-
PDF
- Split View
-
Views
-
Cite
Cite
Teppo Särkämö, Mari Tervaniemi, Sari Laitinen, Anita Forsblom, Seppo Soinila, Mikko Mikkonen, Taina Autti, Heli M. Silvennoinen, Jaakko Erkkilä, Matti Laine, Isabelle Peretz, Marja Hietanen, Music listening enhances cognitive recovery and mood after middle cerebral artery stroke, Brain, Volume 131, Issue 3, March 2008, Pages 866–876, https://doi.org/10.1093/brain/awn013
Close - Share Icon Share
Abstract
We know from animal studies that a stimulating and enriched environment can enhance recovery after stroke, but little is known about the effects of an enriched sound environment on recovery from neural damage in humans. In humans, music listening activates a wide-spread bilateral network of brain regions related to attention, semantic processing, memory, motor functions, and emotional processing. Music exposure also enhances emotional and cognitive functioning in healthy subjects and in various clinical patient groups. The potential role of music in neurological rehabilitation, however, has not been systematically investigated. This single-blind, randomized, and controlled trial was designed to determine whether everyday music listening can facilitate the recovery of cognitive functions and mood after stroke. In the acute recovery phase, 60 patients with a left or right hemisphere middle cerebral artery (MCA) stroke were randomly assigned to a music group, a language group, or a control group. During the following two months, the music and language groups listened daily to self-selected music or audio books, respectively, while the control group received no listening material. In addition, all patients received standard medical care and rehabilitation. All patients underwent an extensive neuropsychological assessment, which included a wide range of cognitive tests as well as mood and quality of life questionnaires, one week (baseline), 3 months, and 6 months after the stroke. Fifty-four patients completed the study. Results showed that recovery in the domains of verbal memory and focused attention improved significantly more in the music group than in the language and control groups. The music group also experienced less depressed and confused mood than the control group. These findings demonstrate for the first time that music listening during the early post-stroke stage can enhance cognitive recovery and prevent negative mood. The neural mechanisms potentially underlying these effects are discussed.
Introduction
During the first weeks and months of recovery after a stroke, the brain can undergo dramatic plastic changes (Witte, 1998; Kreisel et al., 2006) that can be further enhanced by stimulation provided by the environment. Post-stroke motor and somatosensory environmental enrichment (Johansson, 2004; Nithianantharajah and Hannan, 2006), virtual environments (You et al., 2005), and electrical cortical and peripheral stimulation (Hummel and Cohen, 2005) have all been shown to improve motor recovery. Interestingly, multimodal stimulation, including auditory, visual and olfactory stimuli, combined to the enriched motor environment enhanced motor and cognitive recovery more than the enriched motor environment alone (Maegele et al., 2005). Evidence from developmental animal studies also suggests that an enriched sound environment can enhance auditory cortical functions (Engineer et al., 2004) as well as learning and memory (Chikahisa et al., 2006; Kim et al., 2006; Angelucci et al., 2007a). In humans, the effects of an enriched sound environment on recovery from neural damage have, however, not been systematically studied.
In the human brain, one of the most powerful sources of auditory stimulation is provided by music (Sacks, 2006). Listening to music is a complex process for the brain, since it triggers a sequel of cognitive and emotional components with distinct neural substrates (Peretz and Zatorre, 2005). Recent brain imaging studies have shown that neural activity associated with music listening extends well beyond the auditory cortex involving a wide-spread bilateral network of frontal, temporal, parietal and subcortical areas related to attention, semantic and music-syntactic processing, memory and motor functions (Bhattacharya et al., 2001; Janata et al., 2002; Koelsch et al., 2004; Popescu et al., 2004), as well as limbic and paralimbic regions related to emotional processing (Blood et al., 1999; Blood and Zatorre, 2001; Brown et al., 2004; Koelsch et al., 2006; Menon and Levitin, 2005). Music has a well-documented effect on alleviating anxiety, depression and pain in patients with a somatic illness (Cassileth et al., 2003; Cepeda et al., 2006; Siedliecki and Good, 2006). Recent cognitive and neuropsychological studies suggest that it may also enhance a variety of cognitive functions, such as attention, learning, communication and memory, both in healthy subjects (Wallace, 1994; Thompson et al., 2001; Thompson et al., 2005; Schellenberg et al., 2007) and in clinical conditions, such as dyslexia (Overy, 2003), autism (Gold et al., 2006), schizophrenia (Talwar et al., 2006), multiple sclerosis (Thaut et al., 2005), coronary artery disease (Emery et al., 2003) and dementia (Brotons and Koger, 2000; Foster and Valentine, 2001; Van de Winckel et al., 2004). In stroke rehabilitation, elements of music have previously been used as a part of physiotherapy (Thaut et al., 1997) and speech therapy (Belin et al., 1996) to enhance the recovery of motor and speech functions. In addition, nonverbal auditory stimuli have been shown to temporarily ameliorate left visual neglect after stroke (Hommel et al., 1990). However, the knowledge about the long-term effects of everyday music listening itself on the recovery of cognitive and emotional functions after stroke is very limited.
The purpose of this single-blind, randomized and controlled trial was to determine whether regular self-directed music listening during the first months after middle cerebral artery (MCA) stroke can enhance the recovery of cognitive functions and mood. Since the brain areas involved in music processing are mainly supplied by the MCA (Ayotte et al., 2000) we hypothesized that, in addition to engaging cognitive and emotional networks, music listening would also stimulate both the perilesional and healthy brain areas that normally show increased excitability and adaptability in this subacute recovery phase (Kreisel et al., 2006), and thereby enhance and speed up the spontaneous recovery process. As listening to real music, especially if it contains lyrics, activates the brain bilaterally, we also hypothesized that it would facilitate the recovery from unilateral stroke more than listening to purely verbal material, which activates primarily the left hemisphere (Zatorre et al., 2002; Tervaniemi and Hugdahl, 2003). Thus, we compared the effect of music listening both to the effect of listening to audio books and to normal spontaneous recovery.
Methods
Subjects and procedure
Subjects (n = 60) were stroke patients recruited between March 2004 and May 2006 from the Department of Neurology of the Helsinki University Central Hospital (HUCH) after been admitted to the hospital for treatment of acute stroke. Following inclusion criteria were used: (1) an acute ischaemic MCA stroke in the left or right temporal, frontal, parietal or subcortical brain regions, (2) no prior neurological or psychiatric disease, (3) no drug or alcohol abuse, (4) no hearing deficit, (5) right-handed, (6) ≤75 years old, (7) Finnish-speaking and (8) able to co-operate. Eligible patients were randomly assigned to one of three groups: a music group, a language group or a control group (n = 20 in each) as soon as possible after hospitalization. Randomization was performed with a random number generator by a researcher not involved in the patient enrollment. The study was approved by the HUCH Ethics Committee, and all patients signed an informed consent. All patients received standard treatment for stroke in terms of medical care and rehabilitation. All patients underwent a clinical neuropsychological assessment and a magnetoencephalographic (MEG) measurement 1 week (baseline), 3 months and 6 months post-stroke, and magnetic resonance imaging (MRI) within 2 weeks of the stroke and 6 months post-stroke. The results from the MEG part of the study will be presented in another paper.
Of the 60 subjects originally recruited in to the study, 55 completed the study up to the 3-month follow-up (music group n = 19, language group n = 19 and control group n = 17). Of the five drop-outs, one was due to false diagnosis (transient ischaemic attack), one due to a new stroke, one due to dementia and two due to refusal. One further subject died from myocardial infarction before the 6-month follow-up (music group n = 18, language group n = 19 and control group n = 17 at the 6-month stage).
