-
PDF
- Split View
-
Views
-
Cite
Cite
Laura Lanteaume, Stéphanie Khalfa, Jean Régis, Patrick Marquis, Patrick Chauvel, Fabrice Bartolomei, Emotion Induction After Direct Intracerebral Stimulations of Human Amygdala, Cerebral Cortex, Volume 17, Issue 6, June 2007, Pages 1307–1313, https://doi.org/10.1093/cercor/bhl041
Close - Share Icon Share
Abstract
Very few studies in humans have quantified the effect obtained after direct electrical stimulation of the amygdala, in terms of both emotional and physiological responses. We tested patients with drug-resistant partial epilepsies who were explored with intracerebral electrodes in the setting of presurgical evaluation. We assessed the effects of direct electric stimulations in either the right or the left amygdala on verbally self-reported emotions (Izard scale) and on psychophysiological markers of emotions by recording skin conductance responses (SCRs) and by measuring the electromyographic responses of the corrugator supercilii (EMGc). According to responses on Izard scales, electrical stimulations of the right amygdala induced negative emotions, especially fear and sadness. In contrast, stimulations of the left amygdala were able to induce either pleasant (happiness) or unpleasant (fear, anxiety, sadness) emotions. Unpleasant states induced by electrical stimulations were accompanied by an increase in EMGc activity. In addition, when emotional changes were reported after electrical stimulation, SCR amplitude for the positively valenced emotions was larger than for the negative ones. These findings provide direct in vivo evidence that the human amygdala is involved in emotional experiences and strengthen the hypothesis of a functional asymmetry of the amygdala for valence and arousal processing.
Introduction
Since the early description of the limbic system, many studies have explored the anatomo-functional correlates of emotions. Among the structures forming the limbic system, the amygdala has been the focus of a large number of studies dealing with its role in emotion physiology. The amygdala is particularly involved in the processing of fear because amygdala damage impairs fear conditioning in animals (LeDoux 1993) and in humans (LaBar and others 1995). Moreover, data from neuroimaging-based studies in healthy patients have shown that amygdala activity increases during fear conditioning (Buchel and others 1998; LaBar and others 1998).
The amygdala is also involved in the recognition of fear and other negative emotions. For example, deficits in perception and judgment of negative facial expressions have been found in patients with amygdala damage (Adolphs and others 1994, 1999; Adolphs and Tranel 2004). Other studies have even shown that amygdala activity varies according to the valence of stimuli (pleasant or positive, unpleasant or negative) such as emotional pictures (Garavan and others 2001), facial expressions (Breiter and others 1996; Morris and others 1996), and emotional words (Hamann and Mao 2002). The notion of the amygdala's role in negative emotions is fully demonstrated (Davidson 2002), but it is less clear whether the amygdala may also be involved in the processing of some positive emotions (Schneider and others 1997; Whalen and others 1998).
In addition, some works have suggested a possible lateralization for amygdala functions in emotion perception (Wager and others 2003; Zald 2003). Amygdala activations have been shown to be mostly lateralized to the left, particularly for negative emotions (Morris and others 1996) but the left amygdala also responds to happy faces (Killgore and others 2001). According to the meta-analysis of Wager and others (2003) and Zald (2003), no clear pattern of amygdala lateralization emerges for positive stimuli because both bilateral and unilateral (right or left) amygdala activations have been found in response to pleasant stimuli.
An alternative and classical way to study amygdala function is by direct electrical stimulation of this structure. To date, the direct electrical stimulation of the amygdala has induced behavioral patterns of fear in animals (Aggleton 2000). However, it has also been employed in humans when intracerebral electrodes are required to investigate the epilepsy of patients during a presurgical evaluation phase. The few previous studies using such stimulation have reported the induction of negative emotions (fear and anxiety) after stimulation of the medial temporal lobe, including amygdala and other structures (Bancaud and others 1966, 1968; Halgren and others 1978; Gloor and others 1982) according to verbal reports of the patients. However, these studies were not dedicated to quantifying the induced emotions, both in terms of physiological modifications and of subjective experience. Among the autonomic responses sensitive to emotional stimuli, electrodermal activity, which is a valid and reliable index of sympathetic nervous system, has often been used in terms of skin conductance responses (SCRs).
SCRs have been shown to represent the arousal response (Lang and others 1993, 1998; Bradley and Lang 2000), that is, to reflect the relaxing/stimulating aspect of a stimulus. The electromyographic activity of the corrugator supercilii (EMGc) is associated with the expression of sadness, anger, and fear or with the presentation of an unpleasant stimulus.
The present study was thus designed in order to investigate effects of the direct electrical stimulation of the right and/or left amygdala on 1) emotional reports as measured by a self-reported emotional scale and 2) emotion-related physiological responses measured by SCR and EMGc.
