-
PDF
- Split View
-
Views
-
Cite
Cite
H. Tsao, M. P. Galea, P. W. Hodges, Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain, Brain, Volume 131, Issue 8, August 2008, Pages 2161–2171, https://doi.org/10.1093/brain/awn154
Close - Share Icon Share
Abstract
Many people with recurrent low back pain (LBP) have deficits in postural control of the trunk muscles and this may contribute to the recurrence of pain episodes. However, the neural changes that underlie these motor deficits remain unclear. As the motor cortex contributes to control of postural adjustments, the current study investigated the excitability and organization of the motor cortical inputs to the trunk muscles in 11 individuals with and without recurrent LBP. EMG activity of the deep abdominal muscle, transversus abdominis (TrA), was recorded bilaterally using intramuscular fine-wire electrodes. Postural control was assessed as onset of TrA EMG during single rapid arm flexion and extension tasks. Motor thresholds (MTs) for transcranial magnetic stimulation (TMS) were determined for responses contralateral and ipsilateral to the stimulated cortex. In addition, responses of TrA to TMS over the contralateral cortex were mapped during voluntary contractions at 10% of maximum. MTs and map parameters [centre of gravity (CoG) and volume] were compared between healthy and LBP groups. The CoG of the motor cortical map of TrA in the healthy group was ∼2 cm anterior and lateral to the vertex, but was more posterior and lateral in the LBP group. The location of the CoG and the map volume were correlated with onset of TrA EMG during rapid arm movements. Furthermore, the MT needed to evoke ipsilateral responses was lower in the LBP group, but only on the less excitable hemisphere. These findings provide preliminary evidence of reorganization of trunk muscle representation at the motor cortex in individuals with recurrent LBP, and suggest this reorganization is associated with deficits in postural control.
Introduction
Described as a ‘Western epidemic’, low back pain (LBP) is the most common cause of work-related absence in western society (Blyth et al., 2001). Studies show 50–80% of adults in the general population will suffer this condition at some stage in their lives, and 15–30% will have LBP at any given time (Andersson, 1998). While many individuals will recover within 1 month (Pengel et al., 2003), most people will have recurrence of pain episodes within a 12-month period (Cassidy et al., 2005; Wasiak et al., 2006). A possible contributor to the persistence or recurrence of this condition is changes in postural control of the trunk muscles. Several studies have demonstrated delayed activation of the deep abdominal (Hodges and Richardson, 1996) and back muscles (Leinonen et al., 2001), and increased activity of superficial trunk muscles (Arendt-Nielsen et al., 1996; Radebold et al., 2001) in patients with recurrent LBP. Many of these changes persist after the resolution of symptoms (Hodges and Richardson, 1996) and have been argued to contribute to the recurrence of LBP episodes (Hodges and Moseley, 2003; Cholewicki et al., 2005). However, exactly how the organization of control of these responses in the motor system is changed with pain remains unclear.
The motor cortex provides a critical contribution to postural control (see review Deliagina et al., 2008). For instance, stimulation of the motor cortex in standing cats induced both a flexion movement of the contralateral forelimb and an anticipatory postural change in the supporting forelimb (Gahéry and Nieoullon, 1978). In addition, data from human studies demonstrate that inhibition of the motor cortex can reduce postural activity of the trunk muscles associated with voluntary limb movements (Hodges et al., 2003). As cortical regions contribute to postural control, it could be speculated that deficits in postural activation, such as those observed in people with LBP, may be associated with changes in the excitability and organization of the motor cortex.
There is a tremendous potential for areas of the brain, such as the motor and sensory cortices, to undergo an organizational change that was once thought only possible during early human development (Sanes and Donoghue, 2000). For instance, the motor cortex is extensively reorganized following stroke (Nudo and Milliken, 1996; Bütefisch, 2004). Furthermore, changes in motor cortex organization have been observed in conditions such as phantom limb pain (Flor et al., 1995; Karl et al., 2001) and complex regional pain syndrome (Krause et al., 2006; Maihöfner et al., 2007), where the central nervous system (CNS) remains largely intact. Few studies have examined the plasticity of the sensorimotor cortex in people with recurrent LBP. One study showed an expansion and shift in the representation of the lower back in the somatosensory cortex (Flor et al., 1997). Whether there are similar changes in the motor cortex of individuals with recurrent LBP remains unclear. The only available data suggest higher thresholds to evoke facilitation or inhibition of responses of the erector spinae muscles to transcranial magnetic stimulation (TMS) over the motor cortex compared to healthy individuals (Strutton et al., 2005). In that study, the change in threshold was related to the pain and functional disability experienced by LBP patients. However, it remains unclear whether changes in excitability are related to changes in organization at the motor cortex, or whether the cortical changes are associated with changes in postural control.
