-
PDF
- Split View
-
Views
-
Cite
Cite
Saga Johansson, I-Hui Lee, Lars Olson, Christian Spenger, Olfactory ensheathing glial co-grafts improve functional recovery in rats with 6-OHDA lesions, Brain, Volume 128, Issue 12, December 2005, Pages 2961–2976, https://doi.org/10.1093/brain/awh644
Close - Share Icon Share
Abstract
Olfactory ensheathing cells (OEC) transplanted to the site of a spinal cord injury can promote axonal sparing/regeneration and functional recovery. The purpose of this study was to investigate if OEC enhance the effects of grafted dopamine-neuron-rich ventral mesencephalic tissue (VM) in a rodent model of Parkinson's disease. We co-grafted VM with either OEC or astrocytes derived from the same olfactory bulbs as the OEC to rats with a unilateral 6-hydroxydopamine lesion of the nigrostriatal system. Co-grafting fetal VM with OEC, but not with astrocytes enhanced dopamine cell survival, striatal reinnervation and functional recovery of amphetamine- and apomorphine-induced rotational behaviour compared with grafting embryonic VM alone. Grafting OEC or astrocytes alone had no effects. Intriguingly, only in the presence of OEC co-grafts, did dopamine neurons extend strikingly long neurites that reached peripheral striatal compartments. Comparable results were observed in a co-culture system where OEC promoted dopamine cell survival and neurite elongation through a mechanism involving both releasable factors and direct contact. Cell type analysis of fetal VM grafts suggested that dopamine neurons of the substantia nigra rather than of the ventral tegmental area were increased in the presence of OEC co-grafts. We conclude that the addition of OEC enhances efficacy of grafted immature dopamine neurons in a rat Parkinson's disease model.
Introduction
Parkinson's disease is a progressive neurodegenerative disorder. A cardinal feature is degeneration of dopamine neurons projecting from substantia nigra (SN) to striatum. To date, there is no cure and the causes are largely unknown, although there is increasing evidence for causative genetic factors in familial forms and genetic susceptibility factors in idiopathic forms. Grafting fetal ventral mesencephalic tissue (VM) to the adult dopamine-depleted striatum can partly restore dopamine innervation and counteract functional deficits in animal models of the disorder (Björklund and Stenevi, 1979; Perlow et al., 1979; Dunnett et al., 1997), and in some patients with severe Parkinson's disease (Lindvall et al., 1988; Björklund et al., 2003). Dopamine cell transplantation protocols have been developed and refined in several laboratories but remain experimental (Lindvall and Hagell, 2000). The success of transplantations as a clinical approach is hampered by limited availability of embryonic tissue, limited graft survival, restricted dopaminergic reinnervation of striatum, suboptimal functional effects and, sometimes, negative side-effects (Freed et al., 2001).
Low cell survival can be partially counteracted by neuroprotective (Brundin et al., 2000; Nakao et al., 1994) or neurotrophic agents (Hurelbrink and Barker, 2001; Fernandez-Espejo, 2004) but even when cell survival is improved, reinnervation appears to remain insufficient (Barker et al., 1996; Strömberg and Bickford, 1996). It has been suggested that glial cells play key roles in survival and function of the grafted neurons. Immature mesencephalic glial cells that accompany immature grafted dopamine neurons in most experimental grafting protocols, as well as adult host striatal glial cells, may influence survival and fibre outgrowth from grafted neuroblasts (Schwab and Thoenen, 1985; Gates et al., 1993; Moon et al., 2001; Bradbury et al., 2002; Morgenstern et al., 2002). Olfactory ensheathing cells (OEC) are unique in supporting continuous olfactory nerve fibre growth from the olfactory mucosa into the CNS throughout adulthood (Graziadei and Monti Graziadei, 1980; Barber, 1982; Barber and Lindsay, 1982; Doucette et al., 1983; Doucette, 1984). Several studies have suggested a therapeutic potential of OEC grafted to the injured spinal cord (Li et al., 1997; Ramon-Cueto et al., 1998, 2000; Lu et al., 2001). Denis-Donini and Estenoz (1988) have also observed extraordinary neurite outgrowth from nigral dopamine neurons while co-cultured with OEC.
To determine if OEC improves effects of embryonic dopamine neurons grafted to the adult dopamine-depleted striatum, we implanted single grafts of fetal VM, OEC or astrocytes derived from the same source as the OEC, and compared the outcome of these three types of single grafts to double grafts containing VM + OEC or VM + astrocytes. We show that OEC, but not astrocytes, increase survival of grafted dopamine neurons, enhance dopaminergic reinnervation of host striatum and promote functional recovery compared with grafting embryonic VM alone. Grafts consisting of OEC or astrocytes alone have no effects. Using an in vitro system we further demonstrate that OEC are able to promote dopamine cell survival and neurite elongation through releasable factors.
Materials and methods
6-Hydroxydopamine (6-OHDA) lesions
Adult female Sprague–Dawley rats (Scanbur, Sollentuna, Sweden) were kept on a 12:12-h day:night cycle with free access to food pellets and water. Experiments were approved by the Animal Research Ethics Committee of Stockholm.
Unilateral lesions were performed by stereotaxic injection of 6-OHDA (Sigma, Sweden: 2 μg/μl in 0.9% NaCl containing 0.2 mg/ml ascorbic acid). The rats (5-week-old, 150 g body wt) were anesthetized with halothane and positioned in a stereotaxic frame. Injections into the medial forebrain bundle of the nigrostriatal pathway (coordinates 4.4 mm posterior and 1.2 mm lateral to bregma and 7.8 mm below the dural surface) were performed using a micropump connected to a 10 μl Hamilton syringe and at a rate of 1 μl/min with a total volume of 4 μl, totalling 8 μg of 6-OHDA/animal. The syringe was withdrawn 2 min after the injection was completed. Animals received subcutaneous injections of buprenorphine (Temgesic, 0.3 mg/kg) every twelfth hour during the first 2 days after surgery to reduce pain.
Rotational behaviour
Amphetamine- and apomorphine-induced rotational behaviour was used to confirm completeness of the lesion and to determine effects of engraftment protocols. Rats were placed in plastic rotometer bowls and connected to a computerized system registering the number of turns. After 10 min, when spontaneous rotational behaviour had ceased, amphetamine (2 mg/kg intraperitoneally, diluted in 0.9% NaCl) or apomorphine (0.05 mg/kg subcutaneously in the flank region, diluted in 0.9% NaCl) was injected. Rotational behaviour was followed for another 70 (apomorphine) or 90 (amphetamine) min. Rotational tests were carried out 2 and 4 weeks post-lesion. Amphetamine, which releases dopamine, was used to detect unilateral losses of dopamine fibres in the form of ipsilateral rotations. Since it is difficult to determine completeness of a striatal dopamine denervation by amphetamine (Hefti et al., 1980a, b; Heikkila et al., 1981; Casas et al., 1988; Carman et al., 1991; Hudson et al., 1993; Moore et al., 2001) we also used the D2 receptor agonist apomorphine, which causes contralateral rotations as a way to determine completeness of the 6-OHDA lesions in living animals. To ensure selection of well denervated animals, care was taken to select only animals with at least 7.0 turns/min after amphetamine injection and that responded to apomorphine with totally >450 turns and displayed the characteristic two-peak rotation curve known to be seen only in completely or almost completely denervated animals (Herrera-Marschitz and Ungerstedt, 1984). Behavioural recovery was assessed by amphetamine-induced rotations 2, 3 and 7 weeks after grafting, and by apomorphine-induced rotations 8 weeks after grafting.
OEC and astrocyte cultures
Primary OEC and astrocyte cultures were prepared from the olfactory bulb of adult female Sprague–Dawley rats (5-week-old, 150 g body wt, Scanbur, Sollentuna, Sweden) based on a previously described method (Nash et al., 2001). Isolation of the different types of glia was accomplished by the different rates of cell attachment. Briefly, animals were anaesthetized with isoflurane and decapitated. The olfactory bulbs were removed and transferred into Hank's balanced salt solution (HBSS, Sigma). After removal of the meninges and vessels, the medioventral superficial aspects of the bulbs (mainly olfactory nerve and glomerular layer) were collected, minced, and incubated with 0.1% trypsin at 37°C for 10 min. The trypsination was stopped by addition of culture medium: Dulbecco's modified Eagle's medium (DMEM)/Ham's/F-12 (1:1 mixture, Sigma, St Louis, MO) supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine and penicillin (100 U/ml, Gibco), streptomycin (100 μg/ml, Gibco), amphotericin B (1.75 μg/ml, Gibco). Following two washes in the medium, cells were resuspended, triturated by fire-polished glass Pasteur pipettes, and plated on uncoated Petri dishes. Many fibroblasts attach to the Petri dishes during this first 18-h incubation period (37°C, 5% CO2). The supernatants, enriched for astrocytes and OEC, were transferred to an uncoated culture flask and incubated for another 36 h to allow for the attachment of astrocytes. OEC do not attach to uncoated surfaces for 96–120 h, therefore remain in the supernatant, which was then transferred onto poly-D-lysine-coated (PDL, 0.1 mg/ml, Sigma) culture flasks. OEC and astrocytes were allowed to grow to confluency in 10–14 days on PDL-coated and uncoated flasks, respectively. On the day of transplantation, the cells were trypsinized with 0.25% trypsin for 3 min at 37°C. The detached cells were transferred to DMEM containing FBS and centrifuged at 2800 r.p.m. for 8 min. After washing, the cell pellet was resuspended in 1.5 ml DMEM and the cells were counted. Finally the cells were centrifuged at 6000 r.p.m. for 7.5 min before the volume was adjusted to the appropriate cell concentration for transplantation. More flasks of astrocyte cultures had to be harvested per graft because less number of cells survived on the uncoated surface.
