-
PDF
- Split View
-
Views
-
Cite
Cite
Jan Havlicek, Pavlina Lenochova, The Effect of Meat Consumption on Body Odor Attractiveness, Chemical Senses, Volume 31, Issue 8, October 2006, Pages 747–752, https://doi.org/10.1093/chemse/bjl017
Close - Share Icon Share
Abstract
Axillary body odor is individually specific and potentially a rich source of information about its producer. Odor individuality partly results from genetic individuality, but the influence of ecological factors such as eating habits are another main source of odor variability. However, we know very little about how particular dietary components shape our body odor. Here we tested the effect of red meat consumption on body odor attractiveness. We used a balanced within-subject experimental design. Seventeen male odor donors were on “meat” or “nonmeat” diet for 2 weeks wearing axillary pads to collect body odor during the final 24 h of the diet. Fresh odor samples were assessed for their pleasantness, attractiveness, masculinity, and intensity by 30 women not using hormonal contraceptives. We repeated the same procedure a month later with the same odor donors, each on the opposite diet than before. Results of repeated measures analysis of variance showed that the odor of donors when on the nonmeat diet was judged as significantly more attractive, more pleasant, and less intense. This suggests that red meat consumption has a negative impact on perceived body odor hedonicity.
Introduction
Human body odor is individually specific, similar to our appearance. Odor individuality is established very early in life as was clearly shown by Porter's team and other researchers (for review, see Winberg and Porter 1998). Newborn babies are able to distinguish their mothers' breast and axillary odor within several weeks after delivery (Russell 1976; Cernoch and Porter 1985). Simultaneously, mothers can recognize the odor of their offspring 2 days after delivery (Porter et al. 1983). Fathers can also distinguish their own baby's smell from the smell of a strange baby when babies are aged 3 weeks (Porter et al. 1986). Individual odor recognition is not restricted to early parent–offspring relationships, occurring also in adulthood in sibling recognition for instance (Porter and Moore 1981). In addition, body odor plays a significant role in mate choice (Herz and Cahill 1997; Herz and Inzlicht 2002) and in sexual partners' recognition once the relationship is established (Hold and Schleidt 1977). Based on all the above-mentioned empirical findings, body odor emitted by a specific individual was therefore labeled an “odor signature” (Porter et al. 1985).
The odor signature is to some extent genetically inherited. This view is supported by 3 lines of evidence. First, odors of parents and offspring can be correctly matched by subjects not acquainted to odor donors (Porter et al. 1985). On the other hand, the raters were not able to match odors of spouses, who are not genetically related. This result also excluded the possibility that matching of parents and offspring was due to shared home odor. Second, odors of monozygotic twins are more difficult to distinguish than dizygotic twins (Wallace 1977), and monozygotic twins are matched at rates better than chance, even when they live apart (Roberts et al. 2005). Third, odor preferences are correlated with genes in the major histocompatibility complex (MHC). Products of MHC genes are crucial elements of the immune system, particularly in self/nonself recognition. It was found that women rate the odor of MHC dissimilar men as most attractive (Wedekind et al. 1995; Wedekind and Füri 1997). Such preference may potentially result in more MHC heterozygous, and therefore also more viable, offspring (Penn 2002).
However, not only is body odor preprogrammed by genetic factors but also much variability is due to psychophysiological and ecological influences. For instance, it has been shown repeatedly that body odor changes across women's menstrual cycle, peaking in attractiveness around the time of ovulation (Thornhill et al. 2003; Kuukasjärvi et al. 2004; Havlicek et al. 2006). Other studies found that mood (e.g., fear) of a target person may influence hedonic perception of his/her axillary odor (Chen and Haviland-Jones 2000; Ackerl et al. 2002). Eating habits also may have a crucial impact on body odor composition. However, very little is known about the effect of individual alimentary components on human body odor. Some folk beliefs connect odor hedonicity with meat consumption. For instance, Hindu Indians who are usually vegetarian say that people who eat meat smell bad because of it (S Komarek, personal communication). To our knowledge, however, this effect has not yet been tested under controlled conditions.
