamine is marketed as aerosol (which is slowly being withdrawn), oral and suppository formulations. In some countries, ergotamine can be used alone in an oral formulation, or particularly in the very useful inhalational form, but most often the suppository formulation is compounded and contains 1–2 mg of ergotamine with caffeine.
Clinical studies with ergotamine
Ergotamine is a relatively old drug and thus did not undergo a controlled clinical trial programme as would be expected of a modern drug. Nevertheless, oral ergotamine has been used over the past 30 years as the standard comparative drug in controlled trials of other medicines, although the number of good clinical trials incorporating this widely used drug is not large. A recent review (Dahlof, 1993) stated that `there is little evidence that it is significantly more effective than placebo' and further `the recommended doses of ergotamine cannot be justified'. Despite the limited number of studies with contemporary methodology that involve ergotamine (The International Headache Society Committee on Clinical Trials in Migraine, 1991), there is evidence for the efficacy of ergotamine in the literature, and this will be summarized briefly here.
Randomized controlled clinical trials with ergotamine
A summary of 18 controlled double-blind trials of oral ergotamine, or oral ergotamine plus caffeine, is given in Table 2. In 10 trials (Ostfeld, 1961; Ryan, 1970; Waters, 1970; Hakkarainen et al., 1979; Kinnunen et al., 1988; Sargent et al., 1988; Friedman et al., 1989; Cortelli et al., 1996; McNeely and Goa, 1999; Reches and Eletriptan Steering Committee, 1999) ergotamine was compared with placebo, whereas in eight other trials ergotamine served as the standard comparative drug (Adams et al., 1971; Yuill et al., 1972; Hakkarainen et al., 1978, 1980; Pradalier et al., 1985; The Multinational Oral Sumatriptan and Cafergot Comparative Study Group, 1991; Treves et al., 1992; Le Jeunne et al., 1999) without placebo control. The initial dose of ergotamine varied from 1 to 5 mg, and in several trials repeated intake of test drugs was used (Table 2). The reported parameters for efficacy were not all validated and varied considerably, from benefit based on a clinical interview (Waters, 1970) to use of changes on a verbal headache scale (Yuill et al., 1972; Friedman et al., 1989; The Multinational Oral Sumatriptan and Cafergot Comparative Study Group, 1991). Methodological flaws in some of these trials include the lack of clearly stated inclusion criteria, no reporting of the baseline criteria and randomization procedures, unusual design of some of the crossover trials with a variable number of attacks per patient, and superiority claims without appropriate statistics.
Ergotamine (1–5 mg) was superior to placebo for some parameters in seven trials (Ostfeld, 1961; Ryan, 1970; Hakkarainen et al., 1979; Kinnunen et al., 1988; Sargent et al., 1988; Friedman et al., 1989; Reches and Eletriptan Steering Committee, 1999) and no better than placebo in three studies using a dose of 2–3 mg (Waters, 1970; Cortelli et al., 1999; McNeely and Goa, 1999). In two comparative trials, ergotamine was superior to aspirin (500 mg) (Hakkarainen et al., 1978, 1980), and was inferior to an isometheptene compound in one trial (Yuill et al., 1972) and superior to it in another trial (Adams et al., 1971). As shown in Table 2, the drugs, such as ergocristine, tolfenamic acid, dextropropoxyphene, naproxen sodium, pirprofen and diclofenac, were generally found to be comparable with ergotamine, although there is one recent study of diclofenac which showed superiority of this drug (Cortelli et al., 1999). Exceptions are sumatriptan (100 mg orally) which was superior to 2 mg of ergotamine plus 200 mg of caffeine (The Multinational Oral Sumatriptan and Cafergot Comparative Study Group, 1991), the combination of calcium carbasalate (equivalent to 900 mg of aspirin) and metoclopramide (10 mg), which was superior to a rather small dose of 1 mg of ergotamine plus 100 mg of caffeine (Le Jeunne et al., 1999), and eletriptan at 40 and 80 mg doses which were superior to 2 mg of ergotamine plus caffeine (Reches and Eletriptan Steering Committee, 1999).
These trials of ergotamine, some of them placebo-controlled, suggest that oral ergotamine is efficacious in the treatment of migraine but they do not quantify the benefit effectively. Thus no uniform picture of the utility of oral ergotamine emerges from these trials. Early use of ergotamine in migraine treatment was tried in two of the trials (Hakkarainen et al., 1978, 1980) in which the drugs were administered as soon as the patients felt the onset of an attack. The results from this strategy are not convincing. The use of escape medication is a clinically relevant efficacy parameter (The International Headache Society Committee on Clinical Trials in Migraine, 1991), and this was used by 31% (Kinnunen et al., 1988), 44% (The Multinational Oral Sumatriptan and Cafergot Comparative Study Group, 1991) and 46% (Ryan, 1970) of patients treated with ergotamine. No clinical trial data are available on within-subject consistency, which from results of pharmacokinetic studies and from clinical practice is probably poor compared with the use of triptans (Kramer et al., 1998; Pfaffenrath et al., 1998).
Non-oral routes of administration
Other routes of administration of ergotamine, which from a kinetic point of view should be more efficacious, have scarcely been investigated. In one trial, inhaled ergotamine (maximum dose of 1.8 mg) was found to be superior to sublingual ergotamine (maximum dose of 2 mg) which was no better than a sublingual placebo (Crooks et al., 1964). In a double-blind placebo-controlled study, a suppository of ergotamine (2 mg) was no better than placebo, whereas ketoprofen (100 mg as a suppository) was superior to placebo (Kangasniemi and Kaaja, 1992). In a recent randomized, crossover, double-blind trial including 251 patients, so far published only on the Internet (1998), ergotamine plus caffeine suppositories (2 and 100 mg, respectively) were superior to 25 mg sumatriptan suppositories, with response rates of 73 and 63% respectively, after 2 h. Headache recurrence (see below) occurred more frequently in sumatriptan- (22%) than in ergotamine- (11%) treated patients. However, significantly more patients preferred sumatriptan suppositories (44%) than preferred ergotamine suppositories (36%), due to more side-effects after the latter. Full publication of this study will be of great interest.
Headache recurrence with ergotamine
Headache recurrence can be defined as a return or worsening of the headache and associated migraine symptoms within 24–72 h after an initial medication-induced amelioration. It is a major issue for all acute migraine treatments, but has only been recognized during the clinical trial programme with subcutaneous sumatriptan (Visser et al., 1996c). Recognition was triggered by the often dramatic contrast of an excellent initial improvement, which was followed by a rapid and very disappointing return of the headache after 10–12 h. Subsequently, it has been observed that headache recurrence is common to all acute migraine treatments (Ferrari, 1998), including ergotamine (The Multinational Oral Sumatriptan and Cafergot Comparative Study Group, 1991), although some treatments are better than others in this regard.
The mechanism of headache recurrence is unknown, but breakthrough of a temporarily suppressed migraine generator seems more likely than a new attack (Weiller et al., 1995; Visser et al., 1996a, b, c). A longer drug plasma half-life does not reduce the incidence of headache recurrence, but may delay the time to recurrence (Visser et al., 1996a). Where the risk of headache recurrence has been studied in sumatriptan users, it seems to be a patient-dependent rather than an attack-dependent phenomenon. About one-third of migraine patients using sumatriptan, especially those with long attacks of 2–3 days, will consistently experience headache recurrence in each successfully treated attack, while patients with shorter attacks experience headache recurrence only rarely (Visser et al., 1996b, c).
A major point of discussion, even among the authors of the present review, is whether headache recurrence rates differ between drugs, and whether any differences have clinical implications. The general perception is that, when effective, ergotamine carries a lower risk of headache recurrence than the triptans. However, the questions arise as to whether this impression is correct, whether such a comparison can actually be made and whether this also implies that patients who experience headache recurrence on triptans will not do so on ergotamine. The initial response, since a patient has to respond first in order to be at risk for headache recurrence, and the use of analgesics for early treatment of recurring headache must be taken into account. In addition, the time at which recurrence occurs must be considered, since headache is usually only monitored up to 24 h, although in an early direct comparison of rates of recurrence at 48 h after dosing, a significant difference (P = 0.009), reported to be 41% for oral sumatriptan 100 mg and 30% for patients treated with Cafergot (ergotamine 1 mg plus caffeine, two tablets), was noted (The Multinational Oral Sumatriptan and Cafergot Comparative Study Group, 1991). It is important to bear in mind that headache recurrence is assessed in a non-randomized population (responders to treatment), and therefore an imbalance in the baseline clinical characteristics cannot be excluded. As a result, simple comparison of headache recurrence may be misleading. Instead of reporting response and recurrence rates separately, overall efficacy might be better ascertained with a composite measure which includes all the factors mentioned above.