Materials and Methods
Subjects
Subjects were 8 adult patients (6 males and 2 females, with a mean age of 36.5 ± 11 years) with drug-resistant partial epilepsies. They were prospectively studied in the Epilepsy Unit of the Clinical Neurophysiology Department (Timone Hospital, Marseille, France). All were investigated using stereotactically implanted intracerebral electrodes as part of their planned presurgical evaluation. Each patient had a comprehensive evaluation including detailed history and neurological examination, neuropsychological testing, high resolution magnetic resonance imaging (MRI), interictal/ictal single photon computerized tomography, and video electroencephalography (EEG) recording of seizures. Following these noninvasive investigations, it was sometimes necessary to record intracerebral activity (SEEG, stereoelectroencephalography) to define the distinct role of temporal or extratemporal lobe structures in seizure generation. Patients underwent continuous video SEEG monitoring for 4 days, or longer (up to 2 weeks), if no seizures had been recorded during this period. Antiepileptic drug treatment was reduced to facilitate occurrence and recording of seizures. Fully informed consent was obtained from each patient prior to electrode implantation.
Three of the patients included in this study had an epileptogenic zone located in the left mesial temporal lobe (including the amygdala), 2 in the right mesiotemporal lobe, and 2 in the frontal cortex. Concerning investigation of the amygdala, 2 participants had bilateral electrodes placement, 3 patients had implantation of the right amygdala, and 3 others the left amygdala. In none of the patients did MRI disclose an evident structural lesion of the amygdala. The main clinical characteristics of participants are summarized in Table 1.
Clinical and experimental data of participants
| Patients | Years | Seizures foci | EZ | SEEG implantation | Amygdala stimulation | Threshold of emotional response (mA) | Total number of stimulations | Valence of emotion (%) | ||||||
| Right A | Left A | EZ | nEZ | − | + | |||||||||
| GJ | 35 | Left TL | Amygdala, hippocampus, temporal pole | × | × | 0.5 | 9 | 55 | 0 | |||||
| CM | 33 | Bilateral FL | Cingulate gyrus, area 6 | × | × | × | 0.5 | Right A | Left A | Right A | Left A | Right A | Left A | |
| 10 | 5 | 50 | 0 | 0 | 40 | |||||||||
| TS | 37 | Bilateral TL | Amygdala, hippocampus, entorhinal cortex | × | × | 1.5 | 6 | 50 | 0 | |||||
| FR | 24 | Left TL | Hippocampus, entorhinal cortex | × | × | × | 1 | 4 | 0 | 50 | ||||
| TF | 33 | Right FL | Prefrontal | × | × | 0.75 | 8 | 37.5 | 0 | |||||
| CG | 55 | Left TL | Hippocampus, parahippocampic gyrus | × | × | 1 | 4 | 25 | 0 | |||||
| CV | 44 | Right TL | Amygdala, hippocampus, entorhinal cortex | × | × | 0.5 | 5 | 80 | 0 | |||||
| CL | 31 | Left TL | Amygdala, hippocampus, temporal pole | × | × | 0.5 | 8 | 0 | 75 | |||||
| Patients | Years | Seizures foci | EZ | SEEG implantation | Amygdala stimulation | Threshold of emotional response (mA) | Total number of stimulations | Valence of emotion (%) | ||||||
| Right A | Left A | EZ | nEZ | − | + | |||||||||
| GJ | 35 | Left TL | Amygdala, hippocampus, temporal pole | × | × | 0.5 | 9 | 55 | 0 | |||||
| CM | 33 | Bilateral FL | Cingulate gyrus, area 6 | × | × | × | 0.5 | Right A | Left A | Right A | Left A | Right A | Left A | |
| 10 | 5 | 50 | 0 | 0 | 40 | |||||||||
| TS | 37 | Bilateral TL | Amygdala, hippocampus, entorhinal cortex | × | × | 1.5 | 6 | 50 | 0 | |||||
| FR | 24 | Left TL | Hippocampus, entorhinal cortex | × | × | × | 1 | 4 | 0 | 50 | ||||
| TF | 33 | Right FL | Prefrontal | × | × | 0.75 | 8 | 37.5 | 0 | |||||
| CG | 55 | Left TL | Hippocampus, parahippocampic gyrus | × | × | 1 | 4 | 25 | 0 | |||||
| CV | 44 | Right TL | Amygdala, hippocampus, entorhinal cortex | × | × | 0.5 | 5 | 80 | 0 | |||||
| CL | 31 | Left TL | Amygdala, hippocampus, temporal pole | × | × | 0.5 | 8 | 0 | 75 | |||||
Note: TL, temporal pole; FL, frontal pobe; A, amygdala; EZ, epileptogenic zone; nEZ, nonepileptogenic zone.
