-
PDF
- Split View
-
Views
-
Cite
Cite
Oluwole O Awosika, Saira Matthews, Emily J Staggs, Pierce Boyne, Xiao Song, Bridget A Rizik, Heidi J Sucharew, Christina Zhang, Gabrielle Mungcal, Rohitha Moudgal, Amit Bhattacharya, Kari Dunning, Daniel Woo, Brett M Kissela, Backward locomotor treadmill training combined with transcutaneous spinal direct current stimulation in stroke: a randomized pilot feasibility and safety study, Brain Communications, Volume 2, Issue 1, 2020, fcaa045, https://doi.org/10.1093/braincomms/fcaa045
Close - Share Icon Share
Abstract
Walking impairment impacts nearly 66% of stroke survivors and is a rising cause of morbidity worldwide. Despite conventional post-stroke rehabilitative care, the majority of stroke survivors experience continued limitations in their walking speed, temporospatial dynamics and walking capacity. Hence, novel and comprehensive approaches are needed to improve the trajectory of walking recovery in stroke survivors. Herein, we test the safety, feasibility and preliminary efficacy of two approaches for post-stroke walking recovery: backward locomotor treadmill training and transcutaneous spinal direct current stimulation. In this double-blinded study, 30 chronic stroke survivors (>6 months post-stroke) with mild-severe residual walking impairment underwent six 30-min sessions (three sessions/week) of backward locomotor treadmill training, with concurrent anodal (N = 19) or sham transcutaneous spinal direct current stimulation (N = 11) over the thoracolumbar spine, in a 2:1 stratified randomized fashion. The primary outcomes were: per cent participant completion, safety and tolerability of these two approaches. In addition, we collected data on training-related changes in overground walking speed, cadence, stride length (baseline, daily, 24-h post-intervention, 2 weeks post-intervention) and walking capacity (baseline, 24-h post-intervention, 2 weeks post-intervention), as secondary exploratory aims testing the preliminary efficacy of these interventions. Eighty-seven per cent (N = 26) of randomized participants completed the study protocol. The majority of the study attrition involved participants with severe baseline walking impairment. There were no serious adverse events in either the backward locomotor treadmill training or transcutaneous spinal direct current stimulation approaches. Also, both groups experienced a clinically meaningful improvement in walking speed immediately post-intervention that persisted at the 2-week follow-up. However, in contrast to our working hypothesis, anodal-transcutaneous spinal direct current stimulation did not enhance the degree of improvement in walking speed and capacity, relative to backward locomotor treadmill training + sham, in our sample. Backward locomotor treadmill training and transcutaneous spinal direct current stimulation are safe and feasible approaches for walking recovery in chronic stroke survivors. Definitive efficacy studies are needed to validate our findings on backward locomotor treadmill training-related changes in walking performance. The results raise interesting questions about mechanisms of locomotor learning in stroke, and well-powered transcutaneous spinal direct current stimulation dosing studies are needed to understand better its potential role as a neuromodulatory adjunct for walking rehabilitation.
Introduction
Walking impairment after a stroke is primarily due to the loss of adequate lower extremity function and is a significant cause of disability, with nearly two-thirds of stroke survivors having significant limitations in walking (Dobkin, 2005). This impairment results in an increased risk for falls, fractures and a progressive decline in mobility (Duncan et al., 2011; Langhorne et al., 2011). With the increasing survival rate after stroke, walking impairment is becoming an even greater public health issue. Hence, novel neurorehabilitative approaches are needed to improve the potential and trajectory of walking recovery after stroke. This study explores non-body weight supported backward locomotion treadmill training (BLTT) as a novel rehabilitation approach, and investigates transcutaneous spinal direct current stimulation (tsDCS) as an adjunct for walking rehabilitation in stroke.
Backward walking
The network control of forward and backward walking in humans remains an area of high interest. While the precise relationship of these networks is not fully understood (Winter et al., 1989; Choi and Bastian, 2007; Jansen et al., 2012; Musienko et al., 2012; Hoogkamer et al., 2014), recent physiologic and rehabilitation studies suggest that forward and backward locomotor networks, while independent, may interact such that backward training could improve performance with forward locomotion (Yang et al., 2005; Hao and Chen, 2011; Michaelsen et al., 2014; El-Basatiny and Abdel-Aziem, 2015; Foster et al., 2016; Rose et al., 2018). For example, kinematic studies, performed in neurologically intact individuals, suggest that backward walking training improves forward walking ability to a greater extent than forward walking training alone, because backward walking training incorporates supplementary core and lower extremity muscle groups which are less active during forward walking (Winter et al., 1989; Grasso et al., 1998; Błażkiewicz, 2013). Also, backward walking has been suggested to improve walking symmetry by targeting the maladaptive flexor-synergy gait pattern associated with central nervous system injury (Thorstensson, 1986; Winter et al., 1989; Duysens et al., 2013; Rose et al., 2018). Since backward walking relies more heavily on proprioception and sensorineural integration, to know where the foot is in space, backwards training could also theoretically improve walking stability and balance over time (Hao and Chen, 2011; Fritz et al., 2013; Ordway et al., 2016; Rose et al., 2018).
Backward locomotion treadmill training
Our training approach in this study differs from typical overground backward walking because the entirety of the training is performed on a non-body weight supported treadmill which facilitates high repetition of practice while enabling real-time control of training speed. Moreover, the absence of body weight support provides even a greater challenge because it forces participants to bear more weight on their paretic limb (Wernig and Wernig, 2010). In addition, the presence of sensors underneath the belt enables for collection of temporospatial data comparable over sessions (Yeon-Gyu and Jung-Wan, 2016; Zachary et al., 2017).