Ideally, one would like to know how many patients require only one dose of medication to treat a migraine attack effectively. This could be assessed with the so-called `complete response', which is the proportion of patients who become pain-free within 2 h after drug administration and do not experience headache recurrence nor use analgesics in the subsequent 24–72 h (sustained relief).
Side-effect issues with ergotamine use
Ergotamine has a low degree of receptor selectivity which increases the risk of experiencing a drug-induced side-effect (see above). Ergotamine often causes nausea and vomiting in a migraine sufferer and these are major clinical disadvantages given the high prevalence of these symptoms during the migraine attack. Nausea and vomiting occur in ~10% of patients after oral administration of ergotamine and in about twice that number after parenteral administration. Nausea is most probably caused by a direct effect on CNS emetic centres.
General side-effects
Weakness in the legs has been reported, and occasionally severe muscle pains have occurred in the extremities following ergotamine use. Numbness and tingling of the fingers and toes are other reminders of the ergotism that this alkaloid may cause. Localized oedema and itching may occur in an occasional hypersensitive patient. Most of these effects are not alarming and ordinarily do not necessitate interruption of ergotamine therapy.
In doses used in the treatment of migraine, the rectal administration of ergotamine produces little change in blood pressure but does cause a slowly progressing increase in peripheral arterial constriction that persists for up to 24 h (Bulow et al., 1986).
Cardiovascular side-effects
Ergotamine usually induces bradycardia even when the blood pressure is not increased (Hoffman and Lefkowitz, 1996). This is due predominantly to increased vagal activity, but a reduction in sympathetic tone (by a central as well as peripheral presynaptic action) and direct myocardial depression may also be involved (Saxena and Cairo-Rawlins, 1979; Hoffman and Lefkowitz, 1996). Ergotamine can produce coronary vasoconstriction, often with associated ischaemic changes and anginal pain in patients with coronary artery disease (Galer et al., 1991). In contrast to triptans, the contractile effect of ergotamine in the human isolated coronary artery is long-lasting and persists even after repeated washings (Fig. 1) (MaassenVanDenBrink et al., 1998). Similarly, administration of ergotamine (0.25 mg) intravenously caused a reduction in coronary microcirculatory blood flow as measured by PET (Gnecchi-Ruscone et al., 1998) where, by contrast, sumatriptan produced no such change (Lewis et al., 1997).
Ergotamine doses that produce peripheral vasoconstriction can also damage the capillary endothelium. The mechanism of this toxic action is not clearly understood. Vascular stasis, thrombosis and gangrene are prominent features of ergot poisoning. The propensity of ergotamine to cause gangrene appears to parallel its vasoconstrictor activity (Peroutka, 1996).
Uterine effects
Ergotamine increases the motor activity of the uterus. After small doses, contractions are increased in force or frequency, or both, but are followed by a normal degree of relaxation. As the dose is increased, contractions become more forceful and prolonged, resting tonus is markedly increased, and sustained contracture can result (Graves, 1996).
Contraindications
Ergotamine is contraindicated in women who are or may become pregnant, since the drugs may cause foetal harm. Ergotamine is also contraindicated in patients with peripheral vascular disease, coronary heart disease, uncontrolled hypertension, stroke, impaired hepatic or renal function, and sepsis. Based on the theoretical additive pharmacological effects of the drugs, ergotamine should not be taken within 6 h of the use of triptans, and similarly triptans should not be administered within 24 h of ergotamine. It also is recommended that ergotamine should not be used in complicated migraine (Peroutka, 1996), migraine with prolonged aura, basal migraine or familial hemiplegic migraine.
Daily headache and ergotamine overuse
It seems likely that any medication used for the treatment of migraine attacks can be misused by being taken daily or almost daily (Diener and Tfelt-Hansen, 1993; Kaube et al., 1994; Limmroth et al., 1999). The problem with ergotamine overuse with rebound headache was recognized by Graham in the late 1940s (Wolfson and Graham, 1949) and further clarified by Peters and Horton (Peters and Horton, 1951) and Friedman and colleagues (Friedman et al., 1955). Why some patients are more prone to develop abuse and daily headache than others is unclear. Genetic and psychological factors seem to be involved. Analgesic abuse as a major cause of chronic daily headache was recognized in the 1980s (Mathew et al., 1982; Dichgans et al., 1984).
Migraine patients taking ergotamine daily suffer from several kinds of headaches (Diener and Tfelt-Hansen, 1993): (i) a constant, diffuse, dull headache; (ii) a frequent throbbing headache in the early morning, sometimes combined with nausea, which disappears within 1 h after the intake of ergotamine and is probably a minor withdrawal headache; (iii) migraine attacks; and (iv) a withdrawal headache resembling a severe and prolonged migraine attack with gradual return over weeks to the underlying headache pattern if ergotamine is stopped. In addition, the patients often have constant nausea, acrocyanosis and intermittent claudication due to ergotamine toxicity (von Storch, 1938). Some authors (Mathew et al., 1982) have argued that ergotamine can change the primary headache pattern into what has been termed transformed migraine, but there is no consensus on this issue.
When patients are abusing ergotamine, they fear the withdrawal headache and keep on taking ergotamine. This withdrawal headache is most likely a rebound headache since it occurs ~24 h after the last intake of ergotamine, the normal duration of the vascular effect of a single dose (Bulow et al., 1986; Tfelt-Hansen and Johnson, 1993). The withdrawal headache is often so severe that the ergotamine abusers have to be hospitalized in the withdrawal phase. Spontaneous improvement is common after the medication is discontinued (Diener and Tfelt-Hansen, 1993). When ergotamine is discontinued, the prophylactic medications that previously have been largely without benefit become more effective. The prevention of ergotamine abuse is achieved primarily by restricting the frequency of intake to once per week, as a general rule. Furthermore, patients should be carefully instructed to use ergotamine only for migraine attacks and not for tension-type headaches.
When to use ergotamine—consensus recommendations
Despite the lack of consistent evidence for the efficacy of ergotamine, we as clinicians are left to place the drug in a therapeutic context. Should ergotamine ever be used and if so, when? The writers take the view that there remains a place for ergotamine in modern clinical practice but only when used carefully. Many patients who would have received ergotamine in the `pre-triptan' era are probably now better off not being prescribed the drug. The recommendations for ergotamine use are a distillation of the views of the authors as they emerged during consideration of the data in this review and reflect our clinical practice. Ergotamine remains useful in certain patients, such as those with prolonged attacks or in whom headache recurrence is a substantial issue. It no doubt has cost advantages, but in the use of medicines there is a need to balance cost with clinical outcome. When ergotamine is ineffective, a repeated dosing within half an hour is sometimes recommended, but we do not support this recommendation. This is partly for the reason that one simply cannot expect onset of efficacy within this short time frame, and thus this approach increases the risk for drug-induced side-effects. Table 3 summarizes a prudent use of ergotamine.
Ultimately, physicians will decide to whom ergotamine will be given. Clearly those patients taking ergotamine who have a satisfactory response, as judged by the patient, and who have infrequent headache and no medical contraindication can usefully continue to use ergotamine. Those patients, as with all migraine sufferers, need medical review from time to time to ensure there are no issues of concern arising that would necessitate a change of medication, such as increased headache frequency. Migraine is not unlike hypertension in terms of the attitude to follow-up that we must adopt. The real question is what to do with a patient who has failed to improve with analgesics and NSAIDs (non-steroidal anti-inflammatory drugs), with prokinetics. In the first instance, should they be advanced to triptans immediately or channelled through ergotamine first? This question assumes a stepped care model, where each patient is moved systematically through each level of care; this assumption is now being tested in clinical trials (Lipton et al., 1998). Putting aside financial considerations, moving patients straight to triptans and by-passing ergotamine would be ideal practice as we consider it highly likely that most patients who take ergotamine will be more satisfied with triptans and end up taking them eventually.
