-
PDF
- Split View
-
Views
-
Cite
Cite
Sridhar V Basavaraju, Monica E Patton, Kacie Grimm, Mohammed Ata Ur Rasheed, Sandra Lester, Lisa Mills, Megan Stumpf, Brandi Freeman, Azaibi Tamin, Jennifer Harcourt, Jarad Schiffer, Vera Semenova, Han Li, Bailey Alston, Muyiwa Ategbole, Shanna Bolcen, Darbi Boulay, Peter Browning, Li Cronin, Ebenezer David, Rita Desai, Monica Epperson, Yamini Gorantla, Tao Jia, Panagiotis Maniatis, Kimberly Moss, Kristina Ortiz, So Hee Park, Palak Patel, Yunlong Qin, Evelene Steward-Clark, Heather Tatum, Andrew Vogan, Briana Zellner, Jan Drobeniuc, Matthew R P Sapiano, Fiona Havers, Carrie Reed, Susan Gerber, Natalie J Thornburg, Susan L Stramer, Serologic Testing of US Blood Donations to Identify Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–Reactive Antibodies: December 2019–January 2020, Clinical Infectious Diseases, Volume 72, Issue 12, 15 June 2021, Pages e1004–e1009, https://doi.org/10.1093/cid/ciaa1785
Close - Share Icon Share
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), was first identified in Wuhan, China, in December 2019, with subsequent worldwide spread. The first US cases were identified in January 2020.
To determine if SARS-CoV-2–reactive antibodies were present in sera prior to the first identified case in the United States on 19 January 2020, residual archived samples from 7389 routine blood donations collected by the American Red Cross from 13 December 2019 to 17 January 2020 from donors resident in 9 states (California, Connecticut, Iowa, Massachusetts, Michigan, Oregon, Rhode Island, Washington, and Wisconsin) were tested at the Centers for Disease Control and Prevention for anti–SARS-CoV-2 antibodies. Specimens reactive by pan-immunoglobulin (pan-Ig) enzyme-linked immunosorbent assay (ELISA) against the full spike protein were tested by IgG and IgM ELISAs, microneutralization test, Ortho total Ig S1 ELISA, and receptor-binding domain/ACE2 blocking activity assay.
Of the 7389 samples, 106 were reactive by pan-Ig. Of these 106 specimens, 90 were available for further testing. Eighty-four of 90 had neutralizing activity, 1 had S1 binding activity, and 1 had receptor-binding domain/ACE2 blocking activity >50%, suggesting the presence of anti–SARS-CoV-2–reactive antibodies. Donations with reactivity occurred in all 9 states.
These findings suggest that SARS-CoV-2 may have been introduced into the United States prior to 19 January 2020.
(See the Major Article by Reese et al on pages e1010–7 and the Editorial Commentary by Rosenberg and Bradley on pages e1018–20)
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes the novel coronavirus disease 2019 (COVID-19), was first identified in Wuhan, China, with notification to the World Health Organization on 31 December 2019, about a cluster of pneumonia cases of unknown etiology and release of the genomic sequence on 10 January 2020 [1]. Subsequent reports have identified a patient with confirmed SARS-CoV-2 hospitalized in Wuhan with symptom onset as early as 1 December 2019 [2]. In the United States, the first COVID-19 infection was reported on 19 January 2020 in a returned traveler from China, 2 days after domestic testing was initiated [3]. While the first confirmed case had a symptom-onset date of 19 January 2020, 2 others within the first 12 US cases identified had illness-onset dates of 14 January 2020 [4]. Some reports have suggested the introduction of SARS-CoV-2 into the United States may have occurred earlier than initially recognized, although widespread community transmission was not likely until late February [5–7].
Simulation models used to predict COVID-19 case burden, subsequent healthcare utilization, and fatalities are reliant on accurately assessing date(s) of introduction of a pathogen into susceptible populations [8]. A number of strategies have been used to estimate the introduction of SARS-CoV-2, including retrospective molecular testing of clinical respiratory samples, nucleic acid testing (NAT), and, in some circumstances, phylogenetic analyses [6, 9–12]. Early phylogenetic analyses suggest that SARS-CoV-2 may have evolved between October and December 2019 [9–11]. While the first recorded COVID-19 case outside of China was identified in Thailand on 13 January 2020 [13], retrospective NAT identified a respiratory specimen with molecular evidence of SARS-CoV-2 from a patient hospitalized in France on 27 December 2019 [12]. Similarly, in the United States, retrospective NAT of archived respiratory samples in the Seattle region have suggested introduction of SARS-CoV-2 virus into the Seattle, Washington, area between 18 January and 9 February 2020 [6].