Interventions
After agreeing to participate in the study, all patients were individually contacted by a music therapist (author S.L. or A.F.) who interviewed them about their pre-stroke leisure activities and hobbies, such as music listening and reading, and informed them about the group allocation. The music therapists provided the patients in the music group with portable CD players and CDs of their own favourite music in any musical genre. Similarly, they provided the patients in the language group with portable cassette players and narrated audio books on cassette selected by the patients from a collection of the Finnish Celia library for the visually impaired (http://www.celialib.fi). The patients in both groups were then trained in using the players and were instructed to listen to the material by themselves daily (minimum 1 h per day) for the following 2 months while still in the hospital or at home. The patients were also asked to keep a listening diary. During the 2-month period, the music therapists kept close weekly contact with the patients to encourage listening, to provide more material, and to give practical aid in using the equipment, if needed. Also nursing staff of the hospital wards and relatives of the patients were informed about the study, and were asked to help the patients in using the equipment, if needed. The protocol in the music and language groups was therefore identical with the only difference being the type of listening material used. The control group was not given any listening material. All patients were interviewed about their leisure activities again after the 2-month intervention and at the 6-month follow-up.
In order to test for differences between the emotional responses and preferences to music and verbal material, a short behavioural listening experiment was also performed by the music therapists at the acute stage, before the intervention. In this experiment, two short musical pieces and narrated poems, which were either happy or sad (as judged by the therapists), were first presented to the patients. Thereafter they were interviewed about the emotions, thoughts and memories evoked by those. From these qualitative data, we scored individually for each patient whether the stimulus (happy or sad) evoked any emotions, and which stimulus type (music or poems) was preferred.
Structural brain imaging
MRI was performed within 2 weeks of stroke onset and 6 months post-stroke using the 1.5 T Siemens Vision scanner of the HUCH Department of Radiology. The first MRI was used to verify the stroke diagnosis and the second to evaluate the size and location of the lesion without the interfering effect of the acute stage oedema. Size was evaluated from fluid-attenuated inversion recovery (FLAIR) images by measuring the maximum diameter of the lesion, or in case of multiple lesions the sum of the diameters, in the sagittal, coronal or horizontal plane. Following subcategories were used in classifying the location(s) of the lesion(s) within the damaged hemisphere: frontal lobe, temporal lobe, parietal lobe, insula and subcortical structures or white matter.
Outcome measures
Clinical neuropsychological assessment was performed on all patients at the baseline (1 week from stroke onset), and repeated again 3 months and 6 months post-stroke. The researchers involved in these studies (authors T.S. and M.M.) were blinded to the group allocation of the patients. An extensive neuropsychological test battery was used to evaluate the following cognitive domains: verbal memory, short-term and working memory, language, visuospatial cognition, music cognition, executive functions, focused attention and sustained attention. Summary scores of the tests measuring each cognitive domain were used in the statistical analyses. Parallel test versions of the memory tests were used in different testing occasions to minimize practice effects. Reaction time (RT) tests were always performed using the better, non-paretic hand. All assessments were carried out in a quiet room reserved for neuropsychological studies. The baseline assessment was carried out in two or three testing sessions to avoid interference due to fatigue.
Verbal memory was evaluated with the story recall subtest from the Rivermead Behavioural Memory Test (RBMT; Wilson et al., 1985) and an auditory list-learning task. In the story recall, both immediate and delayed recall scores were used. In the list-learning task, a 10-word list was presented orally three times, and after each presentation the subjects were requested to recall as many words as they could. Total score of the three trials and delayed recall score were used. Short-term and working memory was assessed with the digit span subtest from the Wechsler Memory Scale—Revised (WMS-R; Wechsler, 1987) and a memory interference task, in which the subjects were first orally presented with three words, then asked to perform a short mental arithmetic or verbal task, and then asked to recall the words again. Language was evaluated with the word and sentence repetition and reading subtests from the Finnish version (Laine et al., 1997) of the Boston Diagnostic Aphasia Examination (BDAE; Goodglass and Kaplan, 1983), the verbal fluency and naming subtests from the CERAD battery (Morris et al., 1989), and a shortened version of the Token Test (De Renzi and Faglioni, 1978). Visuospatial cognition was assessed with a clock task (Lezak et al., 2004), in which both setting clock hands and recognition of time was evaluated; a copying task (Lezak et al., 2004), in which copying of four geometric drawings (triangle, flag, cube, cross) was evaluated; a shortened version of the Benton Visual Retention Test (BVRT; Benton, 1974); and subtest B from the Balloons Test (Edgeworth et al., 1998). Music cognition was evaluated with the scale and rhythm subtests from the shortened version of the Montreal Battery of Evaluation of Amusia (MBEA; Peretz et al., 2003), which was administered at baseline and 3 months post-stroke. Executive functions were assessed with the Frontal Assessment Battery (FAB; Dubois et al., 2000). Attention was evaluated with the CogniSpeed© reaction time software (Revonsuo and Portin, 1995), which has previously been used, for example, in studies of multiple sclerosis (Kujala et al., 1994) and brain tumors (Lilja et al., 2001). Focused attention, the executive ability to control and perform mental operations and resolve conflicts among responses (Raz and Buhle, 2006), was assessed with summed correct responses and summed RTs of the mental subtraction and Stroop subtests. Sustained attention, the ability to achieve and maintain an alert state (Raz and Buhle, 2006), was evaluated with the percentage of correct responses in the vigilance subtest and summed RTs in the vigilance and simple reaction time subtests.
In addition to cognitive functions, also mood was evaluated at baseline and 3 and 6 months post-stroke using the shortened Finnish version (Hänninen, 1989) of the Profile of Mood States (POMS; McNair et al., 1981). It contains 38 items that form following eight subscales: tension, depression, irritability, vigor, fatigue, inertia, confusion and forgetfulness. Also quality of life (QOL) was assessed 3 and 6 months post-stroke with both a self-reported and a proxy-reported Stroke and Aphasia Quality Of Life Scale-39 (SAQOL-39; Hilari et al., 2003) questionnaire.
Data analysis
Group differences in the baseline characteristics of the patients and in the amount of rehabilitation received during the follow-up were analysed with one-way analyses of variance (ANOVA), Kruskal–Wallis tests, t-tests and chi-square tests. Group differences in mood and QOL 3 and 6 months post-stroke were analysed with one-way ANOVAs. Recovery in the cognitive domains and mood was analysed using a mixed-model ANOVA with a within-subjects factor of time (baseline, 3-month stage and 6-month stage) and between-subjects factors of group (music, language and control) and lesion laterality (left and right). The Greenhouse–Geisser epsilon was used to correct for sphericity. Main effects of time and group as well as time × group and time × group × lesion laterality interactions are reported. All post hoc analyses were performed with Tukey's honestly significant difference test. For the mixed-model ANOVA, post hoc tests were performed on change scores from the baseline to the 3-month stage and from the baseline to the 6-month stage. Relationships between the cognitive domains were also analysed with Pearson's correlation coefficients. The level of statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 14.0). Missing values in test scores were considered missing at random.
Results
There were no statistically significant differences between the groups in the baseline demographic or clinical variables or in relevant leisure activities prior to stroke (Table 1). There were also no significant group differences in the baseline cognitive performance or mood (Table 2). The groups did not differ significantly in the antidepressant medication received at the acute stage or in rehabilitation received in public health care during the follow-up period (Tables 1 and 3). In the short pre-intervention listening experiment, emotions were evoked in the majority of both music and language group patients after both music listening (63% versus 81%; χ2(Yates’ correction) = 0.3, P = 0.567) and poem listening (72% versus 94%; χ2 (Yates’ correction) = 1.4, P = 0.233), and the proportion of patients who preferred music was highly similar in both groups (50% versus 56%; χ2 = 0.1, P = 0.716). Thus, the emotional response to and preference for music and verbal material were comparable at baseline.