The aim of this study was to test the effect of red meat consumption on axillary odor hedonicity. For this purpose, we used a balanced within-subject design with a relatively long period (2 weeks) of diet control. The results may improve our rather poor understanding of how axillary odor is determined and also may be of methodological importance for dietary control in olfactory studies.
Materials and methods
Subjects
Odor donors
Seventeen male students of Charles University, Prague, agreed to participate in the study. Their mean age was 22.5 years (minimum 19 years, maximum 31 years), body weight 75.5 kg (minimum 63 kg, maximum 88 kg), and body height 182 cm (minimum 171 cm; maximum 200 cm). All of them were nonsmokers, reported no dermatological or other diseases and did not shave their armpits. The donors were given CZK 2000 (approximately GBP 45) as compensation for their time and potential inconvenience caused by the prescribed diet.
Raters
Raters were contacted by e-mail or personally by the authors. Thirty-two female students (mean age 23.3 years, minimum 19 years, maximum 32 years) took part in the study. Two of them, however, did not finish both sessions and were excluded from all analyses. None of the women were using hormonal contraception, and all reported having “normal” menstrual cycle length (25–40 days). They were also asked about the date of the onset of their last menstrual bleeding (day 1). Women in days 9–15 of their cycle on the testing day were judged to be in fertile phase and others to be in nonfertile phases of the cycle. As the experiment was composed of 2 rating sessions, we decided to perform the second session 28 days after the first one to minimize the possible effect of menstrual cycle on the raters' olfactory sensitivity (Doty et al. 1981; Caruso et al. 2001; Navarrete-Palacios et al. 2003). The raters were not paid for participation; however, they received a perfume tester after the first session and a 100 g chocolate bar after the second session.
Odor sampling procedure
We used a balanced within-subject design in which odor donors were randomly assigned to one of 2 groups (A, B). Odor donors in group A were in the “meat” condition for the first session, whereas those in group B were in the “nonmeat” condition. Groups were reversed for the second session.
The odor donors followed our diet protocol for 2 weeks prior to the odor sampling. They were also asked to keep a “diet diary” in which they recorded all food eaten during the day and alcoholic beverages and level of stress, fatigue, and general mood using a 7-point scale. The diet protocol comprised 2 stages. In the first stage (days 1–10), the odor donors received a list of 33 meals; they had to choose at least one main dish out of the given list every day. The diet protocol was built in 2 versions and composed only of meat or “nonmeat” dishes. The individual dishes were elaborated to differ in meat content only (e.g., vegetable risotto/pork risotto). For the list of meals offered, see the Appendix (Supplementary material). Each participant received instructions and restrictions in a written form. They were instructed to refrain from 1) using perfumes, deodorants, antiperspirants, aftershave, and shower gels, 2) eating meals containing garlic, onion, chilli, pepper, vinegar, blue cheese, cabbage, radish, fermented milk products, and marinated fish, 3) drinking alcoholic beverages or using other drugs, and 4) smoking. As this very strict procedure had to be maintained for a relatively long period, during the first stage (days 1–10), nonserious violations were tolerated (e.g., one 0.5l beer or one glass of wine).
In the second stage (days 11–14), all food (3 meals and 2 snacks a day) were provided to odor donors in order to precisely control their dietary intake. The meat group members were served a 100-g red meat dish for each main course (i.e., lunch and dinner); the nonmeat group again differed only in the absence of meat. For particular meals served to the odor donors, see the Appendix (Supplementary material). Odor donors were reminded not to break the restrictions given in the first stage. For the second stage, they were provided with nonperfumed soap (Sara Lee Household & Body Care, Stockholm, Sweden) and were asked to avoid exaggerated physical activities, sexual activity, and sleeping in the same bed as their partner.