Serologic testing has been previously used to estimate the introduction of viral infections into populations, including for human immunodeficiency virus (HIV) [14]. Retrospective serologic testing may augment results obtained from testing archived respiratory specimens with molecular methods when trying to identify the introduction of SARS-CoV-2 into a population. For several reasons, infections may not be fully captured by surveillance conducted using respiratory specimens collected from symptomatic people in healthcare settings. Patients infected with SARS-CoV-2 may not seek medical care because infections could be mild or asymptomatic [15]. For those with symptomatic infections who may have sought medical care before SARS-CoV-2 was known to be circulating in the United States, clinical samples may not have been collected and therefore respiratory virus testing may not have been performed; even fewer specimens would likely be archived and available for retrospective molecular testing. To determine whether serologic testing can provide further insight into SARS-CoV-2 introduction into the United States, US blood donation specimens from an existing repository collected by the American Red Cross from 13 December 2019 to 17 January 2020 were sent to the Centers for Disease Control and Prevention (CDC) for retrospective testing for SARS-CoV-2–reactive antibodies. Implications for future SARS-CoV-2 seroprevalence surveys are discussed.
METHODS
Ethical Considerations
The study was approved by the American Red Cross Institutional Review Board. Data for this report were collected as part of public health emergency response and determined by the CDC Office of the Associate Director for Science to not require additional CDC Institutional Review Board review. All blood donations were de-identified prior to shipment to CDC and subsequent testing.
Blood Donor Sample Description
Whole blood or blood products intended for transfusion are collected from volunteer donors in either fixed collection sites or as part of mobile collection drives. All blood donors are subjected to medical and social history questionnaires to ascertain risk factors associated with transfusion-transmissible infectious diseases, such as HIV [16]. Donors are questioned regarding travel outside of the United States and are deferred for travel to malaria-affected areas [16]. SARS-CoV-2 risk-based donor deferral for travel to China was not implemented until February 2020 [17]. As part of the donation evaluation, donors undergo a basic physical examination, which includes temperature, blood pressure, and heart rate measurements. Persons presenting to donate blood with signs or symptoms consistent with bacterial or viral respiratory infections, including influenza, are deferred and instructed to return for donation once symptoms have resolved. Serum specimens from all blood donations are tested for infectious-disease markers as required by the Food and Drug Administration [18].
Archived, residual serum specimens from routine donations collected by the American Red Cross from 13 December 2019 to 17 January 2020 from donors resident in California, Connecticut, Iowa, Massachusetts, Michigan, Oregon, Rhode Island, Washington, and Wisconsin were sent to CDC (Atlanta, Georgia) for additional testing (n = 7389). All donations collected during this period, for which residual serum specimens were available, were included in this study. These specimens were previously archived for potential future studies to identify emerging transfusion-transmissible infections but were re-purposed for the present study.
Laboratory Methods
Once at CDC, sera were screened using a pan-immunoglobulin (pan-Ig) enzyme-linked immunosorbent assay (ELISA) against the pre-fusion stabilized ectodomain of the spike protein (S) that includes both S1 and S2 domains [19, 20]. To ensure high-throughput screening capability, initial screening did not include background correction. Initial reactive specimens, defined as having an optical density (OD) of 0.5 or greater in the screening ELISA (tested at a 1:100 dilution), were then confirmed by reflex testing at 1:100 and 1:400 dilutions using the same ELISA with background correction. Specimens were considered confirmed reactive if there was a signal to threshold ratio of 1 or greater at a background-corrected OD of 0.4. At a background-corrected OD of 0.4 with serum diluted 1:100, specificity of this assay is 99.3% (95% confidence interval, 98.32–99.88%) and sensitivity is 96% (95% confidence interval, 89.98–98.89%) [20]. When paired sera from polymerase chain reaction (PCR)–confirmed infections with other common coronaviruses were tested, 4 of 42 exhibited increasing signal between the acute and convalescent timepoints, but all were below the assay cutoff [20]. Isotype-specific tests were performed using the same ELISA technique, but with IgG- or IgM-specific secondary antibody (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
Confirmed-reactive specimens were further tested using a microneutralization test with live SARS-CoV-2 USA-WA1/2020 [21], SARS-CoV-1 S1 pan-Ig ELISA (Ortho Clinical Diagnostics, Raritan, NJ), and a surrogate neutralization assay that measures the ability of sera to block the interaction between the S receptor-binding domain (RBD) and the cellular receptor ACE2 (Genscript). For microneutralization, sera were serially diluted 2-fold between 1:20 and 1:640, incubated with virus for 30 minutes at 37°C, and used to inoculate Vero CCL-81 cells. After 5 days, cells were fixed and stained with formalin–crystal violet to observe live/dead cells. The highest dilution at which sera blocked viral infection was determined to be the neutralizing titer, with more than 40 designated as positive. For the Ortho ELISA and surrogate neutralization assays, the manufacturers’ instructions were followed.