Receptor profile of ergotamine compared with dihydroergotamine and sumatriptan
| Receptor type . | pKi value on human cloned receptors in radioligand-binding assaya . | ||
|---|---|---|---|
| . | Ergotamine . | Dihydroergotamine . | Sumatriptan . |
| aUnless otherwise stated; ? = species and test not specified; ND = not determined. bP. J. Pauwels, personal communication to P.R.S.; cLeysen et al., 1996; dAdham et al., 1993; eHoyer, 1998; fGlusa and Roos, 1996; gHoyer et al., 1994; hLeysen and Gommeren, 1984. | |||
| 5-HT1A | 7.89b | 9.30c | 6.43c |
| 5-HT1B | 7.88b | 9.22c | 7.82c |
| 5-HT1D | 8.36b | 8.60c | 8.46c |
| 5-HT1E | 6.22d | 6.22c | 5.80c |
| 5-HT1F | 6.77d | 6.96c | 7.86c |
| 5-HT2A | 7.69e | 8.54c | < 5.0 (pIC50)c |
| 5-HT2B | 8.17 (pEC50, pig, functional)f | 7.70 (pEC50, pig,ND functional)f | |
| 5-HT2C | 7.25 (pig, native)e | 7.43 (pig)c | < 5.0 (pIC50, pig)c |
| 5-HT3 | ND | < 5.0 (pIC50, mouse)c | < 5.0 (pIC50, mouse)c |
| 5-HT4 | ND | 6.52 (guinea pig)c | < 5.0 (pIC50, guinea pig)c |
| 5-HT5A | 7.26b | 7.34b | 5.50b |
| 5-HT5B | 8.50 (pKd, rat)g | ND | ND |
| 5-HT6 | ND | 6.78b | 5.31b |
| 5-HT7 | 7.49 (pKd, rat)g | 7.17b | 6.51b |
| α1 adrenoceptor | 8.00 (?)h | 8.00 (rat)c | < 5.0 (pIC50, rat)c |
| α2 adrenoceptor | 8.20 (?)h | 8.00 (rat)c | < 5.0 (pIC50, rat)c |
| β1 adrenoceptor | ND | 5.27c | < 5.0 (pIC50)c |
| β2 adrenoceptor | ND | < 5.0 (pIC50)c | < 5.0 (pIC50)c |
| Dopamine D1 | ND | 5.32 (rat)c | < 5.0 (pIC50, rat)c |
| Dopamine D2 | 8.50 (?)h | 8.21c | < 5.0 (pIC50)c |
| Receptor type . | pKi value on human cloned receptors in radioligand-binding assaya . | ||
|---|---|---|---|
| . | Ergotamine . | Dihydroergotamine . | Sumatriptan . |
| aUnless otherwise stated; ? = species and test not specified; ND = not determined. bP. J. Pauwels, personal communication to P.R.S.; cLeysen et al., 1996; dAdham et al., 1993; eHoyer, 1998; fGlusa and Roos, 1996; gHoyer et al., 1994; hLeysen and Gommeren, 1984. | |||
| 5-HT1A | 7.89b | 9.30c | 6.43c |
| 5-HT1B | 7.88b | 9.22c | 7.82c |
| 5-HT1D | 8.36b | 8.60c | 8.46c |
| 5-HT1E | 6.22d | 6.22c | 5.80c |
| 5-HT1F | 6.77d | 6.96c | 7.86c |
| 5-HT2A | 7.69e | 8.54c | < 5.0 (pIC50)c |
| 5-HT2B | 8.17 (pEC50, pig, functional)f | 7.70 (pEC50, pig,ND functional)f | |
| 5-HT2C | 7.25 (pig, native)e | 7.43 (pig)c | < 5.0 (pIC50, pig)c |
| 5-HT3 | ND | < 5.0 (pIC50, mouse)c | < 5.0 (pIC50, mouse)c |
| 5-HT4 | ND | 6.52 (guinea pig)c | < 5.0 (pIC50, guinea pig)c |
| 5-HT5A | 7.26b | 7.34b | 5.50b |
| 5-HT5B | 8.50 (pKd, rat)g | ND | ND |
| 5-HT6 | ND | 6.78b | 5.31b |
| 5-HT7 | 7.49 (pKd, rat)g | 7.17b | 6.51b |
| α1 adrenoceptor | 8.00 (?)h | 8.00 (rat)c | < 5.0 (pIC50, rat)c |
| α2 adrenoceptor | 8.20 (?)h | 8.00 (rat)c | < 5.0 (pIC50, rat)c |
| β1 adrenoceptor | ND | 5.27c | < 5.0 (pIC50)c |
| β2 adrenoceptor | ND | < 5.0 (pIC50)c | < 5.0 (pIC50)c |
| Dopamine D1 | ND | 5.32 (rat)c | < 5.0 (pIC50, rat)c |
| Dopamine D2 | 8.50 (?)h | 8.21c | < 5.0 (pIC50)c |
Receptor profile of ergotamine compared with dihydroergotamine and sumatriptan
| Receptor type . | pKi value on human cloned receptors in radioligand-binding assaya . | ||
|---|---|---|---|
| . | Ergotamine . | Dihydroergotamine . | Sumatriptan . |
| aUnless otherwise stated; ? = species and test not specified; ND = not determined. bP. J. Pauwels, personal communication to P.R.S.; cLeysen et al., 1996; dAdham et al., 1993; eHoyer, 1998; fGlusa and Roos, 1996; gHoyer et al., 1994; hLeysen and Gommeren, 1984. | |||
| 5-HT1A | 7.89b | 9.30c | 6.43c |
| 5-HT1B | 7.88b | 9.22c | 7.82c |
| 5-HT1D | 8.36b | 8.60c | 8.46c |
| 5-HT1E | 6.22d | 6.22c | 5.80c |
| 5-HT1F | 6.77d | 6.96c | 7.86c |
| 5-HT2A | 7.69e | 8.54c | < 5.0 (pIC50)c |
| 5-HT2B | 8.17 (pEC50, pig, functional)f | 7.70 (pEC50, pig,ND functional)f | |
| 5-HT2C | 7.25 (pig, native)e | 7.43 (pig)c | < 5.0 (pIC50, pig)c |
| 5-HT3 | ND | < 5.0 (pIC50, mouse)c | < 5.0 (pIC50, mouse)c |
| 5-HT4 | ND | 6.52 (guinea pig)c | < 5.0 (pIC50, guinea pig)c |
| 5-HT5A | 7.26b | 7.34b | 5.50b |
| 5-HT5B | 8.50 (pKd, rat)g | ND | ND |
| 5-HT6 | ND | 6.78b | 5.31b |
| 5-HT7 | 7.49 (pKd, rat)g | 7.17b | 6.51b |
| α1 adrenoceptor | 8.00 (?)h | 8.00 (rat)c | < 5.0 (pIC50, rat)c |
| α2 adrenoceptor | 8.20 (?)h | 8.00 (rat)c | < 5.0 (pIC50, rat)c |
| β1 adrenoceptor | ND | 5.27c | < 5.0 (pIC50)c |
| β2 adrenoceptor | ND | < 5.0 (pIC50)c | < 5.0 (pIC50)c |
| Dopamine D1 | ND | 5.32 (rat)c | < 5.0 (pIC50, rat)c |
| Dopamine D2 | 8.50 (?)h | 8.21c | < 5.0 (pIC50)c |
| Receptor type . | pKi value on human cloned receptors in radioligand-binding assaya . | ||
|---|---|---|---|
| . | Ergotamine . | Dihydroergotamine . | Sumatriptan . |
| aUnless otherwise stated; ? = species and test not specified; ND = not determined. bP. J. Pauwels, personal communication to P.R.S.; cLeysen et al., 1996; dAdham et al., 1993; eHoyer, 1998; fGlusa and Roos, 1996; gHoyer et al., 1994; hLeysen and Gommeren, 1984. | |||
| 5-HT1A | 7.89b | 9.30c | 6.43c |
| 5-HT1B | 7.88b | 9.22c | 7.82c |
| 5-HT1D | 8.36b | 8.60c | 8.46c |
| 5-HT1E | 6.22d | 6.22c | 5.80c |
| 5-HT1F | 6.77d | 6.96c | 7.86c |
| 5-HT2A | 7.69e | 8.54c | < 5.0 (pIC50)c |
| 5-HT2B | 8.17 (pEC50, pig, functional)f | 7.70 (pEC50, pig,ND functional)f | |
| 5-HT2C | 7.25 (pig, native)e | 7.43 (pig)c | < 5.0 (pIC50, pig)c |
| 5-HT3 | ND | < 5.0 (pIC50, mouse)c | < 5.0 (pIC50, mouse)c |
| 5-HT4 | ND | 6.52 (guinea pig)c | < 5.0 (pIC50, guinea pig)c |
| 5-HT5A | 7.26b | 7.34b | 5.50b |
| 5-HT5B | 8.50 (pKd, rat)g | ND | ND |
| 5-HT6 | ND | 6.78b | 5.31b |
| 5-HT7 | 7.49 (pKd, rat)g | 7.17b | 6.51b |
| α1 adrenoceptor | 8.00 (?)h | 8.00 (rat)c | < 5.0 (pIC50, rat)c |
| α2 adrenoceptor | 8.20 (?)h | 8.00 (rat)c | < 5.0 (pIC50, rat)c |
| β1 adrenoceptor | ND | 5.27c | < 5.0 (pIC50)c |
| β2 adrenoceptor | ND | < 5.0 (pIC50)c | < 5.0 (pIC50)c |
| Dopamine D1 | ND | 5.32 (rat)c | < 5.0 (pIC50, rat)c |
| Dopamine D2 | 8.50 (?)h | 8.21c | < 5.0 (pIC50)c |
Double-blind randomized trials with pure oral ergotamine (Erg) or an ergotamine compound with caffeine (ErgC) in the treatment of migraine attacks
| Trial . | Drug . | Initial (maximum) dosage (mg) . | Study design . | No. of attacks treateda . | No. of patients (no. evaluated) . | Result of trial . |
|---|---|---|---|---|---|---|
| The table is modified from Tfelt-Hansen and Johnson (1993). ASA = aspirin; CASA + M = calcium carbasalate (equivalent to 900 mg of ASA) plus metoclopramide; DextC = dextropropoxyphene compound; Diclo = diclofenac; Erg = ergotamine; ErgC = ergotamine compound with caffeine (1 mg of ergotamine + 100 mg of caffeine); Ergs = ergostine (+ caffeine); Ele = eletriptan; IsomC = isometheptene compound; Napxs = naproxen sodium; Pirp = pirprofen; Sum = sumatriptan; Tfa = tolfenamic acid; Pl = placebo; CO = crossover; Pa = parallel group; NS or = = no statistical significant difference; > = more effective than. aMaximum number of attacks treated; bapproximately one-quarter of patients did not have migraine (74); conly dose of isometheptene given (for other components, see reference); dverbal scale : 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe; eonly doses for dextropropoxyphene [65 mg of the chloride (9) or 100 mg of the napsylate (10)] are indicated (for other components, see references); fcontains other components in addition to caffeine, see references; gstudy conclusions weakened by the lack of use of double dummy technique; hpatients refractory to ergot therapy were excluded; ia decrease from severe or moderate headache to no or mild headache. | ||||||