Analysis of OEC and astrocyte cultures
To determine purity of OEC and astrocytes in the primary cultures, additional cell cultures were plated onto 2-well glass chamber slides (PDL-coated and uncoated, respectively; LabTek, Naperville IL) and processed for immunohistochemistry. Following a week in culture, cells were fixed for 20 min with 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) containing 0.4% picric acid, washed several times in PBS, and preincubated in blocking solution containing 10% donkey serum (Sigma) and 0.3% Triton X-100 in PBS for 1 h at room temperature. Triple staining was performed using primary antibodies raised against the low affinity neurotrophin receptor p75 (p75, mouse, 1:300, Abcam), glial fibrillary acidic protein (GFAP; chicken, 1:50, Abcam), and fibronectin (rabbit, 1:100, Chemicon) diluted in blocking solution. Following an overnight incubation at 4°C, cells were washed three times in PBS and incubated with Cy3/Cy2/Cy5 conjugated secondary antibodies (1:300/1:50/1:50, Jackson ImmunoResearch Lab.) diluted in blocking solution for 1 h at room temperature. Slides were coverslipped with mounting medium containing DAPI (Vectashield/4,6-diamidino-2-phenylindole, Vector, Burlingame, CA) before microscopy. The purity of the cell cultures was determined by counting the immunoreactive cell types attached to the slides (Fig. 1). Cells with p75 immunoreactivity were designated OEC, these cells can also be diffuse GFAP or weak fibronectin immunoreactive, as has been reported previously (Ramon-Cueto and Nieto-Sampedro, 1992; Nash et al., 2001); cells labelled strongly by fibronectin-immunohistochemistry alone were designated fibroblasts/meningeal cells; GFAP+ but p75-negative cells were designated astrocytes. Five sample areas per well and six wells totally at 20× magnification were used to calculate the average percentage of each cell type. Using this protocol, out of the cell types that could be observed, we obtained 43% astrocytes, 56% fibroblasts and 1% OEC in the astrocyte culture and 44% OEC, 51% fibroblasts and 5% astrocytes in the OEC culture. Thus fibroblasts/meningeal cells are abundant and present in the same relative amounts in both types of cultures, while the two types of cell preparations are relatively pure with respect to containing either OEC or astrocytes. While fibroblasts/meningeal cells together with OEC have been reported beneficial in spinal cord injury (Li et al., 1998; Lakatos et al., 2003b), these cells do not appear critical for the different results obtained in the present in vivo experiments.
Examples of cell types found in OEC cultures from the adult olfactory bulb. Confocal images of triple-labelled cell cultures (A) and (E). As expected, many cells display p75 immunoreactivity located in a Golgi-like perinuclear pattern and as puncta in the cell membrane (B) and (F). Other cells have strong fibrillar GFAP immunoreactivity typical of astrocytes (C). OEC can be weakly GFAP + (C) or fibronectin + (D). Cells characterized by fibronectin immunoreactivity, presumably fibroblasts/meningeal cells (H) are also found. Circle = OEC, asterisk = astrocyte, square = fibroblast/meningeal cell. Scale bar = 50 μm.
Transplantations
Animals were anaesthetized with halothane. A stereotaxic frame was used for implantation. VM from fetuses was dissected in DMEM from embryonic day 14.5. The tissue from 20 fetuses was pooled and trypsinated in 100 μl 0.5% trypsin for 5 min in 37°C. After addition of 1.4 ml DMEM with 10% FBS the tissue was centrifuged at 1000 r.p.m. for 5 min. The cell pellet was washed with DMEM, centrifuged at 2000 r.p.m. for 2 min, resuspended in 500 μl DMEM and mechanically dissociated by trituration (10–20 strokes) using a Pasteur pipette, and finally a 23G needle. Thereafter the cells were counted in a Bürker chamber and the cell suspension was centrifuged for 7.5 min at 6000 r.p.m.. The cell pellet was resuspended in DMEM. For co-transplantation, the cell suspension from the cultured OEC or astrocytes was mixed with the embryonic VM suspension.
In one experiment, as illustrated in Table 1, animals received either 100 000 embryonic cells together with 200 000 OEC (OEC/VMco-grafts) or with 200 000 astrocytes (astrocyte/VM co-grafts), or 200 000 OEC alone (OEC grafts) or 200 000 astrocytes alone or 100 000 embryonic cells alone (VM grafts). In a second experiment, the number of VM cells was increased from 100 000 to 250 000, and the animals therefore received 250 000 embryonic cells plus 200 000 OEC (OEC/VM co-grafts), or only 250 000 embryonic cells (VM grafts) or only 200 000 OEC (OEC grafts).
Experimental groups
| Groupsa . | Grafted cells . | . | . | Number of grafted animals . | ||
|---|---|---|---|---|---|---|
. | VM cells . | OEC . | Astrocytes . | . | ||
| Experiment 1 | ||||||
| a | 100 000 | 15 | ||||
| b* | 200 000 | 15 | ||||
| c | 100 000 | 200 000 | 18 | |||
| d | 200 000 | 5 | ||||
| e | 100 000 | 200 000 | 8 | |||
| f** | 5 | |||||
| Experiment 2 | ||||||
| a | 250 000 | 10 | ||||
| b* | 200 000 | 10 | ||||
| c | 250 000 | 200 000 | 12 | |||
| d** | 5 | |||||
| Groupsa . | Grafted cells . | . | . | Number of grafted animals . | ||
|---|---|---|---|---|---|---|
. | VM cells . | OEC . | Astrocytes . | . | ||
| Experiment 1 | ||||||
| a | 100 000 | 15 | ||||
| b* | 200 000 | 15 | ||||
| c | 100 000 | 200 000 | 18 | |||
| d | 200 000 | 5 | ||||
| e | 100 000 | 200 000 | 8 | |||
| f** | 5 | |||||
| Experiment 2 | ||||||
| a | 250 000 | 10 | ||||
| b* | 200 000 | 10 | ||||
| c | 250 000 | 200 000 | 12 | |||
| d** | 5 | |||||
Well dopamine-denervated rats, as determined by amphetamine- and apomorphine-induced rotational behaviour, were randomly selected to six groups in Experiment 1. Similarly, such animals were randomly selected to four groups in Experiment 2.
Animals grafted with OEC from the two experiments were pooled for behavioural and histological analyses. The pooled group thus consists of 25 animals.
Sham-operated controls were pooled for behavioural and histological analyses. The pooled sham group thus consists of 10 animals.
Experimental groups
| Groupsa . | Grafted cells . | . | . | Number of grafted animals . | ||
|---|---|---|---|---|---|---|
. | VM cells . | OEC . | Astrocytes . | . | ||
| Experiment 1 | ||||||
| a | 100 000 | 15 | ||||
| b* | 200 000 | 15 | ||||
| c | 100 000 | 200 000 | 18 | |||
| d | 200 000 | 5 | ||||
| e | 100 000 | 200 000 | 8 | |||
| f** | 5 | |||||
| Experiment 2 | ||||||
| a | 250 000 | 10 | ||||
| b* | 200 000 | 10 | ||||
| c | 250 000 | 200 000 | 12 | |||
| d** | 5 | |||||
| Groupsa . | Grafted cells . | . | . | Number of grafted animals . | ||
|---|---|---|---|---|---|---|
. | VM cells . | OEC . | Astrocytes . | . | ||
| Experiment 1 | ||||||
| a | 100 000 | 15 | ||||
| b* | 200 000 | 15 | ||||
| c | 100 000 | 200 000 | 18 | |||
| d | 200 000 | 5 | ||||
| e | 100 000 | 200 000 | 8 | |||
| f** | 5 | |||||
| Experiment 2 | ||||||
| a | 250 000 | 10 | ||||
| b* | 200 000 | 10 | ||||
| c | 250 000 | 200 000 | 12 | |||
| d** | 5 | |||||
Well dopamine-denervated rats, as determined by amphetamine- and apomorphine-induced rotational behaviour, were randomly selected to six groups in Experiment 1. Similarly, such animals were randomly selected to four groups in Experiment 2.
Animals grafted with OEC from the two experiments were pooled for behavioural and histological analyses. The pooled group thus consists of 25 animals.
Sham-operated controls were pooled for behavioural and histological analyses. The pooled sham group thus consists of 10 animals.
The grafts were placed in the lower lateral part of the upper medial quadrant of striatum (coordinates 0.5 mm anterior and 2.0 mm lateral to bregma, and 5.0 mm below the dural surface). Injections were performed at a rate of 1 μl/min. The syringe was slowly withdrawn 5 min after the injection was completed to minimize back-flow. Non-grafted controls received an injection of DMEM at the same coordinates used for grafting. The animals received subcutaneous injections of buprenorphine every twelfth hour during the first 2 days after surgery to reduce pain, and they were sacrificed 8.5 weeks after transplantation. From the day prior to transplantation, the rats received a daily injection of cyclosporin A (10 mg/kg body wt, Sandimmun, diluted in Ringer's solution with an addition of 2 mg/kg doxycycline) to avoid graft rejection.
In vitro experiments
OEC cultures were seeded onto PDL-coated 3.24 cm2 cover slips placed in 6-well culture plates or onto PDL-coated membrane inserts suspended 0.8 mm above the well's bottom (4.2 cm2 with pore size 1.0 μm, Falcon). After 7 days in culture the OEC had reached 70% confluency and a cell suspension of fetal VM was added to the cover slips. The mesencephalic cell suspension was prepared as described above for transplantation, 75 000 VM cells were added to each cover slip. The cultures were divided into three groups: Group 1, VM cells that were in direct contact with OEC (n = 10); Group 2, VM cells on PDL-coated cover slips beneath a membrane with an OEC monolayer (n = 10) and Group 3, VM cells on PDL-coated cover slips with no OEC in the culture (n = 11). The culture medium was switched from DMEM with 10% FBS, to a culture medium consisting of two-thirds DMEM and one-third HBSS supplemented with 10% FBS, 1.5% glucose and 1% HEPES when VM cells were added to the cultures. The medium was changed every third day. After 8 days in culture, cover slips were fixed in 2% paraformaldehyde for 30 min, rinsed in PBS and processed for immunohistochemistry.