Baseline demographic and clinical characteristics of the three patient groups
| Variable . | Music group (n = 19) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Age (years) | 56.1 (9.6) | 59.3 (8.3) | 61.5 (8.0) | 0.178 (F) |
| Gender (male/female) | 12/7 | 9/10 | 8/9 | 0.531 (χ2) |
| Education (years) | 11.2 (4.3) | 11.8 (3.0) | 9.7 (3.3) | 0.198 (F) |
| Living alone (yes/no) | 4/15 | 5/14 | 3/14 | 0.817 (χ2) |
| Music listening prior to strokea | 4.0 (1.5) | 3.2 (1.4) | 3.4 (1.6) | 0.115 (K) |
| Radio listening prior to strokea | 4.5 (1.1) | 4.1 (1.2) | 4.3 (1.2) | 0.560 (K) |
| Reading prior to strokea | 4.0 (0.9) | 4.0 (0.7) | 4.2 (0.9) | 0.558 (K) |
| Time from stroke to baseline (days) | 5.6 (2.3) | 7.1 (3.9) | 5.9 (3.0) | 0.313 (F) |
| Time from stroke to treatment (days) | 7.4 (2.8) | 9.6 (3.4) | 9.2 (5.4) | 0.191 (F) |
| Motor deficit severityb | 1.4 (1.0) | 1.2 (1.0) | 1.4 (1.2) | 0.849 (K) |
| Aphasiac (yes/no) | 7/12 | 6/13 | 6/11 | 0.941 (χ2) |
| Aphasia severityc | 2.6 (1.4) | 2.8 (1.5) | 2.7 (1.0) | 0.938 (F) |
| Visual neglectd (yes/no) | 5/14 | 7/12 | 4/13 | 0.644 (χ2) |
| Antidepressantse (yes/no) | 6/13 | 5/14 | 2/15 | 0.356 (χ2) |
| Lesion laterality (left/right) | 10/9 | 8/11 | 8/9 | 0.809 (χ2) |
| Lesion sizef | 5.4 (2.7) | 5.0 (2.1) | 5.8 (2.4) | 0.543 (F) |
| Lesion in frontal lobe (yes/no) | 16/3 | 12/7 | 13/4 | 0.322 (χ2) |
| Lesion in temporal lobe (yes/no) | 11/8 | 15/4 | 14/3 | 0.195 (χ2) |
| Lesion in parietal lobe (yes/no) | 10/9 | 12/7 | 10/7 | 0.804 (χ2) |
| Lesion in insula (yes/no) | 11/8 | 13/6 | 12/5 | 0.686 (χ2) |
| Lesion in subcortical or WM areas (yes/no) | 9/10 | 11/8 | 8/9 | 0.753 (χ2) |
| Variable . | Music group (n = 19) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Age (years) | 56.1 (9.6) | 59.3 (8.3) | 61.5 (8.0) | 0.178 (F) |
| Gender (male/female) | 12/7 | 9/10 | 8/9 | 0.531 (χ2) |
| Education (years) | 11.2 (4.3) | 11.8 (3.0) | 9.7 (3.3) | 0.198 (F) |
| Living alone (yes/no) | 4/15 | 5/14 | 3/14 | 0.817 (χ2) |
| Music listening prior to strokea | 4.0 (1.5) | 3.2 (1.4) | 3.4 (1.6) | 0.115 (K) |
| Radio listening prior to strokea | 4.5 (1.1) | 4.1 (1.2) | 4.3 (1.2) | 0.560 (K) |
| Reading prior to strokea | 4.0 (0.9) | 4.0 (0.7) | 4.2 (0.9) | 0.558 (K) |
| Time from stroke to baseline (days) | 5.6 (2.3) | 7.1 (3.9) | 5.9 (3.0) | 0.313 (F) |
| Time from stroke to treatment (days) | 7.4 (2.8) | 9.6 (3.4) | 9.2 (5.4) | 0.191 (F) |
| Motor deficit severityb | 1.4 (1.0) | 1.2 (1.0) | 1.4 (1.2) | 0.849 (K) |
| Aphasiac (yes/no) | 7/12 | 6/13 | 6/11 | 0.941 (χ2) |
| Aphasia severityc | 2.6 (1.4) | 2.8 (1.5) | 2.7 (1.0) | 0.938 (F) |
| Visual neglectd (yes/no) | 5/14 | 7/12 | 4/13 | 0.644 (χ2) |
| Antidepressantse (yes/no) | 6/13 | 5/14 | 2/15 | 0.356 (χ2) |
| Lesion laterality (left/right) | 10/9 | 8/11 | 8/9 | 0.809 (χ2) |
| Lesion sizef | 5.4 (2.7) | 5.0 (2.1) | 5.8 (2.4) | 0.543 (F) |
| Lesion in frontal lobe (yes/no) | 16/3 | 12/7 | 13/4 | 0.322 (χ2) |
| Lesion in temporal lobe (yes/no) | 11/8 | 15/4 | 14/3 | 0.195 (χ2) |
| Lesion in parietal lobe (yes/no) | 10/9 | 12/7 | 10/7 | 0.804 (χ2) |
| Lesion in insula (yes/no) | 11/8 | 13/6 | 12/5 | 0.686 (χ2) |
| Lesion in subcortical or WM areas (yes/no) | 9/10 | 11/8 | 8/9 | 0.753 (χ2) |
Data are mean (SD) unless otherwise stated. WM = white matter; F = one-way ANOVA; χ2 = chi-square test; K = Kruskal–Wallis test.
aNumbers denote values on a Likert scale with a range 0 (does never) to 5 (does daily). bNumbers denote values on a Likert scale with a range 0 (no deficit) to 3 (hemiplegia). cBDAE Aphasia Severity Rating Scale: scores 0–4 = aphasia, score 5 = no aphasia. For aphasic patients, the mean score (range 0–4) is shown. dCut-off from the Lateralized Inattention Index of the Balloons Test. eAntidepressant medication (citalopram or mirtazapin) used in the acute post-stroke phase. fMaximum lesion diameter in cm (see Methods for details).
Baseline demographic and clinical characteristics of the three patient groups
| Variable . | Music group (n = 19) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Age (years) | 56.1 (9.6) | 59.3 (8.3) | 61.5 (8.0) | 0.178 (F) |
| Gender (male/female) | 12/7 | 9/10 | 8/9 | 0.531 (χ2) |
| Education (years) | 11.2 (4.3) | 11.8 (3.0) | 9.7 (3.3) | 0.198 (F) |
| Living alone (yes/no) | 4/15 | 5/14 | 3/14 | 0.817 (χ2) |
| Music listening prior to strokea | 4.0 (1.5) | 3.2 (1.4) | 3.4 (1.6) | 0.115 (K) |
| Radio listening prior to strokea | 4.5 (1.1) | 4.1 (1.2) | 4.3 (1.2) | 0.560 (K) |
| Reading prior to strokea | 4.0 (0.9) | 4.0 (0.7) | 4.2 (0.9) | 0.558 (K) |
| Time from stroke to baseline (days) | 5.6 (2.3) | 7.1 (3.9) | 5.9 (3.0) | 0.313 (F) |
| Time from stroke to treatment (days) | 7.4 (2.8) | 9.6 (3.4) | 9.2 (5.4) | 0.191 (F) |
| Motor deficit severityb | 1.4 (1.0) | 1.2 (1.0) | 1.4 (1.2) | 0.849 (K) |
| Aphasiac (yes/no) | 7/12 | 6/13 | 6/11 | 0.941 (χ2) |
| Aphasia severityc | 2.6 (1.4) | 2.8 (1.5) | 2.7 (1.0) | 0.938 (F) |
| Visual neglectd (yes/no) | 5/14 | 7/12 | 4/13 | 0.644 (χ2) |
| Antidepressantse (yes/no) | 6/13 | 5/14 | 2/15 | 0.356 (χ2) |
| Lesion laterality (left/right) | 10/9 | 8/11 | 8/9 | 0.809 (χ2) |
| Lesion sizef | 5.4 (2.7) | 5.0 (2.1) | 5.8 (2.4) | 0.543 (F) |
| Lesion in frontal lobe (yes/no) | 16/3 | 12/7 | 13/4 | 0.322 (χ2) |
| Lesion in temporal lobe (yes/no) | 11/8 | 15/4 | 14/3 | 0.195 (χ2) |
| Lesion in parietal lobe (yes/no) | 10/9 | 12/7 | 10/7 | 0.804 (χ2) |
| Lesion in insula (yes/no) | 11/8 | 13/6 | 12/5 | 0.686 (χ2) |
| Lesion in subcortical or WM areas (yes/no) | 9/10 | 11/8 | 8/9 | 0.753 (χ2) |
