-
PDF
- Split View
-
Views
-
Cite
Cite
Andrea E. Cavanna, Michael R. Trimble, The precuneus: a review of its functional anatomy and behavioural correlates, Brain, Volume 129, Issue 3, March 2006, Pages 564–583, https://doi.org/10.1093/brain/awl004
Close - Share Icon Share
Abstract
Functional neuroimaging studies have started unravelling unexpected functional attributes for the posteromedial portion of the parietal lobe, the precuneus. This cortical area has traditionally received little attention, mainly because of its hidden location and the virtual absence of focal lesion studies. However, recent functional imaging findings in healthy subjects suggest a central role for the precuneus in a wide spectrum of highly integrated tasks, including visuo-spatial imagery, episodic memory retrieval and self-processing operations, namely first-person perspective taking and an experience of agency. Furthermore, precuneus and surrounding posteromedial areas are amongst the brain structures displaying the highest resting metabolic rates (hot spots) and are characterized by transient decreases in the tonic activity during engagement in non-self-referential goal-directed actions (default mode of brain function). Therefore, it has recently been proposed that precuneus is involved in the interwoven network of the neural correlates of self-consciousness, engaged in self-related mental representations during rest. This hypothesis is consistent with the selective hypometabolism in the posteromedial cortex reported in a wide range of altered conscious states, such as sleep, drug-induced anaesthesia and vegetative states. This review summarizes the current knowledge about the macroscopic and microscopic anatomy of precuneus, together with its wide-spread connectivity with both cortical and subcortical structures, as shown by connectional and neurophysiological findings in non-human primates, and links these notions with the multifaceted spectrum of its behavioural correlates. By means of a critical analysis of precuneus activation patterns in response to different mental tasks, this paper provides a useful conceptual framework for matching the functional imaging findings with the specific role(s) played by this structure in the higher-order cognitive functions in which it has been implicated. Specifically, activation patterns appear to converge with anatomical and connectivity data in providing preliminary evidence for a functional subdivision within the precuneus into an anterior region, involved in self-centred mental imagery strategies, and a posterior region, subserving successful episodic memory retrieval.
Introduction
To our knowledge, in the neuroscientific literature there are no reviews focusing on the structure and function of the posterior region of the medial parietal cortex, alternatively referred to as the precuneus or the mesial extent of Brodmann's area (BA) 7. The anatomical location of the posteromedial parietal cortex (i.e. buried in the interhemispheric fissure and encased by the sagittal sinus and bridging veins) makes this territory especially difficult to study. The precuneus has thus remained one of the less accurately mapped areas of the whole cortical surface. Moreover, the posteromedial parietal cortex has traditionally received little study, since it is rarely lesioned in strokes or accidents, but its strategic location and wide-spread connections suggest the precuneus is a major association area that may subserve a variety of behavioural functions. However, the modern era of neuroimaging has recently made it possible to explore the morphological and functional aspects of this long-neglected part of the brain.
After reviewing current knowledge about the anatomical and cytoarchitectonic structure of the precuneus, along with its cortical and subcortical connectivity patterns, the present paper encompasses the diverse array of its behavioural functions, disclosed mainly by functional imaging studies involving both higher-order cognitive tasks and normal and altered conscious states.
Macroscopic, microscopic and functional anatomy of the precuneus
Despite recent intense interest in the functional significance of the precuneus, the details of its cytoarchitecture and connections have remained a relatively unexplored topic of brain mapping, largely due to its inaccessible location along and in the depths of the longitudinal fissure (Pandya and Seltzer, 1982). Moreover, since in vivo axonal tracing techniques cannot be applied to the human brain, our knowledge about the connectivity of the posteromedial parietal lobe is based mainly on axonal tracing studies in the macaque brain (Zilles et al., 2003).
The associative cortices, to which the precuneus belongs, have undergone a gradual increase in the complexity of their organization during the course of primate and hominid evolution. As a consequence of this, in the cebida (New World monkey) the superior parietal and precuneate regions are poorly developed (Critchley, 1953); on the other hand, the posteromedial cortex of the macaque (Old World monkey) has been shown to share its main architectonic patterns with Homo sapiens (von Bonin and Bailey, 1947; Leichnetz, 2001). Overall, the medial aspect of the parietal lobe of the chimpanzee and other apes closely resembles the general appearance of the same structures in the human brain (Bailey et al., 1950). However, it should be pointed out that the precuneus has received relatively little attention even in the most comprehensive comparative neuroanatomy treatises (e.g. Nieuwenhuys et al., 1998), thus indicating a need for further comparative studies specifically addressing this cortical area.
Topographical anatomy
The medial aspect of the posterior parietal lobe has historically been referred to as the precuneus, or quadrate lobule of Foville (1844). This nomenclature follows the topographical location and geometrical appearance of this cortical area, situated immediately in front of the triangular-shaped convolution of the cuneus, on the medial surface of the occipital lobe. The precuneus is limited anteriorly by the marginal branch of the cingulate sulcus, posteriorly by the medial portion of the parieto-occipital fissure and inferiorly by the subparietal (i.e. postlimbic) sulcus. Figure 1 shows the medial surface of the human brain with the main landmarks of the precuneus according to the traditional anatomical descriptions (Critchley, 1953).
A drawing of the medial surface of the human brain; the precuneus and its traditional anatomical landmarks are labelled [after Critchley (1953)].
The variable boundaries of the precuneus have been recently described in detail by Salamon et al. (2003), who also highlighted some correlations with neuroimaging findings. In summary, the cingulate sulcus ends upward with the ramus marginalis, which marks the division of the brain between the precuneus and the primary sensory and motor areas. The parieto-occipital fissure has a limited extension on the upper part of the brain and ends at the level of the upper bend of the calcarine fissure, or slightly more anteriorly. Its shape is variable: straight, T-shaped or more complex with three branches. The subparietal sulcus constitutes the inferior margin of the precuneus and continues its course around the posterior part of the cingulum. Most often, this sulcus is not represented as a single line, but as a complete or incomplete H shape, with one to three ascending branches along its course.
The vasculature of this region shows remarkable interindividual variability. The main arterial supply of the precuneus stems from the posterior cerebral artery, with predominance from the P2 segment. The occipito-parietal artery, a terminal branch of the internal occipital artery, principally supplies the precuneate and anteromedial occipital cortices.
Cytoarchitectonics
Numerous cytoarchitectonic and myeloarchitectonic maps of the posteromedial portion of the parietal cortex have been proposed since the beginning of the past century. However, its exact parcellation remains the subject of discussion, since the existing maps differ considerably concerning the number and size of individual brain areas. The cytoarchitectonic map of Brodmann (1909) still dominates our present concepts of the structural organization of the human cerebral cortex, since it serves, via a popular brain atlas (Talairach and Tournoux, 1988), as an anatomical reference for functional imaging studies. The territory of the precuneus mainly corresponds to the mesial extent of BA 7, which also occupies most of the lateral parietal cortex above the intraparietal sulcus (IPS) (Leichnetz, 2001; Zilles et al., 2003). In addition, an adjacent cytoarchitectonic region has been proposed to be a part of the precuneus: according to some authors (e.g. van Hoesen et al., 1993; Frackowiak et al., 1997) BA 31, which is positioned between the cingulate and splenial sulci, includes both posterior cingulate and precuneate cortices. However, throughout this study we explicitly confined our analysis to the precuneus in its more restricted sense, i.e. the medial aspect of BA 7. The rationale for choosing BA 7 instead of broader anatomical descriptions, including the superior portion of BA 31, was because different BAs with different cytoarchitectonic structures and connectivity patterns are likely to differ in terms of their subserved functions as well. The medial surface of BA 7 is easily distinguished from adjacent posterior cingulate and retrosplenial cortices by its representative parietal cytoarchitecture, characterized by fully differentiated isocortex: a columnar pattern with conspicuous layers II, IV, V and VI, and a noticeable thinning of cortex as a whole (Pandya and Seltzer, 1982). BA 31, on the other hand, appears to be a cortical transition zone from the medial parietal areas to the posterior cingulate, presenting an apparent shift in cytoarchitecture from parietal isocortex to limbic cortex.
Brodmann described gradual rostrocaudal architectonic changes within area 7; thus, he defined the existence of two main subdivisions, which he named 7a and 7b, although he did not define a clear border between them (Zilles et al., 2003). Von Economo and Koskinas (1925) summarized the previous efforts made by Brodmann and others (Campbell, 1905; Elliot Smith, 1907) and described a practically identical location for their area PE, which was subdivided into the anterior area PEm, with a more pronounced magnocellular appearance, and the relatively smaller-celled posterior area PEp. PEm and PEp are probably equivalent to Brodmann's subdivisions 7a and 7b, respectively; yet, since Brodmann did not provide a cytoarchitectonic description or a micrograph of BA 7, comparisons with the maps of other authors can be performed only on the basis of topography (Zilles et al., 2003). Figure 2 compares the cytoarchitectonic maps of the precuneus as defined for the scope of the present review (i.e. mesial BA 7) and the adjacent areas of the human medial parietal lobe after Brodmann (1909) and von Economo and Koskinas (1925). A number of subsequent cytoarchitectonic studies (von Bonin and Bailey, 1947; Sarkissov et al., 1949; Pandya and Seltzer, 1982) adopted or further developed Brodmann's and von Economo and Koskinas' parcellation schemes. Table 1 summarizes the different labels proposed for the precuneate cortex, according to the major cytoarchitectonic maps. Interested readers are referred to the recent paper by Zilles and Palomero-Gallagher (2001) for a historical overview of the cytoarchitectonic parcellation of human parietal cortex and further anatomical details. It has been pointed out that the classical cortical maps fail to explain the more detailed aerial organization of the posterior parietal cortex, as revealed by recent functional imaging studies (Bremmer et al., 2001). As a consequence, these cytoarchitectonic and myeloarchitectonic studies can only be considered as guidelines for future multimodal and observer-independent quantitative architectonic analyses (Zilles et al., 2003).
Comparison of the cytoarchitectonic maps of the human medial parietal isocortex after Brodmann (1909) (left) and von Economo and Koskinas (1925) (right); for the scope of the present review, the precuneus corresponds to mesial BA 7 and PE, respectively (horizontally shaded areas) [reprinted with permission from Zilles and Palomero-Gallagher (2001)].
Nomenclature of the precuneus according to the major cytoarchitectonic cortical maps
| Author . | Area . |
|---|---|
| Brodmann (1909) | 7 (7a, 7b) |
| von Economo and Koskinas (1925) | PE (PEm, PEp) |
| von Bonin and Bailey (1947) | PE |
| Pandya and Seltzer (1982) | PGm |
| Cavada and Goldman-Rakic (1989) | 7m |
| Author . | Area . |
|---|---|
| Brodmann (1909) | 7 (7a, 7b) |
| von Economo and Koskinas (1925) | PE (PEm, PEp) |
| von Bonin and Bailey (1947) | PE |
| Pandya and Seltzer (1982) | PGm |
| Cavada and Goldman-Rakic (1989) | 7m |
Nomenclature of the precuneus according to the major cytoarchitectonic cortical maps
| Author . | Area . |
|---|---|
| Brodmann (1909) | 7 (7a, 7b) |
| von Economo and Koskinas (1925) | PE (PEm, PEp) |
| von Bonin and Bailey (1947) | PE |
| Pandya and Seltzer (1982) | PGm |
| Cavada and Goldman-Rakic (1989) | 7m |
| Author . | Area . |
|---|---|
| Brodmann (1909) | 7 (7a, 7b) |
| von Economo and Koskinas (1925) | PE (PEm, PEp) |
| von Bonin and Bailey (1947) | PE |
| Pandya and Seltzer (1982) | PGm |
| Cavada and Goldman-Rakic (1989) | 7m |
Cortical and subcortical connectivity
Quite recently, Leichnetz (2001) studied the afferent and efferent connections of the precuneus in Cebus apella (New World monkey) and Macaca fascicularis (Old World monkey) using the retrograde and anterograde capabilities of the horseradish peroxidase technique and compared his findings with those of previous tracing studies (Blum et al., 1950; Pribram and Barry, 1956; Mesulam et al., 1977; Pandya and Seltzer, 1982; Petrides and Pandya, 1984; Goldman-Rakic, 1988). Figure 3 summarizes the main cortical and subcortical projections of the precuneus.
Summary of the cortical (left) and subcortical (right) connections of the precuneus. Bidirectional arrows indicate reciprocal projections; unidirectional arrows indicate afferent/efferent projections.
The precuneus has reciprocal corticocortical connections with the adjacent areas of the posteromedial cortex, namely the posterior cingulate and retrosplenial cortices. This intimate interconnection is also bilateral, bridging homologous components of the two hemispheres, and to some extent providing an anatomical basis for their functional coupling. The precuneus is also selectively connected with other parietal areas, namely the caudal parietal operculum, the inferior and superior parietal lobules (SPLs), and the IPS, known to be involved in visuo-spatial information processing (Selemon and Goldman-Rakic, 1988; Cavada and Goldman-Rakic, 1989; Leichnetz, 2001).