Immunohistochemistry
Animals were intraperitonally injected with an overdose of pentobarbital and intracardially infused with 100 ml Ca2+-free Tyrode's solution containing 0.1 ml heparin followed by 250 ml 4% paraformaldehyde in PBS containing 0.4% picric acid. After perfusion, brains were post-fixed for 1 h and rinsed in cold 10% sucrose in PBS for at least 24 h. The tissue was rapidly frozen with gaseous CO2 and 30 or 14 μm cryostat sections generated. After thawing, slides were rinsed in PBS before processing for immunohistochemistry.
Single, double or triple labelling was performed using primary antibodies raised against tyrosine hydroxylase (TH, mouse, Diasorin, 1 : 5000 or rabbit, 1 : 300, Pelfreeze) to label dopamine neurons, p75 to label OEC (mouse, 1 : 500, Abcam) or GFAP (rabbit, 1 : 100, Sigma, or goat, 1 : 50, Santa Cruz) to label astrocytes. To identify different sub-populations of TH+ neurons in the grafts, sections were double labelled with antibodies raised against TH and the G-protein-gated inwardly rectifying K+ channel subunit (Girk2, rabbit, 1 : 80, Alomone Labs, Israel) or calbindin (CB, mouse, 1 : 500, Sigma). All antibodies were diluted in 0.3 or 0.6% Triton X-100 in PBS for 14 or 30 μm sections, respectively. Incubation with primary antibodies was performed for 48 h at 4°C, and double and triple labelling was performed in a sequence such that one type of antibody was applied at a time. Between incubations, sections was rinsed thrice for 10 min in PBS. Secondary fluorescent antibodies, either conjugated with Cy3 (1 : 400, Jackson ImmunoResearch Lab.), ALEXA454 (1 : 500, Molecular Probes, USA), or Cy5 (1 : 50, Jackson ImmunoResearch Lab.) were applied for 1 h at room temperature. Finally, sections were rinsed in PBS, mounted in 90% glycerol in PBS and coverslipped. The 30 μm thick sections were processed for stereology and histology by pretreatment with 2 M HCl for 30 min in 37°C and rinsing in PBS before incubation. Sections processed for stereology were single labelled and were, after incubation with primary antibodies, incubated with biotinylated anti-mouse secondary antibodies (1 : 200 in 0.6% Triton X-100 in PBS, Vector, USA) at room temperature for 60 min, followed by incubation with an avidin– biotinylated-peroxidase complex (Vectastain ABC kit, Vector, USA, diluted 1 : 100 in 0.6% Triton X-100 in PBS) for 2 h at room temperature. The substrate of 3,3′-diaminobenzidine (DAB; 0.7 mg/ml, Sigma) was used as a chromogen in the presence of 0.01% H2O2. Finally, sections were washed in PBS and dehydrated in alcohol and xylene before mounting with Entellan® (Merck).
Cultures were double or triple labelled with antibodies against TH, GFAP and p75. The staining protocol used was the same as for 14 μm slides except that the antibodies were diluted in 0.6% Triton X-100.
Image analysis
Histological evaluation of co-localization of the different cell types in intrastriatal grafts and cultures were performed using multi-tract scanning with confocal microscopy (Zeiss LSM 510 Meta).
Quantification of different sub-populations of dopamine neurons
The majority of dopamine neurons in the ventral tegmental area (VTA) are small rounded cells that co-express TH and CB, but not Girk2. The dopamine neurons in SN on the other hand are typically larger cells with an elongated morphology, and the majority of the nigral TH+ neurons express Girk2, but not CB (Mendez et al., 2005; Thompson et al., 2005). Counting of TH+/Girk2+ and TH+/CB+ cells was performed on digital images (AxioVison image software) of sections at the level of + 0.2 mm with respect to bregma. Sections from five randomly selected animals from each sub-group from the first experiment (Group 1a–f, Table 1) were analysed. The cell diameter was also measured on all counted neurons, and the position within the graft (central versus peripheral) was also noted.
Stereology
Numbers of TH+ neurons in intrastriatal grafts, lengths of TH-immunoreactive fibres in striatum and numbers of TH+ neurons within the lesioned SN were determined using computerized image analysis (Nikon microscope and Stereologer™, SPA inc.). The software utilizes a stereological three-level fraction-based sampling design based on the fractionator sampling method (Gundersen, 1986). Every fifth section throughout the transplants [section sampling fraction (ssf) = 1/5] and every tenth section (ssf = 1/10) of the ∼1.8 mm long rostro–caudal distribution of the SN (between 4.5 and 6.30 mm posterior to bregma) were systematically sampled using the ‘optical fractionator’ after randomly selecting the first section within the first interval. A rectangular counting frame, ‘the dissector’, with known area was superimposed on the field of view by the computer software, and then the numbers of cell nuclei that come into focus within the height of the optical dissector was counted. Counting frames were systematically distributed with known x and y steps throughout the marked region from a random starting point. The area of the counting frame relative to the area associated with the x and y steps gives the second fraction [area sampling fraction (asf)]. The height of the optical dissector relative to the thickness of the section results in the third fraction [thickness (t)/height (h)]. The total number of neurons is given by Ntotal = ∑Q− (1/ssf) (1/asf) (t/h), where Q− is the number of neurons counted in the dissectors. Optical fractionator estimates are free of assumptions about cellular shape and size and are unaffected by tissue shrinkage.
The area of the intrastriatal graft and the SN was manually outlined using a 10× lens. The area of SN was defined based on neuro-anatomical landmarks, and the position of TH+ neurons on the contralateral intact side. Cell counts were performed with a ×60 lens (numerical aperture = 1.4). For cell counts of the intrastriatal graft, eight animals from each sub-group from the first and second experiment were randomly chosen for stereology with exception of the VM/astrocytes co-grafted and astrocytes grafted group in which intrastriatal cell counts were performed on all eight and five rats, respectively. The measured sections were randomly selected and coded. Cell counts in SN were performed on sections from five randomly selected animals from each treatment sub-group from the first experiment (Group 1a–f, Table 1).
The total length of TH-immunoreactive fibres innervating defined volumes of striatum was measured by superimposing a virtual 3D-probe in the shape of a sphere with a diameter of 10 μm in the rectangular dissector (Mouton et al., 2002; Hofstetter et al., 2005, for further illustration of the stereology method, see supplementary Fig. 1). Fibres that traverse the virtual sphere surface at any angle were counted at the level of +0.2 mm with respect to bregma (Paxinos and Watson). Again, the counting frames, with the virtual spheres, were distributed with known x and y steps throughout the marked region. The total length of fibres/volume is given by Ltotal = 2 · ∑Q− · (v/a) · (1/ssf) · (1/asf) · (t/h), where (v/a) is the volume of the dissector relative to the surface area of the probe and Q− is the number of fibres transversing the probe surface. The length of TH+ fibres was counted in two volumes; the entire caudate-putamen (CPu) on the lesioned side, excluding the area of the graft, to estimate the total amount of fibres in striatum, and the area of CPu most lateral to the ventricle to calculate the amount of fibres furthest away from the graft (illustrated in Fig. 2G). The area of the entire CPu was defined by the borders to the lateral ventricle, corpus callosum, external capsule and the ventral pallidum. The area of the CPu most distal to the graft was determined by the border between CPu and the external capsule and the swelling of the olfactory tubercle (Fig. 2G). This corresponds approximately to the area of CPu between 3.8 and 4.8 mm lateral to bregma. The areas were outlined using a ×4 lens and fibre length was measured with a ×100 lens (numerical aperture = 1.4).
Effect of co-grafting VM with OEC or astrocytes on drug-induced rotational behaviour, survival of dopamine neurons and length of TH-positive fibres in host striatum. Grafting VM cells caused significant reduction of amphetamine- (A) and (C) and apomorphine-induced (B) and (D) rotations (expressed in percentage of pre-grafting values) in all animals. When OEC were mixed with 100 000 VM cells, there was a significant further decrease of both amphetamine- and apomorphine-induced rotations at all time points compared to animals given only 100 000 VM cells or the same number of VM cells mixed with astrocytes, or grafted with OEC or AC alone (A) and (B). When OEC were mixed with 250 000 VM cells there was a significantly larger reduction of amphetamine-induced rotations compared to rats given 250 000 VM cells alone 2 weeks after transplantation (C, D). No such effect of OEC mixed with 250 000 VM cells on amphetamine-induced rotations were seen at later time points; apomorphine-induced rotations were not influenced at any time point (C) and (D). The host SN on the denervated side contained a few percent of the original population of TH+ dopamine neurons. There were no significant differences between the different groups in numbers of surviving TH+ neurons (expressed in percentage of intact contralateral side) in the lesioned SN (E). The number of surviving grafted TH+ neurons was significantly higher in animals in which OEC and 100 000 VM cells were grafted together, compared to groups co-grafted with astrocytes or given 100 000 VM cells alone (F). Increasing the number of grafted VM cells to 250 000 doubled the number of surviving grafted TH+ neurons, but there was no significant difference in cell survival between the group grafted with 250 000 VM cells alone and the group co-grafted with OEC and 250 000 VM cells (F). The amount of TH+ fibres was quantified in the entire CPu on the lesioned side (grey and blue area) and in the distal lateral part of CPu (blue area) in (G). Both the total amount of TH+ fibres in the striatal volume (H) and the amount of TH+ fibres in the lateral part of the host CPu (I) was significantly increased in the group co-grafted with OEC and 100 000 VM cells compared to animals with single VM grafts or co-grafted with astrocytes and 100 000 VM cells. Similarly, co-grafting OEC with 250 000 VM cells significantly increased both the total (H) and lateral (I) amount of TH+ fibres in striatum compared to grafting 250 000 VM cells alone. CPu = caudate-putamen; ec = external capsule; LV = lateral ventricle; Tu = olfactory tubercle; AC = astrocytes. Error bars ± SEM, *P < 0.05, **P < 0.01, ***P < 0.005.