This study investigated changes in postural activation of the deep abdominal muscle, transversus abdominis (TrA) in people with recurrent LBP. Feedforward postural activation of this muscle in association with arm movement is consistently delayed in these individuals compared to healthy controls (Hodges and Richardson, 1996; Hodges and Richardson, 1997). Although changes in trunk muscle activation are not restricted to the TrA, deficits in activation of this muscle provide an useful marker of motor control dysfunction as they are observed relatively consistently despite differences in LBP presentation. The study aimed to investigate the excitability and organization of cortical networks in the motor cortex that induce activation of TrA when excited by TMS in healthy individuals, and to compare these parameters to individuals with recurrent LBP. If changes in cortical parameters were observed, a further aim was to determine whether the extent of cortical reorganization was associated with changes in postural activation of the trunk muscles.
Methods
Participants
Eleven right-handed individuals with recurrent non-specific LBP lasting longer than 3 months and 11 right-handed healthy individuals with no history of LBP were recruited (Table 1). Individuals were included in the LBP group if they experienced pain in the low back region with or without accompanying buttock pain and of sufficient intensity to have limited activities of daily living. This group was selected as previous studies have consistently shown delays in postural activation of TrA (Hodges and Richardson, 1996). Subjects in the LBP group had minimal pain at time of testing and symptoms were not aggravated by the experimental procedures, as variability in the level of pain during testing could increase the variability of the data. Subjects were excluded if they had any major circulatory, orthopaedic, neurological or respiratory conditions, a history or family history of epilepsy, recent or current pregnancies, previous surgery to the abdomen or back, or if they had undertaken any form of abdominal exercises in the preceding 12 months. The study conformed to the Declaration of Helsinki and was approved by the Institutional Medical Research Ethics Committee.
Subject demographics (mean ± SD)
| . | Healthy (n = 11) . | LBP (n = 11) . |
|---|---|---|
| Age (years) | 23 ± 3 | 24 ± 7 |
| Gender (M/F) | 4/7 | 5/6 |
| Height (cm) | 170 ± 10 | 172 ± 9 |
| Weight (kg) | 67 ± 16 | 68 ± 9 |
| Pain VAS (/10) | – | 5.5 ± 2.0 |
| Pain duration (years) | – | 5.6 ± 4.2 |
| Oswestry Disability Index (/100) | – | 26.2 ± 9.9 |
| . | Healthy (n = 11) . | LBP (n = 11) . |
|---|---|---|
| Age (years) | 23 ± 3 | 24 ± 7 |
| Gender (M/F) | 4/7 | 5/6 |
| Height (cm) | 170 ± 10 | 172 ± 9 |
| Weight (kg) | 67 ± 16 | 68 ± 9 |
| Pain VAS (/10) | – | 5.5 ± 2.0 |
| Pain duration (years) | – | 5.6 ± 4.2 |
| Oswestry Disability Index (/100) | – | 26.2 ± 9.9 |
Pain visual analogue scale (VAS) represents the worst pain reported in the last month.
Subject demographics (mean ± SD)
| . | Healthy (n = 11) . | LBP (n = 11) . |
|---|---|---|
| Age (years) | 23 ± 3 | 24 ± 7 |
| Gender (M/F) | 4/7 | 5/6 |
| Height (cm) | 170 ± 10 | 172 ± 9 |
| Weight (kg) | 67 ± 16 | 68 ± 9 |
| Pain VAS (/10) | – | 5.5 ± 2.0 |
| Pain duration (years) | – | 5.6 ± 4.2 |
| Oswestry Disability Index (/100) | – | 26.2 ± 9.9 |
| . | Healthy (n = 11) . | LBP (n = 11) . |
|---|---|---|
| Age (years) | 23 ± 3 | 24 ± 7 |
| Gender (M/F) | 4/7 | 5/6 |
| Height (cm) | 170 ± 10 | 172 ± 9 |
| Weight (kg) | 67 ± 16 | 68 ± 9 |
| Pain VAS (/10) | – | 5.5 ± 2.0 |
| Pain duration (years) | – | 5.6 ± 4.2 |
| Oswestry Disability Index (/100) | – | 26.2 ± 9.9 |
Pain visual analogue scale (VAS) represents the worst pain reported in the last month.
EMG
EMG activity of TrA was recorded bilaterally using intramuscular fine-wire electrodes (Teflon-coated stainless steel wire, 75 µm diameter with 1 mm of Teflon removed and tips bent back ∼1 and ∼2 mm to form hooks). Wires were threaded into a hypodermic needle and inserted with real-time ultrasound guidance (Hodges and Richardson, 1997). Pairs of surface electrodes (Ag/AgCl discs, Grass Telefactor, USA) were placed over the anterior and posterior deltoid muscles for assessment of an arm movement task (see below). The ground electrode was placed over the lower lateral rib cage. EMG data were pre-amplified 2000 times, band-pass filtered between 20 and 1000 Hz, and sampled at 2000 Hz using a Power1401 Data Acquisition System with Signal2 and Spike2 software (CED, UK).