Clinical and experimental data of participants
| Patients | Years | Seizures foci | EZ | SEEG implantation | Amygdala stimulation | Threshold of emotional response (mA) | Total number of stimulations | Valence of emotion (%) | ||||||
| Right A | Left A | EZ | nEZ | − | + | |||||||||
| GJ | 35 | Left TL | Amygdala, hippocampus, temporal pole | × | × | 0.5 | 9 | 55 | 0 | |||||
| CM | 33 | Bilateral FL | Cingulate gyrus, area 6 | × | × | × | 0.5 | Right A | Left A | Right A | Left A | Right A | Left A | |
| 10 | 5 | 50 | 0 | 0 | 40 | |||||||||
| TS | 37 | Bilateral TL | Amygdala, hippocampus, entorhinal cortex | × | × | 1.5 | 6 | 50 | 0 | |||||
| FR | 24 | Left TL | Hippocampus, entorhinal cortex | × | × | × | 1 | 4 | 0 | 50 | ||||
| TF | 33 | Right FL | Prefrontal | × | × | 0.75 | 8 | 37.5 | 0 | |||||
| CG | 55 | Left TL | Hippocampus, parahippocampic gyrus | × | × | 1 | 4 | 25 | 0 | |||||
| CV | 44 | Right TL | Amygdala, hippocampus, entorhinal cortex | × | × | 0.5 | 5 | 80 | 0 | |||||
| CL | 31 | Left TL | Amygdala, hippocampus, temporal pole | × | × | 0.5 | 8 | 0 | 75 | |||||
| Patients | Years | Seizures foci | EZ | SEEG implantation | Amygdala stimulation | Threshold of emotional response (mA) | Total number of stimulations | Valence of emotion (%) | ||||||
| Right A | Left A | EZ | nEZ | − | + | |||||||||
| GJ | 35 | Left TL | Amygdala, hippocampus, temporal pole | × | × | 0.5 | 9 | 55 | 0 | |||||
| CM | 33 | Bilateral FL | Cingulate gyrus, area 6 | × | × | × | 0.5 | Right A | Left A | Right A | Left A | Right A | Left A | |
| 10 | 5 | 50 | 0 | 0 | 40 | |||||||||
| TS | 37 | Bilateral TL | Amygdala, hippocampus, entorhinal cortex | × | × | 1.5 | 6 | 50 | 0 | |||||
| FR | 24 | Left TL | Hippocampus, entorhinal cortex | × | × | × | 1 | 4 | 0 | 50 | ||||
| TF | 33 | Right FL | Prefrontal | × | × | 0.75 | 8 | 37.5 | 0 | |||||
| CG | 55 | Left TL | Hippocampus, parahippocampic gyrus | × | × | 1 | 4 | 25 | 0 | |||||
| CV | 44 | Right TL | Amygdala, hippocampus, entorhinal cortex | × | × | 0.5 | 5 | 80 | 0 | |||||
| CL | 31 | Left TL | Amygdala, hippocampus, temporal pole | × | × | 0.5 | 8 | 0 | 75 | |||||
Note: TL, temporal pole; FL, frontal pobe; A, amygdala; EZ, epileptogenic zone; nEZ, nonepileptogenic zone.
Stereotactic Implantation of Depth EEG Electrodes
Data were obtained from patients implanted with a chronic SEEG electrode in the right or left amygdala. Additionally, 6–12 other electrodes were implanted in various intracerebral sites. SEEG was carried out as part of our patients' normal clinical care, and patients gave informed consent in the usual way. Patients were informed that their data might be used for research purposes.
The technique of SEEG used was described by Talairach and others 1974 and was previously reported (Ostrowsky and others 2002; Bartolomei and others 2004; Liegeois-Chauvel and others 2004). This requires implantation of multiple contact intracerebral electrodes (diameter: 0.8 mm, number of contacts: 5–15, length of each contact: 2 mm, spacing interval: 1.5 mm), using a standard double-grid system fastened to the Talairach stereotactic frame. Planning of the stereotactic trajectory for each electrode was carried out preoperatively with nonstereotactic MRI, using software platforms (Surgiplan, Elektra, Stockholm, Sweden and Anatomist, France). These allow a computerized 3-dimensional (3D) image of the sulci to be created by extraction from other cerebral structures, with automatic labeling and navigation in 3D images. Postoperatively, a CT scan without contrast was performed to check for the absence of bleeding and to confirm the precise topography of each contact. After the period of continuous video SEEG recording, electrodes were removed, and a MRI was performed the day after in order to locate the trace of each electrode in the brain. Combining data from the postoperative CT scan and the postimplantation MRI allows precise anatomical location of each electrode (see more detailed methodology in Balzamo and others 2004).
Intracerebral Stimulations
Direct electrical stimulation was routinely carried out as part of the standard presurgical assessment, to provide additional electroclinical data about the epileptogenic zone (in determining the sites in which seizures can be triggered) as well as functional mapping of brain region (Chauvel and others 1993). Electrical stimulations were produced by a regulated neurostimulator designed for safe diagnostic stimulation of the human brain (Inomed, Teningen, Germany) (Bartolomei and others 2004).
During stimulation, patients were sitting in bed and were asked to read or to count. They were not aware of at which moment stimulation occurred. Each stimulation was triggered by the experimenters. Emotions were studied after each stimulation of the contacts located in the right and left amygdalae. The laterobasal complex of the amygdala is the main target of the implantation. It cannot however be excluded that other nuclei might be stimulated because of the size of the electrodes. The stimulated amygdala was within the epileptogenic zone in 4 patients and outwith the epileptogenic zone in 4 others (see Table 1). High-frequency stimulations (50 Hz, pulse duration 1 ms, 5-s duration) were applied in a bipolar fashion to each contact in the brain structure. The current intensity was gradually increased (0.25 mA increments) ranging from 0 to 2 mA until a clinical effect or an after discharge was obtained. The 0 mA stimulation was used as a control condition, in order to check the absence of emotional changes under these conditions.