To check the odor donors' conformity with the instructions, we carefully examined the “diet diaries.” Two of the donors when on nonmeat diet reported once having a meat dish during the first stage of the diet protocol. All donors when on meat diet reported having at least a small amount of meat every day. Most of the subjects reported having a small amount of alcohol beverages (e.g., 0.5l beer or one glass of wine) on some days during the first stage of the diet protocol. Two donors in nonmeat and 2 donors in meat condition reported beer or wine consumption during the second stage of their diet. No consumption of spirits was reported.
Cotton pads, a T-shirt, plaster, 2 zip-lock plastic bags for storing the pads from left and right armpits, and an instruction sheet were provided to each odor donor 2 days before the rating session (day 15). In the evening of day 13, the odor donors were asked to shower without using even the nonperfumed soap. Odor donors then applied the cotton pads in the morning (7:00 AM) of day 14 and wore them for 24 h in total.
Cotton pads (Ebelin cosmetic pads, DM-drogerie markt, Ceske Budejovice, Czech Republic, http://www.dm-drogeriemarkt.cz) served as stimuli, a method used in several previous studies (e.g., Havlicek et al. 2005, 2006). The pads were 100% cotton, elliptical in shape, approximately 9 × 7 cm at their longest axis. In the morning, the odor donors fixed their cotton pads to both armpits by using 3 M Micropore surgical tape and wore them for 24 h. To avoid odor contamination from odor donors' clothes or background odor, the donors were asked to wear new white 100% cotton T-shirts (previously washed without washing powder) as the first layer of clothes. The next morning they put the pads into the zip-lock plastic bags and handed them back to the experimenters. As we received samples from both armpits from each donor, we randomly chose one of them for the testing session. To avoid possible effects of refrigeration on the stimuli, olfactory rating of the samples started within an hour after collection.
Odor rating procedure
Ratings took place in a quiet, ventilated room. The temperature during the first session was 20.5–21.5 °C (44–48% humidity) and 20.5–21.5 °C (35–37% humidity) during the second session. Raters were asked to attend the session approximately at the same time for both sessions in order to avoid possible temporal changes of rated odors and/or diurnal fluctuation in olfactory abilities. Stimuli (pads) were encased in 250 ml opaque jars labeled by a 2-number code for the first session and by a 2-letter code for the second session. Stimuli were split in 2 sets. Each subject rated both sets (i.e., 17 stimuli). The order of sets and order of stimuli within a set was randomized in the first session. In the second session, the order of sets was the same as in the first session for each subject. However, the order of stimuli within a set was again randomized. This design ensured that each stimulus was assessed by all raters in both conditions and in a balanced order. Stimuli were rated on a 7-point scale for their 1) intensity, 2) pleasantness, 3) sexual attractiveness, and 4) masculinity. Both ends of each scale were anchored by verbal descriptions (e.g., very unpleasant and very pleasant). The ratings were written down immediately after sniffing each stimulus, but the time spent by sniffing was not restricted. Raters had an approximately 10-min break between the 2 sets to avoid possible odor habituation. Coffee, tea, mineral water, and a small snack (i.e., cookies) were offered to raters, and they were also asked to complete an additional questionnaire during the break.
Statistical analysis
As our design was within subject, repeated measures analysis of variance (ANOVA) was used. The data were analyzed in 2 different ways: 1) using mean odor donors ratings and 2) using mean subjects (raters) ratings as the unit of analysis. Each of the approaches has its specific advantages and disadvantages. The statistical package STATISTICA 7.0 was used for all testing. To test the possible effect of repeated testing, we performed repeated measures ANOVAs for each of dependent variables (e.g., pleasantness) using odor donors as unit of the analysis. The same procedure was performed with subjects as unit of the analysis. We did not find a difference between the first and the second session in any of the odor ratings (all P > 0.4). There was also no difference between the sessions in self-evaluated stress, fatigue, and mood of the odor donors (all P > 0.1).