| Variable . | Music group (n = 19) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Age (years) | 56.1 (9.6) | 59.3 (8.3) | 61.5 (8.0) | 0.178 (F) |
| Gender (male/female) | 12/7 | 9/10 | 8/9 | 0.531 (χ2) |
| Education (years) | 11.2 (4.3) | 11.8 (3.0) | 9.7 (3.3) | 0.198 (F) |
| Living alone (yes/no) | 4/15 | 5/14 | 3/14 | 0.817 (χ2) |
| Music listening prior to strokea | 4.0 (1.5) | 3.2 (1.4) | 3.4 (1.6) | 0.115 (K) |
| Radio listening prior to strokea | 4.5 (1.1) | 4.1 (1.2) | 4.3 (1.2) | 0.560 (K) |
| Reading prior to strokea | 4.0 (0.9) | 4.0 (0.7) | 4.2 (0.9) | 0.558 (K) |
| Time from stroke to baseline (days) | 5.6 (2.3) | 7.1 (3.9) | 5.9 (3.0) | 0.313 (F) |
| Time from stroke to treatment (days) | 7.4 (2.8) | 9.6 (3.4) | 9.2 (5.4) | 0.191 (F) |
| Motor deficit severityb | 1.4 (1.0) | 1.2 (1.0) | 1.4 (1.2) | 0.849 (K) |
| Aphasiac (yes/no) | 7/12 | 6/13 | 6/11 | 0.941 (χ2) |
| Aphasia severityc | 2.6 (1.4) | 2.8 (1.5) | 2.7 (1.0) | 0.938 (F) |
| Visual neglectd (yes/no) | 5/14 | 7/12 | 4/13 | 0.644 (χ2) |
| Antidepressantse (yes/no) | 6/13 | 5/14 | 2/15 | 0.356 (χ2) |
| Lesion laterality (left/right) | 10/9 | 8/11 | 8/9 | 0.809 (χ2) |
| Lesion sizef | 5.4 (2.7) | 5.0 (2.1) | 5.8 (2.4) | 0.543 (F) |
| Lesion in frontal lobe (yes/no) | 16/3 | 12/7 | 13/4 | 0.322 (χ2) |
| Lesion in temporal lobe (yes/no) | 11/8 | 15/4 | 14/3 | 0.195 (χ2) |
| Lesion in parietal lobe (yes/no) | 10/9 | 12/7 | 10/7 | 0.804 (χ2) |
| Lesion in insula (yes/no) | 11/8 | 13/6 | 12/5 | 0.686 (χ2) |
| Lesion in subcortical or WM areas (yes/no) | 9/10 | 11/8 | 8/9 | 0.753 (χ2) |
Data are mean (SD) unless otherwise stated. WM = white matter; F = one-way ANOVA; χ2 = chi-square test; K = Kruskal–Wallis test.
aNumbers denote values on a Likert scale with a range 0 (does never) to 5 (does daily). bNumbers denote values on a Likert scale with a range 0 (no deficit) to 3 (hemiplegia). cBDAE Aphasia Severity Rating Scale: scores 0–4 = aphasia, score 5 = no aphasia. For aphasic patients, the mean score (range 0–4) is shown. dCut-off from the Lateralized Inattention Index of the Balloons Test. eAntidepressant medication (citalopram or mirtazapin) used in the acute post-stroke phase. fMaximum lesion diameter in cm (see Methods for details).
Baseline cognitive performance and mood in the three patient groups
| . | Music group (n = 19) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Cognitive domaina | ||||
| Verbal memory (max. 124) | 45.1 (21.2) | 60.7 (21.7) | 50.0 (25.6) | 0.105 (F) |
| Short-term and working memory (max. 42) | 19.7 (9.4) | 23.3 (7.2) | 17.7 (9.5) | 0.164 (F) |
| Language (max. 162) | 109.2 (36.8) | 122.1 (28.3) | 110.7 (31.7) | 0.405 (K) |
| Music cognition (max. 28) | 19.9 (4.5) | 19.2 (5.2) | 17.1 (3.5) | 0.183 (K) |
| Visuospatial cognition (max. 105) | 82.8 (23.4) | 89.2 (13.3) | 77.3 (23.7) | 0.174 (K) |
| Executive functions (max. 18) | 12.6 (3.7) | 13.9 (3.5) | 12.6 (3.6) | 0.344 (K) |
| Focused attention (correct responses) (max. 90) | 74.8 (19.5) | 84.3 (8.5) | 87.3 (3.2) | 0.105 (K) |
| Focused attention (RT, s) | 3.0 (1.1) | 3.4 (1.5) | 3.7 (2.0) | 0.797 (K) |
| Sustained attention (correct responses) (max. 100) | 87.0 (23.0) | 91.1 (12.1) | 95.9 (7.4) | 0.542 (K) |
| Sustained attention (RT, s) | 1.0 (0.3) | 1.2 (0.5) | 1.0 (0.2) | 0.656 (K) |
| Profile of Mood States (POMS) subscale | ||||
| Tension (max. 16) | 3.9 (3.4) | 4.4 (3.6) | 3.9 (2.7) | 0.870 (F) |
| Depression (max. 28) | 7.0 (7.3) | 6.1 (6.7) | 8.5 (7.4) | 0.615 (F) |
| Irritability (max. 28) | 4.4 (6.2) | 4.7 (6.4) | 4.7 (4.2) | 0.987 (F) |
| Vigor (max. 24) | 10.7 (5.6) | 9.1 (5.3) | 10.1 (6.3) | 0.698 (F) |
| Fatigue (max. 12) | 5.4 (2.9) | 4.6 (2.7) | 4.2 (4.1) | 0.514 (F) |
| Inertia (max. 12) | 2.7 (2.4) | 2.8 (2.8) | 3.6 (3.2) | 0.578 (F) |
| Confusion (max. 20) | 7.1 (4.0) | 7.4 (4.5) | 8.8 (4.8) | 0.481 (F) |
| Forgetfulness (max. 12) | 4.3 (2.6) | 4.5 (2.6) | 4.8 (3.1) | 0.862 (F) |
| . | Music group (n = 19) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Cognitive domaina | ||||
| Verbal memory (max. 124) | 45.1 (21.2) | 60.7 (21.7) | 50.0 (25.6) | 0.105 (F) |
| Short-term and working memory (max. 42) | 19.7 (9.4) | 23.3 (7.2) | 17.7 (9.5) | 0.164 (F) |
| Language (max. 162) | 109.2 (36.8) | 122.1 (28.3) | 110.7 (31.7) | 0.405 (K) |
| Music cognition (max. 28) | 19.9 (4.5) | 19.2 (5.2) | 17.1 (3.5) | 0.183 (K) |
| Visuospatial cognition (max. 105) | 82.8 (23.4) | 89.2 (13.3) | 77.3 (23.7) | 0.174 (K) |
| Executive functions (max. 18) | 12.6 (3.7) | 13.9 (3.5) | 12.6 (3.6) | 0.344 (K) |
| Focused attention (correct responses) (max. 90) | 74.8 (19.5) | 84.3 (8.5) | 87.3 (3.2) | 0.105 (K) |
| Focused attention (RT, s) | 3.0 (1.1) | 3.4 (1.5) | 3.7 (2.0) | 0.797 (K) |
| Sustained attention (correct responses) (max. 100) | 87.0 (23.0) | 91.1 (12.1) | 95.9 (7.4) | 0.542 (K) |
| Sustained attention (RT, s) | 1.0 (0.3) | 1.2 (0.5) | 1.0 (0.2) | 0.656 (K) |
| Profile of Mood States (POMS) subscale | ||||
| Tension (max. 16) | 3.9 (3.4) | 4.4 (3.6) | 3.9 (2.7) | 0.870 (F) |
| Depression (max. 28) | 7.0 (7.3) | 6.1 (6.7) | 8.5 (7.4) | 0.615 (F) |
| Irritability (max. 28) | 4.4 (6.2) | 4.7 (6.4) | 4.7 (4.2) | 0.987 (F) |
| Vigor (max. 24) | 10.7 (5.6) | 9.1 (5.3) | 10.1 (6.3) | 0.698 (F) |
| Fatigue (max. 12) | 5.4 (2.9) | 4.6 (2.7) | 4.2 (4.1) | 0.514 (F) |
| Inertia (max. 12) | 2.7 (2.4) | 2.8 (2.8) | 3.6 (3.2) | 0.578 (F) |
| Confusion (max. 20) | 7.1 (4.0) | 7.4 (4.5) | 8.8 (4.8) | 0.481 (F) |
| Forgetfulness (max. 12) | 4.3 (2.6) | 4.5 (2.6) | 4.8 (3.1) | 0.862 (F) |
Data are mean (SD). RT = reaction time; F = one-way ANOVA; K = Kruskal–Wallis test.
aSummary scores of the neuropsychological tests measuring each cognitive domain.