Stimulations that induced an overt seizure (fully developed seizure with loss of consciousness) were not included. Among the 4 patients stimulated within their epileptogenic zone, 3 had one seizure after the stimulation of the amygdala. As an internal control, 11 stimulations to the temporal neocortex (in the middle of the temporal gyrus) were also studied. Finally, a total of 69 stimulations of the amygdala were studied, corresponding to 4–9 stimulations per patient. Thirty-eight (including 10 stimulations at 0 mA) did not induce any emotional modifications, whereas 31 induced emotional changes. The percentage of emotional changes obtained for each patient is indicated in Table 1. None of the 0 mA stimulations evoked changes in emotional responses.
Self-Reported Measures
Emotional responses were investigated and quantified on the basis of affective self-reports using a French version (Ouss and others 1990) of the differential emotion scale created by C. Izard (Izard 1977). Participants were instructed to indicate their emotional intensity experienced by rating each of 9 emotion scales (surprise, fear, disgust, sadness, anger, anxiety, disdain, happiness, and joy) ranging from 1 (no emotion) to 5 (very strong emotion). Ratings were carried out just before the beginning of the experiment (in order to obtain a baseline emotional state) and immediately after each stimulation.
Facial and Electrodermal Measures
A Biopac Student Lab 3.7 data acquisition system was used (Biopac Systems, Santa Barbara, CA). Bipolar electromyographic (μV) recordings were monitored from right corrugator supercilii according to previously described recommendations (Fridlund and Cacioppo 1986). We used miniature surface Ag/AgCl electrodes (diameter: 0.5 mm) filled with electrode paste. Before the electrodes were attached, the skin was cleaned and gently rubbed. A root mean square rectification was performed. Signals were band-pass filtered from 30 to 500 Hz. Change scores were calculated separately for EMGc by subtracting the average activity during the 1 s preceding the stimulation from the average response during 1 s after the electrical stimulation.
Electrodermal activity was recorded in terms of SCRs (mho). Bipolar Ag-AgCl electrodes placed in direct contact with the skin were attached to the distal phalanges of the second and third fingers of the hand ipsilateral to the stimulation side because Mangina and Beuzeron-Mangina (1996) showed a greater SCR in the side ipsilateral to the stimulated amygdala. A 10-min resting period for patients to quieten and relax was required before acquisition so as to establish a baseline of electrodermal conductivity. Each rating on the Izard scales after stimulation was followed by an interval of at least 30 s to allow for skin conductance recovery to baseline before the next stimulation was applied. The measure of electrodermal activity corresponded to the maximal amplitude of the SCR elicited by each stimulation, in relation to the onset level (representing the baseline). For each of the observed SCR, we used the delta value, within an interval of 1–3 s after the onset of the stimulation (Venables and others 1980), which corresponded to the inflection point to peak.
Statistical Analysis
First, the temporal lateral neocortex was considered as a control condition. Stimulation was applied to the most lateral contacts of the electrode reaching the amygdala and located in the middle temporal gyrus (Fig. 1a,b). Descriptive analysis showed that from 11 electric stimulations in the neocortex, only one evoked a concomitant increase for anxiety and surprise, and this increase was weak (only 1 point on 5). Because the neocortex electrical stimulations elicited almost no emotion (9% of stimulations evoking only a weak emotional change), statistical analyses were therefore not performed on these responses. Consequently, statistical analysis was only applied to the results of amygdala electrical stimulations that were superior to 0 mA (52.5% of stimulation evoking emotions, ratio: 31/59).
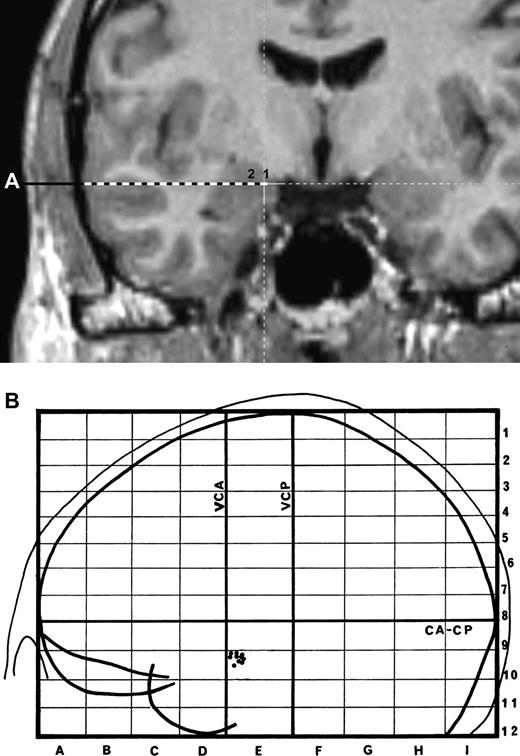
(a) Reconstruction of the amygdala electrode route in the MRI (patient TF). The stimulations were applied in the 2 most internal contacts (within the amygdalae) of the electrode (labeled 1 and 2). (b) Representation of the placement of the electrodes from the 8 patients exploring the amygdala. Right and left electrode positions are projected on a lateral view of the left hemisphere normalized in the proportional stereotactic grid system of Talairach and Tournoux. CA–CP, anterior commissure–posterior commissure plane; VCA, vertical plane through CA; VCP, vertical plane through CP.