| Ostfeld, 1961 | Erg | 5 | CO | 1 | 44 | More than 50% headache relief: Erg (70%) > Pl (39%) |
| Pl | ||||||
| Waters, 1970 | Erg | 2–3 | CO | ?b | 88 (79) | Benefited based on clinical interview: Erg (51%)/Pl (58%) |
| Pl | ||||||
| ErgC | 2 (6) | CO | 1 | 48 | Escape medication: ErgC (22/48) = Ergs (22/46) > Pl (33/46) | |
| Ergs | 2 (6) | |||||
| Pl | ||||||
| Ryan, 1970 | ErgC | 2 (6) | CO | 2 | 54 | Mean headache duration: ErgC > IsomC |
| IsomC | 130 (130) | |||||
| Yuill et al., 1972 | ErgC | 2 (6) | CO | 1 | 38 | Headache intensityd: IsomC (2.8) > ErgC (3.3). Nausead: IsomC (1.1) > ErgC (2.0) |
| IsomCc | 130 (390) | |||||
| Hakkarainen et al., 1979 | Erg | 1 | CO | 2 | 20 | Mean duration of attack in h: Erg (3.8) = Tfa (3.2) = ASA (4.2) > Pl (7.1) Preference: all drugs > Pl |
| Tfa | 200 | |||||
| ASA | 500 | |||||
| Pl | ||||||
| Hakkarainen et al., 1978 | Erg | 1 (3) | CO | 7 | 25 | Mean of attack prevented: Erg (3.6) =DextC (2.6) > Pl (1.1) |
| DextCe | 100 (200) | |||||
| ASA | 500 (1500) | |||||
| Hakkarainen et al., 1980 | Erg | 1 (2) | CO | 7 | 25 | Attack not prevented: Erg (53%) = DextC (59%) > Pl (82%) |
| DextCe | 100 (200) | |||||
| ASA | 500 (1000) | |||||
| Pradalier et al., 1985 | ErgCf | 2 (4) | Pa | 6 | 114 (95) | For test drug taken within 2 h: Napxs > ErgC for headache relief. Later intake of test drug, NSg |
| Napxs | 825 (1375) | |||||
| Sargent et al., 1988 | ErgC | 2 (3) | Pa | 6 | 169 (122) | Relief of headache at 1 h: Napxs > Pl, ErgC = Pl. Overall efficacy: ErgC > Pl, Napxs = Pl |
| Napxs | 825 (1100) | |||||
| Pl | ||||||
| Kinnunen et al., 1988 | ErgCf | 2 (5) | CO | 1 | 67 (61) | Escape medication: ErgC (18/59) = Pirp (18/58) > Pl (32/60). Duration of attacks in h: ErgC (6.5) > Pl (10.5) but versus Pirp NS. For most parameters, ErgC vs Pirp NS |
| Pirp | 200 (500) | |||||
| Pl | ||||||
| Friedman et al., 1989 | ErgCf | 2 (6) | Pa | 2 | ? (104) | Mean improvement from baseline on a 5-point headache scale after 2 h: ErgC (1.0) > Pl (0)h. |
| Pl | ||||||
| The Multinational Oral | ErgC | 2 | Pa | 3 | 580 (577) | Headache reliefi: Sum (66%) > ErgC (48%) |
| Sumatripan and Cafergot | Sum | 100 | ||||
| Comparative Study Group, 1991 | ||||||
| Treves et al., 1992 | Erg | 2 (4) | Pa | 6 | 79 (71?) | Napxs > Erg for overall efficacy rating of treatments on a 6-point scale (none to excellent) .Improvement of headache: Napxs = Erg |
| Napxs | 750 (1750) | |||||
| Le Jeunne et al., 1997 | ErgC | 1 | Pa | 3 | 268 | Headache reliefi: CASA + M (54%) > ErgC (36%) |
| CASA+M | 900+10 | |||||
| Cortelli et al., 1996 | ErgC | 2 (6) | CO | 1 | 63 | Diclo > Pl (–15 mm mean difference for changes on a VAS scale after 1 h). Diclo > ErgC (–11.9 mm mean difference) ErgC = Pl (–2.8 mm mean difference) |
| Diclo | 50 (150) | |||||
| Pl | ||||||
| McNeely and Goa, 1999 | ErgC | 2 (5) | Pa | 1 | 423 | Diclo > Pl (–9 mm mean difference for changes on VAS scale after 2 h). Diclo = (–3.6 mm mean difference). ErgC =ErgC Pl (–5.4 mm mean difference). |
| Diclo | 50 (200) | |||||
| Pl | ||||||
| Reches and Eletriptan | ErgC | 2 (4) | Pa | 1 | Headache reliefi: Ele 80 (68%) > Ele 40 (58%) > ErgC (33%) > Pl (21%) | |
| Steering Committee, 1999 | Ele | 40 (80) | ||||
| Ele | 80 (160) | |||||
| Pl | ||||||
| Trial . | Drug . | Initial (maximum) dosage (mg) . | Study design . | No. of attacks treateda . | No. of patients (no. evaluated) . | Result of trial . |
|---|---|---|---|---|---|---|
| The table is modified from Tfelt-Hansen and Johnson (1993). ASA = aspirin; CASA + M = calcium carbasalate (equivalent to 900 mg of ASA) plus metoclopramide; DextC = dextropropoxyphene compound; Diclo = diclofenac; Erg = ergotamine; ErgC = ergotamine compound with caffeine (1 mg of ergotamine + 100 mg of caffeine); Ergs = ergostine (+ caffeine); Ele = eletriptan; IsomC = isometheptene compound; Napxs = naproxen sodium; Pirp = pirprofen; Sum = sumatriptan; Tfa = tolfenamic acid; Pl = placebo; CO = crossover; Pa = parallel group; NS or = = no statistical significant difference; > = more effective than. aMaximum number of attacks treated; bapproximately one-quarter of patients did not have migraine (74); conly dose of isometheptene given (for other components, see reference); dverbal scale : 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe; eonly doses for dextropropoxyphene [65 mg of the chloride (9) or 100 mg of the napsylate (10)] are indicated (for other components, see references); fcontains other components in addition to caffeine, see references; gstudy conclusions weakened by the lack of use of double dummy technique; hpatients refractory to ergot therapy were excluded; ia decrease from severe or moderate headache to no or mild headache. | ||||||
| Ostfeld, 1961 | Erg | 5 | CO | 1 | 44 | More than 50% headache relief: Erg (70%) > Pl (39%) |
| Pl | ||||||
| Waters, 1970 | Erg | 2–3 | CO | ?b | 88 (79) | Benefited based on clinical interview: Erg (51%)/Pl (58%) |
| Pl | ||||||
| ErgC | 2 (6) | CO | 1 | 48 | Escape medication: ErgC (22/48) = Ergs (22/46) > Pl (33/46) | |
| Ergs | 2 (6) | |||||
| Pl | ||||||
| Ryan, 1970 | ErgC | 2 (6) | CO | 2 | 54 | Mean headache duration: ErgC > IsomC |
| IsomC | 130 (130) | |||||
| Yuill et al., 1972 | ErgC | 2 (6) | CO | 1 | 38 | Headache intensityd: IsomC (2.8) > ErgC (3.3). Nausead: IsomC (1.1) > ErgC (2.0) |
| IsomCc | 130 (390) | |||||
| Hakkarainen et al., 1979 | Erg | 1 | CO | 2 | 20 | Mean duration of attack in h: Erg (3.8) = Tfa (3.2) = ASA (4.2) > Pl (7.1) Preference: all drugs > Pl |
| Tfa | 200 | |||||
| ASA | 500 | |||||
| Pl | ||||||
| Hakkarainen et al., 1978 | Erg | 1 (3) | CO | 7 | 25 | Mean of attack prevented: Erg (3.6) =DextC (2.6) > Pl (1.1) |
| DextCe | 100 (200) | |||||
| ASA | 500 (1500) | |||||
| Hakkarainen et al., 1980 | Erg | 1 (2) | CO | 7 | 25 | Attack not prevented: Erg (53%) = DextC (59%) > Pl (82%) |
| DextCe | 100 (200) | |||||
| ASA | 500 (1000) | |||||
| Pradalier et al., 1985 | ErgCf | 2 (4) | Pa | 6 | 114 (95) | For test drug taken within 2 h: Napxs > ErgC for headache relief. Later intake of test drug, NSg |
| Napxs | 825 (1375) | |||||
| Sargent et al., 1988 | ErgC | 2 (3) | Pa | 6 | 169 (122) | Relief of headache at 1 h: Napxs > Pl, ErgC = Pl. Overall efficacy: ErgC > Pl, Napxs = Pl |
| Napxs | 825 (1100) | |||||
| Pl | ||||||
| Kinnunen et al., 1988 | ErgCf | 2 (5) | CO | 1 | 67 (61) | Escape medication: ErgC (18/59) = Pirp (18/58) > Pl (32/60). Duration of attacks in h: ErgC (6.5) > Pl (10.5) but versus Pirp NS. For most parameters, ErgC vs Pirp NS |
| Pirp | 200 (500) | |||||
| Pl | ||||||