Statistical Analyses
Descriptive analyses were performed to stratify reactive donations by state of residence, date of collection, and donor age and sex. Analysis was performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC). As these donations represent a convenience sample, additional tests to ascertain statistical significance or extrapolate findings to a broader population were not performed.
RESULTS
Serum specimens were sent to CDC for anti–SARS-CoV-2 testing from 7389 unique donations (Table 1). Of these, 106 (1.4%) were confirmed reactive by the pan-Ig S ELISA screen and then a confirmatory assay with background correction (Table 1). These confirmed-reactive sera included 39 of 1912 (2.0%) donations collected between 13 and 16 December 2019 from residents of California (23/1912) and Oregon or Washington (16/1912). Sixty-seven confirmed-reactive (67/5477, 1.2%) donations were collected between 30 December 2019 and 17 January 2020 from residents of Massachusetts (18/5477), Wisconsin or Iowa (22/5477), Michigan (5/5477), and Connecticut or Rhode Island (33/5477). During validation of the assay, 3 of 519 true-negative sera had reactivities above the signal: threshold cutoff of 1 ranging from 1.46 to 2.11. These true-negative sera were collected from healthy adults between 2016 and 2019 (n = 377), patients with suspected Hantavirus between 2016 and 2019 (n = 101), HIV-positive individuals between 2011 and 2012 (n = 10), hepatitis B–positive individuals between 2011 and 2012 (n = 10), or hepatitis C–positive individuals between 2011 and 2012 (n = 10). True positive sera from 99 patients with PCR-confirmed COVID-19 collected more than 10 days post–symptom onset ranged from 0.11 to 6.99, with a median of 6.10 and a standard deviation of 1.91 [20]. Of the 106 confirmed-reactive sera, 67 had threshold cutoffs between 1.0 and 2.11, which is within the same range of true-negative sera that were above the assay cutoff. In contrast, 32 and 4 had threshold cutoffs of 2.12–4.08 and greater than 4.30, respectively, well above the true-negative sera that tested above cutoff limits.
Total Number of Samples Tested, Number of Samples That Were Reactive, Number of Samples With Positive Microneutralization and Surrogate Neutralization, and Number of Samples That Were Reactive for the S1 Ortho Test
| . | No. tested . | No. Reactive (% of Tested) . | No. Reactive With Further Testing (% of Tested) . | No. Reactive With Positive Microneutralization (% of Tested) . | No. With Surrogate Neutralization (% of Reactive With Positive Microneutralization) . | No. S1 Reactive (Ortho) . |
|---|---|---|---|---|---|---|
| All specimens | 7389 | 106 (1.4) | 90 (1.2) | 84 (1.1) | 23 (27.4) | 1 |
| All specimens from 13–16 December 2019 | 1912 | 39 (2.0) | 39 (2.0) | 37 (1.9) | 9 (24.3) | 1 |
| American Red Cross Blood Services region | ||||||
| Northern California (CA) | 508 | 12 (2.4) | 12 (2.4) | 11 (2.2) | 7 (63.6) | 1 |
| Pacific Northwest (OR, WA) | 763 | 16 (2.1) | 16 (2.1) | 15 (2.0) | 1 (6.7) | 0 |
| Southern California (CA) | 641 | 11 (1.7) | 11 (1.7) | 11 (1.7) | 1 (9.1) | 0 |
| Donor sex | ||||||
| Female | 859 | 12 (1.4) | 12 (1.4) | 11 (1.3) | 1 (9.1) | 0 |
| Male | 1053 | 27 (2.6) | 27 (2.6) | 26 (2.5) | 8 (30.8) | 1 |
| Donor age group | ||||||
| 16–29 y | 254 | 3 (1.2) | 3 (1.2) | 2 (0.8) | 2 (100.0) | 1 |
| 30–39 y | 298 | 3 (1.0) | 3 (1.0) | 3 (1.0) | 1 (33.3) | 0 |
| 40–49 y | 291 | 6 (2.1) | 6 (2.1) | 6 (2.1) | 1 (16.7) | 0 |
| 50–59 y | 397 | 9 (2.3) | 9 (2.3) | 8 (2.0) | 2 (25.0) | 0 |
| 60–69 y | 483 | 14 (2.9) | 14 (2.9) | 14 (2.9) | 3 (21.4) | 0 |