Second, verbal reports of emotional changes were studied when the amygdala stimulations induced emotional changes (at least 1 point change on 5), that is, on 31 stimulations. In order to test for the stimulation effects on right and left amygdalae, a repeated measures analysis of variance was performed on Izard scores with stimulation (before and after) as a “within factor,” emotions (happiness, disgust, fear, anger, joy, disdain, anxiety, sadness, surprise) and laterality (right or left) as “between factors.” When effects reached significant levels, multiple comparison tests were performed, and a Bonferroni correction was applied. In addition, percentages of emotional changes after electrical stimulations were calculated.
Third, in order to assess the effect of the electrical stimulations of the amygdala on EMGc, a nonparametric Wilcoxon test was used because EMGc values did not follow the normality law according to the Kolmogorov–Smirnoff test. The tests allowed comparison of EMGc before and after stimulations according to the amygdala side and to the presence or absence of emotional changes (either positive or negative).
Fourth, as the SCRs also did not follow the normality law, Mann–Whitney tests were performed to compare between SCRs when right and left amygdalae were stimulated inducing or not emotional changes (either positive or negative).
Results
Scores of Emotional Changes
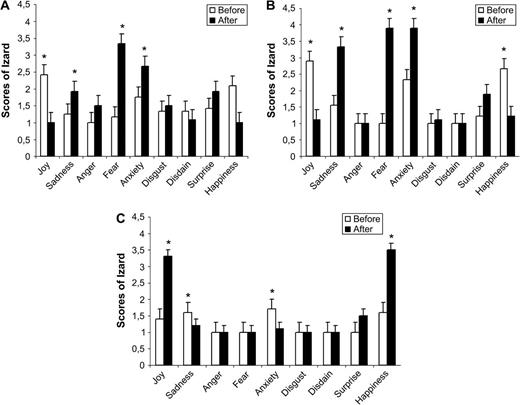
The total of electric stimulations was 69, including controlled stimulations at 0 mA (14.5%, ratio: 10/69 stimulation). After the remaining controlled stimulations, it appears that 52.5% (ratio: 31/59 stimulation) of the amygdala stimulations evoked changes of either negative or positive emotional scores (see Table 1). Among these changes, the stimulation (F1,261 = 16.43, P < 0.0001) as well as the stimulation × emotion interaction were statistically significant (F1,8 = 9.16, P < 0.0001). Indeed, as seen in Figure 2a,b,c, the stimulations increased the average ratings of sadness (t = −3.147, n = 30, P < 0.005) and fear (t = −6.562, n = 30, P < 0.0001). This effect depends upon the stimulation side because significant results were also found for lateralization × emotion (F1,8 = 3.12, P < 0.005) and stimulation × lateralization × emotion interactions (F1,8 = 3.107, P < 0.005). Before amygdala stimulation, no difference in Izard scores was found whatever the side of the stimulations and the category of emotion (P > 0.05). In contrast, after stimulation of the right amygdala, fear, anxiety, and sadness scores were higher than those of anger, disdain, disgust, joy, and happiness (P < 0.05). On the contrary, left amygdala stimulations seemed to evoke negative as well as positive emotions. Indeed, mean ratings of fear, anxiety, sadness, happiness, and joy increased with the stimulations and were superior to the ratings of anger, disdain, and disgust (P < 0.05).
(a) Graphic summarizing the effect of the stimulation on Izard scores (means and standard deviations) for each type of emotion, after right amygdala stimulation. (b) Graphic summarizing the effect of the stimulation on Izard scores (means and standard deviations) for each type of emotion when participants reported to have unpleasant states after left amygdala stimulation. (c) Graphic summarizing the effect of the stimulation on Izard scores (means and standard deviations) for each type of emotion when participants reported to have pleasant states, after left amygdala stimulation.
These results are in accordance with results on the percentages of emotional changes according to stimulations of right and left amygdalae. As described in Table 2, the stimulations of the right amygdala evoked only negative emotions, especially fear, whereas stimulation of the left induced negative (notably fear, anxiety, and sadness) or positive emotions (joy and happiness).