In vitro cell counts and morphometric analysis
Quantification of TH-immunoreactive neuron numbers in cell culture was performed on digital images (AxioVision image software) on 10 cover slips from each group. The cover slip was divided into an 81 square grid; each individual square had an area of 4 mm2. To obtain cell counts the numbers of neurons in 18 squares were counted in a randomized fashion and the total number of neurons on the cover slip was calculated. For quantative morphometry the following parameters were investigated for 30 randomly chosen individual TH+ neurons in each group: number of primary neurites, total number of branch points, and length of the longest neurite.
Statistical analysis
Statistical analysis of rotational data, cell counts, fibre length and morphometric results were performed on mean differences in the treatment groups using one-way analysis of variance (ANOVA) followed by Tukey–Kramer post hoc analysis.
Results
Amphetamine- and apomorphine-induced rotational behaviour
Significant reduction of amphetamine- and apomorphine-induced rotations was seen in all groups that received fetal VM grafts, either alone or in combination with OEC or astrocytes. Reductions were seen at all time points investigated. In the first experiment, when rats where grafted with 100 000 cells derived from fetal VM, OEC/VM co-grafted rats showed a 78% reduction in amphetamine-induced rotations while animals given single VM grafts showed a 55% reduction in amphetamine-induced rotation (P < 0.01) at 7 weeks post-grafting (Fig. 2A and B). Generally, rats with OEC/VM co-grafts demonstrated a significantly larger decrease in both amphetamine and apomorphine-induced rotations compared with animals with astrocyte/VM co-grafts at all times. No significant reduction of rotational behaviour compared with the situation prior to transplantation was observed in animals that received a single astrocyte graft or no graft at all. However, animals that received a single OEC graft showed a modest, but significant reduction (−8%; P < 0.05, Fig. 2A) of amphetamine-induced rotational behaviour 7 weeks after transplantation, but no reduction in the number of apomorphine-induced rotations 8 weeks after transplantation was apparent (Fig. 2B).
When we increased the number of grafted embryonic cells 2.5-fold in the second experiment, a significantly improved reduction of amphetamine-induced rotational behaviour caused by the addition of OEC was seen at 2 weeks after transplantation compared with animals with VM grafts alone (37 and 20% decrease in number of rotations, respectively; P < 0.05, Fig. 2C). However, the difference between OEC/VM co-grafted and VM-grafted animals diminished and was no longer significant at 3 weeks (57 and 52% reduction in rotations, respectively) and 7 weeks (88 and 83% reduction in rotations, respectively) post-grafting due to a ceiling effect. Similarly, co-grafting of OEC with 100 000 or 250 000 VM cells gave a very similar maximal reduction in amphetamine-induced (79 and 88%, respectively) or apomorphine-induced (76 and 79%, respectively, Fig. 2A and C, and B and D, respectively) rotation. However, a significant difference was observed between animals that received single VM grafts of either 100 000 or 250 000 cells (reduction of amphetamine-induced rotation at 7 weeks 55% compared with 83%, P < 0.01; reduction of apomorphine-induced rotation at 8 weeks 44% compared with 76%, P < 0.05).
Graft analysis—general observations
Histological analysis 8 weeks post-grafting revealed viable grafts in striatum in all grafted brains. Immunohistochemistry demonstrated surviving TH+ neurons, GFAP+ astrocytes and p75+ OEC as expected in the respective grafts (Figs. 3 and 5A and B). TH+ neurons with different sizes were observed within all grafts with VM cells. TH+ neurons with large elongated cell bodies were mainly located at the periphery of the grafts and were observed in single VM grafts and co-grafts with OEC or in co-grafts with astrocytes whereas TH+ neurons with smaller rounded cell bodies were mainly located in the centre of the grafts and were observed in single VM grafts and co-grafts with OEC or astrocytes. In OEC co-grafts, small TH+ neurons were also located in the periphery of the graft.
Histology of intrastriatal grafts. All pictures show TH-immunoreactivity except (D) and (L) (p75) and (I) (GFAP). The boxed areas in (A), (E) and (I) are shown in higher magnification in (B), (F) and (J), respectively. Grafts with VM cells had surviving TH+ neurons and TH+ fibres reinnervating the host striatum (A)–(C), (E)–(G), (I) and (J). Animals co-grafted with OEC and 100 000 VM cells (A)–(C) had a more extensive TH+ reinnervation compared to animals grafted with 100 000 VM cells alone (E)–(G) or co-grafted with astrocytes and 100 000 VM cells (I) and (J). There was a striking difference between the amount of TH+ fibres in the peripheral part of striatum in animals co-grafted with OEC (C) and animals with single VM grafts (G). No TH+ fibres were observed at the transplantation site in animals grafted with OEC alone (H). Immunohistochemical labelling of p75 demonstrated surviving OEC in animals grafted with OEC alone (L), shows the area corresponding to panel (H) as well as in OEC/VM co-grafts (D). No p75+ cells were found in non-grafted controls or animals grafted with VM alone, and on rare occasions a few p75+ cells were present in animals grafted with astrocytes alone or co-grafted with astrocytes and VM (data not shown). Animals grafted with astrocytes alone had an intense GFAP+ labelling of the graft area (K). The illustration in (M) shows the location of the graft versus pictures of the peripheral striatum (shown in C and G). AC = astrocytes; LV = lateral ventricle; asterisk = border between cortex and striatum. Scale bars: (A), 300 μm; (E) and (I), 300 μm; (B)–(D), (F)–(H) and (J)–(L), 300 μm.
A dense TH+ fibre network was present within the VM grafts and also extended to variable distances into host striatum (Fig. 3A–C, E–G, I and J). In animals with OEC co-grafts dopamine neurons gave rise to an extensive TH+ fibre network reaching as far as the lateralmost periphery of the striatum (Fig. 3C). This peripheral fibre network though was less dense than that close to the graft site. Only a few, scattered TH+ fibres could be observed in peripheral regions of striatum in animals with single VM grafts and co-grafts with astrocytes (Fig. 3G). As expected, groups grafted with 250 000 VM cells had a more dense TH+ fibre outgrowth compared with groups grafted with 100 000 VM cells. Also, no TH+ cells were observed in animals grafted with astrocytes alone, OEC alone (Fig. 3H) or in non-grafted, sham-injected controls. Neither were there any TH+ fibres observed within the respective graft areas. However, a very small number of TH-immunoreactive fibres, presumably residual fibres spared from the lesion, were occasionally seen randomly scattered in striatum in all groups. These fibres reflect the very low number (1–6%) of remaining dopamine neurons found in SN.
Olfactory ensheathing cells were identified by p75 immunohistochemistry. Co-grafted OEC were present both in the centre and the periphery of the graft (Figs. 3D and 5A). No significant migration of OEC into host striatum was observed in either single grafts (Fig. 3L) or co-grafts (Fig. 3D and 5A). However, it cannot be excluded that some OEC, either inside or outside the graft area, had become p75-negative and thus were not detected. No p75+ cells were found in host striatum in animals grafted with VM alone or in non-grafted controls. A few p75+ cells were occasionally found at the graft site in some animals with a single astrocyte graft or astrocyte/VM co-graft.
Single grafts and co-grafts with astrocytes were labelled with GFAP antibodies. Due to the general and widespread presence of astrocytes in all brain tissues, the origin of GFAP+ cells is not easily determinable. However, single astrocyte grafts (Fig. 3K) were more intensely GFAP+ than areas surrounding the injection tracts in sham-operated controls. Single OEC and VM grafts and co-grafts of OEC with VM were associated with less GFAP+ immunoreactivity than astrocyte/VM co-grafts.
Girk2 and CB immunoreactivity of grafted dopamine neurons
To further characterize TH+ neurons in the grafts we used Girk2 and CB immunoreactivity. Analysis of the number of Girk2+ and CB+ cells in the grafts showed that co-grafting VM with OEC significantly increased the percentage of TH+ neurons that express Girk2 (VM/OEC co-grafts: 69.3%; 68 ± 3 TH+/Girk2+ cells of 98 ± 3 TH+ cells; P < 0.01; VM/astrocyte co-grafts: 48.1%; TH+/Girk2+ cells 37 ± 2 TH+/Girk2+ cells of 74 ± 2 TH+ cells; single VM grafts: 49.6%; 39 ± 2 TH+/Girk2+ cells of 78 ± 2 TH+ cells). The percentage of TH+/CB+ neurons did not significantly differ within different groups (an average of 27 ± 2% of TH+ cells were also CB+ in all groups). The vast majority of TH+/Girk2+ neurons were large cells with a mean diameter of ∼18 μm. These cells were located at the periphery of the graft (Fig. 4A–C). In OEC/VM co-grafts, however, there were also smaller TH+/Girk2+ cells with a diameter between 13 and 15 μm. Most of the TH+/CB+ neurons were small rounded cells with an average diameter of ∼13 μm and located in the centre of the grafts (Fig. 4D–F).
Sub-populations of dopamine neurons in VM grafts. To identify sub-populations of VM dopamine cells, sections were double labelled for TH and Girk2 or CB, which preferentially label SN or VTA dopamine neurons, respectively. Adjacent sections of an OEC/VM co-graft are shown in (A) and (D). The boxed areas in (A) and (D) are shown at higher magnification in (B) and (C) and (E) and (F), respectively. TH+/Girk2+ dopamine neurons cells were located primarily in the peripheral part of the graft area (B) and (C) whereas TH+/CB+ neurons were mainly located at the core of the graft (E) and (F). No or few TH−/Girk2+ cells were observed in the grafts. A population of TH−/CB+ cells were also observed in the grafts (E) and (F). Scale bars: (A, D) 400 μm, (B), (C), (E), (F) 200 μm.