| 70 y or older | 189 | 4 (2.1) | 4 (2.1) | 4 (2.1) | 0 (0.0) | 0 |
| All specimens from 30 December 2019–17 January 2020 | 5477 | 67 (1.2) | 51 (0.9) | 47 (0.9) | 14 (29.8) | 0 |
| American Red Cross Blood Services region | ||||||
| New England (MA) | 1963 | 18 (0.9) | 11 (0.6) | 11 (0.6) | 1 (9.1) | 0 |
| Badger-Hawkeye (WI, IA) | 1556 | 22 (1.4) | 17 (1.1) | 16 (1.0) | 6 (37.5) | 0 |
| Great Lakes (MI) | 416 | 5 (1.2) | 5 (1.2) | 3 (0.7) | 0 (0.0) | 0 |
| Connecticut (CT, RI) | 1542 | 22 (1.4) | 18 (1.2) | 17 (1.1) | 7 (41.2) | 0 |
| Donor sex | ||||||
| Female | 2541 | 23 (0.9) | 19 (0.7) | 16 (0.6) | 6 (37.5) | 0 |
| Male | 2936 | 44 (1.5) | 32 (1.1) | 31 (1.1) | 8 (25.8) | 0 |
| Donor age group | ||||||
| 16–29 y | 641 | 7 (1.1) | 4 (0.6) | 3 (0.5) | 2 (66.7) | 0 |
| 30–39 y | 587 | 9 (1.5) | 8 (1.4) | 8 (1.4) | 3 (37.5) | 0 |
| 40–49 y | 779 | 11 (1.4) | 9 (1.2) | 9 (1.2) | 1 (11.1) | 0 |
| 50–59 y | 1447 | 15 (1.0) | 11 (0.8) | 9 (0.6) | 3 (33.3) | 0 |
| 60–69 y | 1410 | 16 (1.1) | 12 (0.9) | 11 (0.8) | 3 (27.3) | 0 |
| 70 y or older | 613 | 9 (1.5) | 7 (1.1) | 7 (1.1) | 2 (28.6) | 0 |
| . | No. tested . | No. Reactive (% of Tested) . | No. Reactive With Further Testing (% of Tested) . | No. Reactive With Positive Microneutralization (% of Tested) . | No. With Surrogate Neutralization (% of Reactive With Positive Microneutralization) . | No. S1 Reactive (Ortho) . |
|---|---|---|---|---|---|---|
| All specimens | 7389 | 106 (1.4) | 90 (1.2) | 84 (1.1) | 23 (27.4) | 1 |
| All specimens from 13–16 December 2019 | 1912 | 39 (2.0) | 39 (2.0) | 37 (1.9) | 9 (24.3) | 1 |
| American Red Cross Blood Services region | ||||||
| Northern California (CA) | 508 | 12 (2.4) | 12 (2.4) | 11 (2.2) | 7 (63.6) | 1 |
| Pacific Northwest (OR, WA) | 763 | 16 (2.1) | 16 (2.1) | 15 (2.0) | 1 (6.7) | 0 |
| Southern California (CA) | 641 | 11 (1.7) | 11 (1.7) | 11 (1.7) | 1 (9.1) | 0 |
| Donor sex | ||||||
| Female | 859 | 12 (1.4) | 12 (1.4) | 11 (1.3) | 1 (9.1) | 0 |
| Male | 1053 | 27 (2.6) | 27 (2.6) | 26 (2.5) | 8 (30.8) | 1 |
| Donor age group | ||||||
| 16–29 y | 254 | 3 (1.2) | 3 (1.2) | 2 (0.8) | 2 (100.0) | 1 |
| 30–39 y | 298 | 3 (1.0) | 3 (1.0) | 3 (1.0) | 1 (33.3) | 0 |
| 40–49 y | 291 | 6 (2.1) | 6 (2.1) | 6 (2.1) | 1 (16.7) | 0 |
| 50–59 y | 397 | 9 (2.3) | 9 (2.3) | 8 (2.0) | 2 (25.0) | 0 |
| 60–69 y | 483 | 14 (2.9) | 14 (2.9) | 14 (2.9) | 3 (21.4) | 0 |
| 70 y or older | 189 | 4 (2.1) | 4 (2.1) | 4 (2.1) | 0 (0.0) | 0 |
| All specimens from 30 December 2019–17 January 2020 | 5477 | 67 (1.2) | 51 (0.9) | 47 (0.9) | 14 (29.8) | 0 |
| American Red Cross Blood Services region | ||||||
| New England (MA) | 1963 | 18 (0.9) | 11 (0.6) | 11 (0.6) | 1 (9.1) | 0 |
| Badger-Hawkeye (WI, IA) | 1556 | 22 (1.4) | 17 (1.1) | 16 (1.0) | 6 (37.5) | 0 |
| Great Lakes (MI) | 416 | 5 (1.2) | 5 (1.2) | 3 (0.7) | 0 (0.0) | 0 |
| Connecticut (CT, RI) | 1542 | 22 (1.4) | 18 (1.2) | 17 (1.1) | 7 (41.2) | 0 |
| Donor sex | ||||||
| Female | 2541 | 23 (0.9) | 19 (0.7) | 16 (0.6) | 6 (37.5) | 0 |
| Male | 2936 | 44 (1.5) | 32 (1.1) | 31 (1.1) | 8 (25.8) | 0 |
| Donor age group | ||||||
| 16–29 y | 641 | 7 (1.1) | 4 (0.6) | 3 (0.5) | 2 (66.7) | 0 |
| 30–39 y | 587 | 9 (1.5) | 8 (1.4) | 8 (1.4) | 3 (37.5) | 0 |
| 40–49 y | 779 | 11 (1.4) | 9 (1.2) | 9 (1.2) | 1 (11.1) | 0 |
| 50–59 y | 1447 | 15 (1.0) | 11 (0.8) | 9 (0.6) | 3 (33.3) | 0 |
| 60–69 y | 1410 | 16 (1.1) | 12 (0.9) | 11 (0.8) | 3 (27.3) | 0 |
| 70 y or older | 613 | 9 (1.5) | 7 (1.1) | 7 (1.1) | 2 (28.6) | 0 |
Specimens collected between 13th and 16th December 2019 and those collected between 30th December 2019 and 17th January 2020 are summarized separately.