TMS
A single-pulse monophasic MagStim 2002 stimulator (Magstim Company, UK) was used to stimulate the motor cortex. A 7-cm figure-of-eight coil was placed with cross-over position over respective scalp sites and the coil handle oriented at ∼45° from the mid-sagittal plane to induce currents in an anterior-medial direction (Sakai et al., 1997). This coil orientation has been shown to evoke consistent contralateral responses from the abdominal muscles during submaximal voluntary contractions (Tunstill et al., 2001; Strutton et al., 2004; Tsao et al., 2008). The figure-of-eight coil provides better focality of stimulation compared to the standard circular coil and is more ideal for mapping of the motor cortex (Cohen et al., 1990; Brasil-Neto et al., 1992). However, our previous study showed that even at maximum stimulator output with the figure-of-eight coil, it was difficult to evoke ipsilateral responses in TrA during submaximal contractions, or evoke contralateral responses with the TrA muscle relaxed (Tsao et al., 2008). Thus, a 110 mm double-cone coil (Magstim Company, UK) was also used as this coil produces a stronger magnetic field and could induce consistent contralateral and ipsilateral responses of the trunk muscles (Davey et al., 2002; Strutton et al., 2005; Tsao et al., 2008). The double-cone coil was positioned perpendicular to the scalp site with induced current flowing in an anterior direction.
EMG was recorded during maximum voluntary contraction (MVC) performed as a forced expiratory manoeuvre to determine targets for voluntary activation of TrA. Three repetitions, each lasting for at least 3 s, were completed with verbal encouragement. The contraction with the highest root-mean-square (RMS) EMG amplitude was identified and the peak RMS EMG over 1 s was recorded. The target for voluntary contraction of TrA was set at 10% MVC as this was a comfortable level for subjects to maintain and minimized the potential for fatigue. Feedback of real-time RMS EMG of TrA (averaged online over 200 ms windows on a duplicate channel) and the target level was displayed on a monitor. Excitability of the abdominal motoneurons is thought to be modulated throughout the respiratory cycle as a result of central respiratory drive potentials (Gill and Kuno, 1963; Sears, 1964), which would likely affect the amplitude of motor evoked potentials (MEPs; Lissens et al., 1995). To standardize motoneuron excitability, subjects ceased breathing at the end of normal expiration and maintained their glottis open prior to the TMS pulse. The phase of respiration was monitored online using a pressure cuff strapped to the chest to measure rib cage displacement (Hodges et al., 1997).
Subjects sat comfortably in a reclined chair with arms well-supported, hips flexed to ∼70°, and knees flexed to ∼45°. A tight-fitting elastic cap was worn over the head and the location of the vertex was identified using the International 10/20 system (Jasper, 1958). The optimal location was identified (i.e. scalp site that induced the largest contralateral response in TrA) using the figure-of-eight coil set at suprathreshold intensity (∼70–100% maximum stimulator output) during the activation of TrA to 10% MVC. Four motor thresholds (MT) for TrA were identified at the optimal location: MTs were defined as the minimum intensity to evoke five consecutive MEPs in TrA, and these were to be clearly discernible from background EMG activity (Mills and Nithi, 1997). Previous studies from our group have shown that TMS delivered over the optimal location (∼2 cm lateral to the midline in most subjects) minimizes concurrent stimulation of both motor cortices, and evokes contralateral responses that are faster than ipsilateral responses from the same hemisphere (Tsao et al., 2008).
Active MT for contralateral responses using the figure-of-eight coil.
Active MT for contralateral responses using the double-cone coil.
Active MT for ipsilateral responses using the double-cone coil.
Resting MT for contralateral responses using the double-cone coil.
Topography of TrA responses to TMS over the contralateral motor cortex was examined using the figure-of-eight coil (Wassermann et al., 1992; Wilson et al., 1993). The coil intensity was set to 120% active MT, and stimulation was delivered over pre-marked scalp sites on a 5 × 5 cm grid oriented in a Cartesian system. Five stimuli were delivered at each scalp site during 10% MVC with an inter-stimulus interval of at least 5 s (Wilson et al., 1993). Early pilot trials in individuals with recurrent LBP showed that MEPs could also be induced when TMS was delivered at scalp sites posterior to the inter-aural line (i.e. the line that joins the left and right pre-auricular creases and pass through the vertex). Thus stimulation in the LBP group was extended 2 cm posterior to the vertex. This was not possible for the control group as data for that group were collected prior to the LBP group. This does not compromise the data as little or no activation was achieved by stimulation at or behind the inter-aural line in this group.
Rapid arm movements
Subjects performed single rapid arm movements during a choice reaction time task to induce a perturbation to the trunk for assessment of postural activation of TrA (Hodges and Richardson, 1997). Subjects stood comfortably and remained relaxed prior to movement of the left arm into flexion or extension to ∼45° ‘as fast as possible’ in response to auditory tones triggered by the experimenter. Movement direction was indicated by distinct tones and emphasis was placed on the speed of arm movement rather than magnitude. Practice trials were included to familiarize subjects to the task. To ensure arm movements were performed in a similar manner between trials, the left arm was attached to a potentiometer at the wrist. This device restricted motion to the sagittal plane and measured angular displacement. Ten trials of arm flexion and extension were completed as this number of trials has been shown to yield sufficient repeatability of the data.