The principal extraparietal corticocortical connections of the precuneus are with the frontal lobes. The precuneus and prefrontal cortex have been demonstrated to be strongly interconnected, and these projections tend to concentrate at the level of BA 8, 9 and 46. There are also extensive connections between the precuneus and the dorsal premotor area, the supplementary motor area (SMA) and the anterior cingulate cortex (Petrides and Pandya, 1984; Goldman-Rakic, 1988; Cavada and Goldman-Rakic, 1989; Leichnetz, 2001). The results of the tracer injection studies of Leichnetz and colleagues (Leichnetz and Goldberg, 1988; Leichnetz and Gonzalo-Ruiz, 1996; Leichnetz, 2001) and Tian and Lynch (1996a, b) in macaque and cebus monkeys strongly support the existence of a topographical organization in the reciprocal parietofrontal connections, such that the precuneus has connections with oculomotor-related cortical regions, including the frontal eye fields. Neurophysiological studies in non-human primates provided further evidence for the selective functional coupling of medial parietal and frontal cortices. Thier and Andersen (1998) were able to elicit saccades by direct low-current electrostimulation of the medial aspect of the posterior parietal cortex in the monkey, raising the hypothesis that the brain of these primates contains a ‘medial parietal eye field’ (Thier and Andersen, 1993) involved in the control of eye movement and ‘visual reaching’ (Johnson et al., 1996), in addition to the already known ‘lateral parietal eye field’, located in the lateral bank of the IPS (Andersen et al., 1990). Likewise, the corticocortical projections from the precuneus to the lateral parietal areas and premotor cortex (Cavada and Goldman-Rakic, 1989; Johnson et al., 1993, 1996; Wise et al., 1997) seem to play a pivotal role in the visual guidance of hand movements, i.e. hand–eye coordination (Ferraina et al., 1997) and reaching (Caminiti, 1996; Caminiti et al., 1999).
Other reciprocal cortical connections involve the medial prestriate cortex, with the parietooccipital visual area and the caudomedial lobule, and the cortex buried in the superior temporal sulcus, known as temporoparietooccipital cortex (TPO) (Blum et al., 1950; Leichnetz, 2001). The association cortices of the TPO form a heteromodal higher associative cortical network, which is involved in the integration of auditory, somatosensory and visual information.
The thalamic projections of the precuneus target mostly the dorsum of the thalamus, which contains nuclei connected with higher association cortices, the ventrolateral thalamic nucleus, the central nuclei of the intralaminar complex and the lateral pulvinar (Yeterian and Pandya, 1985, 1988; Schmahmann and Pandya, 1990). All these nuclei send projections back to the precuneus; in addition, the precuneus receives unilateral projections from the ‘non-specific’ anterior intralaminar nuclei. Interestingly, the lack of connections with the sensory thalamic nuclei, such as the ventral posterior lateral nucleus, suggests that the precuneus does not share the thalamic connectivity pattern of the parietal somatosensory cortical regions.
Other major subcortical connections of the precuneus include the claustrum, corticostriate projections to the dorsolateral caudate nucleus and putamen, and efferent projections to the zona incerta and brainstem structures with strongly ‘oculomotor’ characteristics, such as the pretectal area, the superior colliculus and the nucleus reticularis tegmenti pontis (Yeterian and Pandya, 1993; Leichnetz, 2001). Finally, projections from each cytoarchitectonic area of the posteromedial cortex to the basis pontis target different domains of this structure, and because each domain of the basis pontis recruits a specific set of cerebellar territories, the precuneus can gain access to multiple cerebellar circuits.
Overall, the extent of the connectivity of the precuneus is widespread and involves higher association cortical and subcortical structures. Notably, no direct connections with the primary sensory regions have been observed. Therefore, it seems reasonable to assume that precuneus activity influences an extensive network of cortical and subcortical structures involved in elaborating highly integrated and associative information, rather than directly processing external stimuli.
Behavioural correlates of the precuneus
The posterior medial parietal cortex has long been known to belong to the associative cortices, which is the widely distributed network sharing connections with other cortical and subcortical regions to permit the brain to integrate both external and self-generated information and to produce much of the mental activity that characterizes Homo sapiens. Furthermore, the precuneus is more highly developed (i.e. comprises a larger portion of the brain volume) in human beings than in non-human primates or other animals, has the most complex columnar cortical organization and is among the last regions to myelinate (Goldman-Rakic, 1987).
Taken together, anatomical and connectivity data seem to suggest a relevant role for the precuneus in the implementation of a wide range of higher-order cognitive functions, the exact nature of which has long been a subject for speculation. In fact, the few lesion studies of both humans and non-human primates have been unsuccessful in illuminating a specific function. Fortunately, the results of lesion studies have been informed by a number of recent functional imaging studies that have demonstrated activity within the medial parietal areas during certain forms of complex behaviours. These studies suggest that the precuneus plays an important role in a diverse array of highly integrated functions that can no longer be regarded as a simple extension of the visuo-spatial processes subserved by the lateral parietal cortices.
In the following section we review the literature on the behavioural correlates of precuneus activity, with special reference to imaging neuroscience. We focus on haemodynamic techniques, namely functional MRI (fMRI) and PET, which investigate neural activity by measuring changes in blood flow, and these have been widely used to explore the functional neuroanatomy of cognitive functions. However, a few magnetoencephalography (MEG) and neuropsychological studies have been included in the discussion, where relevant.
Tables 2–4 summarize the results of fMRI and PET studies demonstrating patterns of activation within the precuneus (BA 7). Neuroimaging studies covering different aspects of cognitive functions have been analysed and arbitrarily classified into four broad categories (visuo-spatial imagery, episodic memory retrieval, self-processing and consciousness), based on similarity of addressed tasks and clusters of cross-references. The studies included in this review have been identified by manual and electronic searches within PubMed and the SCI-Extended database of ISI Web of Knowledge. We excluded publications that (i) were not peer-reviewed, (ii) did not examine the whole brain or (iii) did not report activation foci in 3D Talairach coordinates. However, it should be appreciated that Talairach coordinates in imaging studies do not always refer to the same thing, as some are listed in proper Talairach stereotactic space, others have been spatially normalized to Talairach-type coordinates according to the Montreal Neurological Institute (MNI) template (Evans et al., 1993) and some others again have been converted using daemons. This is a problem for any form of meta-analysis and is made the more difficult because the methods employed by the authors often are not specified. When listing the functional imaging studies in the tables, we have specified the coordinates of the respective activations, along with the indication of the ascribed region and corresponding BA, whenever provided in the original papers, so that the reader can assess the degree of variance. For each category, we listed the publications that, in our view, were designed to study the most general aspects of the function of interest. We minimized redundancy by avoiding listing multiple comparison procedures from individual studies and selected the results that best represent the area under consideration. In some cases, studies could be classified in more than one category. For example, contrasts involving recall of non-verbal materials in the episodic memory retrieval section could also be included in the visuo-spatial imagery section. In such cases, the category into which they seemed to fit best was chosen in light of the other results in the category.
Functional imaging studies of visuo-spatial imagery showing significant activation of the precuneus
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Wenderoth et al. (2005) | fMRI | Coordination of motor behaviour | Bimanual movements versus unimanual subtasks | Precuneus (dorso-anterior) | −10;−52;68 | + | − | 4;−50;70 | + | − | ||||
| Culham et al. (1998) | fMRI | Attentive tracking of moving targets | Attentive tracking versus passive viewing of moving targets | Precuneus | −18;−70;69 | − | + | 10;−63;76 | + | + | ||||
| Simon et al. (2002) | fMRI | Attention orientation | Attention only versus calculation/language/grasping tasks | Precuneus | −4;−52;52 | + | − | − | − | |||||
| Le et al. (1998) | fMRI | Attention orientation | Shifting versus sustained visual attention | Precuneus/cuneus | −3;−74;34 | − | + | 9;−70;36 | − | + | ||||
| Nagahama et al. (1999) | fMRI | Attention shift between object features | Card sorting task with versus without attention shift | Precuneus | −8;−78;36 | − | + | 4;−70;32 | − | + | ||||
| Hanakawa et al. (2003) | fMRI | Motor imagery | Imagery versus execution of finger tapping sequences | Precuneus/post sup. parietal cortex | −18;−53;54 | + | − | 9;−63;58 | + | + | ||||
| Malouin et al. (2003) | PET | Motor imagery | Mental representation (MR) of walking with obstacles versus MR of walking | Precuneus | −17;−59;54 | + | − | 12;−64;65 | + | + | ||||
| Suchan et al. (2002) | PET | Mental rotation | Visuo-spatial matrix rotation versus matrix comparison | Precuneus (BA 7) | −23;−61;51 | + | − | 6;−62;51 | + | − | ||||
| Knauff et al. (2003) | fMRI | Mental imagery in deductive reasoning | Deductive reasoning inferences versus rest interval | Precuneus (BA 7) | −18;−58;55 | + | − | 15;−65;45 | + | + | ||||
| Platel et al. (1997) | PET | Music perception | Detection of high versus low pitch tones | Precuneus/cuneus | −14;−70;28 | − | + | − | − | |||||
| Satoh et al. (2001) | PET | Music perception | Alto part versus whole harmony listening | Precuneus | −1;−60;38 | + | − | 8;−69;45 | − | + | ||||
| Ghaem et al. (1997) | PET | Mental navigation | Mental simulation of memorized routes versus silent rest | Precuneus | −4;−82;40 | − | + | − | − | |||||
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Wenderoth et al. (2005) | fMRI | Coordination of motor behaviour | Bimanual movements versus unimanual subtasks | Precuneus (dorso-anterior) | −10;−52;68 | + | − | 4;−50;70 | + | − | ||||
| Culham et al. (1998) | fMRI | Attentive tracking of moving targets | Attentive tracking versus passive viewing of moving targets | Precuneus | −18;−70;69 | − | + | 10;−63;76 | + | + | ||||
| Simon et al. (2002) | fMRI | Attention orientation | Attention only versus calculation/language/grasping tasks | Precuneus | −4;−52;52 | + | − | − | − | |||||
| Le et al. (1998) | fMRI | Attention orientation | Shifting versus sustained visual attention | Precuneus/cuneus | −3;−74;34 | − | + | 9;−70;36 | − | + | ||||
| Nagahama et al. (1999) | fMRI | Attention shift between object features | Card sorting task with versus without attention shift | Precuneus | −8;−78;36 | − | + | 4;−70;32 | − | + | ||||
| Hanakawa et al. (2003) | fMRI | Motor imagery | Imagery versus execution of finger tapping sequences | Precuneus/post sup. parietal cortex | −18;−53;54 | + | − | 9;−63;58 | + | + | ||||
| Malouin et al. (2003) | PET | Motor imagery | Mental representation (MR) of walking with obstacles versus MR of walking | Precuneus | −17;−59;54 | + | − | 12;−64;65 | + | + | ||||
| Suchan et al. (2002) | PET | Mental rotation | Visuo-spatial matrix rotation versus matrix comparison | Precuneus (BA 7) | −23;−61;51 | + | − | 6;−62;51 | + | − | ||||
| Knauff et al. (2003) | fMRI | Mental imagery in deductive reasoning | Deductive reasoning inferences versus rest interval | Precuneus (BA 7) | −18;−58;55 | + | − | 15;−65;45 | + | + | ||||
| Platel et al. (1997) | PET | Music perception | Detection of high versus low pitch tones | Precuneus/cuneus | −14;−70;28 | − | + | − | − | |||||
| Satoh et al. (2001) | PET | Music perception | Alto part versus whole harmony listening | Precuneus | −1;−60;38 | + | − | 8;−69;45 | − | + | ||||
| Ghaem et al. (1997) | PET | Mental navigation | Mental simulation of memorized routes versus silent rest | Precuneus | −4;−82;40 | − | + | − | − | |||||
Ant = anterior; Post = posterior; + = significant activation detected; − = no significant activation detected. Studies are listed according to their order of appearance in text. Stereotactic coordinates of local maxima of activation are expressed as x;y;z values in proper Talairach space and/or Talairach-type MNI space; however, caution is required since the method used is sometimes not specified in the original papers.