Total Number of Samples Tested, Number of Samples That Were Reactive, Number of Samples With Positive Microneutralization and Surrogate Neutralization, and Number of Samples That Were Reactive for the S1 Ortho Test
| . | No. tested . | No. Reactive (% of Tested) . | No. Reactive With Further Testing (% of Tested) . | No. Reactive With Positive Microneutralization (% of Tested) . | No. With Surrogate Neutralization (% of Reactive With Positive Microneutralization) . | No. S1 Reactive (Ortho) . |
|---|---|---|---|---|---|---|
| All specimens | 7389 | 106 (1.4) | 90 (1.2) | 84 (1.1) | 23 (27.4) | 1 |
| All specimens from 13–16 December 2019 | 1912 | 39 (2.0) | 39 (2.0) | 37 (1.9) | 9 (24.3) | 1 |
| American Red Cross Blood Services region | ||||||
| Northern California (CA) | 508 | 12 (2.4) | 12 (2.4) | 11 (2.2) | 7 (63.6) | 1 |
| Pacific Northwest (OR, WA) | 763 | 16 (2.1) | 16 (2.1) | 15 (2.0) | 1 (6.7) | 0 |
| Southern California (CA) | 641 | 11 (1.7) | 11 (1.7) | 11 (1.7) | 1 (9.1) | 0 |
| Donor sex | ||||||
| Female | 859 | 12 (1.4) | 12 (1.4) | 11 (1.3) | 1 (9.1) | 0 |
| Male | 1053 | 27 (2.6) | 27 (2.6) | 26 (2.5) | 8 (30.8) | 1 |
| Donor age group | ||||||
| 16–29 y | 254 | 3 (1.2) | 3 (1.2) | 2 (0.8) | 2 (100.0) | 1 |
| 30–39 y | 298 | 3 (1.0) | 3 (1.0) | 3 (1.0) | 1 (33.3) | 0 |
| 40–49 y | 291 | 6 (2.1) | 6 (2.1) | 6 (2.1) | 1 (16.7) | 0 |
| 50–59 y | 397 | 9 (2.3) | 9 (2.3) | 8 (2.0) | 2 (25.0) | 0 |
| 60–69 y | 483 | 14 (2.9) | 14 (2.9) | 14 (2.9) | 3 (21.4) | 0 |
| 70 y or older | 189 | 4 (2.1) | 4 (2.1) | 4 (2.1) | 0 (0.0) | 0 |
| All specimens from 30 December 2019–17 January 2020 | 5477 | 67 (1.2) | 51 (0.9) | 47 (0.9) | 14 (29.8) | 0 |
| American Red Cross Blood Services region | ||||||
| New England (MA) | 1963 | 18 (0.9) | 11 (0.6) | 11 (0.6) | 1 (9.1) | 0 |
| Badger-Hawkeye (WI, IA) | 1556 | 22 (1.4) | 17 (1.1) | 16 (1.0) | 6 (37.5) | 0 |
| Great Lakes (MI) | 416 | 5 (1.2) | 5 (1.2) | 3 (0.7) | 0 (0.0) | 0 |
| Connecticut (CT, RI) | 1542 | 22 (1.4) | 18 (1.2) | 17 (1.1) | 7 (41.2) | 0 |
| Donor sex | ||||||
| Female | 2541 | 23 (0.9) | 19 (0.7) | 16 (0.6) | 6 (37.5) | 0 |
| Male | 2936 | 44 (1.5) | 32 (1.1) | 31 (1.1) | 8 (25.8) | 0 |
| Donor age group | ||||||
| 16–29 y | 641 | 7 (1.1) | 4 (0.6) | 3 (0.5) | 2 (66.7) | 0 |
| 30–39 y | 587 | 9 (1.5) | 8 (1.4) | 8 (1.4) | 3 (37.5) | 0 |
| 40–49 y | 779 | 11 (1.4) | 9 (1.2) | 9 (1.2) | 1 (11.1) | 0 |
| 50–59 y | 1447 | 15 (1.0) | 11 (0.8) | 9 (0.6) | 3 (33.3) | 0 |
| 60–69 y | 1410 | 16 (1.1) | 12 (0.9) | 11 (0.8) | 3 (27.3) | 0 |
| 70 y or older | 613 | 9 (1.5) | 7 (1.1) | 7 (1.1) | 2 (28.6) | 0 |