Data analysis
Data analysis was undertaken using MATLAB 7 (The Mathsworks, USA). For mapping, TrA activity from individual trials was full-wave rectified. Trials at each scalp site were averaged, and the onset and offset of the MEP (or onset of silent period) were visually identified from the averaged full-wave rectified traces. As recordings were made using intramuscular electrodes, peak-to-peak amplitude is more variable due to recordings of a small population of motor units. Thus, the amplitude of TrA MEPs was measured as the RMS EMG amplitude between the onset and offset of the MEP, and background RMS EMG was removed (55 to 5 ms prior to stimulation). TrA EMG amplitudes were superimposed over respective scalp sites to produce a topographical map of responses of the muscle and normalized to the amplitude of the peak response. As current spread during magnetic stimulation enlarges the motor cortical map, it is difficult to define map boundaries due to small amplitude MEPs at the map edge (Uy et al., 2002). To minimize this problem, normalized values below 25% of the peak response were removed. Remaining responses were rescaled from 0 to 100%.
Two parameters were calculated from the rescaled normalized maps. Map volume, which is a measure of the total excitability of cortical representation, was calculated as the sum of normalized MEP amplitudes recorded at all scalp sites where responses were evoked (Wassermann et al., 1992). The centre of gravity (CoG) was calculated using the formula: 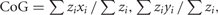 where xi and yi are medial–lateral and anterior–posterior locations, and zi is amplitude (Wassermann et al., 1992). This measure gives an amplitude-weighted indication of map position. Although map volume can substantially change with changes in motor cortical excitability, studies have shown that the CoG is a valid and repeatable measure of motor cortical representation (Boroojerdi et al., 1999; Uy et al., 2002). Shifts in map position were also examined through calculation of the absolute distance between the location of the averaged CoG for the healthy group, and CoG for each individual in the healthy and LBP groups.
where xi and yi are medial–lateral and anterior–posterior locations, and zi is amplitude (Wassermann et al., 1992). This measure gives an amplitude-weighted indication of map position. Although map volume can substantially change with changes in motor cortical excitability, studies have shown that the CoG is a valid and repeatable measure of motor cortical representation (Boroojerdi et al., 1999; Uy et al., 2002). Shifts in map position were also examined through calculation of the absolute distance between the location of the averaged CoG for the healthy group, and CoG for each individual in the healthy and LBP groups.
For rapid arm movement tasks, the onsets of TrA and deltoid EMG were visually identified as the point at which the EMG increased above baseline levels. Visual identification of onset of EMG activation has been shown to be reliable and is preferred to computer-based methods as it is less affected by factors such as amplitude of background EMG or rate of increase in EMG activity (Hodges and Bui, 1996). Onsets of TrA EMG relative to that of the prime mover deltoid were calculated for the left and right TrA muscles. In addition, as onset of trunk muscle activity depends on the acceleration of the arm (Hodges, 2001); angular acceleration for each trial was calculated. Data of arm displacement at the shoulder were smoothed at 10 Hz and twice differentiated to yield angular acceleration. Peak acceleration was identified.
Statistical analysis
Statistica 7 (Statsoft, USA) was used for statistical analysis. Map volume and CoG location (lateral and anterior to the vertex) were compared between groups (healthy versus LBP) and muscles (left versus right TrA) using a repeated-measures analysis of variance (ANOVA). Significant interactions were further analysed using post hoc Duncan's multiple range test. MTs identified using the double-cone coil were compared between groups, muscles and conditions (contralateral, ipsilateral and resting MT) with a repeated-measures ANOVA. MTs identified using the figure-of-eight coil were compared between groups and muscles. In addition, onset of TrA EMG relative to that of the prime mover deltoid was compared between individuals with and without recurrent LBP using repeated-measures ANOVA with two repeated measures [group and direction (flexion versus extension)] and one independent factor (muscle). To determine whether CoG, map volume and MT were associated with the relative onset of TrA EMG in the arm movement task, the relationship between MT and map parameters, and relative onset of TrA activation during arm movement tasks were examined using Pearson's correlation. Significance was set at P < 0.05.