Results
First, we used individual odor donors as a unit of the analysis. Mean ratings for each odor donor in experimental (meat) and control (nonmeat) conditions were calculated. These means were entered into ANOVA as repeated measures. Odors from individuals in the nonmeat condition were rated as more pleasant, attractive, and less intense. However, none of the tests reached formal level of statistical significance.
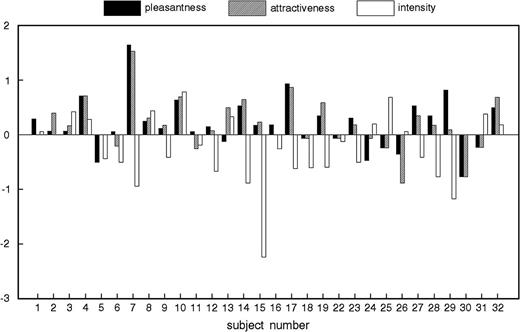
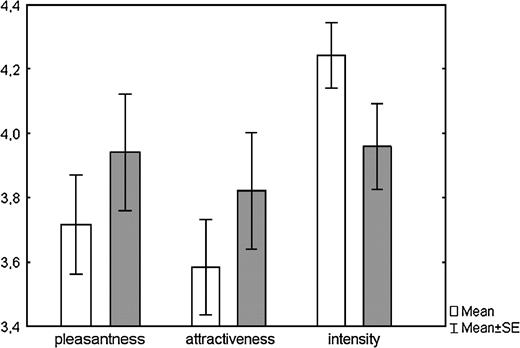
The first type of the analysis is rather crude as it uses only overall means of each donor and does not take into account the differences in individual ratings across the experimental conditions. Thus, for the subsequent analyses, we used individual raters as units of analysis (Figure 1). This approach was possible due to the fact that all raters assessed all samples in both conditions in a balanced design. Samples in the nonmeat condition were rated more pleasant (F1,29 = 7.0; P = 0.01), more attractive (F1,29 = 7.7; P < 0.01), and less intense (F1,29 = 7.0; P = 0.01) (Figure 2).
Mean difference between nonmeat and meat diet conditions in ratings of body odor pleasantness, attractiveness, and intensity for each subject (rater). Values above zero indicate higher ratings in nonmeat condition.
Mean ratings (±SE) of axillary odor pleasantness, attractiveness, and intensity when body odor donors were on meat diet (white bars) and when on nonmeat diet (gray bars). Differences are significant at P = 0.01 (repeated measures ANOVA).
No difference was found in masculinity rating. We further analyzed the potential influence of physiological (menstrual cycle phase) or social conditions (coupled/single status of raters) by including them as independent variables into the analysis. Both the menstrual cycle phase and partnership status were entered as binomial variables. There was neither any effect of menstrual cycle phase or partnership status on any of the dependent variables (e.g., attractiveness) nor any interaction with dietary condition observed.
The relations between rated variables were analyzed by simple correlation analysis using mean subject's value as a unit. We found highly significant correlations between all but one rated variable within one session. The only nonsignificant correlation was between attractiveness and masculinity during the second session. None of the correlations were, however, significant between the 2 sessions. All correlation coefficients are shown in Table 1.