Baseline cognitive performance and mood in the three patient groups
| . | Music group (n = 19) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Cognitive domaina | ||||
| Verbal memory (max. 124) | 45.1 (21.2) | 60.7 (21.7) | 50.0 (25.6) | 0.105 (F) |
| Short-term and working memory (max. 42) | 19.7 (9.4) | 23.3 (7.2) | 17.7 (9.5) | 0.164 (F) |
| Language (max. 162) | 109.2 (36.8) | 122.1 (28.3) | 110.7 (31.7) | 0.405 (K) |
| Music cognition (max. 28) | 19.9 (4.5) | 19.2 (5.2) | 17.1 (3.5) | 0.183 (K) |
| Visuospatial cognition (max. 105) | 82.8 (23.4) | 89.2 (13.3) | 77.3 (23.7) | 0.174 (K) |
| Executive functions (max. 18) | 12.6 (3.7) | 13.9 (3.5) | 12.6 (3.6) | 0.344 (K) |
| Focused attention (correct responses) (max. 90) | 74.8 (19.5) | 84.3 (8.5) | 87.3 (3.2) | 0.105 (K) |
| Focused attention (RT, s) | 3.0 (1.1) | 3.4 (1.5) | 3.7 (2.0) | 0.797 (K) |
| Sustained attention (correct responses) (max. 100) | 87.0 (23.0) | 91.1 (12.1) | 95.9 (7.4) | 0.542 (K) |
| Sustained attention (RT, s) | 1.0 (0.3) | 1.2 (0.5) | 1.0 (0.2) | 0.656 (K) |
| Profile of Mood States (POMS) subscale | ||||
| Tension (max. 16) | 3.9 (3.4) | 4.4 (3.6) | 3.9 (2.7) | 0.870 (F) |
| Depression (max. 28) | 7.0 (7.3) | 6.1 (6.7) | 8.5 (7.4) | 0.615 (F) |
| Irritability (max. 28) | 4.4 (6.2) | 4.7 (6.4) | 4.7 (4.2) | 0.987 (F) |
| Vigor (max. 24) | 10.7 (5.6) | 9.1 (5.3) | 10.1 (6.3) | 0.698 (F) |
| Fatigue (max. 12) | 5.4 (2.9) | 4.6 (2.7) | 4.2 (4.1) | 0.514 (F) |
| Inertia (max. 12) | 2.7 (2.4) | 2.8 (2.8) | 3.6 (3.2) | 0.578 (F) |
| Confusion (max. 20) | 7.1 (4.0) | 7.4 (4.5) | 8.8 (4.8) | 0.481 (F) |
| Forgetfulness (max. 12) | 4.3 (2.6) | 4.5 (2.6) | 4.8 (3.1) | 0.862 (F) |
| . | Music group (n = 19) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Cognitive domaina | ||||
| Verbal memory (max. 124) | 45.1 (21.2) | 60.7 (21.7) | 50.0 (25.6) | 0.105 (F) |
| Short-term and working memory (max. 42) | 19.7 (9.4) | 23.3 (7.2) | 17.7 (9.5) | 0.164 (F) |
| Language (max. 162) | 109.2 (36.8) | 122.1 (28.3) | 110.7 (31.7) | 0.405 (K) |
| Music cognition (max. 28) | 19.9 (4.5) | 19.2 (5.2) | 17.1 (3.5) | 0.183 (K) |
| Visuospatial cognition (max. 105) | 82.8 (23.4) | 89.2 (13.3) | 77.3 (23.7) | 0.174 (K) |
| Executive functions (max. 18) | 12.6 (3.7) | 13.9 (3.5) | 12.6 (3.6) | 0.344 (K) |
| Focused attention (correct responses) (max. 90) | 74.8 (19.5) | 84.3 (8.5) | 87.3 (3.2) | 0.105 (K) |
| Focused attention (RT, s) | 3.0 (1.1) | 3.4 (1.5) | 3.7 (2.0) | 0.797 (K) |
| Sustained attention (correct responses) (max. 100) | 87.0 (23.0) | 91.1 (12.1) | 95.9 (7.4) | 0.542 (K) |
| Sustained attention (RT, s) | 1.0 (0.3) | 1.2 (0.5) | 1.0 (0.2) | 0.656 (K) |
| Profile of Mood States (POMS) subscale | ||||
| Tension (max. 16) | 3.9 (3.4) | 4.4 (3.6) | 3.9 (2.7) | 0.870 (F) |
| Depression (max. 28) | 7.0 (7.3) | 6.1 (6.7) | 8.5 (7.4) | 0.615 (F) |
| Irritability (max. 28) | 4.4 (6.2) | 4.7 (6.4) | 4.7 (4.2) | 0.987 (F) |
| Vigor (max. 24) | 10.7 (5.6) | 9.1 (5.3) | 10.1 (6.3) | 0.698 (F) |
| Fatigue (max. 12) | 5.4 (2.9) | 4.6 (2.7) | 4.2 (4.1) | 0.514 (F) |
| Inertia (max. 12) | 2.7 (2.4) | 2.8 (2.8) | 3.6 (3.2) | 0.578 (F) |
| Confusion (max. 20) | 7.1 (4.0) | 7.4 (4.5) | 8.8 (4.8) | 0.481 (F) |
| Forgetfulness (max. 12) | 4.3 (2.6) | 4.5 (2.6) | 4.8 (3.1) | 0.862 (F) |
Data are mean (SD). RT = reaction time; F = one-way ANOVA; K = Kruskal–Wallis test.
aSummary scores of the neuropsychological tests measuring each cognitive domain.
Music and audio book listening and other rehabilitation in the three patient groups at the three month and the six month post-stroke stage
| Variable . | Music group (n = 19/18) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Music listeninga | ||||
| 3 m | 5.0 (0) | 1.6 (1.9) | 1.7 (2.2) | <0.001 (K) |
| 6 m | 4.5 (0.6) | 2.8 (1.7) | 2.0 (1.8) | <0.001 (K) |
| Audio book listeninga | ||||
| 3 m | 0.1 (0.2) | 4.5 (1.2) | 0.4 (1.2) | <0.001 (K) |
| 6 m | 0.3 (1.0) | 3.3 (1.8) | 0.4 (1.0) | <0.001 (K) |
| Physical therapyb | ||||
| 3 m | 14.5 (23.9) | 11.7 (21.1) | 6.8 (10.5) | 0.810 (K) |
| 6 m | 21.1 (34.9) | 21.2 (34.4) | 11.6 (19.5) | 0.922 (K) |
| Occupational therapyb | ||||
| 3 m | 6.2 (10.0) | 3.2 (5.1) | 4.0 (6.2) | 0.827 (K) |
| 6 m | 10.4 (16.7) | 5.7 (11.8) | 7.1 (14.3) | 0.753 (K) |
| Speech therapyb | ||||
| 3 m | 6.1 (7.4) | 3.2 (7.7) | 2.7 (4.6) | 0.403 (K) |
| 6 m | 8.3 (14.0) | 2.9 (6.7) | 5.4 (9.3) | 0.476 (K) |
| Neuropsychological rehabilitationb | ||||
| 3 m | 3.2 (6.1) | 2.0 (3.2) | 0.6 (1.6) | 0.269 (K) |
| 6 m | 4.3 (7.8) | 5.2 (7.6) | 2.4 (4.2) | 0.849 (K) |
| Variable . | Music group (n = 19/18) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Music listeninga | ||||
| 3 m | 5.0 (0) | 1.6 (1.9) | 1.7 (2.2) | <0.001 (K) |
| 6 m | 4.5 (0.6) | 2.8 (1.7) | 2.0 (1.8) | <0.001 (K) |
| Audio book listeninga | ||||
| 3 m | 0.1 (0.2) | 4.5 (1.2) | 0.4 (1.2) | <0.001 (K) |
| 6 m | 0.3 (1.0) | 3.3 (1.8) | 0.4 (1.0) | <0.001 (K) |
| Physical therapyb | ||||
| 3 m | 14.5 (23.9) | 11.7 (21.1) | 6.8 (10.5) | 0.810 (K) |
| 6 m | 21.1 (34.9) | 21.2 (34.4) | 11.6 (19.5) | 0.922 (K) |
| Occupational therapyb | ||||
| 3 m | 6.2 (10.0) | 3.2 (5.1) | 4.0 (6.2) | 0.827 (K) |
| 6 m | 10.4 (16.7) | 5.7 (11.8) | 7.1 (14.3) | 0.753 (K) |
| Speech therapyb | ||||
| 3 m | 6.1 (7.4) | 3.2 (7.7) | 2.7 (4.6) | 0.403 (K) |
| 6 m | 8.3 (14.0) | 2.9 (6.7) | 5.4 (9.3) | 0.476 (K) |
| Neuropsychological rehabilitationb | ||||
| 3 m | 3.2 (6.1) | 2.0 (3.2) | 0.6 (1.6) | 0.269 (K) |
| 6 m | 4.3 (7.8) | 5.2 (7.6) | 2.4 (4.2) | 0.849 (K) |
Data are mean (SD). 3 m = 3 month post-stroke stage; 6 m = 6 month post-stroke stage; K = Kruskal–Wallis test.