Past studies have demonstrated that BLTT is feasible in young, and neurologically intact adults; however, its safety and feasibility have not been tested in chronic stroke survivors. A few physiological considerations could make BLTT particularly challenging in this population. For example, stroke commonly impacts chronologically older individuals, which is associated with a decline in gait speed, joint range of motion and spatiotemporal ability (Stacy et al., 2007). Moreover, functional neuroimaging studies have reported an increased tendency for prefrontal compensatory recruitment during normal walking in this population (Kurz et al., 2012; Chatterjee et al., 2019). In addition, backward walking is characteristically more physically demanding than walking forward (Flynn et al., 1994; Terblanche et al., 2005) and requires movement patterns that tend to be particularly difficult after stroke (i.e. knee flexion and ankle dorsiflexion with hip extension) (Nilsson et al., 2001). Hence, it is possible that BLTT may be too cerebrally and physically demanding for chronic stroke survivors to complete. Therefore, the primary objective of this study was to investigate the safety and feasibility of BLTT, while in parallel obtaining preliminary outcome data for training-related effects BLTT on overground walking in the chronic stroke population.
Direct current stimulation
The secondary objective of this study was to explore if the concurrent application of a direct current stimulation over the thoracolumbar region of the spinal cord could enhance training-related changes.
Over the last 25 years, direct current stimulation has gained traction as a promising non-invasive neuromodulatory tool for stroke neurorehabilitation (Stagg et al., 2009; Schlaug and Cohen, 2010; Kang et al., 2016). Early studies in the young, elderly, and stroke populations have suggested that its application over the scalp (tDCS), for multiple sessions, may enhance the effects of training by facilitating the acquisition rate and retention of the learned task (Reis et al., 2009; Antal et al., 2010; Fritsch et al., 2010; Kadosh et al., 2010; Dayan et al., 2013; Snowball et al., 2013). However, reports on the effect of tDCS with lower extremity or locomotor training have been less encouraging (Madhavan and Stinear, 2010; Geroin et al., 2011; Geiger et al., 2017). Some have suggested that the inefficacy of tDCS to modulate lower extremity and walking recovery may be a result of the inefficient distribution of direct current to reach critical regions involved in human locomotion, such as the lower extremity region of the motor cortex, subcortical locomotor regions and spinal cord (Jeffery et al., 2007; Jones et al., 2016). Hence, an alternative approach to modulate the central locomotor network termed ‘transcutaneous spinal direct current stimulation (tsDCS)’ has been suggested (Priori et al., 2014).
Supported by electrical current modelling (Parazzini et al., 2014; Fregni et al., 2015; Fiocchi et al., 2016; Kuck et al., 2017), preclinical (Zaghloul, 2014, 2016; Weiguo et al., 2015), neurophysiologic (Cogiamanian et al., 2012; Priori et al., 2014) and neuroimaging studies (Schweizer et al., 2017), a growing body of literature suggests that tsDCS can modulate activity at multiple levels of the central nervous system, including the segmental spinal cord (Winkler et al., 2010; Lamy et al., 2012; Hubli et al., 2013), ascending lemniscal and nociceptive pathways (Cogiamanian et al., 2008; Cogiamanian et al., 2011; Truini et al., 2011), as well as cortical regions (Bocci et al., 2014, 2015a, b, c; Marangolo et al., 2017; Schweizer et al., 2017). In addition, a recent proof-of-concept study from our group, in young and neurologically intact individuals, found that anodal tsDCS applied over the lower thoracic region (T-11) concurrently with BLTT, increased the acquisition rate and retention of backward walking speed up to 2 weeks post-training (Awosika et al., 2019). Therefore, this study explores if tsDCS could comparably enhance the effect of BLTT on forward walking in chronic stroke survivors.
Based on the completion rates of past stroke recovery trials from our group (Kluding et al., 2013; Boyne et al., 2016; Harvey et al., 2018), we anticipated that 30 patients could be enrolled and randomized within 24 months, and predicted that greater than 70% of those participants would complete the study. In line with past neuromodulation studies using direct current stimulation (Antal et al., 2017), we anticipated that tsDCS would be well-tolerated. Lastly, while this study was not powered to detect a significance between the two groups (BLTT + sham tsDCS vs. BLTT + anodal tsDCS), we hypothesized that anodal tsDCS would demonstrate a trend towards greater improved walking performance.
Materials and methods
Participants
This study was conducted at the University of Cincinnati Neurorecovery Lab from 5 September 2017, to 4 February 2019. Community-dwelling individuals between 18 and 80 years of age, with mild to severe gait impairment due to chronic stroke (>6 months), either ischaemic or haemorrhagic were recruited for this study. Prior to group randomization, study participants had to demonstrate the ability to: provide consent (Mini-Mental State Exam Score >23), ambulate without a walker and maintain ≥0.13 m/s speed on the treadmill while walking backwards for 6 min. They were additionally asked to abstain from both formal physiotherapy and botulinum toxin treatments at least 2 weeks prior to enrolment and for the duration of training and follow-up. The exclusion criteria ruled-out individuals with an unstable cardiopulmonary status which may preclude participation in a moderate-high intensity exercise programme, severe lower extremity spasticity (modified Ashworth >2/4), significant language barrier which might prevent the participant from following instructions during training and testing, and untreated depression [>10 on the Patient Health Questionnaire (PHQ9)].
Study design
Potential study participants were screened until the enrolment goal of 30 randomized participants was reached. Participants meeting the inclusion and exclusion criteria were randomized in a 1:2 stratified fashion to either sham (N = 11) or anodal tsDCS (N = 19), respectively—based on baseline 10-m walk test (10MWT) speed, prior to BLTT initiation, on Day 2 (D2) of the study (Table 1). This stratification ratio was used to maximize the number of participants receiving anodal tsDCS, in an effort to reduce the variance in the estimated treatment effect. Group allotment was performed by an independent research coordinator, removed from training and outcomes testing. In addition, the patient, therapists and outcome assessors were blinded to the group allocation.