| . | No. tested . | No. Reactive (% of Tested) . | No. Reactive With Further Testing (% of Tested) . | No. Reactive With Positive Microneutralization (% of Tested) . | No. With Surrogate Neutralization (% of Reactive With Positive Microneutralization) . | No. S1 Reactive (Ortho) . |
|---|---|---|---|---|---|---|
| All specimens | 7389 | 106 (1.4) | 90 (1.2) | 84 (1.1) | 23 (27.4) | 1 |
| All specimens from 13–16 December 2019 | 1912 | 39 (2.0) | 39 (2.0) | 37 (1.9) | 9 (24.3) | 1 |
| American Red Cross Blood Services region | ||||||
| Northern California (CA) | 508 | 12 (2.4) | 12 (2.4) | 11 (2.2) | 7 (63.6) | 1 |
| Pacific Northwest (OR, WA) | 763 | 16 (2.1) | 16 (2.1) | 15 (2.0) | 1 (6.7) | 0 |
| Southern California (CA) | 641 | 11 (1.7) | 11 (1.7) | 11 (1.7) | 1 (9.1) | 0 |
| Donor sex | ||||||
| Female | 859 | 12 (1.4) | 12 (1.4) | 11 (1.3) | 1 (9.1) | 0 |
| Male | 1053 | 27 (2.6) | 27 (2.6) | 26 (2.5) | 8 (30.8) | 1 |
| Donor age group | ||||||
| 16–29 y | 254 | 3 (1.2) | 3 (1.2) | 2 (0.8) | 2 (100.0) | 1 |
| 30–39 y | 298 | 3 (1.0) | 3 (1.0) | 3 (1.0) | 1 (33.3) | 0 |
| 40–49 y | 291 | 6 (2.1) | 6 (2.1) | 6 (2.1) | 1 (16.7) | 0 |
| 50–59 y | 397 | 9 (2.3) | 9 (2.3) | 8 (2.0) | 2 (25.0) | 0 |
| 60–69 y | 483 | 14 (2.9) | 14 (2.9) | 14 (2.9) | 3 (21.4) | 0 |
| 70 y or older | 189 | 4 (2.1) | 4 (2.1) | 4 (2.1) | 0 (0.0) | 0 |
| All specimens from 30 December 2019–17 January 2020 | 5477 | 67 (1.2) | 51 (0.9) | 47 (0.9) | 14 (29.8) | 0 |
| American Red Cross Blood Services region | ||||||
| New England (MA) | 1963 | 18 (0.9) | 11 (0.6) | 11 (0.6) | 1 (9.1) | 0 |
| Badger-Hawkeye (WI, IA) | 1556 | 22 (1.4) | 17 (1.1) | 16 (1.0) | 6 (37.5) | 0 |
| Great Lakes (MI) | 416 | 5 (1.2) | 5 (1.2) | 3 (0.7) | 0 (0.0) | 0 |
| Connecticut (CT, RI) | 1542 | 22 (1.4) | 18 (1.2) | 17 (1.1) | 7 (41.2) | 0 |
| Donor sex | ||||||
| Female | 2541 | 23 (0.9) | 19 (0.7) | 16 (0.6) | 6 (37.5) | 0 |
| Male | 2936 | 44 (1.5) | 32 (1.1) | 31 (1.1) | 8 (25.8) | 0 |
| Donor age group | ||||||
| 16–29 y | 641 | 7 (1.1) | 4 (0.6) | 3 (0.5) | 2 (66.7) | 0 |
| 30–39 y | 587 | 9 (1.5) | 8 (1.4) | 8 (1.4) | 3 (37.5) | 0 |
| 40–49 y | 779 | 11 (1.4) | 9 (1.2) | 9 (1.2) | 1 (11.1) | 0 |
| 50–59 y | 1447 | 15 (1.0) | 11 (0.8) | 9 (0.6) | 3 (33.3) | 0 |
| 60–69 y | 1410 | 16 (1.1) | 12 (0.9) | 11 (0.8) | 3 (27.3) | 0 |
| 70 y or older | 613 | 9 (1.5) | 7 (1.1) | 7 (1.1) | 2 (28.6) | 0 |
Specimens collected between 13th and 16th December 2019 and those collected between 30th December 2019 and 17th January 2020 are summarized separately.