aNumbers denote values on a Likert scale with a range 0 (does never) to 5 (does daily). bNumber of therapy sessions.
Music and audio book listening and other rehabilitation in the three patient groups at the three month and the six month post-stroke stage
| Variable . | Music group (n = 19/18) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Music listeninga | ||||
| 3 m | 5.0 (0) | 1.6 (1.9) | 1.7 (2.2) | <0.001 (K) |
| 6 m | 4.5 (0.6) | 2.8 (1.7) | 2.0 (1.8) | <0.001 (K) |
| Audio book listeninga | ||||
| 3 m | 0.1 (0.2) | 4.5 (1.2) | 0.4 (1.2) | <0.001 (K) |
| 6 m | 0.3 (1.0) | 3.3 (1.8) | 0.4 (1.0) | <0.001 (K) |
| Physical therapyb | ||||
| 3 m | 14.5 (23.9) | 11.7 (21.1) | 6.8 (10.5) | 0.810 (K) |
| 6 m | 21.1 (34.9) | 21.2 (34.4) | 11.6 (19.5) | 0.922 (K) |
| Occupational therapyb | ||||
| 3 m | 6.2 (10.0) | 3.2 (5.1) | 4.0 (6.2) | 0.827 (K) |
| 6 m | 10.4 (16.7) | 5.7 (11.8) | 7.1 (14.3) | 0.753 (K) |
| Speech therapyb | ||||
| 3 m | 6.1 (7.4) | 3.2 (7.7) | 2.7 (4.6) | 0.403 (K) |
| 6 m | 8.3 (14.0) | 2.9 (6.7) | 5.4 (9.3) | 0.476 (K) |
| Neuropsychological rehabilitationb | ||||
| 3 m | 3.2 (6.1) | 2.0 (3.2) | 0.6 (1.6) | 0.269 (K) |
| 6 m | 4.3 (7.8) | 5.2 (7.6) | 2.4 (4.2) | 0.849 (K) |
| Variable . | Music group (n = 19/18) . | Language group (n = 19) . | Control group (n = 17) . | P-value . |
|---|---|---|---|---|
| Music listeninga | ||||
| 3 m | 5.0 (0) | 1.6 (1.9) | 1.7 (2.2) | <0.001 (K) |
| 6 m | 4.5 (0.6) | 2.8 (1.7) | 2.0 (1.8) | <0.001 (K) |
| Audio book listeninga | ||||
| 3 m | 0.1 (0.2) | 4.5 (1.2) | 0.4 (1.2) | <0.001 (K) |
| 6 m | 0.3 (1.0) | 3.3 (1.8) | 0.4 (1.0) | <0.001 (K) |
| Physical therapyb | ||||
| 3 m | 14.5 (23.9) | 11.7 (21.1) | 6.8 (10.5) | 0.810 (K) |
| 6 m | 21.1 (34.9) | 21.2 (34.4) | 11.6 (19.5) | 0.922 (K) |
| Occupational therapyb | ||||
| 3 m | 6.2 (10.0) | 3.2 (5.1) | 4.0 (6.2) | 0.827 (K) |
| 6 m | 10.4 (16.7) | 5.7 (11.8) | 7.1 (14.3) | 0.753 (K) |
| Speech therapyb | ||||
| 3 m | 6.1 (7.4) | 3.2 (7.7) | 2.7 (4.6) | 0.403 (K) |
| 6 m | 8.3 (14.0) | 2.9 (6.7) | 5.4 (9.3) | 0.476 (K) |
| Neuropsychological rehabilitationb | ||||
| 3 m | 3.2 (6.1) | 2.0 (3.2) | 0.6 (1.6) | 0.269 (K) |
| 6 m | 4.3 (7.8) | 5.2 (7.6) | 2.4 (4.2) | 0.849 (K) |
Data are mean (SD). 3 m = 3 month post-stroke stage; 6 m = 6 month post-stroke stage; K = Kruskal–Wallis test.
aNumbers denote values on a Likert scale with a range 0 (does never) to 5 (does daily). bNumber of therapy sessions.
There were, however, highly significant differences between the groups in the frequency of listening to music and audio books both at the 3-month and at the 6-month post-stroke stage (Table 3): the music group listened to music more than the language group or the control group, whereas the language group listened to audio books more than the music group or the control group (P < 0.005 in all pair-wise comparisons). This indicates that the study protocol worked well. One might expect that the patients with damage to the language-dominant hemisphere would have more difficulties in listening to audio books than music, and would thus spend less time listening to them. However, a further group comparison within the left hemisphere-lesioned patients showed that the amount of daily listening (hours per day) in the music group (M = 1.6, SD = 0.7) and in the language group (M = 1.3, SD = 0.5) did not differ significantly [t(13) = 0.68, P = 0.511]. Analysis of the listening diaries kept by the music group patients showed that 62% of all music selections were popular music (pop, rock or rhythm and blues), 10% was jazz, 8% was folk music and 20% was classical or spiritual music. All in all, 63% of the music contained lyrics in a language that the patients could understand (mostly Finnish or English).
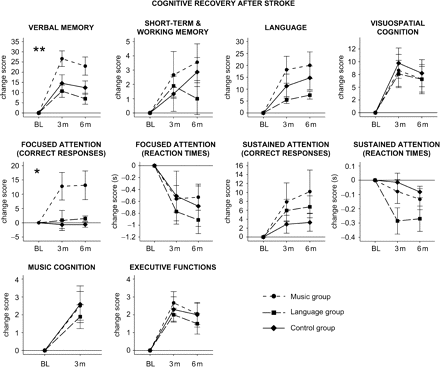
Figure 1 illustrates the recovery in the 10 cognitive domains in all three patient groups. In a mixed-model ANOVA, the within-subjects main effect of time was significant in the domains of verbal memory [F(2, 96) = 56.5, P < 0.001], short-term and working memory [F(2, 90) = 8.7, P < 0.001], language [F(1.2, 51.9) = 26.5, P < 0.001], visuospatial cognition [F(1.4, 58.5) = 18.6, P < 0.001], focused attention (correct responses) [F(1.6, 59.3) = 3.5, P = 0.045], focused attention (RT) [F(1.2, 45.7) = 15.7, P < 0.001], sustained attention (correct responses) [F(1.3, 57.3) = 8.8, P = 0.002], sustained attention (RT) [F(1.2, 51.4) = 8.5, P = 0.003], music cognition [F(1, 47) = 20.6, P < 0.001] and executive functions [F(1.8, 82.8) = 30.6, P < 0.001]. The between-subjects main effect of group was not significant in any cognitive domain.