Main emotional changes (% increase) obtained after a right amygdala and left amygdala stimulations
| Right amygdala | Left amygdala | |
| Increase (%) | Increase (%) | |
| Joy | 0 | 52.6 |
| Sadness | 58.3 | 47.4 |
| Anger | 17 | 0 |
| Fear | 100 | 47.4 |
| Anxiety | 58.3 | 47.4 |
| Disgust | 50 | 5.2 |
| Disdain | 8.3 | 0 |
| Surprise | 33 | 26.3 |
| Happiness | 0 | 52.6 |
| Right amygdala | Left amygdala | |
| Increase (%) | Increase (%) | |
| Joy | 0 | 52.6 |
| Sadness | 58.3 | 47.4 |
| Anger | 17 | 0 |
| Fear | 100 | 47.4 |
| Anxiety | 58.3 | 47.4 |
| Disgust | 50 | 5.2 |
| Disdain | 8.3 | 0 |
| Surprise | 33 | 26.3 |
| Happiness | 0 | 52.6 |
Main emotional changes (% increase) obtained after a right amygdala and left amygdala stimulations
| Right amygdala | Left amygdala | |
| Increase (%) | Increase (%) | |
| Joy | 0 | 52.6 |
| Sadness | 58.3 | 47.4 |
| Anger | 17 | 0 |
| Fear | 100 | 47.4 |
| Anxiety | 58.3 | 47.4 |
| Disgust | 50 | 5.2 |
| Disdain | 8.3 | 0 |
| Surprise | 33 | 26.3 |
| Happiness | 0 | 52.6 |
| Right amygdala | Left amygdala | |
| Increase (%) | Increase (%) | |
| Joy | 0 | 52.6 |
| Sadness | 58.3 | 47.4 |
| Anger | 17 | 0 |
| Fear | 100 | 47.4 |
| Anxiety | 58.3 | 47.4 |
| Disgust | 50 | 5.2 |
| Disdain | 8.3 | 0 |
| Surprise | 33 | 26.3 |
| Happiness | 0 | 52.6 |
Therefore, the left amygdala electrical stimulations evoked either positive or negative emotions, whereas right amygdala stimulation only evoked negative emotions. Consequently, to precisely test for the stimulation effects for each emotion, Izard scores for negative emotions in right amygdala, negative emotions in left amygdala, and positive emotions in left amygdala were considered separately as represented by Figure 2a,b,c.
For the right amygdala, 100% of the stimulations with emotional changes evoked negative emotions, that is, fear (z = −3.08, n = 11, P < 0.05), anxiety (z = −2.2, n = 11, P < 0.05), and sadness (z = −2.53, n = 11, P < 0.05). These increases of negative feelings were accompanied by a significant decrease of joy (z = −2.4, n = 11, P < 0.05) and a tendency for happiness decrease (z = −1.9, n = 11, P = 0.058).
The same results were observed for 47.3% of left stimulations because negative emotions were induced, especially fear (z = −2.7, n = 8, P < 0.05), anxiety (z = −2.72, n = 8, P < 0.05), and sadness (z = −2.716, n = 8, P < 0.05), whereas, happiness and joy scores decreased (z = −2.81, n = 8, P < 0.05; z = −2.716, n = 8, P < 0.05) (See Fig. 2a). In contrast, it is striking to note that 52.6% of left amygdala stimulations evoked positive emotions because the scores for joy (z = −3.05, n = 9, P < 0.05) and happiness (z = −2.836, n = 9, P < 0.05) were higher than before stimulation, whereas the scores for sadness (z = −2, n = 9, P < 0.05) and anxiety (z = 2.44, n = 9, P < 0.05) diminished (See Fig. 2b).
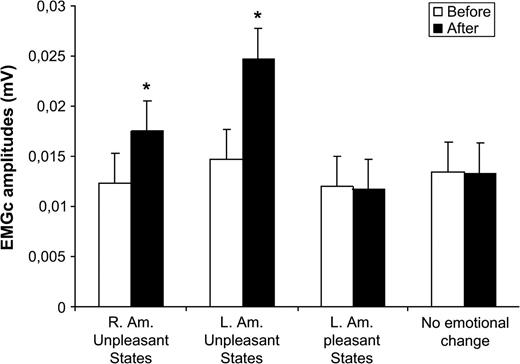
Facial Responses
Emotional changes were accompanied by specific facial modifications as represented in Figure 3. EMGc activity significantly increased when participants felt unpleasant states after both right (z = −3.059, n = 12, P < 0.05) and left (z = −2.66, n = 9, P < 0.05) amygdala stimulations. In contrast, no EMGc modification was observed for positive states (z = −1.34, n = 10, P > 0.05) (when left amygdala was stimulated) and when the stimulations did not induce emotional changes (z = −0.55, n = 28, P > 0.05).
Mean changes and standard deviations for EMGc (mV) when participants reported pleasant, unpleasant, or no emotions.