Baseline demographic and gait variables per intervention group
| . | BLTT + sham tsDCS (n = 11) . | BLTT + anodal tsDCS (n = 19) . | P-value . |
|---|---|---|---|
| Demographic variables | |||
| Age (years) | 54.74 ± 10.9 | 58.55 ± 7.61 | 0.269 |
| Stroke age (months) | 62.25 ± 72.0 | 62.22 ± 66.3 | 0.999 |
| Gender | |||
| Male | 6 (55%) | 10 (53%) | 0.917 |
| Female | 5 (45%) | 9 (47%) | |
| Hemiplegic side | |||
| Right | 7 (64%) | 11 (58%) | 0.750 |
| Left | 4 (36%) | 8 (42%) | |
| Stroke type | |||
| Ischaemic | 9 (82%) | 15 (79%) | 0.845 |
| Haemorrhagic | 2 (18%) | 4 (21%) | |
| Behavioural-Cognitive Scales | |||
| Patient Health Questionnaire (PHQ9) | 3.909 ± 4.53 | 3.053 ± 2.93 | 0.534 |
| Mini-Mental Status Exam | 28.27 ± 1.85 | 27.88 ± 2.87 | 0.690 |
| Gait variables | |||
| 10-m walking speed (fast)-m/s | 1.105 ± 0.31 | 0.982 ± 0.53 | 0.490 |
| Cadence (steps/min) | 124.2 ± 26.9 | 104.9 ± 31.6 | 0.101 |
| Stride length (cm) | 118.8 ± 25.5 | 118.9 ± 44.0 | 0.995 |
| Gait impairment severity | |||
| Mild | 3 (27%) | 4 (21%) | 0.712 |
| Moderate | 7 (64%) | 10 (53%) | 0.564 |
| Severe | 1 (9%) | 5 (26%) | 0.268 |
| . | BLTT + sham tsDCS (n = 11) . | BLTT + anodal tsDCS (n = 19) . | P-value . |
|---|---|---|---|
| Demographic variables | |||
| Age (years) | 54.74 ± 10.9 | 58.55 ± 7.61 | 0.269 |
| Stroke age (months) | 62.25 ± 72.0 | 62.22 ± 66.3 | 0.999 |
| Gender | |||
| Male | 6 (55%) | 10 (53%) | 0.917 |
| Female | 5 (45%) | 9 (47%) | |
| Hemiplegic side | |||
| Right | 7 (64%) | 11 (58%) | 0.750 |
| Left | 4 (36%) | 8 (42%) | |
| Stroke type | |||
| Ischaemic | 9 (82%) | 15 (79%) | 0.845 |
| Haemorrhagic | 2 (18%) | 4 (21%) | |
| Behavioural-Cognitive Scales | |||
| Patient Health Questionnaire (PHQ9) | 3.909 ± 4.53 | 3.053 ± 2.93 | 0.534 |
| Mini-Mental Status Exam | 28.27 ± 1.85 | 27.88 ± 2.87 | 0.690 |
| Gait variables | |||
| 10-m walking speed (fast)-m/s | 1.105 ± 0.31 | 0.982 ± 0.53 | 0.490 |
| Cadence (steps/min) | 124.2 ± 26.9 | 104.9 ± 31.6 | 0.101 |
| Stride length (cm) | 118.8 ± 25.5 | 118.9 ± 44.0 | 0.995 |
| Gait impairment severity | |||
| Mild | 3 (27%) | 4 (21%) | 0.712 |
| Moderate | 7 (64%) | 10 (53%) | 0.564 |
| Severe | 1 (9%) | 5 (26%) | 0.268 |
Baseline demographic and gait variables per intervention group
| . | BLTT + sham tsDCS (n = 11) . | BLTT + anodal tsDCS (n = 19) . | P-value . |
|---|---|---|---|
| Demographic variables | |||
| Age (years) | 54.74 ± 10.9 | 58.55 ± 7.61 | 0.269 |
| Stroke age (months) | 62.25 ± 72.0 | 62.22 ± 66.3 | 0.999 |
| Gender | |||
| Male | 6 (55%) | 10 (53%) | 0.917 |
| Female | 5 (45%) | 9 (47%) | |
| Hemiplegic side | |||
| Right | 7 (64%) | 11 (58%) | 0.750 |
| Left | 4 (36%) | 8 (42%) | |
| Stroke type | |||
| Ischaemic | 9 (82%) | 15 (79%) | 0.845 |
| Haemorrhagic | 2 (18%) | 4 (21%) | |
| Behavioural-Cognitive Scales | |||
| Patient Health Questionnaire (PHQ9) | 3.909 ± 4.53 | 3.053 ± 2.93 | 0.534 |
| Mini-Mental Status Exam | 28.27 ± 1.85 | 27.88 ± 2.87 | 0.690 |
| Gait variables | |||
| 10-m walking speed (fast)-m/s | 1.105 ± 0.31 | 0.982 ± 0.53 | 0.490 |
| Cadence (steps/min) | 124.2 ± 26.9 | 104.9 ± 31.6 | 0.101 |
| Stride length (cm) | 118.8 ± 25.5 | 118.9 ± 44.0 | 0.995 |
| Gait impairment severity | |||
| Mild | 3 (27%) | 4 (21%) | 0.712 |
| Moderate | 7 (64%) | 10 (53%) | 0.564 |
| Severe | 1 (9%) | 5 (26%) | 0.268 |
| . | BLTT + sham tsDCS (n = 11) . | BLTT + anodal tsDCS (n = 19) . | P-value . |
|---|---|---|---|
| Demographic variables | |||
| Age (years) | 54.74 ± 10.9 | 58.55 ± 7.61 | 0.269 |
| Stroke age (months) | 62.25 ± 72.0 | 62.22 ± 66.3 | 0.999 |
| Gender | |||
| Male | 6 (55%) | 10 (53%) | 0.917 |
| Female | 5 (45%) | 9 (47%) | |
| Hemiplegic side | |||
| Right | 7 (64%) | 11 (58%) | 0.750 |
| Left | 4 (36%) | 8 (42%) | |
| Stroke type | |||
| Ischaemic | 9 (82%) | 15 (79%) | 0.845 |
| Haemorrhagic | 2 (18%) | 4 (21%) | |
| Behavioural-Cognitive Scales | |||
| Patient Health Questionnaire (PHQ9) | 3.909 ± 4.53 | 3.053 ± 2.93 | 0.534 |
| Mini-Mental Status Exam | 28.27 ± 1.85 | 27.88 ± 2.87 | 0.690 |
| Gait variables | |||
| 10-m walking speed (fast)-m/s | 1.105 ± 0.31 | 0.982 ± 0.53 | 0.490 |
| Cadence (steps/min) | 124.2 ± 26.9 | 104.9 ± 31.6 | 0.101 |
| Stride length (cm) | 118.8 ± 25.5 | 118.9 ± 44.0 | 0.995 |
| Gait impairment severity | |||
| Mild | 3 (27%) | 4 (21%) | 0.712 |
| Moderate | 7 (64%) | 10 (53%) | 0.564 |
| Severe | 1 (9%) | 5 (26%) | 0.268 |
Impairment classification
To determine the impact of baseline walking impairment level on BLTT completion and outcome, participants were categorized into mild, moderate or severe walking impairment using the self-selected 10MWT (self-selected) at screening (D1), where ≥0.8–1.2 m/s was classified as mild, ≥0.4 to <0.8 m/s as moderate and <0.4 m/s as severe (Perry et al., 1995).