Results
MT
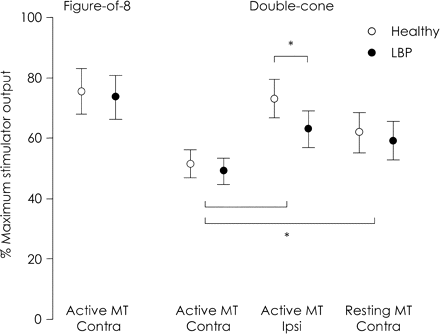
Figure 1 shows group data for MTs during different stimulation conditions. For all subjects, MTs could be identified for all conditions over at least one motor cortex. However, contralateral responses using the figure-of-eight coil could not be evoked over one hemisphere in five subjects from the healthy group and four subjects from the LBP group, even at maximum stimulator output. Ipsilateral responses using the double-cone coil could not be evoked over one hemisphere in four healthy and one LBP individual. Responses evoked ipsilateral to the side of stimulation at the optimal location were on average 3 ± 1 ms (mean ± SD) slower than MEPs evoked on the contralateral side. This confirms that ipsilateral responses were not due to concurrent excitation of the faster contralateral pathways from the opposite motor cortex, and originated from the stimulated motor cortex (Ziemann et al., 1999; Tsao et al., 2008).
Active and resting MTs for contralateral (contra) and ipsilateral (ipsi) responses in healthy and LBP group. Mean and 95% CI are illustrated. For double-cone coil, lower MT for ipsilateral responses in LBP group was observed compared to healthy controls (*P < 0.05). No differences were detected between groups for MT to elicit contralateral responses using the double-cone coil or figure-of-eight coils.
Using the double-cone coil, the lowest MT was found for responses contralateral to the stimulated cortex (main effect for condition P < 0.001; post hoc: P < 0.001; Fig. 1). No differences in MT were detected between the left and right TrA for any condition using the double-cone coil (main effect for muscle P = 0.84), or for MT using the figure-of-eight coil (t = 0.35, P = 0.98).
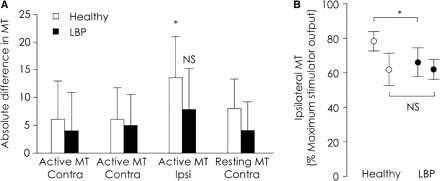
MT for ipsilateral responses in the LBP group was significantly lower compared to healthy controls (interaction between condition and group P = 0.009; post hoc: P = 0.04). Inspection of individual data showed a tendency for greater asymmetry of ipsilateral MTs between hemispheres in most healthy individuals, but the lowest threshold was not always on the dominant or non-dominant side. A lesser difference between hemispheres was observed for individual data for the LBP group. To quantify this observation, the absolute difference in MT between left and right TrA was calculated for each subject (Fig. 2A). There was a greater difference in MTs for ipsilateral responses compared to contralateral responses in the healthy group (P = 0.01), but not for the LBP group (P > 0.14). Furthermore, absolute difference in MTs for ipsilateral responses was greater for the healthy group compared to LBP participants (P = 0.043), i.e. less asymmetrical. To examine this further, ipsilateral MT for each individual were rearranged into either a ‘lower’ or ‘higher’ MT to evoke ipsilateral responses (Fig. 2B). When analysed in this way, the MT for the less excitable hemisphere was higher for the control subjects compared to those with LBP (P < 0.001), but there was no difference between groups in the MT for the more excitable hemisphere (P > 0.066). Together, these data suggest that the reduced MT of ipsilateral responses in the LBP group is likely due to reduced MT of a less excitable side.
(A) Absolute difference in MT between the left and right TrAs. Note absolute difference in MT was greater for ipsilateral responses compared to that for contralateral responses in the healthy group. However, comparisons of the absolute difference between ipsilateral and contralateral MTs were not significant (NS) for the LBP group. (B) MT for ipsilateral responses in healthy and LBP group, re-arranged into the side with higher or lower MT. Mean and 95% CI are displayed. Note MT in LBP group on the less-excitable side is less than MT in the control group. (*P < 0.05).
Motor cortical map
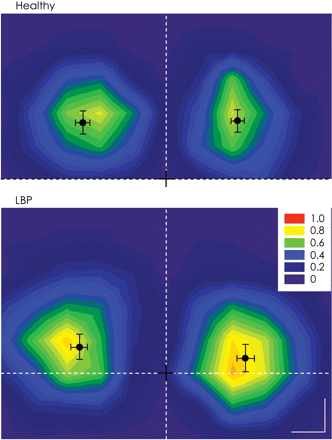
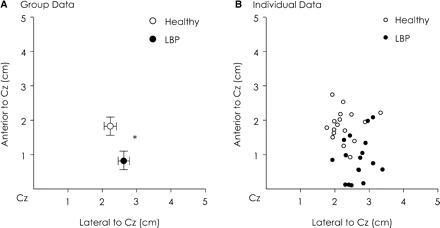
Average maps of TrA responses to TMS for healthy and LBP groups are illustrated in Fig. 3. The time of onset and offset of contralateral MEPs were not different between healthy and LBP groups [main effect for group P = 0.63; mean ± SD onset 16 ± 1 ms, mean ± SD offset 35 ± 5 ms (averaged across healthy and LBP groups)]. No differences in map volume were detected between the left and right hemispheres for either group (main effect for side P = 0.89). However, the volume of TMS maps for the LBP group was greater compared to healthy controls (healthy 5.2 ± 1.2, LBP 7.6 ± 1.2; main effect for group P < 0.001). Furthermore, the location of TrA CoG for LBP was located posterior and lateral to the CoG location in healthy individuals (interaction for location and group P < 0.001; post hoc: P < 0.011, Fig. 4). No difference was observed between the left and right motor cortices (main effect for muscle P = 0.08). The TrA CoG in individuals with recurrent LBP were located further away from the mean location of TrA CoG in healthy controls than the individual data for the control group (healthy: 0.45 ± 0.22 cm; LBP: 1.24 ± 0.52 cm; P < 0.001).