Functional imaging studies of visuo-spatial imagery showing significant activation of the precuneus
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Wenderoth et al. (2005) | fMRI | Coordination of motor behaviour | Bimanual movements versus unimanual subtasks | Precuneus (dorso-anterior) | −10;−52;68 | + | − | 4;−50;70 | + | − | ||||
| Culham et al. (1998) | fMRI | Attentive tracking of moving targets | Attentive tracking versus passive viewing of moving targets | Precuneus | −18;−70;69 | − | + | 10;−63;76 | + | + | ||||
| Simon et al. (2002) | fMRI | Attention orientation | Attention only versus calculation/language/grasping tasks | Precuneus | −4;−52;52 | + | − | − | − | |||||
| Le et al. (1998) | fMRI | Attention orientation | Shifting versus sustained visual attention | Precuneus/cuneus | −3;−74;34 | − | + | 9;−70;36 | − | + | ||||
| Nagahama et al. (1999) | fMRI | Attention shift between object features | Card sorting task with versus without attention shift | Precuneus | −8;−78;36 | − | + | 4;−70;32 | − | + | ||||
| Hanakawa et al. (2003) | fMRI | Motor imagery | Imagery versus execution of finger tapping sequences | Precuneus/post sup. parietal cortex | −18;−53;54 | + | − | 9;−63;58 | + | + | ||||
| Malouin et al. (2003) | PET | Motor imagery | Mental representation (MR) of walking with obstacles versus MR of walking | Precuneus | −17;−59;54 | + | − | 12;−64;65 | + | + | ||||
| Suchan et al. (2002) | PET | Mental rotation | Visuo-spatial matrix rotation versus matrix comparison | Precuneus (BA 7) | −23;−61;51 | + | − | 6;−62;51 | + | − | ||||
| Knauff et al. (2003) | fMRI | Mental imagery in deductive reasoning | Deductive reasoning inferences versus rest interval | Precuneus (BA 7) | −18;−58;55 | + | − | 15;−65;45 | + | + | ||||
| Platel et al. (1997) | PET | Music perception | Detection of high versus low pitch tones | Precuneus/cuneus | −14;−70;28 | − | + | − | − | |||||
| Satoh et al. (2001) | PET | Music perception | Alto part versus whole harmony listening | Precuneus | −1;−60;38 | + | − | 8;−69;45 | − | + | ||||
| Ghaem et al. (1997) | PET | Mental navigation | Mental simulation of memorized routes versus silent rest | Precuneus | −4;−82;40 | − | + | − | − | |||||
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Wenderoth et al. (2005) | fMRI | Coordination of motor behaviour | Bimanual movements versus unimanual subtasks | Precuneus (dorso-anterior) | −10;−52;68 | + | − | 4;−50;70 | + | − | ||||
| Culham et al. (1998) | fMRI | Attentive tracking of moving targets | Attentive tracking versus passive viewing of moving targets | Precuneus | −18;−70;69 | − | + | 10;−63;76 | + | + | ||||
| Simon et al. (2002) | fMRI | Attention orientation | Attention only versus calculation/language/grasping tasks | Precuneus | −4;−52;52 | + | − | − | − | |||||
| Le et al. (1998) | fMRI | Attention orientation | Shifting versus sustained visual attention | Precuneus/cuneus | −3;−74;34 | − | + | 9;−70;36 | − | + | ||||
| Nagahama et al. (1999) | fMRI | Attention shift between object features | Card sorting task with versus without attention shift | Precuneus | −8;−78;36 | − | + | 4;−70;32 | − | + | ||||
| Hanakawa et al. (2003) | fMRI | Motor imagery | Imagery versus execution of finger tapping sequences | Precuneus/post sup. parietal cortex | −18;−53;54 | + | − | 9;−63;58 | + | + | ||||
| Malouin et al. (2003) | PET | Motor imagery | Mental representation (MR) of walking with obstacles versus MR of walking | Precuneus | −17;−59;54 | + | − | 12;−64;65 | + | + | ||||
| Suchan et al. (2002) | PET | Mental rotation | Visuo-spatial matrix rotation versus matrix comparison | Precuneus (BA 7) | −23;−61;51 | + | − | 6;−62;51 | + | − | ||||
| Knauff et al. (2003) | fMRI | Mental imagery in deductive reasoning | Deductive reasoning inferences versus rest interval | Precuneus (BA 7) | −18;−58;55 | + | − | 15;−65;45 | + | + | ||||
| Platel et al. (1997) | PET | Music perception | Detection of high versus low pitch tones | Precuneus/cuneus | −14;−70;28 | − | + | − | − | |||||
| Satoh et al. (2001) | PET | Music perception | Alto part versus whole harmony listening | Precuneus | −1;−60;38 | + | − | 8;−69;45 | − | + | ||||
| Ghaem et al. (1997) | PET | Mental navigation | Mental simulation of memorized routes versus silent rest | Precuneus | −4;−82;40 | − | + | − | − | |||||
Ant = anterior; Post = posterior; + = significant activation detected; − = no significant activation detected. Studies are listed according to their order of appearance in text. Stereotactic coordinates of local maxima of activation are expressed as x;y;z values in proper Talairach space and/or Talairach-type MNI space; however, caution is required since the method used is sometimes not specified in the original papers.
Functional imaging studies of episodic memory retrieval showing significant activation of the precuneus
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Tulving et al. (1994) | PET | Auditory sentence recognition | Listening to 'old' versus 'new' sentences | Medial BA 7 | −14;−58;32 | + | − | − | − | |||||
| Shallice et al. (1994) | PET | Verbal episodic memory retrieval | Cued retrieval of word-pairs versus word repetition | Precuneus | −6;−68;36 | − | + | 12;−72;28 | − | + | ||||
| Fletcher et al. (1995) | PET | Memory-related imagery | Cued recall of imageable versus non-imageable verbal paired associates | Precuneus | −2;−54;32 | + | − | 6;−46;36 | + | − | ||||
| Henson et al. (1999) | fMRI | Recognition memory judgements | Recollection of studied words versus judgements for unstudied words | Precuneus (BA 7) | 0;−66;33 | + | + | 0;−66;33 | + | + | ||||
| Krause et al. (1999) | PET | Imagery content-dependent retrieval | Retrieval of abstract word-pairs versus nonsense words | Precuneus | −8;−76;32 | − | + | 12;−76;32 | − | + | ||||
| Schmidt et al. (2002) | PET | Visually/auditorily presented items recall | Retrieval of word-pairs with low imagery content versus nonsense words | Precuneus (BA 7) | −6;−76;28 | − | + | 12;−76;36 | − | + | ||||
| Platel et al. (2003) | PET | Musical episodic memory retrieval | Melodic tunes recognition versus perceptive control tasks | Precuneus (BA 7) | − | − | 2;−62;33 | + | − | |||||
| Lundstrom et al. (2003) | fMRI | Episodic source memory retrieval | Source memory versus item recognition of imagined word-picture pairs | Precuneus (BA 7) | −12;−64;46 | + | + | − | − | |||||
| Lundstrom et al. (2005) | fMRI | Episodic source memory retrieval | Correct source attribution versus false alarm trials | Precuneus (BA 7) | − | − | 12;−74;54 | − | + | |||||
| Gilboa et al. (2004) | fMRI | Autobiographical events recollection | Remote and recent family photographs versus unknown people photographs | Precuneus (BA 7) | − | − | 1;−64;40 | + | + | |||||
| Addis et al. (2005) | fMRI | Autobiographical memory retrieval | Title-cued retrieval of specific events versus general autobiographical memories | Precuneus (BA 7) | 0;−64;44 | + | + | − | − | |||||
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Tulving et al. (1994) | PET | Auditory sentence recognition | Listening to 'old' versus 'new' sentences | Medial BA 7 | −14;−58;32 | + | − | − | − | |||||
| Shallice et al. (1994) | PET | Verbal episodic memory retrieval | Cued retrieval of word-pairs versus word repetition | Precuneus | −6;−68;36 | − | + | 12;−72;28 | − | + | ||||
| Fletcher et al. (1995) | PET | Memory-related imagery | Cued recall of imageable versus non-imageable verbal paired associates | Precuneus | −2;−54;32 | + | − | 6;−46;36 | + | − | ||||
| Henson et al. (1999) | fMRI | Recognition memory judgements | Recollection of studied words versus judgements for unstudied words | Precuneus (BA 7) | 0;−66;33 | + | + | 0;−66;33 | + | + | ||||
| Krause et al. (1999) | PET | Imagery content-dependent retrieval | Retrieval of abstract word-pairs versus nonsense words | Precuneus | −8;−76;32 | − | + | 12;−76;32 | − | + | ||||
| Schmidt et al. (2002) | PET | Visually/auditorily presented items recall | Retrieval of word-pairs with low imagery content versus nonsense words | Precuneus (BA 7) | −6;−76;28 | − | + | 12;−76;36 | − | + | ||||
| Platel et al. (2003) | PET | Musical episodic memory retrieval | Melodic tunes recognition versus perceptive control tasks | Precuneus (BA 7) | − | − | 2;−62;33 | + | − | |||||
| Lundstrom et al. (2003) | fMRI | Episodic source memory retrieval | Source memory versus item recognition of imagined word-picture pairs | Precuneus (BA 7) | −12;−64;46 | + | + | − | − | |||||
| Lundstrom et al. (2005) | fMRI | Episodic source memory retrieval | Correct source attribution versus false alarm trials | Precuneus (BA 7) | − | − | 12;−74;54 | − | + | |||||
| Gilboa et al. (2004) | fMRI | Autobiographical events recollection | Remote and recent family photographs versus unknown people photographs | Precuneus (BA 7) | − | − | 1;−64;40 | + | + | |||||
| Addis et al. (2005) | fMRI | Autobiographical memory retrieval | Title-cued retrieval of specific events versus general autobiographical memories | Precuneus (BA 7) | 0;−64;44 | + | + | − | − | |||||
Ant = anterior; Post = posterior; + = significant activation detected; − = no significant activation detected.
Functional imaging studies of episodic memory retrieval showing significant activation of the precuneus
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Tulving et al. (1994) | PET | Auditory sentence recognition | Listening to 'old' versus 'new' sentences | Medial BA 7 | −14;−58;32 | + | − | − | − | |||||
| Shallice et al. (1994) | PET | Verbal episodic memory retrieval | Cued retrieval of word-pairs versus word repetition | Precuneus | −6;−68;36 | − | + | 12;−72;28 | − | + | ||||
| Fletcher et al. (1995) | PET | Memory-related imagery | Cued recall of imageable versus non-imageable verbal paired associates | Precuneus | −2;−54;32 | + | − | 6;−46;36 | + | − | ||||
| Henson et al. (1999) | fMRI | Recognition memory judgements | Recollection of studied words versus judgements for unstudied words | Precuneus (BA 7) | 0;−66;33 | + | + | 0;−66;33 | + | + | ||||
| Krause et al. (1999) | PET | Imagery content-dependent retrieval | Retrieval of abstract word-pairs versus nonsense words | Precuneus | −8;−76;32 | − | + | 12;−76;32 | − | + | ||||
| Schmidt et al. (2002) | PET | Visually/auditorily presented items recall | Retrieval of word-pairs with low imagery content versus nonsense words | Precuneus (BA 7) | −6;−76;28 | − | + | 12;−76;36 | − | + | ||||
| Platel et al. (2003) | PET | Musical episodic memory retrieval | Melodic tunes recognition versus perceptive control tasks | Precuneus (BA 7) | − | − | 2;−62;33 | + | − | |||||
| Lundstrom et al. (2003) | fMRI | Episodic source memory retrieval | Source memory versus item recognition of imagined word-picture pairs | Precuneus (BA 7) | −12;−64;46 | + | + | − | − | |||||
| Lundstrom et al. (2005) | fMRI | Episodic source memory retrieval | Correct source attribution versus false alarm trials | Precuneus (BA 7) | − | − | 12;−74;54 | − | + | |||||
| Gilboa et al. (2004) | fMRI | Autobiographical events recollection | Remote and recent family photographs versus unknown people photographs | Precuneus (BA 7) | − | − | 1;−64;40 | + | + | |||||
| Addis et al. (2005) | fMRI | Autobiographical memory retrieval | Title-cued retrieval of specific events versus general autobiographical memories | Precuneus (BA 7) | 0;−64;44 | + | + | − | − | |||||
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Tulving et al. (1994) | PET | Auditory sentence recognition | Listening to 'old' versus 'new' sentences | Medial BA 7 | −14;−58;32 | + | − | − | − | |||||
| Shallice et al. (1994) | PET | Verbal episodic memory retrieval | Cued retrieval of word-pairs versus word repetition | Precuneus | −6;−68;36 | − | + | 12;−72;28 | − | + | ||||
| Fletcher et al. (1995) | PET | Memory-related imagery | Cued recall of imageable versus non-imageable verbal paired associates | Precuneus | −2;−54;32 | + | − | 6;−46;36 | + | − | ||||
| Henson et al. (1999) | fMRI | Recognition memory judgements | Recollection of studied words versus judgements for unstudied words | Precuneus (BA 7) | 0;−66;33 | + | + | 0;−66;33 | + | + | ||||
| Krause et al. (1999) | PET | Imagery content-dependent retrieval | Retrieval of abstract word-pairs versus nonsense words | Precuneus | −8;−76;32 | − | + | 12;−76;32 | − | + | ||||
| Schmidt et al. (2002) | PET | Visually/auditorily presented items recall | Retrieval of word-pairs with low imagery content versus nonsense words | Precuneus (BA 7) | −6;−76;28 | − | + | 12;−76;36 | − | + | ||||
| Platel et al. (2003) | PET | Musical episodic memory retrieval | Melodic tunes recognition versus perceptive control tasks | Precuneus (BA 7) | − | − | 2;−62;33 | + | − | |||||
| Lundstrom et al. (2003) | fMRI | Episodic source memory retrieval | Source memory versus item recognition of imagined word-picture pairs | Precuneus (BA 7) | −12;−64;46 | + | + | − | − | |||||
| Lundstrom et al. (2005) | fMRI | Episodic source memory retrieval | Correct source attribution versus false alarm trials | Precuneus (BA 7) | − | − | 12;−74;54 | − | + | |||||
| Gilboa et al. (2004) | fMRI | Autobiographical events recollection | Remote and recent family photographs versus unknown people photographs | Precuneus (BA 7) | − | − | 1;−64;40 | + | + | |||||
| Addis et al. (2005) | fMRI | Autobiographical memory retrieval | Title-cued retrieval of specific events versus general autobiographical memories | Precuneus (BA 7) | 0;−64;44 | + | + | − | − | |||||
Ant = anterior; Post = posterior; + = significant activation detected; − = no significant activation detected.