| Friedman et al., 1989 | ErgCf | 2 (6) | Pa | 2 | ? (104) | Mean improvement from baseline on a 5-point headache scale after 2 h: ErgC (1.0) > Pl (0)h. |
| Pl | ||||||
| The Multinational Oral | ErgC | 2 | Pa | 3 | 580 (577) | Headache reliefi: Sum (66%) > ErgC (48%) |
| Sumatripan and Cafergot | Sum | 100 | ||||
| Comparative Study Group, 1991 | ||||||
| Treves et al., 1992 | Erg | 2 (4) | Pa | 6 | 79 (71?) | Napxs > Erg for overall efficacy rating of treatments on a 6-point scale (none to excellent) .Improvement of headache: Napxs = Erg |
| Napxs | 750 (1750) | |||||
| Le Jeunne et al., 1997 | ErgC | 1 | Pa | 3 | 268 | Headache reliefi: CASA + M (54%) > ErgC (36%) |
| CASA+M | 900+10 | |||||
| Cortelli et al., 1996 | ErgC | 2 (6) | CO | 1 | 63 | Diclo > Pl (–15 mm mean difference for changes on a VAS scale after 1 h). Diclo > ErgC (–11.9 mm mean difference) ErgC = Pl (–2.8 mm mean difference) |
| Diclo | 50 (150) | |||||
| Pl | ||||||
| McNeely and Goa, 1999 | ErgC | 2 (5) | Pa | 1 | 423 | Diclo > Pl (–9 mm mean difference for changes on VAS scale after 2 h). Diclo = (–3.6 mm mean difference). ErgC =ErgC Pl (–5.4 mm mean difference). |
| Diclo | 50 (200) | |||||
| Pl | ||||||
| Reches and Eletriptan | ErgC | 2 (4) | Pa | 1 | Headache reliefi: Ele 80 (68%) > Ele 40 (58%) > ErgC (33%) > Pl (21%) | |
| Steering Committee, 1999 | Ele | 40 (80) | ||||
| Ele | 80 (160) | |||||
| Pl | ||||||
Double-blind randomized trials with pure oral ergotamine (Erg) or an ergotamine compound with caffeine (ErgC) in the treatment of migraine attacks
| Trial . | Drug . | Initial (maximum) dosage (mg) . | Study design . | No. of attacks treateda . | No. of patients (no. evaluated) . | Result of trial . |
|---|---|---|---|---|---|---|
| The table is modified from Tfelt-Hansen and Johnson (1993). ASA = aspirin; CASA + M = calcium carbasalate (equivalent to 900 mg of ASA) plus metoclopramide; DextC = dextropropoxyphene compound; Diclo = diclofenac; Erg = ergotamine; ErgC = ergotamine compound with caffeine (1 mg of ergotamine + 100 mg of caffeine); Ergs = ergostine (+ caffeine); Ele = eletriptan; IsomC = isometheptene compound; Napxs = naproxen sodium; Pirp = pirprofen; Sum = sumatriptan; Tfa = tolfenamic acid; Pl = placebo; CO = crossover; Pa = parallel group; NS or = = no statistical significant difference; > = more effective than. aMaximum number of attacks treated; bapproximately one-quarter of patients did not have migraine (74); conly dose of isometheptene given (for other components, see reference); dverbal scale : 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe; eonly doses for dextropropoxyphene [65 mg of the chloride (9) or 100 mg of the napsylate (10)] are indicated (for other components, see references); fcontains other components in addition to caffeine, see references; gstudy conclusions weakened by the lack of use of double dummy technique; hpatients refractory to ergot therapy were excluded; ia decrease from severe or moderate headache to no or mild headache. | ||||||
| Ostfeld, 1961 | Erg | 5 | CO | 1 | 44 | More than 50% headache relief: Erg (70%) > Pl (39%) |
| Pl | ||||||
| Waters, 1970 | Erg | 2–3 | CO | ?b | 88 (79) | Benefited based on clinical interview: Erg (51%)/Pl (58%) |
| Pl | ||||||
| ErgC | 2 (6) | CO | 1 | 48 | Escape medication: ErgC (22/48) = Ergs (22/46) > Pl (33/46) | |
| Ergs | 2 (6) | |||||
| Pl | ||||||
| Ryan, 1970 | ErgC | 2 (6) | CO | 2 | 54 | Mean headache duration: ErgC > IsomC |
| IsomC | 130 (130) | |||||
| Yuill et al., 1972 | ErgC | 2 (6) | CO | 1 | 38 | Headache intensityd: IsomC (2.8) > ErgC (3.3). Nausead: IsomC (1.1) > ErgC (2.0) |
| IsomCc | 130 (390) | |||||
| Hakkarainen et al., 1979 | Erg | 1 | CO | 2 | 20 | Mean duration of attack in h: Erg (3.8) = Tfa (3.2) = ASA (4.2) > Pl (7.1) Preference: all drugs > Pl |
| Tfa | 200 | |||||
| ASA | 500 | |||||
| Pl | ||||||
| Hakkarainen et al., 1978 | Erg | 1 (3) | CO | 7 | 25 | Mean of attack prevented: Erg (3.6) =DextC (2.6) > Pl (1.1) |
| DextCe | 100 (200) | |||||
| ASA | 500 (1500) | |||||
| Hakkarainen et al., 1980 | Erg | 1 (2) | CO | 7 | 25 | Attack not prevented: Erg (53%) = DextC (59%) > Pl (82%) |
| DextCe | 100 (200) | |||||
| ASA | 500 (1000) | |||||
| Pradalier et al., 1985 | ErgCf | 2 (4) | Pa | 6 | 114 (95) | For test drug taken within 2 h: Napxs > ErgC for headache relief. Later intake of test drug, NSg |
| Napxs | 825 (1375) | |||||
| Sargent et al., 1988 | ErgC | 2 (3) | Pa | 6 | 169 (122) | Relief of headache at 1 h: Napxs > Pl, ErgC = Pl. Overall efficacy: ErgC > Pl, Napxs = Pl |
| Napxs | 825 (1100) | |||||
| Pl | ||||||
| Kinnunen et al., 1988 | ErgCf | 2 (5) | CO | 1 | 67 (61) | Escape medication: ErgC (18/59) = Pirp (18/58) > Pl (32/60). Duration of attacks in h: ErgC (6.5) > Pl (10.5) but versus Pirp NS. For most parameters, ErgC vs Pirp NS |
| Pirp | 200 (500) | |||||
| Pl | ||||||
| Friedman et al., 1989 | ErgCf | 2 (6) | Pa | 2 | ? (104) | Mean improvement from baseline on a 5-point headache scale after 2 h: ErgC (1.0) > Pl (0)h. |
| Pl | ||||||
| The Multinational Oral | ErgC | 2 | Pa | 3 | 580 (577) | Headache reliefi: Sum (66%) > ErgC (48%) |
| Sumatripan and Cafergot | Sum | 100 | ||||
| Comparative Study Group, 1991 | ||||||
| Treves et al., 1992 | Erg | 2 (4) | Pa | 6 | 79 (71?) | Napxs > Erg for overall efficacy rating of treatments on a 6-point scale (none to excellent) .Improvement of headache: Napxs = Erg |
| Napxs | 750 (1750) | |||||
| Le Jeunne et al., 1997 | ErgC | 1 | Pa | 3 | 268 | Headache reliefi: CASA + M (54%) > ErgC (36%) |
| CASA+M | 900+10 | |||||
| Cortelli et al., 1996 | ErgC | 2 (6) | CO | 1 | 63 | Diclo > Pl (–15 mm mean difference for changes on a VAS scale after 1 h). Diclo > ErgC (–11.9 mm mean difference) ErgC = Pl (–2.8 mm mean difference) |
| Diclo | 50 (150) | |||||
| Pl | ||||||
| McNeely and Goa, 1999 | ErgC | 2 (5) | Pa | 1 | 423 | Diclo > Pl (–9 mm mean difference for changes on VAS scale after 2 h). Diclo = (–3.6 mm mean difference). ErgC =ErgC Pl (–5.4 mm mean difference). |
| Diclo | 50 (200) | |||||
| Pl | ||||||
| Reches and Eletriptan | ErgC | 2 (4) | Pa | 1 | Headache reliefi: Ele 80 (68%) > Ele 40 (58%) > ErgC (33%) > Pl (21%) | |
| Steering Committee, 1999 | Ele | 40 (80) | ||||
| Ele | 80 (160) | |||||
| Pl | ||||||
| Trial . | Drug . | Initial (maximum) dosage (mg) . | Study design . | No. of attacks treateda . | No. of patients (no. evaluated) . | Result of trial . |
|---|---|---|---|---|---|---|
| The table is modified from Tfelt-Hansen and Johnson (1993). ASA = aspirin; CASA + M = calcium carbasalate (equivalent to 900 mg of ASA) plus metoclopramide; DextC = dextropropoxyphene compound; Diclo = diclofenac; Erg = ergotamine; ErgC = ergotamine compound with caffeine (1 mg of ergotamine + 100 mg of caffeine); Ergs = ergostine (+ caffeine); Ele = eletriptan; IsomC = isometheptene compound; Napxs = naproxen sodium; Pirp = pirprofen; Sum = sumatriptan; Tfa = tolfenamic acid; Pl = placebo; CO = crossover; Pa = parallel group; NS or = = no statistical significant difference; > = more effective than. aMaximum number of attacks treated; bapproximately one-quarter of patients did not have migraine (74); conly dose of isometheptene given (for other components, see reference); dverbal scale : 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe; eonly doses for dextropropoxyphene [65 mg of the chloride (9) or 100 mg of the napsylate (10)] are indicated (for other components, see references); fcontains other components in addition to caffeine, see references; gstudy conclusions weakened by the lack of use of double dummy technique; hpatients refractory to ergot therapy were excluded; ia decrease from severe or moderate headache to no or mild headache. | ||||||