Skin Conductance Responses
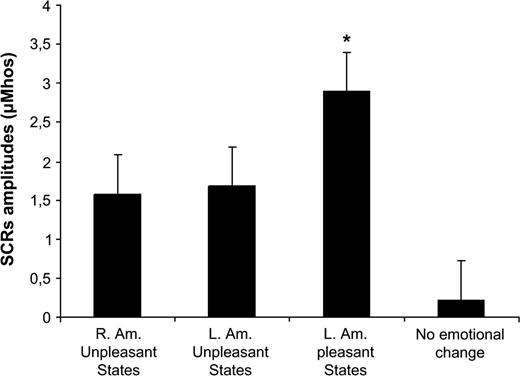
As expected, the stimulation of the amygdala induced electrodermal modifications but responses were higher and systematic when the stimulations were accompanied by emotional changes. A descriptive analysis showed that 43% of stimulations (ratio: 12/28 stimulations), without emotional changes, evoked a SCR, whereas 100% of the stimulations with emotional modifications induced SCR.
This in accordance with the finding that the SCRs amplitudes were significantly larger when stimulations induced emotional changes, either pleasant for the left amygdala stimulation (U = 6, P < 0.0001) or unpleasant for both right (U = 38.5, P < 0.0001) and left amygdala stimulations (U = 17, P < 0.0001) compared with the stimulations without emotional change (see Fig. 4). In addition, SCRs were larger following positive emotions (elicited by left amygdala) compared with SCRs following unpleasant emotions (elicited by the right amygdala) (U = 23.5, P < 0.05). There was no correlation between SCR magnitudes and predominant emotional ratings after each stimulation (fear for negative emotions and joy + happiness for positive emotions) (r = −0.22, n = 31, P = 0.23).
Mean changes and standard deviations for SCR (μmho) magnitude when participants reported pleasant, unpleasant, or no emotions.
Discussion
In this study, we were interested by the effects of stimulation of the amygdala on emotional experiences. Our results support direct in vivo evidence that electrical stimulations of the amygdala involve both positive and negative emotional states. In addition, data clearly demonstrate that positive emotions were only induced by stimulations of the left amygdala, whereas negative emotions were evoked by stimulations of both amygdalae.
From a methodological point of view, it should be noted that emotional modifications appeared after stimulation intensity ranging from 0.5 to 2 mA. These intensities were in the same range as those used in some previous intracerebral stimulation studies. For example, in one study, SCR changes were studied after amygdala stimulations at 0.5 and 0.75 mA (Mangina and Beuzeron-Mangina 1996). In previous clinical studies, clinical responses after stimulation of the amygdala were obtained between 1 and 3 mA (Gloor and others 1982) or 0.5 and 2.5 mA (Bartolomei and others 2004).
When negative emotions were induced, fear, sadness, and anxiety were preferentially evoked over anger, disgust, and disdain, whatever the stimulation side. For Halgren and others (1978), the stimulation of amygdala also evoked fear and anxiety, but in line with our results, these authors rarely observed feelings of anger (only one observation). In addition, Gloor and others (1982) have shown that right amygdala stimulations evoked mostly fear, and less frequently, emotional distress (guilt), or less definable unpleasant emotional states. Therefore, these authors agreed with the idea that the amygdala is essentially involved in fear/anxiety responses (Aggleton 2000). In the present experiment, we also demonstrated that amygdala stimulation may induce feelings of sadness. These findings support previous studies involving activation of the amygdala during self-induced sad mood (Schneider and others 1997) or during presentation of sad pictures (Wang and others 2005). However, in contrast with other reports studying subjects with bilateral amygdala lesions (Adolphs and others 1999) or neuroimaging with healthy participants (Gur and others 2002), our results do not demonstrate an amygdala involvement in anger and disgust. This result does not support the hypothesis of a general role of the amygdala in negative emotions, whatever the subtype of emotion.
Moreover, the amygdala involvement in negative emotions seems to depend upon amygdala side. Wager and others (2003) have already observed in a meta-analysis that amygdala activations are mostly lateralized toward the left side, especially for negative emotions. Baas and others (2004) also found the predominant left amygdala activation in negative emotions. On the contrary, our results demonstrated a preferential role of the right rather than the left amygdala in evoking negative emotions because negative emotions were more often evoked by right than by left amygdala stimulations. These discrepancies between previous studies and the present experiment may be due to the differences between methods employed and thus between processing. Indeed, we assessed the role of the amygdala on emotional affects by direct electric amygdala stimulation in humans, whereas data from the literature have been essentially focused on amygdala involvement in encoding and recognition of external emotional stimuli. It is all the more likely that our results are in agreement with the study by Gloor and others (1982) in which direct right amygdala stimulation elicited negative emotions, whereas this was less frequent for the left amygdala. The right amygdala would thus have a predominant role in processing negative emotion, whereas the left amygdala would play a role in both positive and negative emotions. The more striking finding of this experiment is that half of the left amygdala stimulations evoked positive emotions. This result has never been found when using direct electrical stimulations of the amygdala in humans (Halgren and others 1978; Gloor and others 1982). However, a possible involvement of the amygdala in positive emotions and its lateralization according to the valence of emotions have recently been discussed. For example, in a functional MRI study, Garavan and others (2001) showed bilateral amygdala activation after viewing both negative and positive pictures. The amygdala plays a critical role in negative emotion processing. On the contrary, a recent review (Zald 2003) has mentioned that across studies, emotional implications to positively valenced stimuli are less consistent than those observed with negatively valenced stimuli. In addition, no clear pattern of amygdala lateralization for positive stimuli has seemed to emerge from the literature (Wager and others 2003; Zald 2003). Our study used a unique model of direct amygdala stimulations in humans and allows us to demonstrate a clear amygdala involvement in positive emotions (joy and happiness) as well as a clear left lateralization for these positive emotions. These results are in accordance with the study of Schneider and others (1997) who have shown that happy mood induction increases left amygdala activation. In the same way, a positron emission tomography study by Hamann and others (2002) has shown left amygdala activation during the presentation of positive photographs, whereas there was a bilateral activation for the negative ones.