Functional imaging studies of self-processing showing significant activation of the precuneus
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Kircher et al. (2000) | fMRI | Self-relevant information processing | Judgements on one's own versus another person's face/personality traits | Precuneus (BA 7) | −3;−47;31 | + | − | 9;−64;20 | + | + | ||||
| Kircher et al. (2002) | fMRI | Intentional self-processing | Judgements on self-descriptive versus non-self-descriptive personality traits | Precuneus (BA 7) | −6;−53;31 | + | − | 6;−53;37 | + | − | ||||
| Kjaer et al. (2002) | PET | Reflective self-awareness | Reflections on one's own versus neutral reference person's personality traits | Precuneus (BA 7) | 0;−56;56 | + | − | 0;−56;56 | + | − | ||||
| Lou et al. (2004) | PET | Representation of the mental self | Retrieval of judgements on mental self/other versus syllables counting task | Precuneus/postcingulate | −4;−52;24 | + | − | 4;−50;30 | + | − | ||||
| Vogeley et al. (2001) | fMRI | Perspective taking in story processing | First-person versus third-person short stories processing | Precuneus | −10;−48;64 | + | − | 8;−46;64 | + | − | ||||
| den Ouden et al. (2005) | fMRI | Self-related intentional causality processing | Judgements on intentional versus physical causality | Precuneus/postcingulate | 0;−48;33 | + | − | 0;−48;33 | + | − | ||||
| Ruby and Decety (2001) | PET | Perspective taking in simulation of action | Mental simulation of others versus self-generated action | Precuneus | 0;−66;34 | + | + | 0;−66;34 | + | + | ||||
| Farrer and Frith (2002) | fMRI | Experience of agency | Attribution of visualized action to the experimenter versus self | Precuneus | −6;−58;50 | + | − | 2;−50;44 | + | − | ||||
| Vogeley et al. (2004) | fMRI | Visuo-spatial perspective taking | Third-person versus first-person visual point of reference | Precuneus | − | − | 2;−60;56 | + | + | |||||
| Farrow et al. (2001) | fMRI | Social cognition | Empathic/forgivability versus social reasoning judgements | Precuneus (BA 7) | −4;−64;32 | + | + | − | − | |||||
| Ochsner et al. (2004) | fMRI | Emotional state attribution | Judgements on one's own/another person's emotions versus neutral judgement | Precuneus (BA 7) | −6;−60;30 | + | − | − | − | |||||
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Kircher et al. (2000) | fMRI | Self-relevant information processing | Judgements on one's own versus another person's face/personality traits | Precuneus (BA 7) | −3;−47;31 | + | − | 9;−64;20 | + | + | ||||
| Kircher et al. (2002) | fMRI | Intentional self-processing | Judgements on self-descriptive versus non-self-descriptive personality traits | Precuneus (BA 7) | −6;−53;31 | + | − | 6;−53;37 | + | − | ||||
| Kjaer et al. (2002) | PET | Reflective self-awareness | Reflections on one's own versus neutral reference person's personality traits | Precuneus (BA 7) | 0;−56;56 | + | − | 0;−56;56 | + | − | ||||
| Lou et al. (2004) | PET | Representation of the mental self | Retrieval of judgements on mental self/other versus syllables counting task | Precuneus/postcingulate | −4;−52;24 | + | − | 4;−50;30 | + | − | ||||
| Vogeley et al. (2001) | fMRI | Perspective taking in story processing | First-person versus third-person short stories processing | Precuneus | −10;−48;64 | + | − | 8;−46;64 | + | − | ||||
| den Ouden et al. (2005) | fMRI | Self-related intentional causality processing | Judgements on intentional versus physical causality | Precuneus/postcingulate | 0;−48;33 | + | − | 0;−48;33 | + | − | ||||
| Ruby and Decety (2001) | PET | Perspective taking in simulation of action | Mental simulation of others versus self-generated action | Precuneus | 0;−66;34 | + | + | 0;−66;34 | + | + | ||||
| Farrer and Frith (2002) | fMRI | Experience of agency | Attribution of visualized action to the experimenter versus self | Precuneus | −6;−58;50 | + | − | 2;−50;44 | + | − | ||||
| Vogeley et al. (2004) | fMRI | Visuo-spatial perspective taking | Third-person versus first-person visual point of reference | Precuneus | − | − | 2;−60;56 | + | + | |||||
| Farrow et al. (2001) | fMRI | Social cognition | Empathic/forgivability versus social reasoning judgements | Precuneus (BA 7) | −4;−64;32 | + | + | − | − | |||||
| Ochsner et al. (2004) | fMRI | Emotional state attribution | Judgements on one's own/another person's emotions versus neutral judgement | Precuneus (BA 7) | −6;−60;30 | + | − | − | − | |||||
Ant = anterior; Post = posterior; + = significant activation detected; − = no significant activation detected.
Functional imaging studies of self-processing showing significant activation of the precuneus
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Kircher et al. (2000) | fMRI | Self-relevant information processing | Judgements on one's own versus another person's face/personality traits | Precuneus (BA 7) | −3;−47;31 | + | − | 9;−64;20 | + | + | ||||
| Kircher et al. (2002) | fMRI | Intentional self-processing | Judgements on self-descriptive versus non-self-descriptive personality traits | Precuneus (BA 7) | −6;−53;31 | + | − | 6;−53;37 | + | − | ||||
| Kjaer et al. (2002) | PET | Reflective self-awareness | Reflections on one's own versus neutral reference person's personality traits | Precuneus (BA 7) | 0;−56;56 | + | − | 0;−56;56 | + | − | ||||
| Lou et al. (2004) | PET | Representation of the mental self | Retrieval of judgements on mental self/other versus syllables counting task | Precuneus/postcingulate | −4;−52;24 | + | − | 4;−50;30 | + | − | ||||
| Vogeley et al. (2001) | fMRI | Perspective taking in story processing | First-person versus third-person short stories processing | Precuneus | −10;−48;64 | + | − | 8;−46;64 | + | − | ||||
| den Ouden et al. (2005) | fMRI | Self-related intentional causality processing | Judgements on intentional versus physical causality | Precuneus/postcingulate | 0;−48;33 | + | − | 0;−48;33 | + | − | ||||
| Ruby and Decety (2001) | PET | Perspective taking in simulation of action | Mental simulation of others versus self-generated action | Precuneus | 0;−66;34 | + | + | 0;−66;34 | + | + | ||||
| Farrer and Frith (2002) | fMRI | Experience of agency | Attribution of visualized action to the experimenter versus self | Precuneus | −6;−58;50 | + | − | 2;−50;44 | + | − | ||||
| Vogeley et al. (2004) | fMRI | Visuo-spatial perspective taking | Third-person versus first-person visual point of reference | Precuneus | − | − | 2;−60;56 | + | + | |||||
| Farrow et al. (2001) | fMRI | Social cognition | Empathic/forgivability versus social reasoning judgements | Precuneus (BA 7) | −4;−64;32 | + | + | − | − | |||||
| Ochsner et al. (2004) | fMRI | Emotional state attribution | Judgements on one's own/another person's emotions versus neutral judgement | Precuneus (BA 7) | −6;−60;30 | + | − | − | − | |||||
| Study . | Modality . | Task . | Contrast . | Ascribed region of activation . | Left precuneus . | . | . | Right precuneus . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | . | . | x;y;z . | Ant . | Post . | x;y;z . | Ant . | Post . | ||||
| Kircher et al. (2000) | fMRI | Self-relevant information processing | Judgements on one's own versus another person's face/personality traits | Precuneus (BA 7) | −3;−47;31 | + | − | 9;−64;20 | + | + | ||||
| Kircher et al. (2002) | fMRI | Intentional self-processing | Judgements on self-descriptive versus non-self-descriptive personality traits | Precuneus (BA 7) | −6;−53;31 | + | − | 6;−53;37 | + | − | ||||
| Kjaer et al. (2002) | PET | Reflective self-awareness | Reflections on one's own versus neutral reference person's personality traits | Precuneus (BA 7) | 0;−56;56 | + | − | 0;−56;56 | + | − | ||||
| Lou et al. (2004) | PET | Representation of the mental self | Retrieval of judgements on mental self/other versus syllables counting task | Precuneus/postcingulate | −4;−52;24 | + | − | 4;−50;30 | + | − | ||||
| Vogeley et al. (2001) | fMRI | Perspective taking in story processing | First-person versus third-person short stories processing | Precuneus | −10;−48;64 | + | − | 8;−46;64 | + | − | ||||
| den Ouden et al. (2005) | fMRI | Self-related intentional causality processing | Judgements on intentional versus physical causality | Precuneus/postcingulate | 0;−48;33 | + | − | 0;−48;33 | + | − | ||||
| Ruby and Decety (2001) | PET | Perspective taking in simulation of action | Mental simulation of others versus self-generated action | Precuneus | 0;−66;34 | + | + | 0;−66;34 | + | + | ||||
| Farrer and Frith (2002) | fMRI | Experience of agency | Attribution of visualized action to the experimenter versus self | Precuneus | −6;−58;50 | + | − | 2;−50;44 | + | − | ||||
| Vogeley et al. (2004) | fMRI | Visuo-spatial perspective taking | Third-person versus first-person visual point of reference | Precuneus | − | − | 2;−60;56 | + | + | |||||
| Farrow et al. (2001) | fMRI | Social cognition | Empathic/forgivability versus social reasoning judgements | Precuneus (BA 7) | −4;−64;32 | + | + | − | − | |||||
| Ochsner et al. (2004) | fMRI | Emotional state attribution | Judgements on one's own/another person's emotions versus neutral judgement | Precuneus (BA 7) | −6;−60;30 | + | − | − | − | |||||
Ant = anterior; Post = posterior; + = significant activation detected; − = no significant activation detected.
When plotting the Talairach coordinates of activation maxima from a number of studies, we distinguished between activations occurring in the anterior (y closer to −60 mm) and in the posterior (y closer to −70 mm) precuneus. We chose this subdivision (in addition to the left–right lateralization of the activation focus) with due consideration of the imperfect spatial resolution of the techniques and of the enormous anatomical variability among subjects. The rationale behind this division was based on both existing functional imaging evidence and microstructural findings (i.e. Brodmann's subdivisions 7a and 7b, or von Economo and Koskinas' PEm and PEp).
Precuneus and visuo-spatial imagery
Studies in non-human primates have led to the proposal that the precuneus is part of ‘a neural network functionally specialized for the process of spatially guided behaviour’ (Selemon and Goldman-Rakic, 1988). Based on the findings of neurophysiological and functional imaging studies in healthy humans, it has been argued that the posteromedial parietal cortex acts in concert with the lateral parietal areas in elaborating information about egocentric and allocentric spatial relations for body movement control (motor imagery), as well as higher-order processes such as voluntary attention shift and more abstract mental imagery tasks. Table 2 summarizes the most relevant findings from visuo-spatial imagery studies involving precuneus activation.
The lateral aspect of the posterior parietal cortex—the SPL and IPS, especially in the right hemisphere—has traditionally been considered a higher-order area that is generally involved in controlling spatial aspects of motor behaviour (Grafton et al., 1996; Connolly et al., 2000; Seitz and Binkofski, 2003; Grefkes et al., 2004). Likewise, severe disturbances of visually goal-directed hand movements not related to motor, sensory, visual acuity or visual field disorders (so-called optic ataxia) have traditionally been ascribed to lesions of the SPL and/or the IPS. However, using lesion subtraction methods, Karnath et al. (2005) have recently re-evaluated this view investigating the typical lesion location in a large group of unilateral stroke patients with optic ataxia. In both left hemisphere- and right hemisphere-damaged patients they found optic ataxia to be associated with a lesion area that extended, via the underlying parietal white matter, to the medial cortical aspect, where it affected the precuneus close to the occipito-parietal junction. These observations suggested that both lateral and medial parietal structures could be integral to the control of visually guided reaching in humans.