Correlation coefficients between rated variables in first (1) and second sessions (2)
| Pleasantness 1 | Attractiveness 1 | Masculinity 1 | Intensity 1 | Pleasantness 2 | Attractiveness 2 | Masculinity 2 | Intensity 2 | |
| Pleasantness 1 | 0.98 | −0.86 | −0.81 | 0.20 | 0.21 | 0.37 | −0.17 | |
| Attractiveness 1 | 0.98 | −0.82 | −0.79 | 0.20 | 0.22 | 0.40 | −0.16 | |
| Masculinity 1 | −0.86 | −0.82 | 0.83 | −0.11 | −0.12 | −0.33 | 0.13 | |
| Intensity 1 | −0.81 | −0.79 | 0.83 | −0.23 | −0.23 | −0.15 | 0.40 | |
| Pleasantness 2 | 0.20 | 0.20 | −0.11 | −0.23 | 0.96 | −0.52 | −0.87 | |
| Attractiveness 2 | 0.21 | 0.22 | −0.12 | −0.23 | 0.96 | −0.41 | −0.81 | |
| Masculinity 2 | 0.37 | 0.40 | −0.33 | −0.15 | −0.52 | −0.41 | 0.66 | |
| Intensity 2 | −0.17 | −0.16 | 0.13 | 0.40 | −0.87 | −0.81 | 0.66 |
| Pleasantness 1 | Attractiveness 1 | Masculinity 1 | Intensity 1 | Pleasantness 2 | Attractiveness 2 | Masculinity 2 | Intensity 2 | |
| Pleasantness 1 | 0.98 | −0.86 | −0.81 | 0.20 | 0.21 | 0.37 | −0.17 | |
| Attractiveness 1 | 0.98 | −0.82 | −0.79 | 0.20 | 0.22 | 0.40 | −0.16 | |
| Masculinity 1 | −0.86 | −0.82 | 0.83 | −0.11 | −0.12 | −0.33 | 0.13 | |
| Intensity 1 | −0.81 | −0.79 | 0.83 | −0.23 | −0.23 | −0.15 | 0.40 | |
| Pleasantness 2 | 0.20 | 0.20 | −0.11 | −0.23 | 0.96 | −0.52 | −0.87 | |
| Attractiveness 2 | 0.21 | 0.22 | −0.12 | −0.23 | 0.96 | −0.41 | −0.81 | |
| Masculinity 2 | 0.37 | 0.40 | −0.33 | −0.15 | −0.52 | −0.41 | 0.66 | |
| Intensity 2 | −0.17 | −0.16 | 0.13 | 0.40 | −0.87 | −0.81 | 0.66 |
Correlations in bold are significant at P < 0.05, N = 17. Means for odor donors served as unit of the analysis.
Correlation coefficients between rated variables in first (1) and second sessions (2)
| Pleasantness 1 | Attractiveness 1 | Masculinity 1 | Intensity 1 | Pleasantness 2 | Attractiveness 2 | Masculinity 2 | Intensity 2 | |
| Pleasantness 1 | 0.98 | −0.86 | −0.81 | 0.20 | 0.21 | 0.37 | −0.17 | |
| Attractiveness 1 | 0.98 | −0.82 | −0.79 | 0.20 | 0.22 | 0.40 | −0.16 | |
| Masculinity 1 | −0.86 | −0.82 | 0.83 | −0.11 | −0.12 | −0.33 | 0.13 | |
| Intensity 1 | −0.81 | −0.79 | 0.83 | −0.23 | −0.23 | −0.15 | 0.40 | |
| Pleasantness 2 | 0.20 | 0.20 | −0.11 | −0.23 | 0.96 | −0.52 | −0.87 | |
| Attractiveness 2 | 0.21 | 0.22 | −0.12 | −0.23 | 0.96 | −0.41 | −0.81 | |
| Masculinity 2 | 0.37 | 0.40 | −0.33 | −0.15 | −0.52 | −0.41 | 0.66 | |
| Intensity 2 | −0.17 | −0.16 | 0.13 | 0.40 | −0.87 | −0.81 | 0.66 |
| Pleasantness 1 | Attractiveness 1 | Masculinity 1 | Intensity 1 | Pleasantness 2 | Attractiveness 2 | Masculinity 2 | Intensity 2 | |
| Pleasantness 1 | 0.98 | −0.86 | −0.81 | 0.20 | 0.21 | 0.37 | −0.17 | |
| Attractiveness 1 | 0.98 | −0.82 | −0.79 | 0.20 | 0.22 | 0.40 | −0.16 | |
| Masculinity 1 | −0.86 | −0.82 | 0.83 | −0.11 | −0.12 | −0.33 | 0.13 | |
| Intensity 1 | −0.81 | −0.79 | 0.83 | −0.23 | −0.23 | −0.15 | 0.40 | |
| Pleasantness 2 | 0.20 | 0.20 | −0.11 | −0.23 | 0.96 | −0.52 | −0.87 | |
| Attractiveness 2 | 0.21 | 0.22 | −0.12 | −0.23 | 0.96 | −0.41 | −0.81 | |
| Masculinity 2 | 0.37 | 0.40 | −0.33 | −0.15 | −0.52 | −0.41 | 0.66 | |
| Intensity 2 | −0.17 | −0.16 | 0.13 | 0.40 | −0.87 | −0.81 | 0.66 |
Correlations in bold are significant at P < 0.05, N = 17. Means for odor donors served as unit of the analysis.