Intervention
Backward locomotion treadmill training
Screening (D1)
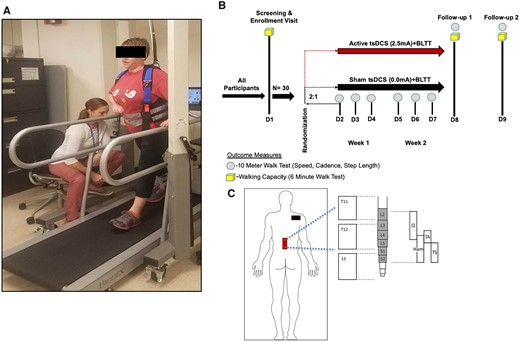
All study participants underwent 6 min of BLTT on screening day (D1) for orientation and to screen out individuals who were not able to achieve ≥0.13 m/s on the treadmill—the minimum belt speed needed to demonstrate adequate neuromotor capacity for participating in aerobic training (Macko et al., 2005; Ivey et al., 2008). For safety, participants were connected to a non-body weight supported safety harness, with their backs facing the head of the treadmill (Fig. 1A). Participants were permitted to hold-on to one handrail for support. The belt speed was started at slowest possible speed (≥0.04 m/s) and increased, based on the participant’s level of comfort, during the 6 min (Supplementary Table 1). A physical therapist remained next to the participant’s paretic side to provide assistance during backward leg extension, as needed. A second therapist was available during unique instances when a participant needed assistance on the non-paretic side (Supplementary Table 2).
Training schematic. Study participants underwent six (D2–D7), 30-min sessions of non-bodyweight supported BLTT, with concurrent sham or anodal tsDCS, applied over T-11/12 (red rectangle) and cathode electrode placed over the right shoulder (black rectangle).
Training (D2–D7)
Participants who completed the ≥0.13 m/s inclusion threshold advanced to the first day of training (D2) (Fig. 1B). The training consisted of four 6-min blocks. The self-selected treadmill speed, established on D1, was used as the starting speed on the first block of D2. Likewise, for D3–D7, the training treadmill speed used on the fourth block of the preceding day was used as the starting speed for the subsequent session. In communication with the participant, the therapist continuously adjusted the speed to maintain a sustainable challenge. To reduce fatigue, all participants received 2-min seated rest breaks between each of the 4, 6-min training blocks, totalling ∼30 min. Six of the 30 study participants needed physical assistance over the 6 days of training (minimal to maximal). For safety and to ensure adequate effort (at least 50% of the predicted maximal heart rate), a Polar H7 (POLAR®, USA) heart rate monitor was worn at all times during training (see Supplementary Table 3). Participants were also offered chocolate milk after training to reduce training-associated muscle soreness (Pritchett and Pritchett, 2012).
Transcutaneous spinal direct current stimulation
TsDCS (2.5 mA, 30 min) was delivered, during BLTT, from a battery-driven programmable direct current stimulator (Soterix, USA) connected to surface electrodes (saline-soaked synthetic sponge of 7 × 5 cm, and 0.6 cm depth). Prior to the initiation of training, the anode/sham electrode was centred on the T-11 spinous process of the thoracic spine with the major axis parallel to the spinal cord, a second electrode was placed over the right shoulder, aligned with previous studies demonstrating modulation of segmental spinal reflex excitability with this montage (Vergari et al., 2008; Truini et al., 2011; Lamy et al., 2012). The second electrode was placed over the right shoulder (Fig. 1C). A tsDCS lumbar body strap (Soterix, USA) was used to secure electrode positioning in place. Computerized modelling of this electrode montage and stimulation parameters estimates a current density of 0.071 mA/cm2, delivering a total charge density of 85.7 mC/cm2 (Cogiamanian et al., 2008), which is well within safety levels. The direct current stimulator was programmed to ramp up current to 2.5 mA over a 30-s period and similarly ramped down at the end of the stimulation. Sham tsDCS was achieved by delivering a 2.5-mA current over a period of 30 s at the beginning and end of the stimulation period.
Outcomes measures
Safety, feasibility and tolerability
A tolerability, activity and safety questionnaire (Supplementary Fig. 1) was completed by the patient at the first post-training follow-up (D8). Information regarding study enrolment, attrition and adverse events were documented throughout the study. The primary outcome was the proportion of participants who completed the BLTT study. Completion was defined as finishing the entirety of the training protocol (180 min) and returning for the two follow-up visit days [24-h post-training Day 6 (D8), and 2-week post-training Day 6 (D9)].
10-m walk test
Community ambulation is correlated with gait speed (Perry et al., 1995), and changes in gait speed that result in a transition to a higher category of ambulation classification are associated with improved function and quality of life. Therefore, 10MWT is the gold standard measure of post-stroke walking function that reflects overall mobility (Lord et al., 2004; Schmid et al., 2007) and health status (Studenski et al., 2003). Training-related changes in gait speed were assessed with the 10MWT. This test was administered at screening, before each BLTT session (D2–D7), and at follow-up (D8–D9). Participants were instructed to walk as fast as possible, with or without an assistive device (single pint cane or quad cane), with three attempts. The fastest of the three trials was used in the analysis. To limit the influence of D1 orientation training-effect on outcomes, the 10MWT speed for D2 was set as the baseline.
Gait dynamics
Temporal (cadence) and spatial dynamics (Δ stride length) were acquired using the Zeno Walkway gait analysis mat (Protokinetics, PA, USA) during the10MWT. These data were captured and recorded with the Protokinetics Movement Analysis Software and later exported for offline analysis.