Average normalized motor cortical maps for the healthy and LBP groups on the left and right hemisphere. Mean and SD of the CoG is displayed. The black cross represents the location of vertex, horizontal dotted line denotes the inter-aural line and vertical dotted line denotes the line that connects the nasion and inion (Calibration –1 cm).
Group (A) and individual data (B) of the CoG of TrA. Note that for group data, the location of TrA CoG in LBP group was located posterior and lateral to TrA CoG in healthy groups (*P < 0.05). This was observed in most subjects.
Rapid arm movements
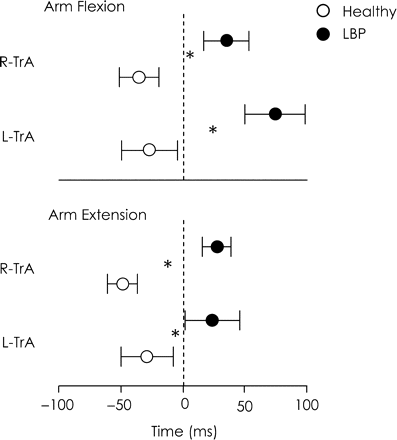
Figure 5 illustrates the onset of TrA EMG relative to that of the deltoid during rapid arm flexion and extension tasks. When LBP individuals moved their arm rapidly into flexion or extension, activation of TrA EMG was significantly delayed compared to healthy individuals (interaction between group, muscle and direction: F = 7.8, P = 0.007; post hoc: P < 0.001). No differences were observed between onsets for the left and right TrA muscles (post hoc: P > 0.065). There was no difference in peak arm acceleration between healthy and LBP group for arm flexion (healthy: 2411 ± 622°/s2; LBP: 2208 ± 938°/s2; P = 0.32) or extension (healthy: 1802 ± 436°/s2; LBP: 1751 ± 509°/s2; P = 0. 41). This suggests the arm was moved in a similar manner by both groups.
Relative onset of TrA activation (mean and 95% CI) during rapid arm flexion and extension tasks for the healthy and LBP groups. Dotted line represents onset of prime mover activation. Data show slower activation of TrA in LBP group for both the left and right TrA muscles and during both arm flexion and extension tasks (*P < 0.05 between healthy and LBP group).
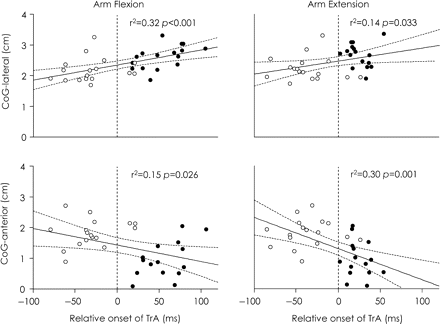
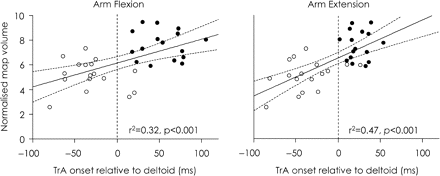
The location of TrA CoG was associated with onset of TrA EMG during rapid arm flexion and extension tasks (r > 0.37, P < 0.033; Fig. 6). That is, slower activation of TrA was likely when the CoG was located more posterior and lateral. Similar correlations were found for map volume; increased map area was associated with slower onset of TrA EMG (r > 0.57, P < 0.001; Fig. 7). There were no significant correlations between MTs and timing of TrA EMG onset (all: P > 0.17).
Relationship between location of the CoG; (distance anterior and lateral from the vertex) and timing of TrA activation during arm flexion and extension. Linear regressions are shown with 95% CI. Vertical dotted line represents activation of prime mover deltoid. Circles represent data of individuals from the healthy group (white) and LBP group (black). Data showed that individuals with slower TrA activation (mostly individuals from LBP group) tended to have TrA CoG located more posterior and lateral to the vertex.
Relationship between normalized map volume and timing of TrA activation during arm flexion and extension. Linear regressions are shown with 95% confidence interval. Vertical dotted line represents activation of prime mover deltoid. Circles represent data of individuals from the healthy group (white) and LBP group (black). There was a positive correlation between normalized map volume and relative onset of TrA activation.