| Ostfeld, 1961 | Erg | 5 | CO | 1 | 44 | More than 50% headache relief: Erg (70%) > Pl (39%) |
| Pl | ||||||
| Waters, 1970 | Erg | 2–3 | CO | ?b | 88 (79) | Benefited based on clinical interview: Erg (51%)/Pl (58%) |
| Pl | ||||||
| ErgC | 2 (6) | CO | 1 | 48 | Escape medication: ErgC (22/48) = Ergs (22/46) > Pl (33/46) | |
| Ergs | 2 (6) | |||||
| Pl | ||||||
| Ryan, 1970 | ErgC | 2 (6) | CO | 2 | 54 | Mean headache duration: ErgC > IsomC |
| IsomC | 130 (130) | |||||
| Yuill et al., 1972 | ErgC | 2 (6) | CO | 1 | 38 | Headache intensityd: IsomC (2.8) > ErgC (3.3). Nausead: IsomC (1.1) > ErgC (2.0) |
| IsomCc | 130 (390) | |||||
| Hakkarainen et al., 1979 | Erg | 1 | CO | 2 | 20 | Mean duration of attack in h: Erg (3.8) = Tfa (3.2) = ASA (4.2) > Pl (7.1) Preference: all drugs > Pl |
| Tfa | 200 | |||||
| ASA | 500 | |||||
| Pl | ||||||
| Hakkarainen et al., 1978 | Erg | 1 (3) | CO | 7 | 25 | Mean of attack prevented: Erg (3.6) =DextC (2.6) > Pl (1.1) |
| DextCe | 100 (200) | |||||
| ASA | 500 (1500) | |||||
| Hakkarainen et al., 1980 | Erg | 1 (2) | CO | 7 | 25 | Attack not prevented: Erg (53%) = DextC (59%) > Pl (82%) |
| DextCe | 100 (200) | |||||
| ASA | 500 (1000) | |||||
| Pradalier et al., 1985 | ErgCf | 2 (4) | Pa | 6 | 114 (95) | For test drug taken within 2 h: Napxs > ErgC for headache relief. Later intake of test drug, NSg |
| Napxs | 825 (1375) | |||||
| Sargent et al., 1988 | ErgC | 2 (3) | Pa | 6 | 169 (122) | Relief of headache at 1 h: Napxs > Pl, ErgC = Pl. Overall efficacy: ErgC > Pl, Napxs = Pl |
| Napxs | 825 (1100) | |||||
| Pl | ||||||
| Kinnunen et al., 1988 | ErgCf | 2 (5) | CO | 1 | 67 (61) | Escape medication: ErgC (18/59) = Pirp (18/58) > Pl (32/60). Duration of attacks in h: ErgC (6.5) > Pl (10.5) but versus Pirp NS. For most parameters, ErgC vs Pirp NS |
| Pirp | 200 (500) | |||||
| Pl | ||||||
| Friedman et al., 1989 | ErgCf | 2 (6) | Pa | 2 | ? (104) | Mean improvement from baseline on a 5-point headache scale after 2 h: ErgC (1.0) > Pl (0)h. |
| Pl | ||||||
| The Multinational Oral | ErgC | 2 | Pa | 3 | 580 (577) | Headache reliefi: Sum (66%) > ErgC (48%) |
| Sumatripan and Cafergot | Sum | 100 | ||||
| Comparative Study Group, 1991 | ||||||
| Treves et al., 1992 | Erg | 2 (4) | Pa | 6 | 79 (71?) | Napxs > Erg for overall efficacy rating of treatments on a 6-point scale (none to excellent) .Improvement of headache: Napxs = Erg |
| Napxs | 750 (1750) | |||||
| Le Jeunne et al., 1997 | ErgC | 1 | Pa | 3 | 268 | Headache reliefi: CASA + M (54%) > ErgC (36%) |
| CASA+M | 900+10 | |||||
| Cortelli et al., 1996 | ErgC | 2 (6) | CO | 1 | 63 | Diclo > Pl (–15 mm mean difference for changes on a VAS scale after 1 h). Diclo > ErgC (–11.9 mm mean difference) ErgC = Pl (–2.8 mm mean difference) |
| Diclo | 50 (150) | |||||
| Pl | ||||||
| McNeely and Goa, 1999 | ErgC | 2 (5) | Pa | 1 | 423 | Diclo > Pl (–9 mm mean difference for changes on VAS scale after 2 h). Diclo = (–3.6 mm mean difference). ErgC =ErgC Pl (–5.4 mm mean difference). |
| Diclo | 50 (200) | |||||
| Pl | ||||||
| Reches and Eletriptan | ErgC | 2 (4) | Pa | 1 | Headache reliefi: Ele 80 (68%) > Ele 40 (58%) > ErgC (33%) > Pl (21%) | |
| Steering Committee, 1999 | Ele | 40 (80) | ||||
| Ele | 80 (160) | |||||
| Pl | ||||||
Recommendations for the use of ergotamine
| Recommendation . | Limitations and comments . |
|---|---|
| Which patients? | |
| • Patients requiring migraine-specific therapy | • When a migraine-specific therapy is indicated, a triptan is a better choice than ergotamine for most patients |
| • Patients established on ergotamine | • Patients established on ergotamine who are responding satisfactorily, with no contraindications to its use and with no signs of dose escalation, should not usually be switched to a triptan |
| Special cases | |
| • Patients with very long attacks | • Attacks lasting > 48 h may be usefully treated with ergotamine |
| • Patients with frequent headache recurrence | • Headache recurrence is probably less likely with ergotamine |
| Frequency of dosing: 1/week or 6/month | • A major problem with ergotamine is ergotamine-induced headache and rebound headache associated with frequent use. This can be limited by restricting ergotamine consumption and encouraging use of a preventative medication as headache becomes more frequent. |
| • May be modified to four consecutive doses for menstrual migraine | |
| • May be modified for use in cluster headache | |
| Dose per attack: single dose (0.5–2 mg) | Ergotamine should be dosed at one time as early as practicable in the attack at a dose that produces a response with as few side-effects as possible. It is useful to test this dose for tolerability for nausea between attacks |
| Preferred route: rectal | Although still useful orally, ergotamine is generally better used, provided it is acceptable to the patient, by the rectal route because of improved absorption. Where it is available, the ergotamine puffer is preferred to the oral route for the same reasons |
| Recommendation . | Limitations and comments . |
|---|---|
| Which patients? | |
| • Patients requiring migraine-specific therapy | • When a migraine-specific therapy is indicated, a triptan is a better choice than ergotamine for most patients |
| • Patients established on ergotamine | • Patients established on ergotamine who are responding satisfactorily, with no contraindications to its use and with no signs of dose escalation, should not usually be switched to a triptan |
| Special cases | |
| • Patients with very long attacks | • Attacks lasting > 48 h may be usefully treated with ergotamine |
| • Patients with frequent headache recurrence | • Headache recurrence is probably less likely with ergotamine |
| Frequency of dosing: 1/week or 6/month | • A major problem with ergotamine is ergotamine-induced headache and rebound headache associated with frequent use. This can be limited by restricting ergotamine consumption and encouraging use of a preventative medication as headache becomes more frequent. |
| • May be modified to four consecutive doses for menstrual migraine | |
| • May be modified for use in cluster headache | |
| Dose per attack: single dose (0.5–2 mg) | Ergotamine should be dosed at one time as early as practicable in the attack at a dose that produces a response with as few side-effects as possible. It is useful to test this dose for tolerability for nausea between attacks |