Walking capacity
Walking capacity, as determined by performance on the 6-min walk test (6MWT), is the most influential individual predictor of limited versus full community ambulation (Fulk et al., 2017). Participants were instructed to walk as fast as possible back and forth in a 23-m corridor, with or without an assistive device (single-point cane or quad cane) for 6 min. The total distance travelled was measured and documented by the blinded therapist after the test. The 6MWT was administered at screening, D8 follow-up (∼24 h following the sixth day of training), and D9 (2-week post-training).
Statistical analysis
The enrolment goal of 30 was determined based on the site recruitment rate from past protocols from our group. Normality assumption was tested by the Shapiro–Wilk method, and the significance level was set at P = 0.05 for all measures. Between-group differences in the proportion who completed the study and tolerability outcomes were determined using the Chi-squared test. Linear mixed-effects models were used to test for within-group change and between-group differences in change for gait speed, cadence and stride length (Δ = D8–D2), and for walking capacity (Δ = D8–D1). These models included each gait measure (separately) as the dependent variable, with fixed effects for time point, group and their interaction and a random effect for participant, to account for the correlated nature of repeated measures from the same person. Also, to account for the relative imbalance of participants with severe walking impairment in the anodal versus sham groups, we performed a secondary analysis adjusting for baseline gait speed. The retention of effect within- and between-groups on each walking measure was determined by comparing the change between D9 and D8. Since walking outcomes were exploratory, adjustment for multiple comparisons was not performed. Walking data from one participant, in the control group, were excluded at 5-timepoints (D5–D9) for 10MWT, and 6MWT (D8, D9), due to interspersed periods of ‘walk-jogging’, characterized by absence of double support time during walking trials on the gait analysis mat.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Feasibility
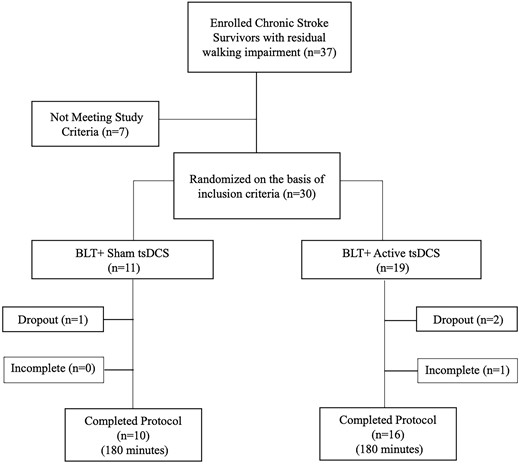
From 5 September 2017, to 7 January 2019, 37 chronic stroke survivors with residual walking impairment were enrolled, with 30 randomized (Fig. 2). There were no significant baseline differences, in age, behavioural-cognitive scales, stroke type, or severity, or gait metrics, between groups (Table 1).
Completion rate
Twenty-six of the 30 (87%) randomized participants completed the study (Fig. 2). All seven randomized participants (100%) with mild walking impairment completed the study, irrespective of group allocation. Sixteen of 17 (94%) of participants with moderate walking impairment completed the study. The dropout from this cohort was in the anodal-tsDCS group and discontinued due to a viral gastrointestinal illness. Three out of six (50%) of participants with severe walking impairment completed the study. There was one dropout in the sham group, on D9, due to a family illness. There were two dropouts in the anodal tsDCS group: one participant discontinued the study on D2 due to the development of leg and back spasms during block 1 of training, and the second did not meet treatment fidelity to satisfy the ‘completion’ criteria on D3 and D6, due to fatigue and transient acute on chronic arthritic knee pain.
Tolerability, activity and safety questionnaire
BLTT was well tolerated by participants in both groups (anodal tsDCS and sham tsDCS). Participants from both groups reported similar improvements in activity level (P = 0.152), strength (P = 0.188), energy level (P = 0.370) and mood (P = 0.238). On a 0–10 scale, both groups similarly scored <1, for headache (P = 0.207), neck pain (P = 0.449) or pain (P = 0.290), tingling (P = 0.423), itching (P = 0.280), burning (P = 1.000) or electric shock sensation (P = 0.754) related to tsDCS. Reports of soreness or fatigue were minimal and similar between groups, P = 0.086, P = 0.472, respectively (Table 2).
Safety and tolerability questionnaire
| Questionsa . | |||
|---|---|---|---|
| . | Sham tsDCS + BLTT . | Anodal tsDCS + BLTT . | P-value . |
| Areas of improvement | |||
| 6.818 ± 3.46 (median: 8) | 4.889 ± 3.39 (median: 5) | 0.152 |
| 6.727 ± 3.04 (median: 7) | 5.111 ± 3.18 (median: 5) | 0.188 |
| 6.454 ± 3.33 (median: 7) | 4.611 ± 3.03 (median: 5) | 0.137 |
| 6.545 ± 3.11 (median: 7) | 4.833 ± 4.02 (median: 5.5) | 0.238 |
| Symptom questions not specific to one intervention | |||
| 0.455 ± 1.51 (median: 0) | 0.000 ± 0.00 (median: 0) | 0.207 |
| 0.000 ± 0.00 (median: 0) | 0.056 ± 0.24 (median: 0) | 0.449 |
| Symptom severity questions related to BLTT | |||
| 1.818 ± 1.72 (median: 2) | 0.778 ± 1.40 (median: 0) | 0.086 |
| 1.818 ± 2.89 (median: 0 ) | 1.222 ± 1.52 (median: 0.5) | 0.472 |
| Symptom severity questions related to tsDCS | |||
| 0.000 ± 0.00 (median: 0) | 0.167 ± 0.51 (median: 0) | 0.290 |
| 0.182 ± 0.40 (median: 0) | 0.389 ± 0.78 (median: 0) | 0.423 |
| 0.000 ± 0.00 (median: 0) | 0.278 ± 0.83 (median: 0) | 0.280 |
| 0.000 ± 0.00 (median: 0) | 0.000 ± 0.00 (median: 0) | |
| 0.091 ± 0.30 (median: 0) | 0.059 ± 0.24 (median: 0) | 0.754 |
| Questionsa . | |||
|---|---|---|---|
| . | Sham tsDCS + BLTT . | Anodal tsDCS + BLTT . | P-value . |
| Areas of improvement | |||
| 6.818 ± 3.46 (median: 8) | 4.889 ± 3.39 (median: 5) | 0.152 |
| 6.727 ± 3.04 (median: 7) | 5.111 ± 3.18 (median: 5) | 0.188 |
| 6.454 ± 3.33 (median: 7) | 4.611 ± 3.03 (median: 5) | 0.137 |
| 6.545 ± 3.11 (median: 7) | 4.833 ± 4.02 (median: 5.5) | 0.238 |
| Symptom questions not specific to one intervention | |||
| 0.455 ± 1.51 (median: 0) | 0.000 ± 0.00 (median: 0) | 0.207 |
| 0.000 ± 0.00 (median: 0) | 0.056 ± 0.24 (median: 0) | 0.449 |
| Symptom severity questions related to BLTT | |||
| 1.818 ± 1.72 (median: 2) | 0.778 ± 1.40 (median: 0) | 0.086 |
| 1.818 ± 2.89 (median: 0 ) | 1.222 ± 1.52 (median: 0.5) | 0.472 |
| Symptom severity questions related to tsDCS | |||
| 0.000 ± 0.00 (median: 0) | 0.167 ± 0.51 (median: 0) | 0.290 |
| 0.182 ± 0.40 (median: 0) | 0.389 ± 0.78 (median: 0) | 0.423 |
| 0.000 ± 0.00 (median: 0) | 0.278 ± 0.83 (median: 0) | 0.280 |
| 0.000 ± 0.00 (median: 0) | 0.000 ± 0.00 (median: 0) | |
| 0.091 ± 0.30 (median: 0) | 0.059 ± 0.24 (median: 0) | 0.754 |
Based on 0–10 written analogue scale (0 = No change, 10 = Severe/Significant), values represent the mean and standard deviation.