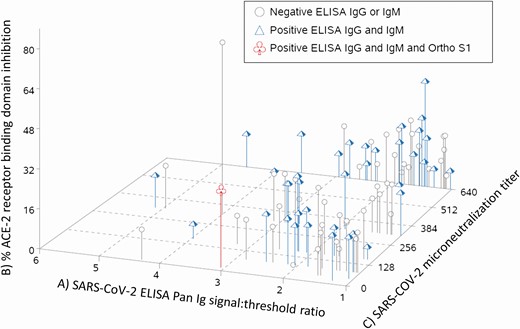
Of the 106 confirmed-reactive specimens, 90 were available for further testing. These sera were tested using isotype-specific spike protein ELISAs, Ortho pan-Ig S1 assay, microneutralization tests, and a surrogate neutralization assay that detects the ability of sera to block RBD binding to ACE2. Of the 90 sera tested by microneutralization, 84 had an endpoint titer greater than 40. When the anti–spike protein isotype responses were examined, 39 of the 90 had both S-reactive IgG and IgM (43.3%), 8 were IgM positive but IgG negative, 29 were IgG positive but IgM negative, and the remaining 14 were just positive using a pan-Ig secondary. By the Ortho S1 pan Ig assay, 1 reactive serum sample had a signal-to-threshold cutoff of 1.89 (with a retest of 1.10), and by surrogate neutralization 21 sera exhibited 20–30% inhibition, 1 at 45% inhibition, and 1 at 71% inhibition. When results in all tests were compared by individual specimen, there was not an obvious pattern of specimens with higher signals in ELISA, surrogate neutralization, or live virus microneutralization tests clustering together (Figure 1), indicating that each donation had a unique pattern of test results. These data might indicate that there is no clear delineation between potentially cross-reactive specimens and those that were obviously from SARS-CoV-2–infected individuals.
Combined results of confirmatory tests from 90 spike-reactive routine blood donations collected in 9 US states between 13 December 2019 and 17 January 2020. Each line indicates a single serum that was already confirmed to bind SARS-CoV-2 spike by ELISA. A, Signal-to-threshold ratios of anti-spike ELISA assay using a pan-Ig secondary antibody are shown on the x axis. A signal:threshold >1.0 is positive, and greater values indicate more reactivity. B, The y axis shows surrogate neutralization data. ACE-2 and spike receptor binding domain binding were assay in the presence and absence of sera. The percent inhibition was calculated by comparing the interaction with and without sera. C, Endpoint microneutralization titers are shown on the z axis. The number indicates the dilution at which serum blocked live virus–induced CPE in all 3 replicative wells. Higher numbers indicate more neutralizing activity. The shape and color of each line indicate isotype-specific spike ELISA results and results using Ortho Vitros total Ig S1 assay. The ELISAs were performed the same as the pan-Ig assay but isotype-specific secondary antibodies were used. Gray circles indicate that the serum was negative by Ortho Vitros total Ig, positive for either IgG or IgM, but not both. Blue triangles indicate negative by Ortho Vitros total Ig and positive for both IgG and IgM spike ELISA. The red cloverleaf indicates positive for all 3. Abbreviations: CPE, cytopathic effects; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The mean age of repeat reactive donors was 52 years (range, 16–95 years). More donations occurred among males than females (55.1% male specimens from 13–16 December 2019; 53.6% male specimens from 30 December 2019 and 17 January 2020). The proportion of reactive donations was higher among males than females among donations from 13–16 December 2019 (2.6% among males, 1.4% among females) and from 30 December 2019–17 January 2020 (1.1% among males, 0.7% among females). Among donations collected in California, Washington, and Oregon, the proportion of reactive donations was higher among donors aged 40 years or older.
DISCUSSION
These findings indicate that SARS-CoV-2–reactive antibodies were detected in 106 specimens, a small percentage of blood donations from California, Oregon, and Washington, as early as 13–16 December 2019. The presence of these serum antibodies indicate that isolated SARS-CoV-2 infections may have occurred in the western portion of the United States earlier than previously recognized or that a small portion of the population may have pre-existing antibodies that bind the SARS-CoV-2 S protein [3]. Similarly, antibodies to SARS-CoV-2 were identified among donations occurring in early January in Connecticut, Iowa, Massachusetts, Michigan, Rhode Island, and Wisconsin prior to known introduction of SARS-CoV-2 into those states.