In fact, a cluster of activation in the SPL extending medially to the anterior precuneus has often been reported in functional imaging studies involving the execution (e.g. Kawashima et al., 1995) or preparation (e.g. Astafiev et al., 2003) of spatially guided behaviours, such as pointing and reaching, and, in particular, when two limbs have to be coordinated in accordance with a complex spatiotemporal pattern. A recent fMRI study by Wenderoth et al. (2005) demonstrated that the execution of spatially complex bimanual coordination tasks as compared with the unimanual subtasks selectively activates the dorso-anterior precuneus as well as the anterior cingulate cortex in both hemispheres. However, in this experiment precuneus activation could also be attributed to its involvement in shifting attention between different locations in space, which is necessary, for example, for monitoring the trajectories of the left and right wrists when both limbs move in parallel. Converging evidence then suggests that the SPL and the precuneus cooperate in directing attention in space not only during the execution of goal-directed movements, but also in the absence of overt motor responses. In an fMRI study by Culham et al. (1998), subjects viewed a display of bouncing balls and used attention to mentally track a subset of them while fixating. Comparison between attentive tracking and passive viewing revealed bilateral activation of the lateral parietal cortex (SPL, IPS, post-central sulcus) extending to the precuneus and the frontal eye fields.
The hypothesis that the medial parietal cortex plays a key role in attentive tracking is supported by neuropsychological evidence. A patient who displayed both a left parietal lesion centred around the precuneus and a posterior split of the corpus callosum was tested by Michel et al. (1997) in a bouncing balls tracking task similar to the one used in the study by Culham et al. (1998). As expected from the isolated left hemisphere damage (with no possible compensation from the intact right hemisphere because of the callosal disconnection), the patient showed a severely impaired attentive-tracking performance in the right visual hemifield. Simon et al. (2002) collected fMRI data while subjects performed several different tasks, including attention, pointing, grasping, saccades, calculation and phoneme detection. Bilateral SPL and precuneus were activated in both saccades and pointing tasks, but a cluster of voxels situated in the left anterior precuneus showed greater activation for attention only. Moreover, in an fMRI study, Le et al. (1998) reported that shifting attention for visual stimuli, when compared with sustained attention, produced bilateral activation of the precuneus and the SPL. These findings are in accordance with the classical clinical picture of Balint's syndrome, the cardinal feature of which is the inability to perceive the visual field as a whole—a fixed form of tunnel vision usually referred to as simultanagnosia (Balint, 1909). The most common cause for this condition is bilateral occipitoparietal damage, often extending medially to include the precuneus (Critchley, 1953; Raichle et al., 2001).
An fMRI study by Nagahama et al. (1999) showed that the precuneus may process not only spatial attention, but also attention shift between object features. In this study, healthy subjects underwent a card-sorting task and had to shift the sorting principle in response to an ‘incorrect’ feedback. Transient increase of neural activity time locked with attention shift phases was detected in the precuneus bilaterally and in the rostral SMA. These results are consistent with those of a previous PET study by Fink et al. (1997), in which the left medial parietal cortex and left SMA co-varied with the number of attention switches between local and global levels of complex visual figures. Furthermore, activation of the posterior parietal cortex extending into the precuneus has been reported in tasks involving covert shifts of spatial attention, i.e. shifts of attention without eye movements (Gitelman et al., 1999; Beauchamp et al., 2001).
Following preliminary reports of precuneus activation during tasks requiring spatial information about the direction of movements in an imaginary field (Bonda et al., 1995; Parsons et al., 1995), the precuneus was observed to be more responsive during motor imagery than during real execution of joystick (Stephan et al., 1995) and finger (Gerardin et al., 2000) movements. More recently, Hanakawa et al. (2003) used fMRI to compare the functional neuroanatomy of motor execution and imagery with a task that objectively assessed imagery performance. Subjects were required to perform sequential finger-tapping movements according to visually presented stimuli in either a movement or an imagery mode. Imagery-predominant areas included the frontal precentral sulcus zone and the posterior superior parietal cortex, extending to the precuneus, bilaterally. Thus, exchange of information between the premotor and parietal areas appears to be necessary when the visuo-spatial stimulus is processed even only mentally, without the execution of motor activity and is independent of perceptual modalities (mental imagery). Malouin et al. (2003) used PET to determine the cerebral regions associated with the mental simulation of increasingly complex locomotor tasks. In this study, the subtraction of the ‘walking’ condition from the ‘walking with obstacles’ condition revealed the existence of a distinct neural network that involves the bilateral precuneus, the right parietal cortex and the left SMA for the construction of an internal representation of the specific location of the obstacles and their position relative to themselves while imagining walking through the virtual environment.
The role of the precuneus in motor imagery has been confirmed by an electromagnetic study in which MEG was applied to subjects who imagined themselves hurdling in self-centred space (Ogiso et al., 2000). Analysis of MEG waveforms revealed that the activation of the precuneus preceded the beginning of imagined movement, thus indicating that the precuneus may be involved in the generation of the spatial information necessary for imagined whole body movements. Supporting evidence has been provided by a recent neuropsychological study by Wiest et al. (2004), in which they described a patient with recurrent episodes of what they referred to as ‘epileptic linear self-motion perception’ caused by a circumscribed ependymoma in the right paramedian precuneus, as revealed by intracranial seizure monitoring.
Notably, functional imaging studies reporting activation of the precuneus in cognitive tasks requiring mental imagery are not limited to motor imagery, but include a few other examples, namely visual rotation, deductive reasoning, music processing and mental navigation. In a PET study by Suchan et al. (2002), visuo-spatial matrix rotation led to activation of the right dorsolateral prefrontal cortex and bilateral superior and inferior parietal lobe, extending to the anterior precuneus. An fMRI study by Knauff et al. (2003) investigated the neurocognitive processes of mental imagery in deductive reasoning. In the absence of any correlated visual input (problems were presented acoustically via headphones), different types of reasoning problems evoked activity in the right superior parietal cortex and bilaterally in the precuneus.
Functional imaging studies addressing music-brain interaction have suggested that music processing and visual imagery are closely interconnected and that the precuneus plays a key role in both these cognitive tasks. A landmark PET investigation that sought to disentangle the different components of music perception in non-musicians found that the left precuneus and cuneus were the main areas active during detection of pitch changes in a sequence of sounds (Platel et al., 1997). This pattern of activation was observed by the authors as a consequence of the mental imagery strategy employed to perform the pitch discrimination task, since subjects had to write the tones on a ‘mental stave’in terms of ‘high’ and ‘low’ pitch. Furthermore, in order to clarify whether different regions of the brain are activated according to the mode of listening, Satoh et al. (2001) studied changes in regional cerebral blood flow (rCBF) with PET in music students concentrating on the alto-part within a piece of music, compared with listening to its harmony as a whole. The alto-part-listening condition was associated with rCBF increases in the left precuneus, bilateral SPL, premotor area and orbital frontal cortex. It is likely that activation of the left precuneus was related to mental imagery processing of the alto part, rather like writing tones of the alto part on a mental score.
In a study by Ghaem et al. (1997), PET was used to investigate the functional anatomy of mental simulation of routes on an internal map which had been previously studied by actual navigation. This task appears to be subserved by a specific mental navigation network, comprising the left posterior precuneus, insula and medial part of the hippocampal regions. According to the authors, both visuo-spatial imagery and retrieval processes could be related to the posterior precuneus activation. Suzuki et al. (1998) described the case of a 70-year-old woman who presented with pure topographical disorientation following haemorrhage in the right medial parietal lobe, located mainly in the precuneus. She could not navigate in the real world despite good performance on visuo-spatial learning tests, indicating a selective impairment of mental navigation-related networks.
In summary, the premotor–posteromedial parietal connections have proven likely to subserve abstract cognitive processes involving visuo-spatial information and, more specifically, voluntary attention shifts between targets. One possibility is that the visuo-spatial tasks activating posterior locations within the precuneus involve more mnemonic visual information processing (especially spatial representation of sequential movements with reference to memorized patterns), whereas the tasks activating anterior locations required more intuitive imagery representation (e.g. movement coordination, mental rotation, deductive reasoning). However, from Table 2 this needs to be interpreted with caution, and further studies are needed to rule out the possibility that visuo-spatial information processing and spatially guided behaviour tasks primarily activate lateral parietal areas with the areas of (co)activation spreading into other parts of the parietal cortex and thus extending into the precuneus.
Precuneus and episodic memory retrieval
Various different systems of memory have been described. It is commonly agreed that a major distinction exists between ‘implicit’ or ‘non-declarative’ memory, which allows for some types of skill learning and conditioning processes, and ‘explicit’ or ‘declarative’ memory, which enables remembering of past events. The latter has been subdivided by Tulving (1972) into ‘episodic’ and ‘semantic’ memory. Episodic memory is employed for storage and recall of previously experienced events (episode = event, Greek), which are sequentially ordered in time. This kind of memory has autobiographical reference (Tulving, 1983), since it entails the recollection of information that is linked to an individual's personal experiences. Moreover, it has been argued that the capacity to place events in time and to reference them to oneself may form the basis for a special awareness for subjective time called ‘autonoetic consciousness’ (Andreasen et al., 1995; Wheeler, 2000; Baddeley, 2001; Tulving, 2002). Episodic memory is contrasted with semantic memory, which corresponds roughly with general knowledge about the world, without any autobiographical context (Gardiner, 2001). Semantic memory is sometimes identified with ‘noetic awareness’ as opposed to autonoetic consciousness, and is measured by ‘know’ rather than ‘remember’ responses (Tulving, 1983).
In 1983 Tulving introduced the concept that episodic and semantic memory corresponded with functionally distinct, though overlapping, mind-brain systems, and even in early neuroimaging studies of memory, the precuneus, together with the interlinked cingulate and prefrontal cortices, has been selectively implicated in episodic memory retrieval-related tasks. Table 3 summarizes the most relevant findings.
A region of increased blood flow situated in the left precuneus, together with a predominantly right-sided prefrontal activation, was described in a PET study by Tulving et al. (1994) for the recognition of meaningful sentences that had been presented 24 h previously. Shallice et al. (1994) examined episodic memory retrieval using verbal paired associates. Scans were performed during the cued retrieval of word-pairs while a control task employed word repetition. A direct comparison between the episodic and the semantic retrieval conditions showed specific engagement of the bilateral precuneus and the right prefrontal cortex in association with episodic retrieval. The importance of this dissociation is that it provided for the first time functional anatomical support for the taxonomic distinction between episodic and semantic memory (Frackowiak et al., 1997).
Earlier functional imaging studies addressed episodic memory, which almost invariably involved the use of concrete highly imageable words. In a landmark PET study, Fletcher et al. (1995) used a mnemonic strategy during retrieval to test a hypothesis about the possible relationship of precuneus activity to visual imagery. Their results led them to label the precuneus as the ‘mind's eye’. In this study, rCBF was measured in six right-handed, healthy male volunteers engaged in the cued recall of either imageable verbal paired associates (e.g. River … Stream) or non-imageable paired associates (e.g. Justice … Law). Memory-related imagery was associated with significant bilateral activation of the anterior precuneus, thus providing strong evidence that the precuneus is a key part of the neural substrate of visual imagery occurring in episodic memory recall (Buckner et al., 1995; Fletcher et al., 1996; Halsband et al., 1998). Likewise, in an fMRI episodic retrieval study by Henson et al. (1999), the precuneus region showed consistent activation for recollection judgements on previously studied words, thus providing further support for the hypothesis that precuneus activation may reflect reinstatement of visual images associated with remembered words.
However, a PET study by Krause et al. (1999) apparently contradicted this view, since they found significant bilateral activation of the posterior precuneus during a paired word associate memory task employing both concrete and abstract nouns. Such activation of the precuneus using abstract and therefore non-imageable words suggests a role in episodic memory retrieval irrespective of the imagery content of the items that are remembered. Another PET study by Schmidt et al. (2002) showed that the precuneus activation was not changed during imagery manipulation, thus providing further evidence that posteromedial parietal cortex involvement could not be restricted to processes involving imagery. In a PET study of musical episodic memory (Platel et al., 2003), melodic tunes recognition tasks were contrasted with perceptive control tasks, resulting in activation of the classic episodic memory network, namely the prefrontal cortex, the anterior cingulate gyrus and the precuneus. Although bilateral, these activations were more prominent in the right hemisphere. Again, precuneus involvement was likely to be related to the success of episodic recall, rather than a process of mental imagery, since the musical material used in this experiment did not involve particularly imageable features, and no subject mentioned had employed a specific mental representation strategy.
Taken together, functional imaging data on activation patterns in episodic memory retrieval tasks suggest an antero–posterior functional segregation within the precuneus. Activation of the posterior precuneus exhibits the strongest correlation with successful retrieval of remembered episodes, regardless of imageable characteristics; whereas, the more anterior portion of the precuneus reveals increased rCBF in the context of the retrieval mode, i.e. polymodal imagery (Kapur et al., 1995; Nyberg, 1999; Naghavi and Nyberg, 2005).