Safety and tolerability questionnaire
| Questionsa . | |||
|---|---|---|---|
| . | Sham tsDCS + BLTT . | Anodal tsDCS + BLTT . | P-value . |
| Areas of improvement | |||
| 6.818 ± 3.46 (median: 8) | 4.889 ± 3.39 (median: 5) | 0.152 |
| 6.727 ± 3.04 (median: 7) | 5.111 ± 3.18 (median: 5) | 0.188 |
| 6.454 ± 3.33 (median: 7) | 4.611 ± 3.03 (median: 5) | 0.137 |
| 6.545 ± 3.11 (median: 7) | 4.833 ± 4.02 (median: 5.5) | 0.238 |
| Symptom questions not specific to one intervention | |||
| 0.455 ± 1.51 (median: 0) | 0.000 ± 0.00 (median: 0) | 0.207 |
| 0.000 ± 0.00 (median: 0) | 0.056 ± 0.24 (median: 0) | 0.449 |
| Symptom severity questions related to BLTT | |||
| 1.818 ± 1.72 (median: 2) | 0.778 ± 1.40 (median: 0) | 0.086 |
| 1.818 ± 2.89 (median: 0 ) | 1.222 ± 1.52 (median: 0.5) | 0.472 |
| Symptom severity questions related to tsDCS | |||
| 0.000 ± 0.00 (median: 0) | 0.167 ± 0.51 (median: 0) | 0.290 |
| 0.182 ± 0.40 (median: 0) | 0.389 ± 0.78 (median: 0) | 0.423 |
| 0.000 ± 0.00 (median: 0) | 0.278 ± 0.83 (median: 0) | 0.280 |
| 0.000 ± 0.00 (median: 0) | 0.000 ± 0.00 (median: 0) | |
| 0.091 ± 0.30 (median: 0) | 0.059 ± 0.24 (median: 0) | 0.754 |
| Questionsa . | |||
|---|---|---|---|
| . | Sham tsDCS + BLTT . | Anodal tsDCS + BLTT . | P-value . |
| Areas of improvement | |||
| 6.818 ± 3.46 (median: 8) | 4.889 ± 3.39 (median: 5) | 0.152 |
| 6.727 ± 3.04 (median: 7) | 5.111 ± 3.18 (median: 5) | 0.188 |
| 6.454 ± 3.33 (median: 7) | 4.611 ± 3.03 (median: 5) | 0.137 |
| 6.545 ± 3.11 (median: 7) | 4.833 ± 4.02 (median: 5.5) | 0.238 |
| Symptom questions not specific to one intervention | |||
| 0.455 ± 1.51 (median: 0) | 0.000 ± 0.00 (median: 0) | 0.207 |
| 0.000 ± 0.00 (median: 0) | 0.056 ± 0.24 (median: 0) | 0.449 |
| Symptom severity questions related to BLTT | |||
| 1.818 ± 1.72 (median: 2) | 0.778 ± 1.40 (median: 0) | 0.086 |
| 1.818 ± 2.89 (median: 0 ) | 1.222 ± 1.52 (median: 0.5) | 0.472 |
| Symptom severity questions related to tsDCS | |||
| 0.000 ± 0.00 (median: 0) | 0.167 ± 0.51 (median: 0) | 0.290 |
| 0.182 ± 0.40 (median: 0) | 0.389 ± 0.78 (median: 0) | 0.423 |
| 0.000 ± 0.00 (median: 0) | 0.278 ± 0.83 (median: 0) | 0.280 |
| 0.000 ± 0.00 (median: 0) | 0.000 ± 0.00 (median: 0) | |
| 0.091 ± 0.30 (median: 0) | 0.059 ± 0.24 (median: 0) | 0.754 |
Based on 0–10 written analogue scale (0 = No change, 10 = Severe/Significant), values represent the mean and standard deviation.
Serious adverse events
There were no serious adverse events in the study, including cardiac, cerebrovascular, orthopaedic injuries (i.e. fracture or dislocation) or incidences requiring a visit to the emergency room, hospitalization, persistent or significant incapacity, or death.