| Preferred route: rectal | Although still useful orally, ergotamine is generally better used, provided it is acceptable to the patient, by the rectal route because of improved absorption. Where it is available, the ergotamine puffer is preferred to the oral route for the same reasons |
Recommendations for the use of ergotamine
| Recommendation . | Limitations and comments . |
|---|---|
| Which patients? | |
| • Patients requiring migraine-specific therapy | • When a migraine-specific therapy is indicated, a triptan is a better choice than ergotamine for most patients |
| • Patients established on ergotamine | • Patients established on ergotamine who are responding satisfactorily, with no contraindications to its use and with no signs of dose escalation, should not usually be switched to a triptan |
| Special cases | |
| • Patients with very long attacks | • Attacks lasting > 48 h may be usefully treated with ergotamine |
| • Patients with frequent headache recurrence | • Headache recurrence is probably less likely with ergotamine |
| Frequency of dosing: 1/week or 6/month | • A major problem with ergotamine is ergotamine-induced headache and rebound headache associated with frequent use. This can be limited by restricting ergotamine consumption and encouraging use of a preventative medication as headache becomes more frequent. |
| • May be modified to four consecutive doses for menstrual migraine | |
| • May be modified for use in cluster headache | |
| Dose per attack: single dose (0.5–2 mg) | Ergotamine should be dosed at one time as early as practicable in the attack at a dose that produces a response with as few side-effects as possible. It is useful to test this dose for tolerability for nausea between attacks |
| Preferred route: rectal | Although still useful orally, ergotamine is generally better used, provided it is acceptable to the patient, by the rectal route because of improved absorption. Where it is available, the ergotamine puffer is preferred to the oral route for the same reasons |
| Recommendation . | Limitations and comments . |
|---|---|
| Which patients? | |
| • Patients requiring migraine-specific therapy | • When a migraine-specific therapy is indicated, a triptan is a better choice than ergotamine for most patients |
| • Patients established on ergotamine | • Patients established on ergotamine who are responding satisfactorily, with no contraindications to its use and with no signs of dose escalation, should not usually be switched to a triptan |
| Special cases | |
| • Patients with very long attacks | • Attacks lasting > 48 h may be usefully treated with ergotamine |
| • Patients with frequent headache recurrence | • Headache recurrence is probably less likely with ergotamine |
| Frequency of dosing: 1/week or 6/month | • A major problem with ergotamine is ergotamine-induced headache and rebound headache associated with frequent use. This can be limited by restricting ergotamine consumption and encouraging use of a preventative medication as headache becomes more frequent. |
| • May be modified to four consecutive doses for menstrual migraine | |
| • May be modified for use in cluster headache | |
| Dose per attack: single dose (0.5–2 mg) | Ergotamine should be dosed at one time as early as practicable in the attack at a dose that produces a response with as few side-effects as possible. It is useful to test this dose for tolerability for nausea between attacks |
| Preferred route: rectal | Although still useful orally, ergotamine is generally better used, provided it is acceptable to the patient, by the rectal route because of improved absorption. Where it is available, the ergotamine puffer is preferred to the oral route for the same reasons |
Persistent contractile response by ergots, but not triptans, on human isolated coronary arteries. Filled triangles = ergotamine; filled diamonds = dihydroergotamine; filled circles = sumatriptan; open squares = zolmitriptan; stars = rizatriptan; open triangles = naratriptan; open circles = avitriptan. All drugs were administered once at a concentration twice their EC50. Data are displayed as mean ± standard error of the mean (MaassenVanDenBrink et al., 1998).
References
Adams M, Aikman P, Allardyce K. General practitioner clinical trials: treatment of migraine.
Adham N, Kao H-T, Schecter LE, Bard J, Olsen M, Urquhart D, et al. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase.
Andersen AR, Tfelt-Hansen P, Lassen NA. The effect of ergotamine and dihydroergotamine on cerebral blood flow in man.
Bulow PM, Ibraheem JJ, Paalzow G, Tfelt-Hansen P. Comparison of pharmacodynamic effects and plasma levels of oral and rectal ergotamine.
Buzzi MG, Moskowitz MA. Evidence for 5-HT1B/1D receptors mediating the antimigraine effect of sumatriptan and dihydroergotamine.
Buzzi MG, Moskowitz MA, Peroutka SJ, Byun B. Further characterization of the putative 5-HT receptor which mediates blockade of neurogenic plasma extravasation in rat dura mater.
Christians U, Schmidt G, Bader A, Lampen A, Schottmann R, Linck A, et al. Identification of drugs inhibiting the in vitro metabolism of tacrolimus by human liver microsomes.
Cortelli P, Pierangeli G, Corsini R, Prologo G, Limido GL. Pain control in migraine attacks: results from a double-blind, randomized, within-patient, placebo-controlled trial comparing diclofenac-K and ergotamine–caffeine.
Cortijo J, Martí-Cabrera M, Bernabeu E, Domenech T, Bou J, Fernández AG, et al. Characterization of 5-HT receptors on human pulmonary artery and vein: functional and binding studies.
Crooks J, Stephen SA, Brass W. Clinical trial of inhaled ergotamine tartrate in migraine.
Dahlof C. Placebo-controlled clinical trials with ergotamine in the acute treatment of migraine. [Review].
De Vries P, Villalon CM, Heiligers JP, Saxena PR. Characterization of 5-HT receptors mediating constriction of porcine carotid arteriovenous anastomoses; involvement of 5-HT1B/1D and novel receptors.
Dichgans J, Diener H-C, Gerber WD, Verspohl EJ, Kukiolka H, Kluck M. Analgetika-induzierter Dauerkopfschmerz.
Diener H-C, Tfelt-Hansen P. Headache associated with chronic use of substances. In: Olesen J, Tfelt-Hansen P, Welch KM, editors. The headaches. New York: Raven Press; 1993. p. 721–7.
Dixon RM, Meire HB, Evans DH, Watt H, On N, Posner J, et al. Peripheral vascular effects and pharmacokinetics of the antimigraine compound, zolmitriptan, in combination with oral ergotamine in healthy volunteers.
Eulenberg A. Subcutane Injectionen von ergotinin (Tanret): Ergotinum citricum solutum (Gehe).
Francis H, Tyndall A, Webb J. Severe vascular spasm due to erythromycin–ergotamine interaction.
Friedman AP, Brazil P, von Storch TJ. Ergotamine tolerance in patients with migraine.
Friedman AP, Di Serio FJ, Hwang DS. Symptomatic relief of migraine—multicenter comparison of Cafergot P-B, Cafergot, and placebo.
Galer BS, Lipton RB, Solomon S, Newman LC, Spierings EL. Myocardial ischemia related to ergot alkaloids: a case report and literature review. [Review].
Glusa E, Roos A. Endothelial 5-HT receptors mediate relaxation of porcine pulmonary arteries in response to ergotamine and dihydroergotamine.