Co-localization of OEC and TH+ neurons in co-grafts
Sections triple labelled for TH, GFAP and p75 were studied by confocal microscopy to evaluate possible co-localization and cell–cell contacts among the grafted cells. OEC were found in close contact with TH+ cell bodies and fibres within the graft (Fig. 5A and B). More TH-immunoreactive fibres growing into the host brain were observed to emerge from OEC rich areas. Interestingly, there was no detectable migration of p75+ OEC outside the graft area, and therefore no contact between TH-positive fibres innervating striatum and OEC were observed. However, some OEC may lose their p75 immunoreactivity after grafting and we can therefore not exclude the possibility of p75− OEC migrating outside of the graft site.
Co-localization of dopamine neurons and OEC in vivo, and morphology and quantification of cell survival and neurite growth in vitro. Panels (A) and (B) show a section from an OEC/VM co-graft, triple-labelled for TH, GFAP and p75. The boxed area in (A) is enlarged in (B). TH+ fibres were observed within the graft and growing into host striatum [arrow in (A)]. Close contact between TH+ cell bodies and fibres and p75+ OEC were observed within the graft (B). TH+ neurons in control cultures (C) and (D) had a more branched morphology compared to neurons cultured in OEC conditioned medium (E) and (F) or in direct contact with OEC (G) and (H). Dopamine neurons cultured in direct contact with the OEC monolayer had a more elongated bipolar morphology (G) and were often seen growing aligned to p75+ OEC (H). Culturing fetal VM cells in direct contact with OEC or in OEC-conditioned medium increased survival of TH+ neurons significantly compared to control cultures (I). Both direct contact with OEC and conditioned medium also enhanced the length of the longest neurite significantly compared to control (J). TH+ neurons cultured in direct contact with OEC had significantly fewer primary neurites and branching points compared to neurons in control and conditioned medium cultures (K) and (L). OEC-conditioned medium appeared to be intermediate between direct contact and control with respect to cell survival, neurite length and branching points (I)–(L). Scale bars: (A) 100 μm, (B) 20 μm, (C), (E) and (G) 50 μm, (D), (F) and (H) 50 μm. Error bars ± SEM, *P < 0.05, **P < 0.01, ***P < 0.005.
Number of TH+ neurons in host substantia nigra
The number of dopamine nerve cells in SN, as defined by TH immunoreactivity, was determined by stereology. The values obtained on the lesioned side were expressed as a percentage of neurons found on the intact contralateral side. Consistent with the rotational responses to apomorphine, few TH+ neurons were observed within SN on the lesioned side, ranging between 1 and 6% of the number of TH-immunoreactive neurons found on the intact side for individual animals (156–963 dopamine neurons on the lesioned side, an average of 15 644 dopamine neurons on the intact side). There was no significant difference in TH+ cell numbers between the different treatment groups or the denervated non-grafted controls (Fig. 2E). This clearly demonstrates that the lesions in all animals were complete or close to complete. This is in agreement with the classic observation by Ungerstedt (1968) that the majority of the dopamine neurons in SN have died at the time point used for transplantation.
Survival of grafted dopamine neurons
A significantly higher number of TH+ neurons were found within OEC/VM co-grafts (611 ± 30; mean ± SEM), compared with VM single grafts (438 ± 19; mean ± SEM; P < 0.001) and astrocyte/VM co-grafts (mean 438 ± 15; P < 0.001) in animals that received grafts with 100 000 VM cells (Fig. 2F). Approximately 5% of the cells prepared from the dissected VM are dopamine neurons prior to transplantation (Fawcett et al., 1995). The number of neurons found in the grafts corresponds to a survival rate of 12.2% in OEC/VM co-grafts and 8.8% in animals grafted with VM alone or with astrocyte/VM co-grafts.
There was no significant difference in the number of surviving grafted TH+ neurons between animals with OEC/VM co-grafts (1306 ± 146; mean ± SEM) and VM single grafts (1202 ± 144; mean ± SEM) when 250 000 VM cells were grafted (Fig. 2F). The survival rate of the TH+ neurons in the groups that received 250 000 VM cells was 10.5% (OEC/VM) and 9.6% (VM). However, grafts with 250 000 VM cells generated more surviving TH+ neurons than grafts with 100 000 VM cells (1306 compared with 611 for the OEC/VM groups, and 1202 compared with 438 for the VM groups, P < 0.001).
Amount of TH+ fibres innervating the lesioned striatum
VM grafts generated a significant higher total amount of TH-immunoreactive fibres in CPu in all animals compared with non-grafted controls (Fig. 2H). Co-grafting with OEC increased the total fibre amount by 181% (865 ± 73 mm; mean ± SEM) compared with animals with single VM grafts (479 ± 89 mm; mean ± SEM), and by 168% compared with astrocyte co-grafts (515 ± 83 mm; mean ± SEM, P < 0.005) in animals receiving 100 000 VM cells. In animals that received 250 000 VM cells, co-grafting with OEC significantly increased the total fibre amount by 153% (1422 ± 159 mm; mean ± SEM) compared with animals grafted with single VM grafts (928 ± 64 mm; mean ± SEM, Fig. 2H). Increasing the number of grafted neurons by 2.5-fold increased the total amount of fibres by 164 and 194% in the OEC/VM co-grafted group and the VM grafted group, respectively. A total amount of 71 ± 16 mm TH+ fibres/striatum was observed in animals grafted with OEC alone; however, this was not significant compared with rats with astrocyte grafts (13 ± 3 mm; mean ± SEM) or non-grafted controls (10 ± 3 mm; mean ± SEM).
No, or almost no TH+ fibres were located in the lateralmost area of striatum in animals without a VM graft (Fig. 2I). In animals with single VM grafts lateral striatal regions remained essentially non-innervated by the graft. However, when OEC were added to the VM cells, a significant amount of TH+ nerve fibres reached the lateral aspects of striatum, (length of TH-immunoreactive fibres in the lateral striatal area; 74 ± 11 and 181 ± 64 mm mean ± SEM; in animals co-grafted with OEC and 100 000 or 250 000 VM cells, respectively). Co-grafting with OEC strikingly increased the number of fibres found in the lateralmost peripheral portion of striatum 10–14-fold (100 000 VM cells and 250 000 VM cells, respectively) compared with single VM grafts (Fig. 2I).
Effect of OEC on dopamine neurons in vitro
Analysis of TH+ cell numbers showed that both direct contact with OEC (816 ± 132 cells; mean ± SEM, Fig. 5I) and OEC conditioned medium (579 ± 31 cells; mean ± SEM) significantly increased the number of TH+ neurons in culture by 255 (P < 0.001) and 182% (P < 0.05), respectively, compared with control VM cultures (318 ± 27 cells; mean ± SEM).
The number of primary neurites, total number of branch points and length of longest neurite for TH+ neurons revealed that direct contact with OEC increased neurite length and decreased branching (Fig. 5J–L). There was a significant increase in length of neurite extension from neurons cultured in direct contact with OEC monolayers, 947± 43 μm compared with 624 ± 34 μm (mean ± SEM, P < 0.005) in control cultures (Fig. 5J). However, neurons in control cultures had significantly more branch points, 7.4 ± 0.54 branch points compared with 4.4 ± 0.25 (mean ± SEM, P < 0.001) in direct contact co-cultures (Fig. 5K–L). TH+ nerve cells grown in OEC conditioned medium appeared to be intermediate between the control and direct cell contact conditions, with a neurite length of 801 ± 61 μm (mean ± SEM) and 6.0 ± 0.49 (mean ± SEM) branch points. Furthermore, TH-immunoreactive neurons growing in direct contact with OEC tended to display a bipolar morphology and appeared to be growing aligned with p75+ OEC (Fig. 5G–H). The differences in neuronal morphology in the different cultures are illustrated in Fig. 5C–H.
Discussion
Grafted embryonic dopamine neurons can partly reinnervate the dopamine-denervated striatum and counteract some symptoms in experimental animals and patients with Parkinson's disease. A key problem with this approach is yield in terms of numbers of surviving grafted dopamine neurons and amount of axonal arborization. In this study the possible beneficial effect of adding olfactory ensheathing cells to the grafted dopamine neurons was investigated. We find that the addition of OEC leads to significantly better functional effects of grafted VM cells, in terms of both amphetamine- and apomorphine-induced rotational behaviour in the rat 6-OHDA model, than seen with VM cells alone. We further show that this improvement is paralleled by an increased number of surviving grafted dopamine neurons, preferentially of the Girk2+ rather than the CB+ type, and that there is an increased amount of new dopamine nerve fibres in the denervated host striatum when OEC are present. This effect is specific to the OEC, since it is not seen when instead an astrocyte-rich, OEC-poor cell suspension, derived from the same starting material as the OEC-rich preparation, is mixed with the VM cells. We rule out any major direct effect of OEC, since such cells alone have minor or no functional or structural effects. A striking and unique effect of adding OEC is that the grafted dopamine neurons continue to extend axonal branches to lateral regions of host striatum, regions that remain permanently denervated when VM cells alone are grafted. Finally, our in vitro experiments show that the effects of OEC on embryonic dopamine neurons on survival and neurite elongation are exerted by mechanisms that are not exclusively cell–cell contact dependent.