All of the aforementioned neuroimaging studies of memory retrieval investigated memory for standardized laboratory stimuli, such as lists of words or sets of pictures. However, memories for stimuli studied in a laboratory setting are dissimilar in important ways from naturally acquired autobiographical memories, since latter are more likely to involve complex, multimodal and emotionally salient memories embedded in a rich context of personal, social and environmental information (Rubin, 1998). Lundstrom et al. (2003) performed an fMRI study that examined the neural correlates of explicit event-related source memory retrieval of words paired with corresponding imagined or viewed pictures. In contrast to item recognition, source memory tasks demand explicit recall of contextual information, where subjects must remember not only an item but also the context within which it was presented—the spatial and temporal location of dots, words or pictures at presentation. Results showed that the left precuneus and left lateral prefrontal cortex are selectively activated during source memory retrieval due to regeneration of contextual associations, a possible hallmark of rich, personal memory that is dependent on retrieval of source information. These findings have recently been replicated by the same group in a further fMRI study that used a similar source memory paradigm, with longer latency between encoding and retrieval (Lundstrom et al., 2005). In a functional imaging study by Gilboa et al. (2004), fMRI was used to study brain regions implicated in retrieval of remote autobiographical memory through the inspection of family photographs selected by confederates without the participant's involvement. Context-rich memories were associated with activity in the right precuneus and bilateral lingual gyri, independently of their age. Retrieving detailed vivid autobiographical experiences, as opposed to personal semantic information, was interpreted by the authors as a crucial mediating feature that determines the involvement of the posteromedial neocortical regions. Likewise, an fMRI study by Addis et al. (2004) found that activity in the left SPL, left precuneus and right cuneus characterizes retrieval of specific autobiographical events versus general past memories.
Quite recently, functional imaging studies of clinical populations have been corroborating the existing evidence for a selective role of the medial parietal cortex in autobiographical memory. In a PET study by Eustache et al. (2004) a pattern of reduced resting cerebral glucose utilization in the bilateral precuneus, inferior parietal lobule and posterior cingulate was found to correlate with the severity of autobiographical memory impairment in a group of patients with mild to moderate Alzheimer's disease.
In summary, it is likely that different aspects of episodic memory retrieval are represented in distinct regions within the precuneus. Functional neuroimaging studies of episodic memory retrieval showed that the precuneus may be functionally dissociable according to both retrieval mode and retrieval success. Specifically, the posterior precuneus seems to be associated with successful retrieval attempts, while the more anterior portion reveals increased rCBF in the context of retrieval mode (mental imagery). Furthermore, these results provide additional support for the association between precuneus activity and internal imagery as outlined in the visuo-spatial information processing tasks (see the previous section). Finally, real life and autobiographical memories recall seems to implicate the selective participation of the precuneus and posterior cingulate/retrosplenial cortex, possibly through both successful episodic memory retrieval and mental imagery strategies.
Precuneus and self-processing
The interconnected medial prefrontal regions and the posteromedial parietal cortex have been proposed to represent a network through which personal identity and past personal experiences are interlinked with one another, with the net interactions permitting us to move between representation and awareness of the self (Andreasen et al., 1995). Recent research has delineated a network of brain areas involved in self versus non-self representation: self-referential judgements, first- versus third-person perspective taking, perceived agency and mind reading/social cognition. Table 4 summarizes the most relevant neuroimaging studies demonstrating precuneus involvement in self-processing tasks.
Kircher et al. (2000) studied the neural systems involved in self-relevant information processing by comparing the judgement of self-relevant traits with self-irrelevant traits. Individually tailored faces and personality trait words were used as stimuli in an fMRI experiment where subjects were asked to make decisions about psychological trait adjectives previously categorized as describing their own attributes. Activation was present in the bilateral anterior precuneus, left SPL, left lateral prefrontal cortex and left anterior cingulate, suggesting an interlinked neural network engaged in self-processing. The same group investigated the cerebral correlates of self versus non-self judgements by analysing localized MRI signal changes while the subjects processed words describing personality traits and physical features, in two different experimental settings (Kircher et al., 2002). In the first experiment (intentional self-processing), the subjects were presented with personality trait adjectives and they had to categorize them as either accurate or inaccurate of their own personality. In the second (incidental self-processing), subjects categorized words on physical versus psychological attributes, being unaware that these words had been arranged in blocks of self-descriptiveness. Overall, the results of both experiments showed that self-descriptive traits compared with non-self-descriptive traits evoke a unique pattern of neural activation, including the anterior precuneus. Recent PET studies provided supporting evidence for these preliminary findings. Kjaer et al. (2002) asked healthy subjects to think intensely on how they would describe their own personality traits and physical appearance and of a neutral reference person known to all the subjects. Statistical parametric mapping showed differential activity in the anterior precuneus and angular gyri during reflection on their own personality traits and in the anterior cingulate gyri during reflection on their own physical traits.
Lou et al. (2004) compared rCBF changes during retrieval of previous judgements of psychological traits referred to three subjects with different degrees of self-relevance, namely oneself, best friend and a neutral person. Results showed activation decrease in the right inferior and medial parietal region, namely anterior precuneus and posterior cingulate cortex, with decreasing self-reference. These findings were confirmed when transcranial magnetic stimulation (TMS) was used to transiently disrupt normal neural circuitry in the medial parietal region (Pz, midway between vertex and occipital pole) to see whether such disruption would affect the task. As expected, there was a decrease in the efficiency of retrieval of previous judgement of the mental self compared with that of others indicating an effect of TMS for self-reference specifically at a latency of 160 ms.
A few other imaging studies have focused on the role of posteromedial parietal cortex in the manipulation of perceived agency (point of view). Vogeley et al. (2001) performed an fMRI study where subjects were required to read short stories written in the first-person in comparison with a third-person perspective. They demonstrated differential activation in the bilateral precuneus and anterior cingulate cortex, and right temporo-parietal junction, when the test persons were involved as an agent in the particular story (first-person perspective taking).
These results converge in suggesting that the medial parietal cortex is involved in assigning first-person perspective (the viewpoint of the observing self) and interpreting an action as being controlled by oneself versus another person (Vogeley and Fink, 2003). The general pattern of activation evoked by these first-person perspective tasks is schematically represented in Fig. 4.
Schematic representation of the main cortical regions activated during first-person perspective tasks [reprinted with permission from Vogeley and Fink (2003)].
In a fMRI study by den Ouden et al. (2005), comparison between questions related to the causal link between one's own intentions and actions (intentional causality), and questions related to the causal link between physical events and their consequences (physical causality), resulted in significant bilateral activation of the bilateral precuneus, posterior cingulate, prefrontal cortex, superior temporal sulcus, and temporal pole. According to the authors, one possibility is that the precuneus, together with the posterior cingulate cortex, is specifically involved in processing intentions related to the self. However, previous neuroimaging studies on perspective-taking tasks have produced inconsistent results.
In a PET study, the precuneus showed stronger activation bilaterally for third-person perspective than for first-person perspective simulation (Ruby and Decety, 2001). Likewise, in two fMRI studies, differential increases of neural activity were found bilaterally in the precuneus and frontal cortex during third-person relative to first-person perspective (Farrer and Frith, 2002; Vogeley et al., 2004). One interpretation is that an overactivation of regions involved in self-representation occurs during third-person perspective simulation, because the brain creates a particularly vivid representation of the self in order to be able to imagine another person with the same neural resources as the self (Ruby and Decety, 2001). However, another possibility is that both first- and third-person perspective-taking processes entail a common cognitive function, e.g. internal representation through mental imagery, which has been consistently shown to require anterior precuneus activity (see above).
Moreover, the ability of distinguishing the perspectives of the self from those of others is relevant to knowing that the contents of other people's minds can be different from our own (Siegal and Varley, 2002). To take such a third-person perspective, subjects have to be aware of what the other person thinks and intends to do. This kind of awareness has been referred to as the theory of mind (ToM), the capacity to predict and explain other people's behaviour based on a representation of their intentions and mental states. In addition to several cortical and subcortical structures located in the frontal lobes, the medial parietal cortex has been implicated in ToM. In this context, the precuneus has been shown to be bilaterally activated during judgements requiring empathy in a functional imaging study that used fMRI to examine the neural correlates of making forgivability judgements in social scenarios (Farrow et al., 2001). Empathic and forgivability judgements activate specific brain regions, including the left precuneus, the left superior frontal gyrus and the right orbitofrontal gyrus, which are suggested to contribute to successful social interaction, based on the understanding of other people's intentions and actions (mind reading). The possible role played by the precuneus in empathic judgements has been confirmed in a recent fMRI study by Ochsner et al. (2004), in which regions commonly activated by attribution of emotions to the self and other people were identified in the left precuneus, posterior cingulate and prefrontal cortex.
In sum, the precuneus appears to play a crucial role in self-processing tasks, possibly through the involvement of mental imagery strategies, as suggested by consistent anterior precuneus activation patterns (Table 4). Self-related episodic memory retrieval could also contribute to shifting between first- and third-person perspective taking. Overall, it emerges that the intentional self component is an important factor in precuneus involvement. These results fit well with Damasio's hypothesis (1999), according to which medial parietal areas, together with the secondary somatosensory cortices and insula, help subserve the primitive representation of the self in relationship with the outside world (proto-self).
Precuneus and consciousness
It is now almost 10 years since brain functional imaging studies first suggested that cerebral blood flow and metabolism may vary across different cortical regions during the conscious resting state, being somewhat greater in the medial parietal, medial occipital, and mid-dorsolateral prefrontal areas (Gur et al., 1995; Binder et al., 1999). Shulman et al. (1997) performed a meta-analysis of nine functional imaging studies, all of which included the passive viewing of visual stimuli as control tasks, in order to assess the presence of cortical activations across visual tasks. The results showed a pattern of signal decreases, involving the frontoparietal areas and including the precuneus, during the performance of goal-directed actions when compared with passive stimulus viewing.
More recently, a meta-analysis of other nine PET activation protocols dealing with different cognitive tasks further investigated the functional neuroanatomy of the conscious resting state (Mazoyer et al., 2001). Notably, instead of using passive viewing as control tasks, these studies shared an eyes-closed resting condition as a common control state, during which subjects were instructed to relax, refrain from moving and avoid systematic thoughts. Using a reverse-subtraction strategy, this meta-analysis revealed a striking and consistent set of decreases during the performance of various goal-directed actions, compared with rest with eyes closed. Again, and despite the difference in the control condition, such deactivations occurred in a network of heteromodal associative frontoparietal areas, including the precuneus, thus revealing the presence of processes that sustained the conscious resting state, while being attenuated during the performance of the cognitive tasks.
Eventually, Raichle et al. (2001) used the oxygen extraction fraction, a measure that represents the change in the proportion of oxygen delivered to oxygen utilized, to effectively demonstrate that despite changes in cerebral blood flow and oxygen consumption a metabolic equilibrium is reached in terms of neuronal activity when normal subjects are in a resting state, lying quietly with eyes closed. They found that during the baseline resting state, a neural network comprising the precuneus and posteromedial parietal region, along with lateral parietal, ventromedial prefrontal, mid-dorsolateral prefrontal and anterior temporal cortices, exhibits a remarkably high metabolic activity (hot spots). Moreover, the tonic level of activity of the precuneate cortex and of the other hot spots of the brain decreased when subjects were engaged in goal-directed cognitive processing or perceptual tasks (task-induced deactivations, TIDs). In other words, when obliged to perform an active task, the brain typically suspends baseline processes, producing deactivations in the regions subserving those processes (Binder et al., 1999; Gusnard and Raichle, 2001; Mitchell et al., 2003). According to current thinking, such high baseline metabolic rate and predilection for TIDs suggest the existence of an organized baseline state of neural activity, which is referred to as ‘the default mode of brain function’ (Gusnard et al., 2001; Raichle et al., 2001; Greicius et al., 2003; Van Horn, 2004).
Furthermore, the precuneus is of particular interest, because it shows the highest resting metabolic rate among these zones, consuming ∼35% more glucose than any other area of the cerebral cortex in humans (Gusnard and Raichle, 2001) and other species (Harley and Bielajew, 1992). However, the behavioural correlates of this default-mode network activity have proven difficult to identify, and relatively little is known about the purpose and significance of the spontaneous mental processing taking place during rest. One possibility is that when an individual is awake and alert and yet not actively engaged in a particular cognitive task, the precuneus and interconnected posterior cingulate and medial prefrontal cortices are engaged in continuous information gathering and representation of the self and external world (Gusnard and Raichle, 2001). This hypothesis fits nicely with the observed functional TIDs: when non-self-referential goal-directed processes are to be performed, the resting state processes are interrupted, reflecting a necessary reduction in resources devoted to general information gathering and evaluation. It would appear to be a default activity of the brain with rather obvious evolutionary significance. When the successful performance of a task demands focused attention such a broad information gathering activity needs to be curtailed (Gusnard and Raichle, 2001; Raichle et al., 2001, McKiernan et al., 2003). Likewise, Binder et al. (1999) suggested that precuneus activity during conscious resting states supports conceptual processing operating on internal stores of information (endogenous signals) rather than ‘perceptual’ functions (concerned with sources of information external to the brain). Altogether, the hot spots that characterize the default mode of the resting brain seem to be engaged in such processes as retrieval or consolidation of episodic memory, conscious representation of information in the form of mental images and spontaneous thoughts, and manipulation of this information for problem-solving and planning. This model is neuroanatomically acceptable in that the identified regions comprise a network of areas that are relatively distant (as measured by cortico-cortical connections) from primary sensory areas (Felleman and Van Essen, 1991) and could thus be expected to participate primarily in conceptual rather than perceptual functions. Overall, during the baseline resting state this neural system is likely to be engaged in higher mental functions involving something similar to contemplative thought against a background of general body awareness, upon which any extended consciousness is constructed.