Discussion
This study demonstrates that LBP is associated with reorganization of the networks in the motor cortex associated with activation of the deep trunk muscle, TrA. Posterior and lateral shifts in the CoG and greater map volume were observed in individuals with recurrent episodes of LBP compared to healthy individuals. A particularly novel finding was that the location of the CoG and volume of the TrA map at the motor cortex were related to the timing of onset of TrA EMG during a functional task. There were also changes in MT; compared to controls, threshold for ipsilateral responses on the less excitable side was reduced. As changes in control of TrA are consistently observed in individuals with recurrent LBP, these findings suggest that changes at the motor cortex may underlie or at least contribute to alterations in postural strategies.
Reorganization of the motor cortex in recurrent LBP
The current study confirms that topography of the representation of the abdominal muscles on the motor cortex can be evaluated using TMS. In healthy individuals, TrA representation was located at scalp sites ∼2 cm anterior and lateral to the vertex. This location is consistent with the optimal location used in previous studies to evoke responses in the more superficial abdominal muscles recorded with surface EMG electrodes (Fujiwara et al., 2001; Strutton et al., 2004). As expected, based on the distribution of body representation in the motor homunculus (Penfield and Boldrey, 1937; Woolsey et al., 1952), the representation of TrA was located medially compared to that of the upper limb (Wilson et al., 1993; Pascual-Leone et al., 1994), neck (Thompson et al., 1997) and facial muscles (McMillan et al., 1998).
The CoG of TrA at the motor cortex in individuals with recurrent LBP was shifted posteriorly and laterally to that of healthy individuals. Shifts in the motor cortical representation of specific muscle(s) have been reported in people with other recurrent pain conditions. For instance, studies of patients with phantom limb pain following upper limb amputations demonstrated a shift in the optimal location to evoke responses in the facial muscles on the side of the amputation towards the representation of the missing hand (Karl et al., 2001). The CoG is argued to be a robust measure of motor cortical representation and corresponds closely to the area of high excitability of corticomotor neurons that project to the target muscle(s) (Wassermann et al., 1992). Furthermore, TMS CoG closely approximates the CoG identified from functional magnetic resonance imaging (Boroojerdi et al., 1999; Lotze et al., 2003). Thus, shifts in TrA CoG from TMS maps could imply changes in the structural or functional organization of cortical networks associated with activation of TrA at the motor cortex. As this shift was consistently observed in most individuals with recurrent episodes of LBP, we argue that these findings are unlikely to be related to cap displacement, variability in coil placement or orientation, or inaccurate identification of the vertex. Future studies that utilize brain MRI and navigated brain stimulation with TMS mapping are likely to reduce the variability of motor cortical maps and further validate the present findings. In addition, as evidence reveals reduced grey matter density of the prefrontal cortex in patients with chronic LBP (Apkarian et al., 2004), further studies are needed to examine whether these structural changes in the brain are associated with changes in functional organization of the motor cortex, or how this relates to changes in motor behaviour.
Map volume was also increased in the LBP group compared to healthy individuals. This finding is similar to expansion in area of representation at the somatosensory cortex of patients with acute (Soros et al., 2001) and recurrent pain (Flor et al., 1997). However, increases in motor cortical map volume are not consistent with reduced map areas in patients with complex regional pain syndrome (Krause et al., 2006). This could be related to differences in the calculation of map area, as that study measured two-dimensional spread of representation over scalp sites whereas we measured map volume. It is also possible that reduced map areas detected in patients with complex regional pain syndrome relate to the nature of injury sustained in this group of patients, all of which involved forearm fractures that were immobilized for a period of time following post-fracture. As reduced motor cortex representation has been demonstrated with immobilization and disuse (Liepert et al., 1995), reduced map area in that study could be associated with disuse rather than the presence of pain and injury.
Increased map volume is difficult to interpret as stimulation of the cortical cells/interneurons with TMS is accompanied by current spread to produce TMS maps which are larger than the actual area of motor cortical cells that project to the target muscle (Roth et al., 1991; Mortifee et al., 1994; Thickbroom et al., 1998). It could be argued that increases in map volume in the LBP group could correspond to an increase in the total excitability of motor cortical cells and thus excitation by stimulation over a large area of the cortex (Wassermann et al., 1992). However, there were no differences in MT for contralateral MEPs at the optimal location between the LBP and healthy groups. Taken together, increased map volume without changes in MT at the optimal location suggest that alteration of motor cortical maps are not simply due to increased excitability of cortical cells, but involve more complex neural mechanisms that increase the area of the cortical networks involved in the activation of TrA.
Representation at the motor cortex is associated with postural activation of the trunk muscles
During rapid voluntary limb movements, the CNS initiates postural adjustments in advance of predictable perturbations to the body and these involve activation of trunk and limb muscles (Belen'kii et al., 1967). As this activation is initiated before feedback is available, they must be pre-programmed by the nervous system (Bouisset and Zattara, 1981). In the current study, postural activation of TrA, in most trials, occurred either before or <50 ms after the onset of deltoid EMG. Taking into account electromechanical delay and the latency for nerve conduction, even the shortest latency response to feedback from limb movements cannot be initiated before 50 ms after the onset of deltoid EMG (Aruin and Latash, 1995). Thus, the current study confirms previous findings that feedforward activation of TrA is associated with voluntary limb movements in healthy individuals (Hodges and Richardson, 1997), and that activation of TrA is delayed in individuals with recurrent LBP (Hodges and Richardson, 1996).