Changes in the 10 cognitive domains (mean ± SEM) from the baseline (BL; 1-week post-stroke stage) to the 3-month (3 m) and the 6-month (6 m) post-stroke stage (baseline score subtracted from the values) in the three patient groups. **P < 0.01, *P < 0.05 by mixed-model ANOVA.
The time × group interaction was, however, significant in the domains of verbal memory [F(4, 96) = 4.7, P = 0.002] and focused attention (correct responses) [F(3.2, 59.3) = 3.9, P = 0.012]. Post hoc tests of the change scores showed that at the 3-month stage verbal memory recovery was significantly better in the music group than in the control group (P = 0.049) or in the language group (P = 0.006). Focused attention recovery was significantly better in the music group than in the control group (P = 0.049) and also marginally better in the music group than in the language group (P = 0.058). At the 6-month stage, verbal memory recovery was significantly better in the music group than in the language group (P = 0.006), and focused attention recovery was significantly better in the music group than in the control group (P = 0.008) or in the language group (P = 0.016). A further analysis also showed that, across all patients, the correlation between the focused attention (correct responses) score and the verbal memory score was significant at baseline (r = 0.32, P = 0.037) and at the 3-month (r = 0.54, P < 0.001) and 6-month (r = 0.49, P < 0.001) stages.
In addition, the time × group × lesion laterality interaction was significant in focused attention (correct responses) [F(3.2, 59.3) = 4.1, P = 0.009]. Separate mixed-model ANOVAs for the left hemisphere-lesioned patients and the right hemisphere-lesioned patients showed that the time × group interaction was significant only in the left hemisphere-lesioned patients [F(3.0, 25.7) = 4.5, P = 0.011]. Post hoc tests of the change scores showed that in the left hemisphere-lesioned patients focused attention recovery was significantly better in the music group than in the language group (P = 0.019), and also marginally better in the music group than in the control group (P = 0.092) at the 3-month stage. At the 6-month stage, recovery was significantly better in the music group than in the language group (P = 0.036) or in the control group (P = 0.041).
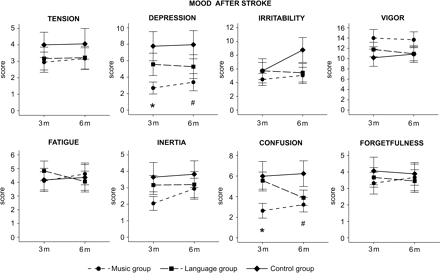
Figure 2 illustrates the 3- and 6-month post-stroke POMS scores in the three patient groups. No significant time × group or time × group × lesion laterality interactions were observed in a mixed-model ANOVA (P = 0.378–0.859 in all subscales), indicating, thus, that the intervention did not induce systematic changes on mood from the baseline to the 3- and 6-month stages. However, the emotional reactions of the patients are typically highly variable in the acute post-stroke stage, encompassing sadness, passivity, withdrawal, crying, catastrophic reactions, lack of adaptation, disinhibition, anosognosia and aggressiveness (Bogousslavsky, 2003). Consequently, the emotional status of the patients can change rapidly during the first days and weeks after the stroke. Thus, the changes that take place between the acute and the 3-month post-stroke stage vary considerably between patients, and more stable effects on mood, such as post-stroke depression, appear usually later, only about 3–4 months after the stroke (Carota et al., 2002). Therefore, directly comparing mood assessed with POMS at the acute and at the 3- and 6-month stages may not be reliable due to the emotional lability of the patients at the acute stage. For this reason, we also analysed group differences in mood cross-sectionally from the 3- and 6-month post-stroke POMS scores. At the 3-month stage, there were significant group differences in the depression [F(2, 51) = 3.7, P = 0.031] and confusion [F(2, 51) = 3.3, P = 0.045] scores. Post hoc tests indicated that the depression score was significantly lower in the music group than in the control group (P = 0.024). Also the confusion score was marginally lower in the music group than in the control group (P = 0.061). At the 6-month stage, the group differences in depression [F(2, 50) = 2.6, P = 0.086] and confusion [F(2, 50) = 2.9, P = 0.064] were still marginally significant with post hoc tests showing a tendency for the music group to experience less depressed (P = 0.071) and confused (P = 0.064) mood than the control group. There were no significant group differences in self-rated or proxy-rated QOL at the 3-month stage or at the 6-month stage (P = 0.094–0.987 in all domains).
Profile of Mood States (POMS) scale scores (mean ± SEM) in the three patient groups at the 3-month (3 m) and the 6-month (6 m) post-stroke stage. *P < 0.05, #P < 0.1 by one-way ANOVA.
Discussion
The novel main finding of this study was that regular self-directed music listening during the early post-stroke stage can enhance cognitive recovery and prevent negative mood. Specifically, after the 2-month intervention period, patients who listened to their favourite music 1–2 h a day showed greater improvement in focused attention and verbal memory than patients who listened to audio books or received no listening material. Moreover, patients who listened to music also experienced less depressed and, to a lesser extent, confused mood after the intervention than patients who received no listening material. Since the patient groups did not differ in demographic and clinical variables at the baseline or in antidepressant medication and rehabilitation received during the intervention, and since any non-specific effects of therapeutic attention were controlled for, these differences observed in cognitive recovery can be directly attributed to the effect of listening to music. Furthermore, the fact that most of the music also contained lyrics, would suggest that it is the musical component (or the combination of music and voice) that plays a crucial role in the observed recovery of these cognitive functions.
By its very nature, music has strong connections to both attention and memory systems. Brain imaging studies have shown that listening to real polyphonic music calls for rule-based analysis and combination of sound patterns from multiple auditory streams, which naturally recruits bilateral temporal, frontal and parietal neural circuits underlying multiple forms of attention, working memory, semantic and syntactic processing, and imagery (Janata et al., 2002; Peretz and Zatorre, 2005). Recent evidence suggests that listening to music that is enjoyable but unrelated to the cognitive task may even temporarily improve performance in tests of spatial-temporal abilities (Thompson et al., 2001), attention (Schellenberg et al., 2007), verbal fluency (Thompson et al., 2005) and creativity (Schellenberg et al., 2007) in healthy subjects. Moreover, auditory stimulation with music has also temporarily improved performance in tests of autobiographical recall in dementia patients (Foster and Valentine, 2001) and in tests of visual neglect in stroke patients (Hommel et al., 1990). Furthermore, in healthy subjects (Wallace, 1994) and in multiple sclerosis patients (Thaut et al., 2005) verbal material presented in a musical modality (as song lyrics) is learned and retrieved more efficiently than one presented verbally. It can also increase the coherence of frontal EEG oscillations during learning more than verbally presented material (Thaut et al., 2005; Peterson and Thaut, 2007). Moreover, aphasic patients repeat and recall more words from novel songs when singing than speaking along an auditory model (Racette et al., 2006). Randomized controlled trials have also shown that active music therapy or music-based exercise improves general cognition and verbal fluency in dementia patients (Van de Winckel et al., 2004), symptom scores in schizophrenia patients (Talwar et al., 2006), and communication skills in autistic children (Gold et al., 2006). Similar studies that have used a within-subject design have also shown that music improves phonological and spelling skills in dyslexic children (Overy, 2003), speech content and fluency in dementia patients (Brotons and Koger, 2000), and verbal fluency in cardiac rehabilitation patients (Emery et al., 2003). Collectively, these findings provide evidence that music engages and facilitates a wide range of cognitive functions.
Music is also closely linked to emotions and arousal. Evidence suggests that music listening modulates emotional arousal as indexed by changes in electrodermal, cardiovascular and respiratory activity (Khalfa et al., 2002; Bernardi et al., 2006; Gomez and Danuser, 2007). Listening to pleasant and relaxing music also enhances the recovery of cardiovascular and respiratory functions and decreases cortisol levels after stress (Khalfa et al., 2003; Leardi et al., 2007; Sokhadze, 2007). Music therapy has been shown to reduce anxiety and depression in patients with a somatic illness (Cassileth et al., 2003; Siedliecki and Good, 2006) and anecdotally also in neurological patients (Magee and Davidson, 2002). These findings suggest that music has an analgesic effect in reducing anxiety and directing attention away from the negative experience, thus helping to cope with emotional stress.