A key question raised by these findings is whether the detection of reactive antibodies in these specimens from December and January indicates infections with SARS-CoV-2 in the US population earlier than currently recognized. As the COVID-19 epidemic has evolved, several serological assays for the detection of SARS-CoV-2 have become available to determine whether persons may have had previous infection. One recent report described cross-reactive serum antibody responses between SARS-CoV-2 and a small proportion of common human coronaviruses, particularly OC43, when using ELISA [22]. Neutralizing activity in sera from individuals with prior common human coronavirus infections has been described against SARS-CoV-2, specifically targeting the S2 portion of the S protein [23, 24]. The S2 subunit of the spike protein is more conserved across coronaviruses and thus may play a role in the cross-reactivity observed during ELISA testing when the whole S protein is used as an antigen [23]. The S2 region is involved in membrane fusion, and cross-neutralizing monoclonal antibodies from SARS-CoV-1 have been identified that bind S2 [25].
In order to better characterize the specimens that were reactive on the pan-Ig ELISA containing whole SARS-CoV-2 spike protein as the capture antigen, and distinguish these from cross-reactivity to common coronaviruses, additional, more specific SARS-CoV-2 testing was performed. The S1 subunit has been reported to be a more specific antigen for SARS-CoV-2 serologic diagnosis than the whole S protein [23]. Furthermore, in recent studies, sera from patients with confirmed human coronavirus infection only contained SARS-CoV-2 S protein–specific IgG antibodies and did not contain IgM or IgA antibodies; neutralizing activity in these sera was found to target only the S2 portion of the spike protein [23, 24]. Therefore, the presence of IgM or IgA antibodies and S1-specific binding activity may distinguish antibodies to SARS-CoV-2 from antibodies to human common coronaviruses [23, 24]. In the present study, 84 of 90 (>93%) reactive sera had neutralizing activity against SARS-CoV-2 virus, 39 (44.3%) had both IgG and IgM SARS-CoV-2 S protein–specific antibodies, 2 (2.2%) sera had surrogate neutralization activities, and 1 of 90 (1.1%) had SARS-CoV-2 S1-specific Ig. Collectively, these data suggest that at least some of the reactive blood donor sera could be due to prior SARS-CoV-2 infection. One serum sample, collected on 10 January 2020 in Connecticut, demonstrated a neutralization titer of 320, a signal-to-threshold ratio of 6.75, and 70% inhibition activity by surrogate neutralization activity, but was Ortho S1 nonreactive. These data indicate that this donation was likely from an individual with a past or active SARS-CoV-2 infection.
In addition to potential cross-reactivity with human common coronavirus infection other than SARS-CoV-2, the findings in this report are subject to the following limitations. First, none of the sera can be considered “true positives.” A true positive would only be collected from an individual with a positive molecular diagnostic test or paired acute–convalescent sera with rising titers [26, 27]. Second, the donations included in this report may not be representative of all blood donors or donations in these states and the findings may not be generalizable to all blood donors during the donation dates reported here. Therefore, population-based seroprevalence estimates or inference on magnitude of infections on a national or state level cannot be made. Third, if some of these samples indicate antibody responses from undetected SARS-CoV-2 infections, it cannot be determined whether these infections were community or travel associated. A previous survey of blood donors to understand travel practices determined that less than 3% of respondents reported travel outside of the United States within the 28 days prior to donation [28]. Of those reporting travel, only 5% traveled to Asia [28]. Fourth, even with a highly specific test, false positives may occur, particularly in low-prevalence areas [29]. However, the number of reactive specimens identified in this study was higher than expected given the specificity of the pan-Ig spike ELISA. Furthermore, additional evidence, including microneutralization, detection of both SARS-CoV-2–specific IgG and IgM, and SARS-CoV-2 S1-specific Ig reactivity, makes it very unlikely that all reactive specimens represent false positives. Further studies involving retrospective analyses of human specimens with molecular or serologic methods are necessary to further corroborate the present findings, which suggest the presence of specific antibodies to SARS-CoV-2 in the United States as early as mid-December 2019.
The findings of this report suggest that SARS-CoV-2 infections may have been present in the United States in December 2019, earlier than previously recognized. These findings also highlight the value of blood donations as a source for conducting SARS-CoV-2 surveillance studies. Data from US blood donation screening have been previously used for population-based incidence and prevalence monitoring during infectious-disease outbreaks, most recently the Zika virus epidemic [30]. The CDC is continuing to work with federal and nongovernmental partners to conduct ongoing surveillance using blood donations and clinical laboratory samples for SARS-CoV-2 infection in multiple sites across the United States. Understanding the dynamics of the SARS-CoV-2 pandemic from early introduction throughout further progression will advance understanding of the epidemiology of this novel virus and inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality associated with COVID-19.
Notes
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
Financial support. This work was conducted as part of US government work with no external funding source.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.





Comments