We also analyzed self-rated levels of stress, fatigue, and general mood, each of which may potentially influence body odor. Each donor wrote down his mood every day when on the prescribed diet (i.e., over 14 days). We computed mean values for each odor donor and compared them between the diets. Results of repeated measures ANOVA showed no difference in stress, fatigue, and mood (all P > 0.5).
Discussion
The results of this study show for the first time that red meat consumption may have a perceivable impact on axillary body odor. Odors of donors on the nonmeat diet were judged as more pleasant, more attractive, and less intense. This pattern was not influenced by raters' menstrual cycle phase or partnership status.
The number of odor donors was relatively small (17 men). However, the nature of the experiment was balanced and within subject. In other words, each rater assessed some donors first in meat condition and some donors first in nonmeat. Moreover, the diet we prescribed to our odor donors was identical except the meat content (e.g., fried pork steak with potatoes, fried Edam cheese with potatoes). This gives us high confidence that our results are due to the studied effect (i.e., meat) and not due to some other variable (e.g., day of the testing). Theoretically, our diet may have influenced the mood of the donors, which may in turn have an impact on quality of body odor (Chen and Haviland-Jones 2000; Ackerl et al. 2002). However, this is probably not the case as we did not find differences in self-rated stress, fatigue, and mood between meat and nonmeat conditions. The reliability of the results is also supported by the fact that mean ratings across the 2 sessions were not significantly different and that there was no change in masculinity rating across the experimental conditions. The relative stability of odor femininity–masculinity was found previously in a sample of women donors (Havlicek et al. 2006).
Potentially, our findings may be a result of different energetic (protein) values. In other words, our diet only differed in meat content and therefore in energetic value. Thus, this factor can be responsible for the effect, not the meat per se. However, if this would be the case, one may expect diet of higher energetic value (i.e., meat diet) to be more attractive as was found in the bank vole study (Ferkin et al. 1997). Our results show higher attractiveness in the nonmeat diet, and we therefore believe that it is caused by the meat effect specifically.
We found a significant positive correlation between odor intensity and masculinity. This phenomenon is consistent with earlier findings on gender discrimination by smell. Doty and colleagues showed that more intense smells are usually judged to be masculine (Doty et al. 1978, 1982). The negative correlation between the rating of pleasantness or attractiveness and subjectively perceived intensity was also observed in earlier studies (Havlicek et al. 2006). One may argue that the higher attractiveness of donors on the nonmeat diet is due to quantitative rather than qualitative changes in their axillary odor. In our view, however, meat consumption changes the composition of some axillary chemicals (see in the second paragraph below), and therefore, we believe that both changes of quantitative and qualitative nature are responsible for the observed effect.