A novel finding was that the cortical reorganization of inputs to TrA was associated with onset of TrA EMG during rapid limb movements. This relationship is consistent with the observation that the motor cortex contributes, at least in part, to postural activation associated with limb movements (Gahéry and Nieoullon, 1978; Hodges et al., 2003). The present data provide evidence that changes in its organization at the motor cortex may contribute to deficits in feedforward postural control. As similar deficits in feedforward control have been demonstrated in other trunk muscles [for instance, the lumbar multifidus muscles (MacDonald et al., 2004)], the current findings suggest the potential for similar reorganization of neural networks at the motor cortex that contribute to the control of these muscles.
Exactly how changes in motor cortical representation are associated with deficits in postural control remains speculative. One possibility is that reorganization in motor cortical map of TrA and potentially other muscles in patients with recurrent LBP could distort the coordination between muscles. Data from people with focal hand dystonia provide evidence that reduced ability to isolate finger movements is associated with reorganization (i.e. reduce differentiation of individual finger representations) of the sensorimotor cortex (Byl et al., 1996). Although TMS maps of other trunk or limb muscles were not evaluated in the current study, similar mechanisms may contribute to changes in postural control of the trunk muscles. Furthermore, several studies have reported atrophy of the paraspinal muscles in human and animal studies of pain and injury to the low back (Hides et al., 1994; Hodges et al., 2006). Thus, it is reasonable to speculate that these morphological changes may be associated with reorganization at the sensorimotor cortex. This warrants further investigation.
In addition, the trunk muscles receive multiple projections from other supraspinal and spinal centres (see review Iscoe, 1998). For example, the reticulospinal and vestibulospinal neurons, which are intricately involved in postural control, have descending projections to the abdominal motoneurons (Miller et al., 1985; Miller et al., 1989). Changes in the excitability and/or organization of these regions of the CNS are also likely to contribute to changes in postural control of the trunk muscles in patients with recurrent LBP.
Reduced MT for ipsilateral corticospinal projections to the abdominal muscles
There was greater asymmetry in MT between the left and right hemispheres for ipsilateral responses compared to contralateral responses in control subjects. The lateralization of ipsilateral MT to one side is consistent with findings from other proximal (MacKinnon et al., 2004) and axial muscles (Strutton et al., 2004), and was unrelated to handedness. Interestingly, individuals with recurrent LBP did not demonstrate this asymmetry in ipsilateral MT. The MT to evoke responses in the left and right TrA in people with recurrent LBP was similar to the more excitable side in healthy individuals. One interpretation of this finding could be that coordination of the activation of the right and left abdominal muscles in healthy individuals is mediated by contralateral and ipsilateral projections from a single hemisphere, that is, the cortex with the lower threshold ipsilateral projections. In contrast, in people with recurrent pain the symmetrical excitability of ipsilateral projections suggests no preference for control by a single hemisphere.
Although changes in MT for ipsilateral responses did not correlate with changes in timing of TrA activation, this does not exclude a contribution of ipsilateral projections to postural control of the trunk muscles. It has been argued that responses evoked ipsilateral to the stimulated cortex using TMS are likely to be mediated via slower conducting uncrossed polysynaptic corticospinal pathways that project via regions in the brain stem (Ziemann et al., 1999). These brainstem pathways are also intricately involved in the coordination of timing and magnitude of postural responses (Inglis and Macpherson, 1995; Prentice and Drew, 2001). Changes in excitability of these pathways may contribute to alterations in activation of the trunk muscles for postural control. However, whether the earliest component of postural responses is related to ipsilateral projections, or how changes in excitability are related to coordination of muscle activation remains unclear.
Clinical implication
The present findings suggest that deficits in postural control in a patient population are associated with reorganization of the motor cortex. In patients with recurrent LBP, these deficits in postural activity can be trained by skilled motor training that involves voluntary contractions of muscle(s) (Tsao and Hodges, 2007, 2008), and is associated with improvements in clinical outcomes (see review Ferreira et al., 2006). Skilled motor training induces greater plastic change at the motor cortex than strength training (see review Adkins et al., 2006). Thus it is reasonable to predict that reorganization of the motor cortex following skilled motor training may be associated with improved postural activity in patients with deficits in postural control. This requires further investigation.
Acknowledgements
Financial support was provided by the National Health and Medical Research Council of Australia (ID351656 to HT and ID401599 to PH) and the Physiotherapy Research Foundation (Grant No: 007/06).
References
Abbreviations:
- LBP
= low back pain
- MVC
= maximum voluntary contraction
- MT
= motor thresholds
- TMS
= transcranial magnetic stimulation
- TrA
= transversus abdominis