In summary, music listening can facilitate a wide variety of cognitive and emotional functions. Whether these effects are truly specific to music, are selective to a few cognitive functions, and are long-lasting is, however, not known due to methodological limitations of most prior studies. Here, we used a single-blind, randomized, longitudinal experimental design with two control groups and extensive neuropsychological outcome measures to evaluate a wide range of cognitive functions. Our results indicate that music, when applied during the most dynamic period of recovery from neural damage, can induce long-term changes on cognition that is indexed by enhanced recovery of focused attention and verbal memory. Interestingly, the facilitating effect of music on focused attention was more pronounced in patients with damage to the language-dominant hemisphere. This most likely reflects the strong verbal component (mental arithmetic and color-word processing) in the tasks we used to evaluate focused attention. Moreover, music listening was associated with less depressed and confused mood, suggesting that music may help to cope with the emotional stress brought about by sudden and severe neurological illness. Here, the possible effect of non-specific therapeutic attention can not, however, be entirely ruled out, since the difference in mood between the music group and the language group was not significant.
An important and difficult question still pertains to the neural mechanisms that can account for the beneficial effect of music on cognition. Most previous studies have attributed the effect to a general positive affective state or enhanced arousal and attention, which, given the wide variability of reported benefits, seems a plausible mechanism. The focused attention and verbal memory scores in our study were also significantly correlated, suggesting that the effect is mostly related to enhanced attention. Thus, current evidence suggests that music has a rather general, non-specific effect on cognition. This is in line with the arousal and mood hypothesis (Thompson et al., 2001), which states that any enjoyable stimuli, such as music, that induces positive affect and heightened arousal can lead to improved performance on cognitive tasks. Recent animal studies and functional neuroimaging studies in humans have shed some light on the neural mechanisms that mediate these effects. Listening to pleasant music activates an interconnected network of subcortical and cortical brain regions, which includes the ventral striatum, nucleus accumbens (NAc), amygdala, insula, hippocampus, hypothalamus, ventral tegmental area (VTA), anterior cingulate, orbitofrontal cortex and ventral medial prefrontal cortex (Blood and Zatorre, 2001; Brown et al., 2004; Menon and Levitin, 2005; Koelsch et al., 2006). VTA produces dopamine and has direct projections to the locus ceruleus (LC), amygdala, hippocampus, anterior cingulate and prefrontal cortex (Ashby et al., 1999). The VTA-NAc responses are suggested to be related to suppression of aversive stimuli and pain (Menon and Levitin, 2005), which would account for the effect of music on coping with stress, whereas LC and hypothalamus mediate arousal. Together, this dopaminergic mesocorticolimbic system is crucial for mediating arousal, emotion, reward, motivation, memory, attention and executive functioning (Ashby et al., 1999). In animals, music listening leads to increased dopamine synthesis in the brain (Panksepp and Bernatzky, 2002; Sutoo and Akiama, 2004). Increased dopamine directly enhances alertness, speed of information processing, attention, and memory in healthy humans (Schück et al., 2002) and also global cognitive functioning in patients with cognitive impairment (Nagaraja and Jayashree, 2001). It is, thus, possible that the music-related enhanced cognitive recovery seen in our study was mediated by positive mood induced by music, and hence the dopaminergic mesocorticolimbic system, especially since the music the patients listened to was their own favourite music and concurrent effects on mood were also observed.
A related topic concerns the pleasurability of listening to music and stories. When comparing the effects of music and narrated story listening on healthy subjects, Nantais and Schellenberg (1999) found that listeners performed better on a cognitive task following the listening condition they preferred. In our study, the music and language groups liked music and story listening to the same degree before the intervention, the material used in both groups was self-selected, and the groups listened to it equally often. This suggests that preference to the type of material did not play a significant role. In general, also anecdotal evidence from the patients’ reports indicated that both music and language groups enjoyed the intervention, although music listening was experienced as easier and less demanding, especially in the early recovery phase. Moreover, it is possible that some aphasic patients in the language group had difficulties in listening to the audio books due to comprehension deficits, and, thus, did not find the intervention as enjoyable as patients in the music group.
In addition to the effect on cognition and mood, music may also have general effects on brain plasticity after stroke. Since our patients had a unilateral MCA stroke, and the brain regions involved in music processing are mainly supplied by the MCA bilaterally (Ayotte et al., 2000), listening to music may well have further stimulated both the peri-infarct regions in the damaged hemisphere as well as regions in the contralesional, healthy hemisphere that normally show increased plasticity at this recovery stage (Witte, 1998; Kreisel et al., 2006). The fact that listening to music, especially with lyrics, is associated with activity of a more widely and bilaterally distributed neural network than listening to verbal material alone (Callan et al., 2006), would also account for the observed superiority of music stimulation over purely verbal stimulation, especially in left hemisphere-lesioned patients (Démonet et al., 2005). Animal studies have shown that an enriched post-stroke recovery environment can induce many structural plastic changes in the recovering brain such as decreased infarct volume and increased dendritic branching, spine density, neurotrophic factors, cell proliferation and neurogenesis (Johansson, 2004; Nithianantharajah and Hannan, 2006). Although the effect of an enriched sound environment on recovery from neural damage has not been directly studied, recent developmental animal studies have shown that exposure to music during development improves auditory cortical functions, learning, and memory (Engineer et al., 2004; Chikahisa et al., 2006; Kim et al., 2006; Angelucci et al., 2007a). Importantly, exposure to music also enhances brain plasticity by increasing neurogenesis in the hippocampus (Kim et al., 2006), modifying the expression of glutamate reseptor GluR2 in the auditory cortex and in the anterior cingulate (Xu et al., 2007), increasing brain-derived neurotrophic factor (BDNF) levels in the hippocampus (Angelucci et al., 2007a) and in the hypothalamus (Angelucci et al., 2007b), and also increasing the levels of tyrosine kinase receptor B (TrkB), a BDNF receptor, in the cortex (Chikahisa et al., 2006). Changes in glutamate transmission in the peri-infarct area (Centonze et al., 2007) and increased BDNF levels (Schäbitz et al., 2007) are also crucial plasticity mechanisms that contribute to recovery from stroke. Thus, it is possible that the music-related enhanced cognitive recovery seen in our study was also due to structural plastic changes induced by music stimulation in the recovering brain. At present, this suggestion is, however, tentative, and further research is clearly needed to elucidate the potential effects of a musically enriched recovery environment on brain plasticity after stroke.
In conclusion, the results of the present study demonstrate, to our knowledge for the first time, that regular self-directed music listening during the 2-month subacute phase of MCA stroke recovery enhanced the recovery of verbal memory and focused attention, and also prevented depressed and confused mood. According to a recent study conducted in European stroke rehabilitation centers (De Wit et al., 2005), stroke patients typically spend >72% of their daily time in non-therapeutic activities, mostly in their rooms, inactive and without any interaction, even though from a plasticity standpoint this time-window is ideal for rehabilitative training (Witte, 1998; Kreisel et al., 2006). We suggest that everyday music listening during early stroke recovery offers a valuable addition to the patients’ care, especially if other active forms of rehabilitation are not yet feasible at this stage, by providing an individually targeted, easy-to-conduct and inexpensive means to facilitate cognitive and emotional recovery.
Acknowledgements
We wish to express our gratitude to the staffs of the HUCH Department of Neurology and other rehabilitation hospitals in the Helsinki metropolitan area for their generous collaboration, and especially to the patient subjects and their families for their participation and effort. This work was supported by Academy of Finland (project no 77322), Jenny and Antti Wihuri Foundation (Helsinki, Finland), National Graduate School of Psychology and Neurology Foundation (Helsinki, Finland). Funding to pay the Open Access publication charges for this article was provided by Cognitive Brain Research Unit, Department of Psychology, University of Helsinki, Finland.
References
Abbreviations:
- FLAIR
fluid-attenuated inversion recovery
- MCA
middle cerebral artery
- MRI
magnetic resonance imaging
- QOL
quality of life
- RT
reaction time