In fact, converging evidence from recent functional imaging studies in healthy subjects indicate that the precuneus may play a role in the internal mentation processes of self-consciousness. Lou et al. (1999) found a medial parietal-prefrontal core in the enhanced consciousness state of yoga meditation, by measuring cerebral blood distribution with the PET technique in experienced yoga teachers. An interaction between precuneus and prefrontal cortex has been postulated in states of consciousness characterized by a high level of reflective self-awareness (Kjaer and Lou, 2000). Moreover, in a functional imaging study aimed at identifying the neural correlates of visual awareness, the same group used brief subliminal and supraliminal verbal stimuli while measuring cerebral blood flow distribution with PET (Kjaer et al., 2001). The major finding of this study was the differential recruiting of precuneus and dorsolateral prefrontal cortex in the right hemisphere when visual-verbal stimulation lasted long enough to elicit awareness, thus suggesting critical involvement of these higher order associative cortices in visual–verbal awareness. Based on these findings, the joint activity of precuneus and frontopolar regions was implicated to represent a prerequisite for both task-elicited and state-dependent awareness, by ensuring a continuing activity of high-level integration between posterior association processes and anterior executive functions.
From a slightly different angle, other functional imaging studies have demonstrated that the precuneus and adjacent posteromedial cortical regions show a profound deactivation in pathophysiological altered states of consciousness, such as slow-wave sleep (SWS) and rapid eye movement (REM) sleep, the hypnotic state, pharmacologically induced general anaesthesia and the persistent vegetative state.
Quite recently, PET studies have yielded original data on the functional neuroanatomy of human sleep. The precuneus, along with lateral parietal and prefrontal cortices, was found to be significantly less active than the rest of the brain during both SWS, or deep sleep (Maquet et al., 1997; Andersson et al., 1998), and REM sleep (Maquet et al., 1996; Braun et al., 1997). The interpretation of this selective deactivation is uncertain. However, since the impaired consciousness of the self and its environment represents a key feature shared by the different sleep stages, these observations might provide further evidence for an active participation of the precuneus in conscious processes (Maquet et al., 1999).
In a PET experiment exploring the neural correlates of hypnosis, rCBF decreases were found in the precuneus, posterior cingulate and right inferior parietal lobule (Rainville et al., 1999). Deactivation of the precuneus, in particular, was considered to be an important metabolic feature of this altered state of consciousness, characterized by temporary loss of high-order body or self representation (Maquet et al., 1999).
Fiset et al. (1999) used PET to investigate changes in rCBF during a general anaesthetic infusion set to produce a gradual transition from the awake state to unconsciousness. In addition to a generalized decrease in global cerebral blood flow, propofol-induced anaesthesia was characterized by marked regional flow decrements in the precuneus, the posterior cingulate, the cuneus, the medial thalamus and frontal cortical regions. These results support the hypothesis that anaesthetics induce behavioural changes via an effect on specific neuronal networks, including the precuneus, that are implicated in the regulation of arousal and performance of associative conscious functions.
The ultimate state of conscious incapacity—the vegetative state—has also been investigated by means of PET and statistical parametric mapping (Laureys et al., 1999). Functional neuroimaging findings from patients in persistent vegetative state identified markedly impaired function of the precuneus and adjacent posterior cingulate cortex, together with prefrontal and parietotemporal association areas. Interestingly enough, the precuneus is among the first regions of the brain to resume its activity if patients regain consciousness. Laureys et al. (2004) reported that the functional relationship between the posteromedial cortex and the thalamus is altered during the vegetative state but regains near-normal values once the patients recover consciousness. Moreover, preliminary data show that overall cerebral metabolism in the minimally conscious state is decreased to values slightly higher but comparable to those observed in the vegetative state. In fact, the precuneus and adjacent posterior cingulate cortex seem to be brain regions that differentiate patients in minimally conscious states from those in vegetative states. Taken together, these findings provide strong, albeit preliminary, evidence that the richly connected multimodal associative area to which precuneus belongs may be part of the neural network subserving self-awareness and conscious experience.
Conclusions
The precuneus is an intriguing cortical area, not only due to its buried location in the posteromedial cortex of the parietal lobe, but also because of its possible role in fundamental cognitive functioning, especially in the human brain. The comparative anatomical studies of the cytoarchitecture and connectivity have partially exposed the neural systems to which the precuneus belongs, a wide-spread network of higher association cortical and subcortical structures, indicating the complexity of its behavioural specializations. As shown in this review, the precuneus has recently received much investigative attention by functional imaging studies as an area of interest in both normal and abnormal brain functioning. Such neuroimaging methods have opened new avenues to disentangle the cerebral structures involved in the different aspects underlying human behaviour through the identification of large-scale activation patterns associated with higher-order cognitive processes. However, results from both fMRI and PET studies only indirectly reflect neural activity and these techniques suffer from several limitations (Raichle, 1998; Logothetis and Wandell, 2004).
First, the resolution of haemodynamic measures is limited both temporally and spatially. Secondly, the studies reviewed in the present paper differ widely in their statistical power and in the criteria they use for defining significant results. Whether a specific activation is actually significant is commonly determined by prespecified statistical thresholding. Thus, the failure of a given region to survive such thresholding does not mean that it can be excluded from consideration in a task. Thirdly, there are problems with the subtraction method involved in the design of most PET and fMRI studies. For example, the ‘pure insertion assumption’ (Friston et al., 1996), i.e. the idea that a task manipulation changes only a single cognitive process, leaving other processes unaffected, has been demonstrated to be not always valid (Jennings et al., 1997). Another important methodological aspect, that represents a general limitation of this kind of review, is that functional neuroimaging as presently employed strongly depends on the statistics used (corrected/uncorrected etc.) and the localization of brain function by looking for local maxima of activity within areas of activation (height versus extent). Some imaging studies mix such different approaches; others look for changes functional connectivity. Inspection of Tables 2–4 clearly shows that some of the local maxima for given cognitive functions lie in different subareas of the precuneus. The question of whether this speaks for a functional specialization within the precuneus or simply highlights a problem of how the data were assessed cannot probably be solved at the present time. Moreover, although functional neuroimaging techniques identify associations between activated regions and cognitive process, they can neither provide information regarding the functional relations between these regions nor determine which of these regions are essential for performing the task.
One intrinsic limitation of the ‘between-study approach’ that forms the basis for this review is that the identification of general activation patterns is critically related to our organization of the results from individual studies. Indeed, the classification we have used may be imperfect at the level of major sections and it can always be questioned whether a particular data point fits well in its context. When reviewing functional imaging studies across different domains, it becomes quite obvious that some brain regions are engaged in a wide variety of cognitive tasks. For example, activations in the precuneus were consistently found in studies of episodic memory, visuo-spatial imagery and self-processing. The most parsimonious account for these kind of activations is that they reflect cognitive processes that are tapped by the tasks in the different domains. However, most functional neuroimaging studies have preferred to interpret activations only within their own domain. Therefore, precuneus activations were usually attributed to episodic memory processes in episodic memory studies, visuo-spatial processes in visuo-spatial studies and so on. These domain-specific interpretations are useful because they allow researchers in each area to refine hypotheses and to design new experiments. At the same time, they do not allow for the development of general theories that account for the involvement of single brain regions in several cognitive tasks. Although these goals are beyond the scope of this review, the section below briefly discusses the possible common role of the precuneus in different cognitive processes.
At first, we emphasized the difficulties in precisely locating the precuneus and the way some authors have used a more extended anatomy than we have chosen in this study. Concentrating on BA 7 anatomical location and its assumed representation in imaging studies, we have noted that the precuneus is involved in visuo-spatial imagery, episodic memory retrieval, self-processing and consciousness (behavioural correlates of the precuneus). With regard to mental imagery, almost all studies show spreading of activation from the lateral parietal cortex (IPS and SPL) to the precuneus. This immediately implicates the precuneus in more widespread parietal functions, at least some of which must relate to the known activity of the lateral parietal cortex in spatial and body image representations. One theory that emerges is a central role for the precuneus in association with more lateral cortex in shifting attention between different targets in space and between different object features, and in motor imagery tasks. Selective activation of the posteromedial parietal cortex represents a ubiquitous finding in the neuroimaging studies of episodic memory retrieval, especially with autobiographical content. These data, taken in conjunction with the studies on self-processing, provide support for some functional division of the precuneus, in particular with mental imagery associated with the anterior portion (Tables 3 and 4). On the other hand, the reviewed studies provide no evidence of interhemispheric specialization for the precuneus, in contrast to the obvious laterality effects that emerge with lesional studies of lateral parietal regions. Of particular interest are the neuroimaging studies seeking to define a physiological baseline state for the normal human brain function, since the precuneus shows one of the highest metabolic activity patterns of all brain regions during the conscious resting state and routinely exhibits decreases from this baseline across a variety of goal-directed behaviours (default mode of brain activity). Moreover, this area has been shown to be somewhat hypoactive in mental states of decreased or abolished consciousness, such as sleep, hypnotic state, pharmacological sedation and vegetative state. Converging evidence therefore suggests that the precuneus may be involved in the integration of multiple neural systems producing a conscious self-percept.
This review has revealed a wide variety of potential functions for the precuneus, although some as we have suggested may well overlap in a coherent way. The review highlights the need for further work to try to reach a consistent account of the role of the precuneus in relation to its activity with the surrounding parietal and other adjacent cortical areas as well as more distributed functions. One possible unifying factor that brings together these findings is that the precuneus belongs to a medial prefrontal-mid-parietal neural network supporting the mental representation of the self. Some of the visuo-spatial imagery studies suggest involvement in internally guided attention and manipulation of mental images, whilst those directed at mental imagery more directly draw upon internal self-representation, which is also implicated in most episodic memory retrieval and first-person perspective-taking tasks. All of this seems compatible with a hypothesis that the precuneus plays a central role in the modulation of conscious processes, a hypothesis that is now being tested in brain imaging studies.
The authors thank Dr Hugo D. Critchley and the two anonymous referees for useful comments and valuable suggestions on earlier versions of the manuscript. Dr Andrea E. Cavanna is a research fellow and is funded by the Raymond Way Research Group, Institute of Neurology, Queen Square, London, and by the Department of Neurology, Amedeo Avogadro University, Novara, Italy. There are no conflicts of interest.
References
Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach.
Andersen RA, Bracewell RM, Barash S, Gnadt JW, Fogassi L. Eye position effects on visual, memory, and saccade-related activity in areas LIP and 7a in the macaque.
Andersson JLR, Onoe H, Hetta J, Lindstrom K, Valind S, Lilja A, et al. Brain networks affected by synchronized sleep visualized by positron emission tomography.
Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins L, et al. Remembering the past: two facets of episodic memory explored with positron emission tomography.
Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing.
Bailey P, von Bonin G, McCullogh WS. The isocortex of the chimpanzee. Urbana: University of Illinois Press;
Balint R. Seelenlähmung des “Schauens” optische Ataxie räumliche, Störung der Aufmerksamkeit Psychiat.
Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuo-spatial attention.
Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study.
Blum JS, Chow KL, Pribram K. A behavioural analysis of the organisation of the parieto-temporo-preoccipital cortex.
Bonda E, Petrides M, Frey S, Evans A. Neural correlates of mental transformations of the body-in-space.
Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, et al. Regional cerebral blood flow throughout the sleep-wake cycle: an (H2O)-O15 PET study.
Bremmer F, Schlack A, Duhamel JR, Graf W, Fink GR. Space coding in primate posterior parietal cortex.
Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: JA Barth;
Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomic studies of explicit and implicit memory retrieval tasks.
Caminiti R. From vision to movement: combinatorial computations in the dorsal stream. In: Caminiti, Hoffman K-P, Lacquaniti F, Altman J, editors. Vision and movement mechanisms in the cerebral cortex. Strasbourg: Human Frontier Science Program;
Caminiti R, Genovesio A, Marconi B, Mayer AB, Onorati P, Ferraina S, et al. Early coding of reaching: frontal and parietal association connections of parieto-occipital cortex.
Campbell AW. Histological studies on the localisation of cerebral function. Cambridge: Cambridge University Press;
Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe.
Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing.
Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RBH. Cortical fMRI activation produced by attentive tracking of moving targets.
Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt Brace;
den Ouden HEM, Frith U, Frith C, Blakemore S-J. Thinking about intentions.
Elliot Smith G. A new topographical survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci.
Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical model from 305 MRI volumes. In: Klaisner LA, editor. Proceedings of the IEEE-Nuclear Science Symposium and Medical Imaging Conference. Piscataway, NJ: IEEE Service Centre;
Eustache F, Piolino P, Giffard B, Viader F, De La Sayette V, Baron J-C, et al. “In the course of time”: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer's disease.
Farrer C, Frith CD. Experiencing oneself vs. another person as being the cause of an action: the neural correlates of the experience of agency.
Farrow TFD, Zheng Y, Wilkinson ID, Spence SA, Deakin JFW, Tarrier N, et al. Investigating the functional anatomy of empathy and forgiveness.
Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex.