OEC/VM co-grafted rats have a 1.5 times higher total nerve fibre length in striatum than VM grafted rats. The nerve fibre length in the lateralmost portion of striatum was remarkably increased by 10–14-fold (100 000 VM cells and 250 000 VM cells, respectively) in OEC/VM co-grafted rats compared with VM grafted rats. Equally remarkably, these fibres were present at distances from the grafted neurons that were almost not at all reached by axons from TH neurons in single VM grafts. Comparable results were observed in the co-culture system; OEC promoted dopamine cell survival and neurite elongation. The extended innervation of the striatum is a long sought-for effect, which has not been reported before in rodent allograft models. Human embryonic dopamine neurons grafted to rodent hosts can show more extended innervation patterns which might be related to the extended period of development in humans as compared with rodents (Strömberg et al., 1986, 1989; Brundin et al., 1988b; Wictorin et al., 1992).
The number of grafted neurons is known to be closely correlated to the resulting decrease of amphetamine- and apomorphine-induced behaviour (Brundin et al., 1985, 1988a; Rioux et al., 1991; Nakao et al., 1994). When we increased the number of grafted VM cells from 100 000 to 250 000, we observed an enhanced reduction of drug-induced rotations in animals that received VM grafts alone. Except for observations 2 weeks after grafting, such an enhanced effect of adding OEC on rotational behaviour was not as evident when we increased the number of grafted dopamine neurons. The reduction in drug-induced rotational behaviour (Brundin et al., 1988b) has been shown to follow a saturation curve, where after a certain degree of dopaminergic reinnervation (Björklund et al., 1980) or neuronal survival (Brundin et al., 1988a), further increases have no additional effect. Presumably, the high number of neurons grafted in the second experiment was sufficient to almost completely reverse the lesion-induced rotational behaviour already in the group that received single VM grafts, thus masking any possible additional effects of OEC co-grafts. Although the survival-promoting effect of co-grafting with OEC is not evident when the number of grafted VM cells increases, the ability to support wide-spread neurite growth was not diminished. Thus the OEC-induced effects on dopamine cell survival and neurite extension are separate events.
There is a wide range in numbers of grafted VM cells, numbers of surviving neurons and effects of grafted neurons on rotational behaviour reported in the literature. Differences can largely be explained by differences in protocols used for cell suspension preparation and transplantation. The survival rate in our study and the subsequent decrease in rotational behaviour are in agreement with several other studies (Costantini and Snyder-Keller, 1997; Sortwell et al., 1998; Wilby et al., 1999; Törnqvist et al., 2000; Ostenfeld et al., 2002). Increased numbers of surviving TH+ cells do not automatically result in better reversal of rotational behaviour (Rosenblad et al., 1999; Wilby et al., 1999; Ostenfeld et al., 2002); the amount and location of graft-derived TH+ intrastriatal innervation is also an important determinant of the functional outcome. It should be noted that while drug-induced rotational behaviour provides a sensitive indicator of graft survival, it only provides limited information about the function of the graft. Other studies have reported a complete reversal of drug-induced rotational behaviour, yet limited effects on complex motoric tasks such as front paw reaching and stepping (Georgievska et al., 2004). Further studies are needed to evaluate the effects of co-grafting VM with OEC on more complex motor tasks.
Interestingly, the presence of OEC led to an increase in the Girk2+ sub-population of TH+ neurons, while there was no increase of the CB+ sub-population. Other studies have demonstrated that the majority of the dopamine neurons in SN express Girk2, whereas the majority of dopamine cells in VTA express CB (Mendez et al., 2005; Thompson et al., 2005). Thompson et al. (2005) further demonstrated that Girk2+ dopamine neurons become mainly located in the periphery of a graft and project preferentially within striatum, while CB+ neurons are found located in the centre of the VM grafts and project to frontal cortex. Our observations of the location of Girk2+ and CB+ TH neurons in the grafts are in agreement with these studies.
The mechanisms underlying the growth-promoting ability of OEC have yet to be clarified. OEC induce little astrocytic response and chondroitin sulphate proteoglycan expression in vitro and in vivo following transplantation into adult CNS, providing a more permissive substrate for nerve outgrowth (Lakatos et al., 2000, 2003). OEC also express membrane surface molecules, neurite-promoting molecules (e.g. amyloid precursor protein), soluble neuregulins and neurotrophic factors that may contribute to the observed effects in the co-grafting situation (Ramon-Cueto and Avila, 1998; Chuah and West, 2002; Moreno-Flores et al., 2002; Lipson et al., 2003; Chung et al., 2004). While co-grafted OEC stimulate TH+ neurites to grow extensively beyond the grafted site, the OEC themselves are primarily confined to the site of engraftment. These are in agreement with others and our previous findings that OEC grafts, identified by p75 immunoreactivity or by lentiviral-transferred green fluorescent protein, exhibit limited migration from a severe lesion site and can hardly extend across an astrocytic barrier (Ruitenberg et al., 2002; Lee et al., 2004). We suggest that OEC grafts in the lesioned striatum are primarily confined close to the injection site, although the possibility that some OEC could have lost their p75 immunoreactivity and migrated outside the graft can not be completely excluded. This phenomenon is somewhat akin to the original observation of Li et al. (1998) that the effect of OEC grafted into the focally lesioned spinal cord was to form a ‘patch’ across which the cut central axons regenerated. We suggest that OEC act on fetal dopamine neurons by providing a cell–cell contact-mediated, and/or paracrine stimulation of dopamine neurons, and/or stimulate neurites to grow longer distances or to grow for a longer-than-normal time to reach the striatal periphery. In our co-culture experiment, dopamine neurons cultured in direct contact with an OEC monolayer were less branched and had a more fusiform morphology, suggesting that OEC may retard dopamine neuroblast maturation, thus prolonging the period of active nerve fibre growth.
The present results are partly seemingly at variance with a recent study by Agrawal et al. (2004) in which OEC and VM cells were grafted together or separately to rats with unilateral 6-OHDA denervations. Agrawal et al. (2004) reported not only that OEC/VM co-grafted rats have better functional recovery than rats with single VM grafts, but also surprisingly that grafting OEC alone is superior to VM alone in terms of striatal dopamine fibre density and that this effect was correlated to an increased number of surviving dopamine neurons within the lesioned SN. The extent of the lesion is critically important in determining the outcome of grafting strategies using the 6-OHDA model (Blanchard et al., 1995, 1996; Hansen et al., 1995; Kirik et al., 1998; Finkelstein et al., 2000; Song and Haber, 2000; Stanic et al., 2003). The most parsimonious explanation of the differences between the present study and that of Agrawal et al. (2004) is that they used rats that were partially denervated, thus allowing for effects of OEC on remaining dopamine fibres in striatum, and possibly trophic stimulation of these neurons reflected also in increased cell counts in SN. Thus our study demonstrates direct effects of OEC on grafted neurons only, while Agrawal et al. noted what appear to be effects of OEC both on grafted neurons and on remaining host dopamine neurons.
Fetal VM has also been co-grafted with a wide variety of other tissues or cells, including peripheral nerve/Schwann cells (van Horne et al., 1991; Collier et al., 1994), immature astrocytes from several regions of CNS (Krobert et al., 1997; Pierret et al., 1998), striatal tissue (Costantini et al., 1994; Costantini and Snyder-Keller, 1997; Emgård-Mattson et al., 1997; Sortwell et al., 1998), carotid body (Shukla et al., 2004), Sertoli cells (Borlongan et al., 1997; Sanberg et al., 1997) or fetal kidney (Granholm et al., 1998; Chiang et al., 2001). Improvements were obtained in all these cases. The common denominator behind the increase in survival and/or fibre density and function in the different studies may be the production of neurotrophic factors. One neurotrophic factor that has been demonstrated to have a strong effect on dopamine cell survival and morphological maturation both in vitro and in vivo is GDNF (Tomac et al., 1995; Widenfalk et al., 1997; Rosenblad et al., 1996; Granholm et al., 1997, Sautter et al., 1998; Espejo et al., 2000; Törnqvist et al., 2000; Widmer et al., 2000; Chaturvedi et al., 2003; Schaller et al., 2005). The increase in survival rate by co-grafting VM with OEC in our study is similar to the increase seen after treatment of VM grafts with GDNF (Wilby et al., 1999; Törnqvist et al., 2000; Ostenfeld et al., 2002). The GDNF treatment can also increase TH+ intrastriatal innervation (Rosenblad et al., 1996; Granholm et al., 1997; Törnqvist et al., 2000) although that has not always been the case (Yurek, 1998). There is, however, no previous study where VM grafts have been treated with neurotrophic factors that has demonstrated a similar remarkable increase in long-distance intrastriatal nerve fibre innervation as seen in the present study.
We observed no effect of co-grafting VM with astrocytes from the adult olfactory nerve. Astrocytes differ in survival and growth-promoting capacity between different regions of the brain and between different states of maturation (Autillo-Touati et al., 1988; Denis-Donini and Estenoz, 1988; Garcia-Abreu et al., 1995; Le Roux and Reh, 1995; Krobert et al., 1997). In previous in vitro studies we have observed the existence of at least two different types of immature astrocytes within the fetal VM tissue, one type associated with elongation of neurites and another type stimulating neurite branching and target innervation (Johansson and Strömberg, 2002, 2003). Since the VM grafts already contained both these types of astrocytes, it appears a priori unlikely that the addition of a third population of astrocytes would have additional effects.
We conclude that the addition of OEC can markedly enhance the effects of fetal dopamine neuron grafts in the completely dopamine-denervated rat striatum. Of particular significance is the fact that neurites from OEC-stimulated grafted dopamine neurons are able to reach a much larger host striatal territory than single grafted dopamine neurons.
Supplementary material
The Supplementary material cited in this article is available at Brain online.
This work was supported by the Swedish Research Council, AFA, NIDA, and Yen Tjing Ling Medical Foundation in Taiwan.