At this point, it is not possible to say how long the meat content in food remains perceptible in axillary odor. Nor do we know how long one must consume meat to produce discernible changes in body odor. Moreover, we can also only speculate about the already perceptible amount of meat consumed. The 2 weeks' diet period we chose for our study was based on results of an analytical study which found compounds of environmental origin in axillary odor after 10 days of hygienic and diet restrictions (Labows et al. 1979). Future studies should address the question of diet duration or quantity of meat consumption and changes in body odor. Further, it should also be tested whether the observed effect is restricted to “red” meat or may be applied also to “white” meat (i.e., poultry and fish).
Current knowledge allows us only to speculate what particular compounds and metabolic processes are responsible for hedonic changes in body odor after the meat consumption. We propose that it could be due to changes in amount and/or relative abundance of aliphatic acids. The axillary region contains abundant numbers of apocrine glands producing milky secretions. Fresh apocrine secretion is odorless but is rapidly converted by axillary microflora to odorous breakdown products. Of particular interest are corynobacteria A as they metabolize fatty acids to short aliphatic acids (James et al. 2004). Chromatographic examination of axillary sweat found a number of both saturated and unsaturated and branched and nonbranched aliphatic acids particularly of C5–C11 length (Zeng et al. 1991). If this is the case, we may expect a correlation between the change in the odor and fat proportion in meat.
Factors influencing axillary odor composition are of a very complex nature. Odor similarity of twins (Wallace 1977) and other relatives suggests that odor individuality (i.e., odor signature) is to some extent under genetic control. Recently it was found that human raters are able to match monozygotic twins but not dizygotic twins who lived apart in a proportion higher than chance (Roberts et al. 2005). Influence of genetic factors is also supported by MHC-related odor preferences (Wedekind et al. 1995; Wedekind and Füri 1997). Environmental factors putatively shaping odor signature are numerous and include eating habits, smoking, using drugs, medicals, some diseases, and infections. Of course, we should not forget that humans also purposely shape their olfactory nature by using cosmetics, perfumes etc. We suggest that the main nongenetic source of axillary odor variation in a healthy human is due to differences in diet. Our opinion is supported by a study which showed that hand odor of twins on different diet was correctly matched; however, this was not true for twins on the same diet (Wallace 1977). Hepper (1988) found that dogs can discriminate between adult monozygotic twins on different diets. On the other hand, the same dogs were not able to distinguish the odor of monozygotic sucklings on the same diet. The diet effect has also been observed in nonhuman animals, for example, in guinea pigs or meadow voles (Beauchamp 1976; Ferkin et al. 1997). Possible effects of some food ingredients were suggested by odor researchers. In most studies, food is controlled to avoid its putative confounding effect. In particular, avoidance of meals containing garlic, onion, chilli, pepper, vinegar, blue cheese, cabbage, radish, fermented milk products, and marinated fish is commonly recommended (e.g., Wedekind and Füri 1997; Chen and Haviland-Jones 2000; Rikowski and Grammer 1999; Platek et al. 2001; Thornhill et al. 2003; Havlicek et al. 2006). In most cases, our knowledge of the above-mentioned variables is based on subjective experience or anecdotal accounts rather than on controlled studies. In fact, we know very little about the effect of particular components and even less about their interactions. Consumption of “red meat,” which may have an impact on body odor, was not controlled in former studies. Thus, we recommend for future studies to control or at least to check whether experimental groups do not systematically differ in the amount of meat consumption. Our results extend our knowledge of how environmental factors influence the body odor.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org.
We thank to all volunteers for their participation in the study, Anna Kotrcova for help with experimental design arrangement and manuscript corrections, Dagmar Kohoutova for helping with data collection, and Jaroslav Flegr, Jitka Hanusova, Jindra Havlickova, Barbara Husarova, Vera Pivonkova, Craig Roberts, and 2 anonymous referees for valuable advices and language corrections. We are also grateful to Optimum Distribution CZ&SK company for providing perfume testers for us. The study was supported by the GAUK 393/2005 grant. The authors declare that they have no competing interest and both are nonvegetarians.