Secondary outcome measures
Overground walking speed
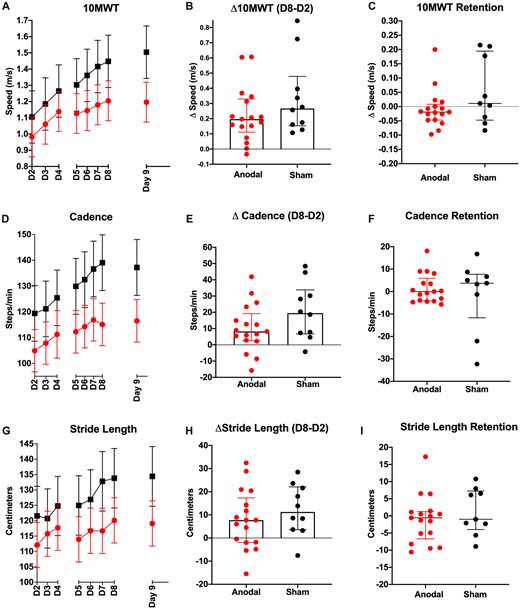
Both groups demonstrated a significant improvement in overground walking speed on the 10MWT after BLTT (P < 0.001), and reached minimal clinically importance difference (MCID = 0.16 m/s) in speed for stroke walking recovery [mean (95% CI): 0.412 m/s (0.213–0.611), sham, 0.215 m/s (0.119, 0.310), anodal], (Schmid et al., 2007). Participants receiving sham tsDCS demonstrated a greater improvement in walking speed (D2–D8), relative to the anodal tsDCS (P = 0.016). This result was still similar, although just non-significant, after adjusting for baseline differences in walking speed (P = 0.054) (Fig. 3B). There was retention in walking speed up to 2 weeks following the period of training in both groups; however, there were no between-group differences in change from D8 to D9 (P = 0.207) (Fig. 3C).
Walking speed and metrics. Mean change in 10MWT speed (A), Cadence (D) and Stride Length (G), during 6 days of BLTT (D2–D7), 24 h, and 2-week follow-up (error bar in SEM). Cumulative training-related changes (D8–D2), for 10MWT (B), Cadence (E), and Stride Length (H), represented as the median and interquartile range. Retention of performance at 2-week follow-up (D9–D8) for 10MWT (C), Cadence (F), represented as the median and interquartile range (I).
Cadence
Both groups demonstrated a significant improvement in cadence on the 10MWT (P < 0.001). Participants receiving sham tsDCS demonstrated a greater improvement in cadence at (D2–D8) (P = 0.046), although this significance was lost after adjusting for baseline differences in walking speed (P = 0.091). Both groups demonstrated retention of cadence gains up to 2 weeks following the period of training, with no between-group differences from D8 to D9 (P = 0.503) (Fig. 3c–E).
Stride length
Similar to speed and cadence, both groups demonstrated a significant improvement in stride length on the 10MWT (P < 0.001). However, there were no between-group differences in stride length change (P = 0.3162). Both groups demonstrated retention of stride length gains up to 2 weeks following the period of training, with no between-group differences from D8 to D9 (P = 0.711) (Fig. 3F and G).
Walking capacity
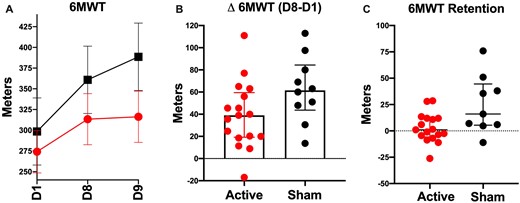
Both groups demonstrated a significant improvement in walking capacity on the 6MWT after BLTT (P < 0.001) and reached clinically meaningful importance difference (MCID = 34.4m) [Mean (95% CI): 92 m (62.70–122.9), sham, 41.96 m (25.74, 58.18), anodal] (Tang et al., 2012). Participants receiving sham tsDCS demonstrated a greater improvement in walking capacity, relative to the anodal tsDCS (P = 0.050), although this significance was lost after adjusting for baseline differences in walking speed (P = 0.082). Both groups demonstrated retention of walking capacity gains up to 2 weeks following the period of training; however, the sham group experienced greater within (P = 0.005) and between-group performance (P = 0.040) (Fig. 4).
Walking capacity. Mean change in the 6-min walk test (6MWT) at screening baseline (D1), 24-h post-BLTT training, and 2-week follow-up (D9) (A), (error bar in SEM). Cumulative training-related change (B), and retention of performance at 2-week follow-up (C) represented as the median and interquartile range.
Discussion
Our findings suggest BLTT and tsDCS are safe, feasible and well-tolerated approaches for walking rehabilitation training in stroke. Moreover, preliminary results on walking speed and capacity demonstrated clinically significant and sustained (at least 2 weeks) improvement following six sessions of BLTT.
Backward locomotion treadmill training
Although, BLTT was not tested head-on with overground walking training, it is understood that treadmill allows for more efficient training by enhancing the number of steps achievable over a fixed unit of time, and providing greater aerobic conditioning (Ivey et al., 2008)—a feature which is particularly advantageous in this era of healthcare constraints and decreasing time allotted for physiotherapy by third-party payers. Therefore, it is encouraging that 87% of randomized participants completed the entirety of the BLTT protocol, with 82% completing the training without assistance from a therapist. It is also notable that three out four participants, who initially needed assistance, were able to perform the BLTT task independently by Day 6 of training (Supplementary Table 2).
Severe walking impairment
Six participants met the classification of severe walking impairment, based on their baseline preferred 10 MW speed (<0.4 m/s). Of these, one participant dropped out entirely from the study on the first day of training, and another stayed in the study but did not meet the criteria for study fidelity. Three others needed assistance with placing the paretic leg backward during training during the early part of the study. In sum, only two of the six participants (33%) were able to independently complete all six BLTT sessions. This finding is akin to previous walking rehabilitation studies that have reported that baseline walking severity level may influence training ability (Burke et al., 2014; Dobkin et al., 2014; Bernhardt et al., 2016; Boyd et al., 2017), which may be associated with the magnitude of post-stroke cognitive and physical limitations in this subgroup, (Kurz et al., 2012; Hawkins et al., 2018; Chatterjee et al., 2019). Therefore, it is possible that our BLTT protocol, in its present form, is too tasking for severely impaired individuals and future protocols will need modification to further accommodate those with severe walking impairment.