References
Agrawal AK, Shukla S, Chaturvedi RK, Seth K, Srivastava N, Ahmad A, et al. Olfactory ensheathing cell transplantation restores functional deficits in rat model of Parkinson's disease: a cotransplantation approach with fetal ventral mesencephalic cells.
Autillo-Touati A, Chamak B, Araud D, Vuillet J, Seite R, Prochiantz A. Region-specific neuro-astroglial interactions: ultrastructural study of the in vitro expression of neuronal polarity.
Barber PC. Neurogenesis and regeneration in the primary olfactory pathway of mammals.
Barber PC, Lindsay RM. Schwann cells of the olfactory nerves contain glial fibrillary acidic protein and resemble astrocytes.
Barker RA, Dunnett SB, Faissner A, Fawcett JW. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum.
Björklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants.
Björklund A, Dunnett SB, Stenevi U, Lewis ME, Iversen SD. Reinnervation of the denervated striatum by substantia nigra transplants: functional consequences as revealed by pharmacological and sensorimotor testing.
Björklund A, Dunnett SB, Brundin P, Stoessl AJ, Freed CR, Breeze RE, et al. Neural transplantation for the treatment of Parkinson's disease.
Blanchard V, Chritin M, Vyas S, Savasta M, Feuerstein C, Agid Y, et al. Long-term induction of tyrosine hydroxylase expression: compensatory response to partial degeneration of the dopaminergic nigrostriatal system in the rat brain.
Blanchard V, Anglade P, Dziewczapolski G, Savasta M, Agid Y, Raisman-Vozari R. Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra.
Borlongan CV, Cameron DF, Saporta S, Sanberg PR. Intracerebral transplantation of testis-derived sertoli cells promotes functional recovery in female rats with 6-hydroxydopamine-induced hemiparkinsonism.
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury.
Brundin P, Barbin G, Isacson O, Mallat M, Chamak B, Prochiantz A, et al. Survival of intracerebrally grafted rat dopamine neurons previously cultured in vitro.
Brundin P, Barbin G, Strecker RE, Isacson O, Prochiantz A, Björklund A. Survival and function of dissociated rat dopamine neurones grafted at different developmental stages or after being cultured in vitro.
Brundin P, Strecker RE, Widner H, Clarke DJ, Nilsson OG, Astedt B. et al. Human fetal dopamine neurons grafted in a rat model of Parkinson's disease: immunological aspects, spontaneous and drug-induced behaviour, and dopamine release.
Brundin P, Karlsson J, Emgård M, Schierle GS, Hansson O, Petersen A, et al. Improving the survival of grafted dopaminergic neurons: a review over current approaches.
Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra: relation between extent of lesion and rotational behavior.
Casas M, Ferre S, Cobos A, Cadafalch J, Grau JM, Jane F. Comparison between apomorphine and amphetamine-induced rotational behaviour in rats with a unilateral nigrostriatal pathway lesion.
Chaturvedi RK, Agrawal AK, Seth K, Shukla S, Chauhan S, Shukla Y, et al. Effect of glial cell line-derived neurotrophic factor (GDNF) co-transplantation with fetal ventral mesencephalic cells (VMC) on functional restoration in 6-hydroxydopamine (6-OHDA) lesioned rat model of Parkinson's disease: neurobehavioral, neurochemical and immunohistochemical studies.
Chiang Y, Morales M, Zhou FC, Borlongan C, Hoffer BJ, Wang Y. Fetal intra-nigral ventral mesencephalon and kidney tissue bridge transplantation restores the nigrostriatal dopamine pathway in hemi-parkinsonian rats.
Chuah MI, West AK. Cellular and molecular biology of ensheathing cells.
Chung RS, Woodhouse A, Fung S, Dickson TC, West AK, Vickers JC, et al. Olfactory ensheathing cells promote neurite sprouting of injured axons in vitro by direct cellular contact and secretion of soluble factors.
Collier TJ, Elsworth JD, Taylor JR, Sladek JR, Jr, Roth RH, Redmond DE Jr Peripheral nerve-dopamine neuron co-grafts in MPTP-treated monkeys: augmentation of tyrosine hydroxylase-positive fibre staining and dopamine content in host systems.
Costantini LC, Snyder-Keller A. Co-transplantation of fetal lateral ganglionic eminence and ventral mesencephalon can augment function and development of intrastriatal transplants.
Costantini LC, Vozza BM, Snyder-Keller AM. Enhanced efficacy of nigral-striatal cotransplants in bilaterally dopamine-depleted rats: an anatomical and behavioral analysis.
Denis-Donini S, Estenoz M. Interneurons versus efferent neurons: heterogeneity in their neurite outgrowth response to glia from several brain regions.
Doucette JR. The glial cells in the nerve fibre layer of the rat olfactory bulb.
Doucette JR, Kiernan JA, Flumerfelt BA. The re-innervation of olfactory glomeruli following transection of primary olfactory axons in the central or peripheral nervous system.
Dunnett SB, Kendall AL, Watts C, Torres EM. Neuronal cell transplantation for Parkinson's and Huntington's diseases.
Emgård-Mattson M, Karlsson J, Nakao N, Brundin P. Addition of lateral ganglionic eminence to rat mesencephalic grafts affects fibre outgrowth but does not enhance function.
Espejo M, Cutillas B, Arenas TE, Ambrosio S. Increased survival of dopaminergic neurons in striatal grafts of fetal ventral mesencephalic cells exposed to neurotrophin-3 or glial cell line-derived neurotrophic factor.
Fawcett JW, Barker RA, Dunnett SB. Dopaminergic neuronal survival and the effects of bFGF in explant, three dimensional and monolayer cultures of embryonic rat ventral mesencephalon.
Fernandez-Espejo E. Pathogenesis of Parkinson's disease: prospects of neuroprotective and restorative therapies.
Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra.
Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease.
Garcia-Abreu J, Moura Neto V, Carvalho SL, Cavalcante LA. Regionally specific properties of midbrain glia: I. Interactions with midbrain neurons.
Gates MA, O'Brien TF, Faissner A, Steindler DA. Neuron-glial interactions during the in vivo and in vitro development of the nigrostriatal circuit.
Georgievska B, Carlsson T, Lacar B, Winkler C, Kirik D. Dissociation between short-term increased graft survival and long-term functional improvements in Parkinsonian rats overexpressing glial cell line-derived neurotrophic factor.
Granholm AC, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, et al. Glial cell line-derived neurotrophic factor improves survival of ventral mesencephalic grafts to the 6-hydroxydopamine lesioned striatum.
Granholm AC, Henry S, Herbert MA, Eken S, Gerhardt GA, van Horne C. Kidney cografts enhance fibre outgrowth from ventral mesencephalic grafts to the 6-OHDA-lesioned striatum, and improve behavioral recovery.
Graziadei PP, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. III. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria in rat.
Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson.
Hansen JT, Sakai K, Greenamyre JT, Moran S. Sprouting of dopaminergic fibres from spared mesencephalic dopamine neurons in the unilateral partial lesioned rat.
Hefti F, Melamed E, Sahakian BJ, Wurtman RJ. Circling behavior in rats with partial, unilateral nigro-striatal lesions: effect of amphetamine, apomorphine, and DOPA.
Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization.
Heikkila RE, Shapiro BS, Duvoisin RC. The relationship between loss of dopamine nerve terminals, striatal [3H]spiroperidol binding and rotational behavior in unilaterally 6-hydroxydopamine-lesioned rats.
Herrera-Marschitz M, Ungerstedt U. Evidence that apomorphine and pergolide induce rotation in rats by different actions on D1 and D2 receptor sites.
Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome.
Hudson JL, van Horne CG, Strömberg I, Brock S, Clayton J, Masserano J, et al. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats.
Hurelbrink CB, Barker RA. Prospects for the treatment of Parkinson's disease using neurotrophic factors.
Johansson S, Strömberg I. Guidance of dopaminergic neuritic growth by immature astrocytes in organotypic cultures of rat fetal ventral mesencephalon.
Johansson S, Strömberg I. Fetal lateral ganglionic eminence attracts one of two morphologically different types of tyrosine hydroxylase-positive nerve fibres formed by cultured ventral mesencephalon.
Kirik D, Rosenblad C, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat.
Krobert K, Lopez-Colberg I, Cunningham LA. Astrocytes promote or impair the survival and function of embryonic ventral mesencephalon co-grafts: effects of astrocyte age and expression of recombinant brain-derived neurotrophic factor.
Lakatos A, Franklin RJ, Barnett SC. Olfactory ensheathing cells and Schwann cells differ in their in vitro interactions with astrocytes.
Lakatos A, Barnett SC, Franklin RJ. Olfactory ensheathing cells induce less host astrocyte response and chondroitin sulphate proteoglycan expression than Schwann cells following transplantation into adult CNS white matter.
Lakatos A, Smith PM, Barnett SC, Franklin RJ. Meningeal cells enhance limited CNS remyelination by transplanted olfactory ensheathing cells.
Lee IH, Bulte JW, Schweinhardt P, Douglas T, Trifunovski A, Hofstetter C, Olson L, Spenger C. In vivo magnetic resonance tracking of olfactory ensheathing glia grafted into the rat spinal cord.
Le Roux PD, Reh TA. Astroglia demonstrate regional differences in their ability to maintain primary dendritic outgrowth from mouse cortical neurons in vitro.
Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells.
Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells.
Lindvall O, Hagell P. Clinical observations after neural transplantation in Parkinson's disease.
Lindvall O, Rehncrona S, Gustavii B, Brundin P, Astedt B, Widner H, et al. Fetal dopamine-rich mesencephalic grafts in Parkinson's disease.
Lipson AC, Widenfalk J, Lindqvist E, Ebendal T, Olson L. Neurotrophic properties of olfactory ensheathing glia.
Lu J, Feron F, Ho SM, Mackay-Sim A, Waite PM. Transplantation of nasal olfactory tissue promotes partial recovery in paraplegic adult rats.
Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Björklund L, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease.
Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC.
Moore AE, Cicchetti F, Hennen J, Isacson O. Parkinsonian motor deficits are reflected by proportional A9/A10 dopamine neuron degeneration in the rat.
Moreno-Flores MT, Diaz-Nido J, Wandosell F, Avila J. Olfactory ensheathing glia: drivers of axonal regeneration in the central nervous system?
Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response.
Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes.
Nakao N, Frodl EM, Duan WM, Widner H, Brundin P. Lazaroids improve the survival of grafted rat embryonic dopamine neurons.
Nash HH, Borke RC, Anders JJ. New method of purification for establishing primary cultures of ensheathing cells from the adult olfactory bulb.
Ostenfeld T, Tai YT, Martin P, Deglon N, Aebischer P, Svendsen CN. Neurospheres modified to produce glial cell line-derived neurotrophic factor increase the survival of transplanted dopamine neurons.
Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science
Pierret P, Quenneville N, Vandaele S, Abbaszadeh R, Lanctot C, Crine P, et al. Trophic and tropic effects of striatal astrocytes on cografted mesencephalic dopamine neurons and their axons.
Ramon-Cueto A, Avila J. Olfactory ensheathing glia: properties and function.
Ramon-Cueto A, Nieto-Sampedro M. Glial cells from adult rat olfactory bulb: immunocytochemical properties of pure cultures of ensheathing cells.
Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants.
Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia.
Rioux L, Gaudin DP, Bui LK, Gregoire L, DiPaolo T, Bedard PJ. Correlation of functional recovery after a 6-hydroxydopamine lesion with survival of grafted fetal neurons and release of dopamine in the striatum of the rat.
Rosenblad C, Martinez-Serrano A, Björklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts.
Rosenblad C, Kirik D, Björklund A. Neurturin enhances the survival of intrastriatal fetal dopaminergic transplants.
Ruitenberg MJ, Plant GW, Christensen CL, Blits B, Niclou SP, Harvey AR, et al. Viral vector-mediated gene expression in olfactory ensheathing glia implants in the lesioned rat spinal cord.
Sanberg PR, Borlongan CV, Othberg AI, Saporta S, Freeman TB, Cameron DF. Testis-derived Sertoli cells have a trophic effect on dopamine neurons and alleviate hemiparkinsonism in rats.
Sautter J, Tseng JL, Braguglia D, Aebischer P, Spenger C, Seiler RW, et al. Implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor improve survival, growth, and function of fetal dopaminergic grafts.
Schaller B, Andres RH, Huber AW, Meyer M, Perez-Bouza A, Ducray AD, et al. Effect of GDNF on differentiation of cultured ventral mesencephalic dopaminergic and non-dopaminergic calretinin-expressing neurons.
Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors.
Shukla S, Agrawal AK, Chaturvedi RK, Seth K, Srivastava N, Sinha C, et al. Co-transplantation of carotid body and ventral mesencephalic cells as an alternative approach towards functional restoration in 6-hydroxydopamine-lesioned rats: implications for Parkinson's disease.
Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting.
Sortwell CE, Collier TJ, Sladek JR. Jr Co-grafted embryonic striatum increases the survival of grafted embryonic dopamine neurons.
Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK. Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat.
Strömberg I, Bickford P. Reduced ageing effects of striatal neuronal discharge rate by aged ventral mesencephalic grafts.
Strömberg I, Bygdeman M, Goldstein M, Seiger A, Olson L. Human fetal substantia nigra grafted to the dopamine-denervated striatum of immunosuppressed rats: evidence for functional reinnervation.
Strömberg I, Almqvist P, Bygdeman M, Finger TE, Gerhardt G, Granholm AC, et al. Human fetal mesencephalic tissue grafted to dopamine-denervated striatum of athymic rats: light- and electron-microscopical histochemistry and in vivo chronoamperometric studies.
Thompson L, Barraud P, Andersson E, Kirik D, Björklund A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections.
Tomac A, Lindqvist E, Lin LF, Ögren SO, Young D, Hoffer BJ, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo.
Törnqvist NBjörklund L, Almqvist P, Wahlberg L, Strömberg I. Implantation of bioactive growth factor-secreting rods enhances fetal dopaminergic graft survival, outgrowth density, and functional recovery in a rat model of Parkinson's disease.
Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons.
van Horne CG, Strömberg I, Young D, Olson L, Hoffer B. Functional enhancement of intrastriatal dopamine-containing grafts by the co-transplantation of sciatic nerve tissue in 6-hydroxydopamine-lesioned rats.
Wictorin K, Brundin P, Sauer H, Lindvall O, Björklund A. Long distance directed axonal growth from human dopaminergic mesencephalic neuroblasts implanted along the nigrostriatal pathway in 6-hydroxydopamine lesioned adult rats.
Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L. Neurturin and glial cell line-derived neurotrophic factor receptor-beta (GDNFR-beta), novel proteins related to GDNF and GDNFR-alpha with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs.
Widmer HR, Schaller B, Meyer M, Seiler RW. Glial cell line-derived neurotrophic factor stimulates the morphological differentiation of cultured ventral mesencephalic calbindin- and calretinin-expressing neurons.
Wilby MJ, Sinclair SR, Muir EM, Zietlow R, Adcock KH, Horellou P, et al. A glial cell line-derived neurotrophic factor-secreting clone of the Schwann cell line SCTM41 enhances survival and fibre outgrowth from embryonic nigral neurons grafted to the striatum and to the lesioned substantia nigra.


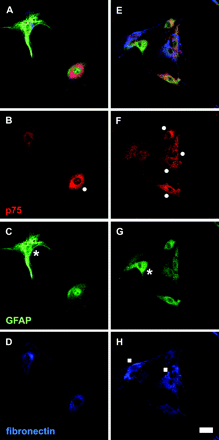
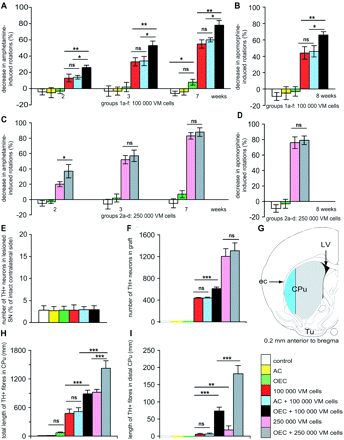
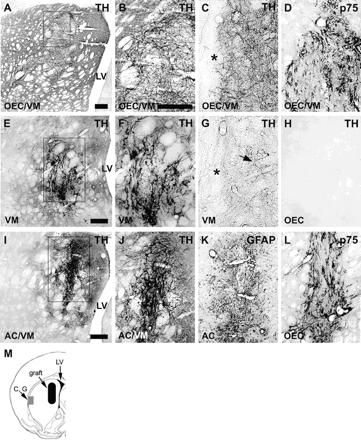
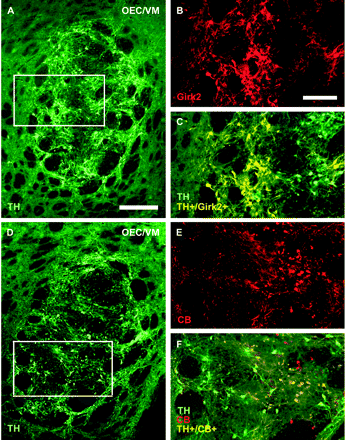
![Co-localization of dopamine neurons and OEC in vivo, and morphology and quantification of cell survival and neurite growth in vitro. Panels (A) and (B) show a section from an OEC/VM co-graft, triple-labelled for TH, GFAP and p75. The boxed area in (A) is enlarged in (B). TH+ fibres were observed within the graft and growing into host striatum [arrow in (A)]. Close contact between TH+ cell bodies and fibres and p75+ OEC were observed within the graft (B). TH+ neurons in control cultures (C) and (D) had a more branched morphology compared to neurons cultured in OEC conditioned medium (E) and (F) or in direct contact with OEC (G) and (H). Dopamine neurons cultured in direct contact with the OEC monolayer had a more elongated bipolar morphology (G) and were often seen growing aligned to p75+ OEC (H). Culturing fetal VM cells in direct contact with OEC or in OEC-conditioned medium increased survival of TH+ neurons significantly compared to control cultures (I). Both direct contact with OEC and conditioned medium also enhanced the length of the longest neurite significantly compared to control (J). TH+ neurons cultured in direct contact with OEC had significantly fewer primary neurites and branching points compared to neurons in control and conditioned medium cultures (K) and (L). OEC-conditioned medium appeared to be intermediate between direct contact and control with respect to cell survival, neurite length and branching points (I)–(L). Scale bars: (A) 100 μm, (B) 20 μm, (C), (E) and (G) 50 μm, (D), (F) and (H) 50 μm. Error bars ± SEM, *P < 0.05, **P < 0.01, ***P < 0.005.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/128/12/10.1093/brain/awh644/2/m_awh644f5.gif?Expires=1716564138&Signature=in9jYhd-Kn8U2cL~kpR5tBDEsfPou6sFEU9MlZHmiKKSYkGCL~4J-BS4Hffua4TfE19amx6zP0xmWiDrM5762zEN9beeO~Bgqm3qwsT9O0zY27T9kKRV5-6xCMgDEb~fx0m7HY2WSO55qbDo4rpJn~0XZVBgY4G20~MbNw5wzs1UyVa7C~R7UTV23hpzbKTnnfV8mQC1tjxT-V2EhKhvQTJBeTPP7W96xyvnucxNLfiuD1QPT5dI5vI5~nWcNI6d-O3Iitxmam0GDxsJj0-JvF8DDS28t69iCor8zOwgLwGJJq2rqwOjH5QAOsjVxqZ9OVhwMDHGh28rR6MiF8zyHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