However, lesion studies provide evidence that amygdala resection does not always disrupt affective states (Anderson and Phelps 2002), arousal ratings, and related autonomic responses to affective words (LaBar and Phelps 1998). By contrast, unilateral amygdala lesions were also shown to evoke cognitive and physiological deficits. Results have demonstrated deficits in emotional learning (LaBar and others 1995), in processing of fear conveyed by music (Gosselin and others 2005), in cognitive ratings of arousal, and in autonomic arousal responses to affective pictures (Glascher and Adolphs 2003). This discrepancy could be explained by differences in the experimental designs, in particular, in the choice of affective material, presentation duration, and tasks.
In addition, even if the amygdala is a key structure in emotion processing, cognitive and autonomic deficits due to its resection could be modulated by the contribution of other cerebral structures of the emotional neural network such as the prefrontal cortex (PFC). Recent advances in neuroanatomical connections of the amygdala showed evidence of an anatomical relation between the human amygdala and the PFC in the primate (Aggleton 2000) as well as functional relationships in humans between amygdala and PFC (Davidson 2002). According to these authors, the PFC could modulate and inhibit the amygdala activity and in a wider sense regulate emotional processes. It is in accordance with the fact that both our results on amygdala and previous results on PFC are in keeping with EEG PFC asymmetries linked to emotional affect. A greater left PFC activity is associated with more positive affect, whereas a greater right PFC activity is associated with more negative affect (Tomarken and others 1992). One may thus wonder whether the emotional reports of our patients are due to amygdala stimulations per se or subsequent PFC activations.
In a second analysis, we investigated the physiological responses related to emotional changes. As predicted, unpleasant states were accompanied by an activation of the corrugator supercilii. This is consistent with the literature because a large body of evidence indicates that corrugator muscles are activated in frowning when an individual feels negative emotions (Lang and others 1998). The negative emotions elicited by amygdala stimulations were therefore strong enough to evoke facial responses.
In addition, our data show that SCRs were essentially due to the emotional responses rather than to the stimulation of the amygdala. As a matter of fact, we found that emotional modifications were systematically accompanied by an increase of SCRs, although 43% of the amygdala stimulations without emotional induction evoked SCRs. Increase in SCRs without emotions has already been demonstrated in one study after amygdala stimulation in humans (Mangina and Beuzeron-Mangina 1996), but the concomitant SCRs and emotion elicitations by amygdala electrical stimulations has never been demonstrated. Thus, this result supports the hypothesis of amygdala involvement in SCRs to emotional pictures (Glascher and Adolphs 2003). In summary, skin conductance is closely dependent on the modulation of the amygdala but is also the expression of emotional responses.
Contrary to the EMGc, the SCR depends upon arousal levels rather than emotional valence (Lang and others 1998; Bradley and Lang 2000). In addition, the nonsignificant correlation between SCRs amplitude and emotional rating does not inevitably involve an absence of relationship between SCRs and arousal. Indeed, the rating was performed on the emotional intensity, and not specifically on the arousal level (stimulating aspect), which is the only value that has already been correlated to SCR (Lang and others 1998). It has also been shown (Khalfa and others 2002) that more stimulating emotions (in music), that is, fear and happiness evoked greater SCRs than less stimulating ones, that is, sadness and peacefulness. Surprisingly, our data showed different SCRs according to the affective valence. We observed greater SCRs to pleasant emotions (happiness and joy) than to negative emotions (fear, sadness, anxiety). However, the presence of sadness among the negative emotions evoked by the amygdala stimulations (anxiety and fear) may indeed decrease the average SCRs because sadness is less stimulating than anxiety, fear, and happiness (Lang and others 1998).
In conclusion, our findings provide major evidence of a link between emotional affect, facial expressions, sympathetic activity, and amygdala stimulations in humans. They also suggest that the induction of emotional experience depends upon the side of the amygdala electrical stimulation. Indeed, our findings reveal a bilateral amygdala involvement in negative emotional states with predominant feelings of fear and a left sided amygdala involvement for positive emotions (joy and happiness). The labeling of emotions during the amygdala stimulations is corroborated by modifications of somatic markers. EMGc increases were present during negative feelings, and SCRs were a reliable index of the emotional experience changes.
We thank Dr Aileen McGonigal for the revision of the English version of this paper and for helpful comments. Conflict of Interest: None declared.