Overground walking performance
While BLTT is, in theory, a task-based training approach, its effects extended beyond the improvement in backward walking ability on the treadmill. Our BLTT paradigm was associated with clinically meaningful improvement in overground forward walking speed, step length, cadence and capacity. Participants also noted an improvement in other aspects in quality of life measures such as increased confidence, strength, agility, sleep and mood (Supplementary Fig. 2). Although this study did not directly probe mechanisms for BLTT-related change in walking function, we speculate that factors such as, reduction in spasticity (Thorstensson, 1986; Winter et al., 1989; Schneider and Capaday, 2003; Duysens et al., 2013; El-Basatiny and Abdel-Aziem, 2015), lower extremity and core strengthening (Straube et al., 2014), enhancement of peripheral somatosensory signals to spinal and supraspinal locomotor centres (Takakusaki, 2013; Clark et al., 2014; Afzal et al., 2017; Takakusaki, 2017) and increased exercise capacity (Flynn et al., 1994; Macko et al., 2005; Terblanche et al., 2005) played a role. Future studies are needed to assess these mechanisms, determine the duration of training-related effects and define which physiologic factors and rehabilitation pathways best predict the training outcome.
Transcutaneous spinal direct current stimulation
From a safety standpoint, tsDCS was well tolerated in this study and did not result in any observable adverse effects. This finding is consistent with previous direct current stimulation studies in the literature (Antal et al., 2017).
Although the study was not sufficiently powered to detect significant group differences, our working hypothesis was that BLTT + anodal tsDCS group would perform better than BLTT alone. However, our results showed the contrary, even after adjusting for baseline differences in walking speed. Past studies with direct current stimulation applied over the scalp suggest that anodal stimulation enhances the rate and retention of learned motor task (Nitsche and Paulus, 2000; Reis et al., 2009; Fritsch et al., 2010; Kadosh et al., 2010; Dayan et al., 2013; Snowball et al., 2013). Likewise, a recent study from our group, albeit in young and neurologically intact individuals, demonstrated that anodal tsDCS over the thoracolumbar vertebra enhanced the acquisition rate and retention of the trained locomotor task (Awosika et al., 2019).
Herein, we propose two potential explanations for this unexpected finding. Firstly, while anodal tsDCS at the spinal segmental level is understood to be facilitatory (Winkler et al., 2010; Lamy et al., 2012; Awosika et al., 2019), its influence on ascending somatosensory pathways has been reported as inhibitory (Cogiamanian et al., 2008; Truini et al., 2011; Lenoir et al., 2018), resulting in a phenomenon known as ‘anodal block’ (Bhadra and Kilgore, 2004; Cogiamanian et al., 2012). Therefore, one possible explanation for our findings was that anodal tsDCS, in stroke patients, resulted in the inhibition of ascending sensory axons of the somatosensory pathway, which may have diminished the degree of proprioceptive feedback reaching supraspinal locomotor centres during BLTT (Hao and Chen, 2011; Takakusaki, 2013; Clark et al., 2014; El-Basatiny and Abdel-Aziem, 2015; Schweizer et al., 2017; Takakusaki, 2017). While anodal-tsDCS does not appear to hinder performance in younger and neurologically intact individuals, it is widely accepted that stroke survivors rely more heavily on somatosensory processing to maintain functional gait and balance (Clark et al., 2014; Afzal et al., 2017); additionally, somatosensation is known to diminish with age (Callisaya et al., 2008; Chu et al., 2015; Seung-Uk et al., 2016); therefore, we speculate that our study population are more likely more susceptible to perturbations of this pathway.
A second explanation may be the inadequate dosing of anodal tsDCS, which may have led to inhibition, rather than excitation of spinally mediated locomotion. While both groups performed similarly in Week 1 training, the tsDCS group began to experience a decline in the rate of training-related improvement by the second week of training, and at follow-up. Along this line, two groups have reported that direct current stimulation over the scalp may exhibit a time-dependent switch in stimulus effect (Batsikadze et al., 2013; Monte-Silva et al., 2013). While active electrode was placed over the spine, this paradoxical effect with prolonged and frequent stimulation may have altered the spinal locomotor physiology, hindering walking performance. Future electrophysiologic and dosing studies would be useful in testing this working hypothesis, and help to better elucidate the effects of tsDCS at the spinal segmental level.
Limitations
Our study could have been strengthened by the addition of a forward walking training control group, which would have helped to determine the magnitude of BLTT-related improvement in forward walking in comparison to regular overground or forward treadmill training. With this said, the average change in walking speed documented in this study was comparable to previous rigorous rehabilitation studies with much longer training sessions (Duncan et al., 2011; Mehrholz et al., 2017).
Our conclusions regarding training changes in walking speed and capacity between groups must be regarded conservatively, given the between-group differences in baseline performance. Although these differences were not statistically significant, the distribution of severity between groups may have skewed our study results in favour of the control group. Furthermore, since the primary aim of this study was on safety and feasibility and was powered based on our recruitment capabilities, our study was not designed to detect statistically significant differences in walking speed and capacity. Based on the observation that stroke survivors with severe walking impairment had more difficulty completing the BLTT protocol as designed and demonstrated less improvement in overground walking, walking severity will be an a priori co-factor in future studies. Moreover, future studies will determine how stroke lesion type, size, location and white matter burden influence training and outcomes.
Conclusion
In summary, this study found that BLTT and tsDCS were both safe and feasible approaches worth further investigation, as possible approaches to optimize walking recovery after stroke. Future, well-powered and dosing-related studies are needed to determine the utility and generalizability of both approaches, alone or combined, in this population.
Competing interests
The authors report no competing interests.
Acknowledgements
We would like to thank the Oliver Family Fund for their generous support of the University of Cincinnati Neurorecovery lab and Leonardo G Cohen for his helpful insights and discussion. In addition, we thank the participants and therapists who gave of their time and effort during experimental testing and training sessions.
Funding
This research was supported by the University of Cincinnati Gardner Neuroscience Institute Pilot Grant and the University of Cincinnati College of Medicine Start-Up Fund.
References
- 6MWT =
6-min walk test
- 10MWT =
10-m walk test
- BLTT =
backward locomotion treadmill training
- PHQ9 =
Patient Health Questionnaire
- TsDCS =
transcutaneous spinal direct current stimulation