Ferraina S, Johnson PB, Garasto MR, Battaglia-Mayer A, Ercolani L, Bianchi L, et al. Combination of hand and gaze signals during reaching: activity in parietal area 7m of the monkey.
Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RSJ, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli.
Fiset P, Paus T, Daloze T, Plourde G, Meuret P, Bonhomme V, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study.
Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind's eye—precuneus activation in memory-related imagery.
Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ. Brain activity during memory retrieval: the influence of imagery and semantic cueing. Brain
Foville AL. Traité complêt de l'anatomie, de la physiologie et de la pathologie du système nerveux cérébro-spinal. Paris: Fortin, Masson;
Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. San Diego: Academic Press;
Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS, Dolan RJ. The trouble with cognitive subtraction.
Gardiner JM. Episodic memory and autonoetic consciousness: a first-person approach. [Review].
Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, et al. Partially overlapping neural networks for real and imagined hand movements.
Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, et al. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula.
Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovicth M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events.
Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim Y-H, Meyer JL, et al. A large-scale distributed network for covert spatial attention.
Goldman-Rakic PS. Development of cortical circuitry and cognitive function.
Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex.
Grafton ST, Fagg AH, Woods RP, Arbib MA. Functional anatomy of pointing and grasping in humans.
Grefkes C, Ritzl A, Zilles K, Fink GR. Human medial intraparietal cortex subserves visuomotor coordinate transformation.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis.
Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, et al. Sex differences in regional cerebral glucose metabolism during a resting state.
Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. [Review].
Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function.
Halsband U, Krause BJ, Schmidt D, Herzog H, Tellman L, Muller-Gartner HW. Encoding and retrieval in declarative learning: a positron emission tomography study.
Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M. Functional properties of brain areas associated with motor execution and imagery.
Harley CA, Bielajew CH. A comparison of glycogen phosphorylase alpha and cytochrome oxidase histochemical staining in rat brain.
Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study.
Jennings JM, McIntosh AR, Kapur S, Tulving E, Houle S. Cognitive subtractions may not add up: the interaction between semantic processing and response mode.
Johnson PB, Ferraina S, Caminiti R. Cortical networks for visual reaching.
Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching. Physiological and anatomical organization of frontal and parietal lobe arm regions.
Kapur S, Craik FIM, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study.
Karnath H-O, Perenin M-T. Cortical control of visually guided reaching: evidence from patients with optic ataxia.
Kawashima R, Roland PE, O'Sullivan BT. Functional anatomy of reaching and visuomotor learning: a positron emission tomography study.
Kircher TTJ, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, et al. Towards a functional neuroanatomy of self-processing: effects of faces and words.
Kircher TTJ, Brammer M, Bullmore E, Simmons A, Bartels M, David AS. The neural correlates of intentional and incidental self-processing.
Kjaer TW, Lou HC. Interaction between precuneus and dorsolateral prefrontal cortex may play a unitary role in consciousness: a principal component analysis of rCBF.
Kjaer TW, Nowak M, Kjaer KW, Lou AR, Lou HC. Precuneus-prefrontal activity during awareness of visual verbal stimuli.
Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core.
Knauff M, Fangmeier T, Ruff CC, Johnson-Laird PN. Reasoning, models, and images: behavioral measures and cortical activity.
Krause BJ, Schmidt D, Mottaghy FM, Taylor J, Halsband U, Herzog H, et al. Episodic retrieval activates the precuneus irrespective of the imagery content of word pair associates: a PET study.
Laureys S, Goldman S, Phillips C, Van Bogaert P, Aerts J, Luxen A, et al. Impaired effective cortical connectivity in vegetative state.
Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. [Review].
Le TH, Pardo JV, Hu X. 4T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions.
Leichnetz GR. Connections of the medial posterior parietal cortex (area 7m) in the monkey.
Leichnetz GR, Goldberg ME. Higher centers concerned with eye movement and visual attention: cerebral cortex and thalamus. In: Buttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. Amsterdam: Elsevier;
Leichnetz GR, Gonzalo-Ruiz A. Prearcuate cortex in the cebus monkey has cortical and subcortical connections like the macaque frontal eye field and projects to fastigial-recipient oculomotor-related brainstem nuclei.
Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, Nowak M. A 15O-H2O PET study of meditation and the resting state of normal consciousness.
Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, et al. Parietal cortex and representation of the mental self.
Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M. Isolating the retrieval of imagined pictures during episode memory: activation of the left precuneus and left prefrontal cortex.
Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval.
Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study.
Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, et al. Functional neuroanatomy of human REM sleep and dreaming.
Maquet P, Degueldre C, Delfiore G, Aerts J, Peters J, Luxen A, et al. Functional neuroanatomy of human slow wave sleep.
Maquet P, Faymonville ME, Degueldre C, Delfiore G, Franck G, Luxen A, et al. Functional neuroanatomy of hypnotic state.
Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man.
McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging.
Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry.
Michel F, Henaff MA, Intriligator J. Posterior callosum split and left parietal lesion reveal visual deficits in the right visual field.
Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge.
Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features.
Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration?
Nieuwenhuys R, Donkelaar HJ, Nicholson C. The central nervous system of vertebrates. Berlin: Springer;
Nyberg L. Imaging episodic memory: implications for cognitive theories and phenomena. [Review].
Ochsner KN, Knierim K, Ludloow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an MRI study of neural systems supporting the attribution of emotion to self and other.
Ogiso T, Kobayashi K, Sugishita M. The precuneus in motor imagery: a magnetoencephalographic study.
Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey.
Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, et al. Use of implicit motor imagery for visual shape discrimination as revealed by PET.
Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey.
Platel H, Price C, Baron J-C, Wise R, Lambert J, Frackowiak RS, et al. The structural components of music perception: a functional anatomic study.
Platel H, Baron J-C, Desgranges B, Bernard F, Eustache F. Semantic and episodic memory of music are subserved by distinct neural networks.
Pribram HB, Barry J. Further behavioural analysis of the parieto-temporo-preoccipital cortex.
Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function.
Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion.
Rubin DC. Beginnings of a theory of autobiographical memory. In: Thompson CP, Herrmann DJ, Bruce D, Read JD, Payne DG, Toglia MP, editors. Autobiographical memory: theoretical and applied perspectives. London: Erlbaum;
Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency.
Salamon G, Salamon-Murayama N, Mongkolwat P, Russell EJ. Magnetic resonance imaging study of the parietal lobe: anatomic and radiologic correlations. [Review].
Sarkissov SA, Filimonoff IN, Preobrashenskaya NS. Cytoarchitecture of the human cortex cerebri. Moskow: Medgiz;
Satoh M, Takeda K, Nagata K, Hatazawa J, Kuzuhara S. Activated brain regions in musicians during an ensamble: a PET study.
Schmahmann JD, Pandya DN. Anatomical investigation of projections from thalamus to posterior parietal cortex in the rhesus monkey: a WGA-HRP and fluorescent tracer study.
Schmidt D. Brain systems engaged in encoding and retrieval of word-pair associates independent of their imagery content or presentation modalities.
Seitz RJ, Binkofski F. Modular organization of parietal lobe functions as revealed by functional activation studies. [Review].
Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior.
Shallice T, Fletcher P, Frith CD, Grasby RS, Frackowiak RSJ, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory.
Shulman GL, Fiez JA, Corbetta M, Buckner RL, Meizin FM, Raichle ME, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex.
Siegal M, Varley R. Neural systems involved in “theory of mind”. [Review].
Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe.
Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, et al. Functional anatomy of the mental representation of upper extremity movements in healthy subjects.
Suchan B, Yágüez L, Wunderlich G, Canavan AGM, Herzog H, Tellmann L, et al. Hemispheric dissociation of visuo-spatial processing and visual rotation.
Suzuki K, Yamadori A, Hayakawa Y, Fujii T. Pure topographical disorientation related to dysfunction of the viewpoint dependent visual system.
Thier P, Andersen RA. Electrophysiological evidence for a second medial “parietal eye field”.
Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex.
Tian J-R, Lynch JC. Functionally defined smooth and saccadic eye movement subregions in the frontal eye field in cebus monkeys.
Tian J-R, Lynch JC. Corticocortical input to the smooth and saccadic eye movement subregions in the frontal eye field in cebus monkeys.
Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press;
Tulving E, Kapur S, Markovitsch HJ, Craik FIM, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition.
Van Hoesen GW, Maddock RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser;
Van Horn JD. The new perspectives in fMRI research award: exploring patterns of default-mode brain activity. [Editorial].
Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, et al. Mind reading: neural mechanisms of theory of mind and self-perspective.
Vogeley K, Fink GR. Neural correlates of the first-person-perspective. [Review].
Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness.
von Economo C, Koskinas GN. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen. Berlin: Springer;
Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour.
Wheeler MA. Episodic memory and autonoetic awareness. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford: Oxford University Press;
Wiest G, Zimprich F, Prayer D, Czech T, Serles W, Baumgartner C. Vestibular processing in human paramedian precuneus as shown by electrical cortical stimulation.
Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations.
Yeterian EH, Pandya DN. Corticothalamic connections of the posterior parietal cortex in the rhesus monkey.
Yeterian EH, Pandya DN. Corticothalamic connections of the paralimbic regions in the rhesus monkey.
Yeterian EH, Pandya DN. Striatal connections of the parietal association cortices in the rhesus monkey.
Zilles K, Palomero-Gallagher N. Cyto-, myelo-, and receptor architectonics of the human parietal cortex.
Author notes
1Institute of Neurology, Queen Square, London, UK and 2Department of Neurology, Amedeo Avogadro University, Novara, Italy


![A drawing of the medial surface of the human brain; the precuneus and its traditional anatomical landmarks are labelled [after Critchley (1953)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/129/3/10.1093/brain/awl004/2/m_awl004f1.gif?Expires=1716312975&Signature=nH5TNzNqPyoujNqRQde9DR4NsavVDdyNgcrwuizcoAQXpk3xno7E3aWZHalK-4xCY9Yliv2tpGkqUI4udAeJTJFAQzdlx0-iayg0oEkqvQB~b8vBYwWKV-8uANl2WyBo8qUCdNp4mavuQ3SM4wUadoXrN7YgKaCczPsQ2lvKnsVoTKvNGyhGnIVp1SypgAJHc8Kan~3VNa3dkeGuERpgXsrDkOU9LoZridI4PLnNdW~kNL6WXFIZqQcmcX4EEcNMbGKpGn4rEcbxCaBV~8xbzSzXtNBPMm-cttOyBi40yid4yQ~EFh0GcAhTfU9ZgIrW2dHI9w88EMN1mevF7r6uHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Comparison of the cytoarchitectonic maps of the human medial parietal isocortex after Brodmann (1909) (left) and von Economo and Koskinas (1925) (right); for the scope of the present review, the precuneus corresponds to mesial BA 7 and PE, respectively (horizontally shaded areas) [reprinted with permission from Zilles and Palomero-Gallagher (2001)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/129/3/10.1093/brain/awl004/2/m_awl004f2.gif?Expires=1716312975&Signature=yQbkJq96F6jZTfoWF0DhgyUuM-ikFWJp6n2Dq6k12DQQLEQePQ-N9xe0fwDjYVnLumzw1VqMxTMFFiY~KMUQ3bKqtAqx7ZQaycOiz6sFDZWBwG45OGGTFSgIKe6h~fRVH2qawy6ubl1QJvav3J0Ac~H3X8KS8ZCoD0aVOrEM9p54rF7soaS47vqFTPOM3LXgY7dCep2QGVYyPNSV3KFybdBrVtKxH~C77Gu2GFxq05aRH6aKpDckDg99e1CUZyS112VRvSyMKHMKrykJvUK94Aq-T3Mix2i9bQIzoSajleu-9qaJ44BwfuPCM6DOaMqurXIiROHqZUD8WXeQrb9e7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
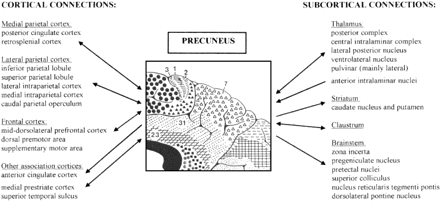
![Schematic representation of the main cortical regions activated during first-person perspective tasks [reprinted with permission from Vogeley and Fink (2003)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/129/3/10.1093/brain/awl004/2/m_awl004f4.gif?Expires=1716312975&Signature=DuL4Fzw9dJ6FtDW4WMnU4WyAaILfiygYKAe2bjX1utbvmmbJVcldwPjwI-tEt2laADYORm~ZA1S6ONI2xvMGoZleov94JG24qQy2XT4v2g3Mx6BmRojisFQOdDAUcG3LgtdSw~TnvtjlGxDsT~jx1zyS9TsBkPjyDKnT3CqCtOXvtK8kO27h-4qIPgUM6FvXnqvWK97EPIhb2qcPi~Afdu3rvh7uiyIVIiOvh6CMm3zOyzpsCSF95fscuXtyJHt5Kx0YYs8haML2o1ku1KmvSRmH1c4N0l6VeqcUVANP43p3T7Ja~U8AGq66WTF4lI3~EfKa0jVq1W1wOHcRly4lew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