Gnecchi-Ruscone T, Lorenzoni R, Anderson D, Legg N, Tousoulis D, Winter PD, et al. Effects of ergotamine on myocardial blood flow in migraineurs without evidence of atherosclerotic coronary artery disease.
Goadsby PJ. Serotonin 5-HT1B/1D receptor agonists in migraine: comparative pharmacology and its therapeutic implications.
Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats.
Graves CR. Agents that cause contraction or relaxation of the uterus. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 9th edn. New York: McGraw-Hill; 1996. p. 939–49.
Hakkarainen H, Gustafsson B, Stockman O. A comparative trial of ergotamine tartrate, acetyl salicylic acid and a dextropropoxyphene compound in acute migraine attacks.
Hakkarainen H, Vapaatalo H, Gothoni G, Parantainen J. Tolfenamic acid is as effective as ergotamine during migraine attacks.
Hakkarainen H, Quiding H, Stockman O. Mild analgesics as an alternative to ergotamine in migraine. A comparative trial with acetylsalicylic acid, ergotamine tartrate, and a dextropropoxyphene compound.
Hoffman BB, Lefkowitz RJ. Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 9th edn. New York: McGraw-Hill; 1996. p. 199–248.
Horowitz RS, Dart RC, Gomez HF. Clinical ergotism with lingual ischemia induced by clarithromycin–ergotamine interaction.
Hoskin KL, Kaube H, Goadsby PJ. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine: a c-Fos and electrophysiological study.
Hoyer D. Functional correlates of serotonin 5-HT1 recognition sites. [Review].
Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Meylcharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). [Review].
Ibraheem JJ, Paalzow L, Tfelt-Hansen P. Low bioavailability of ergotamine tartrate after oral and rectal administration in migraine sufferers.
International Headache Society Committee on Clinical Trials in Migraine. Guidelines for controlled trials of drugs in migraine. 1st edn.
Johnston BM, Saxena PR. The effect of ergotamine on tissue blood flow and the arteriovenous shunting of radioactive microspheres in the head.
Kinnunen E, Erkinjuntti T, Farkkila M, Palomaki H, Porras J, Teirmaa H, et al. Placebo-controlled double-blind trial of pirprofen and an ergotamine tartrate compound in migraine attacks.
Kramer MS, Matzura-Wolfe D, Polis A, Getson A, Amaraneni PG, Solbach MP, et al. A placebo-controlled crossover study of rizatriptan in the treatment of multiple migraine attacks.
Le Jeunne C, Pascual-Gomez J, Pradalier A, Titus i Albareda F, Joffroy A, Liano H, et al. Comparative efficacy and safety of calcium carbasalate plus metoclopramide versus ergotamine tartrate plus caffeine in the treatment of acute migraine attacks.
Lewis PJ, Barrington SF, Marsden PK, Maisey MN, Lewis LD. A study of the effects of sumatriptan on myocardial perfusion in healthy female migraineurs using (NH3)-N-13 positron emission tomography.
Leysen JE, Gommeren W. In vitro receptor binding profile of drugs used in migraine. In: Amery WK, Van Nueten JM, Wauquier A, editors. The pharmacological basis of migraine therapy. London: Pitman; 1984. p. 255–66.
Leysen JE, Gommeren W, Heylen L, Luyten WH, Van de Weyer I, Vanhoenacker P, et al. Alniditan, a new 5-hydroxytryptamine1D agonist and migraine-abortive agent: ligand-binding properties of human 5-hydroxytryptamine1Dα, human 5-hydroxytryptamine1Dβ, and calf 5-hydroxytryptamine1D receptors investigated with [3H]-5-hydroxytryptamine and [3H]alniditan.
Liaudet L, Buclin T, Jaccard C, Eckert P. Drug points: severe ergotism associated with interaction between ritonavir and ergotamine.
Limmroth V, Kazarawa S, Fritsche G, Diener HC. Headache after frequent use of serotonin agonists zolmitriptan and naratriptan [letter].
Lipton RB, Stewart WF, Edmeads J, Sawyer J. Clinical utility of a new instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) score [abstract].
MaassenVanDenBrink A, Reekers M, Bax WA, Ferrari MD, Saxena PR. Coronary side-effect potential of current and prospective antimigraine drugs.
Maier HW. L'ergotamine, inhibiteur du sympathique etudie en clinque, comme moyen d'exploration et comme agent therapeutique.
Mathew NT, Stubits E, Nigam MP. Transformation of episodic migraine into daily headache: analysis of factors.
Moir JC. Ergot: from `St. Anthony's Fire' to isolation of its active principle, ergometrine (ergonovine).
Müller-Schweinitzer E. Ergot alkaloids in migraine: is the effect via 5-HT receptors? In: Olesen J, Saxena PR, editors. 5-Hydroxytryptamine mechanisms in primary headaches. New York: Raven Press; 1992. p. 297–304.
Müller-Schweinitzer E, Weidmann H. Basic pharmacological properties. In: Berde B, Schild HO, editors. Ergot alkaloids and related compounds. Handbook of experimental pharmacology, Vol. 49. Berlin: Springer-Verlag; 1978. p. 87–232.
Multinational Oral Sumatriptan and Cafergot Comparative Study Group. A randomized, double-blind comparison of sumatriptan and Cafergot in the acute treatment of migraine.
Østergaard JR, Mikkelsen E, Voldby B. Effects of 5-hydroxytryptamine and ergotamine on human superficial temporal artery.
Peroutka SJ. Drugs effective in the therapy of migraine. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 9th edn. New York: McGraw-Hill; 1996. p. 487–502.
Peters GA, Horton BT. Headache: with special reference to the excessive use of ergotamine preparations and withdrawal effects.
Pfaffenrath V, Cunin G, Sjonell G, Prendergast S. Efficacy and safety of sumatriptan tablets (25mg, 50mg, and 100mg) in the acute treatment of migraine: defining the optimum doses of oral sumatriptan.
Pradalier A, Rancurel G, Dordain G, Verdure L, Rascol A, Dry J. Acute migraine attack therapy: comparison of naproxen sodium and an ergotamine tartrate compound.
Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: appropriate use of ergotamine tartrate and dihydroergotamine in the treatment of migraine and status migrainosus.
Reches A, Eletriptan Steering Committee. Comparison of the efficacy, safety and tolerability of oral eletriptan and Cafergot[nabla] for the acute treatment of migraine [abstract].
Ryan RE. Double-blind clinical evaluation of the efficacy and safety of ergostine–caffeine, ergotamine–caffeine and placebo in migraine headache.
Sanders SW, Haering N, Mosberg H, Jaeger H. Pharmacokinetics of ergotamine in healthy volunteers following oral and rectal dosing.
Sargent JD, Baumel B, Peters K, Diamond S, Saper JR, Eisner LS, et al. Aborting a migraine attack: naproxen sodium V ergotamine plus caffeine.
Saxena PR, Cairo-Rawlins WI. Presynaptic inhibition by ergotamine of the responses to cardioaccelerator nerve stimulations in the cat.
Saxena PR, Ferrari MD. Pharmacology of antimigraine 5-HT1D receptor agonists.
Swedish Medicines Agency. Sumatriptan versus cafergot suppositories. [Swedish]. 1978. [cited 1999 Aug 27]. Available from: URL: http://www.mpa.se/sve/mono/imig.shtml
Tfelt-Hansen P, Johnson ES. Ergotamine. In: Olesen J, Tfelt-Hansen P, Welch KM, editors. The headaches. New York: Raven Press; 1993. p. 313–22.
Thoms H. John Stearns 1808 Account of the pulvis parturiens, a remedy of quickening childbirth.
Treves TA, Streiffler M, Korczyn AD. Naproxen sodium versus ergotamine tartrate in the treatment of acute migraine attacks.
Villalón CM, De Vries P, Rabelo G, Centurión D, Sánchez-López A, Saxena PR. Canine external carotid vasoconstriction to methysergide, ergotamine and dihydroergotamine: role of 5-HT1B/1D receptors and α2-adrenoceptors.
Visser WH, Burggraaf J, Muller LM, Schoemaker RC, Fowler PA, Cohen AF, et al. Pharmacokinetic and pharmacodynamic profiles of sumatriptan in migraine patients with headache recurrence or no response.
Visser WH, Jaspers NM, de Vriend RH, Ferrari MD. Risk factors for headache recurrence after sumatriptan: a study in 366 migraine patients.
Visser WH, de Vriend RH, Jaspers NM, Ferrari MD. Sumatriptan in clinical practice: a 2-year review of 453 migraine patients.
von Storch TJ. Complications following the use of ergotamine tartrate.
Weiller C, May A, Limmroth V, Jüptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks.
Wolfson WQ, Graham JR. Development of tolerance to ergot alkaloids in a patient with unusually severe migraine.