-
PDF
- Split View
-
Views
-
Cite
Cite
Hannah M Rickman, Tommy Rampling, Karen Shaw, Gema Martinez-Garcia, Leila Hail, Pietro Coen, Maryam Shahmanesh, Gee Yen Shin, Eleni Nastouli, Catherine F Houlihan, Nosocomial Transmission of Coronavirus Disease 2019: A Retrospective Study of 66 Hospital-acquired Cases in a London Teaching Hospital, Clinical Infectious Diseases, Volume 72, Issue 4, 15 February 2021, Pages 690–693, https://doi.org/10.1093/cid/ciaa816
Close - Share Icon Share
Abstract
Coronavirus disease 2019 (COVID-19) can cause deadly healthcare-associated outbreaks. In a major London teaching hospital, 66 of 435 (15%) COVID-19 inpatient cases between 2 March and 12 April 2020 were definitely or probably hospital-acquired, through varied transmission routes. The case fatality was 36%. Nosocomial infection rates fell following comprehensive infection prevention and control measures.
(See the Editorial Commentary by Curry and Salgado on pages 694–6.)
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses unique challenges for infection prevention and control (IPC) within healthcare facilities [1]. Transmission may occur via droplet, fomite, and (following aerosol-generating procedures) airborne routes [2–5] and from individuals who are asymptomatic or yet to develop symptoms [3, 6]. Healthcare users are more likely to be elderly with comorbidities, and therefore particularly vulnerable to severe COVID-19 [7]. Furthermore, efforts to prevent hospital-acquired infection are undertaken in a context of rapidly evolving knowledge and unprecedented demands on healthcare services.
In most hospitals in the United Kingdom, initial IPC responses to COVID-19 followed paradigms established for other respiratory viruses: identification of symptomatic cases meeting a clinical case definition, SARS-CoV-2 real-time polymerase chain reaction (PCR) testing of upper respiratory samples, and isolation or cohorting with enhanced IPC precautions. Most who did not meet the case definition were managed as usual in shared bays of up to 6 patients. However, given the median incubation period of 5 days [8, 9], and high transmissibility before and at the time of symptom onset [3, 6], this strategy may be insufficient to prevent nosocomial transmission.
COVID-19 outbreaks have been reported in varied healthcare settings [6, 7, 10–12]. Of 138 hospitalized COVID-19 cases in Wuhan, 12% were originally admitted for other reasons and were presumed to have acquired COVID-19 in hospital [13]. Of these, 53% required intensive care compared to 22% in the rest of the cohort, suggesting that this group may be particularly susceptible to adverse outcomes.
In March–April 2020, our central London teaching hospital experienced many cases of COVID-19, including apparently hospital-acquired infections. We therefore performed a retrospective analysis to describe the epidemiological and clinical characteristics of hospital-acquired COVID-19, inform knowledge of transmission, and target IPC practices.
METHODS
Setting and Participants
The setting was University College London Hospitals NHS Trust, a tertiary center with 1160 inpatient beds over 4 hospital sites. All admitted patients with a positive SARS-CoV-2 PCR test in the 6-week period 2 March–12 April 2020 were included.
Definitions
The median incubation period of COVID-19 is 5 days [8], with a maximum of around 14 days. Clinical notes were reviewed for documented symptom onset date, and hospital-acquired infection was defined as follows: (1) definite hospital-acquired COVID-19—symptom onset 14 days or more after admission; (2) probable hospital-acquired COVID-19—symptom onset 7 or more days after admission, or symptom onset 5–6 days from admission, with preceding documented contact with a COVID-19 case in hospital.
Epidemiological Analysis
For all definite or probable hospital-acquired cases, electronic hospital systems (EPIC Systems Corporation, Verona, Wisconsin) were used to identify other PCR-confirmed COVID-19 patients admitted into the same bay (room containing 4–6 patient beds, partitioned by curtains, generally sharing the same bathroom) or ward (department comprising multiple bays on the same floor, opening into the same corridors, often sharing staff, equipment, and other facilities). The case with the earlier symptom onset was considered the potential index case. A contact was deemed relevant if it included a time period (1) where the index case was potentially infectious, defined as 2 days prior to 7 days after their symptom onset, and (2) compatible with the incubation period of COVID-19, defined as within the 14 days prior to the secondary case’s symptom onset.
Statistical Analysis
Statistical analysis of anonymized data was performed using Stata version 12 software (StataCorp, College Station, Texas). Nonparametric numerical variables were compared using Wilcoxon rank-sum test. Network visualization was performed using Gephi 0.9.2 software [14].
IPC Procedures
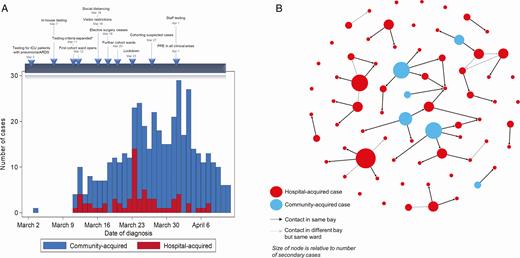
This period saw multiple changes in national and hospital policy (Figure 1A). Guided by Public Health England, local IPC approaches initially focused on prompt recognition, isolation, and testing of patients according to case definitions. Before 11 March, this required an epidemiological link with COVID-19; subsequently it included any admitted patient with acute respiratory distress syndrome, pneumonia, or influenza-like illness. From 27 March, suspected cases were isolated or cohorted according to their risk [15]. Throughout the study period, asymptomatic screening was neither part of national guidelines nor routinely practiced in our hospital (though it has since been introduced). Staff personal protective equipment (PPE) consisted of a minimum of gloves, apron, and surgical mask, with gowns, eye protection, and filtering facepiece class 3 masks for aerosol-generating procedures. PPE was initially used for suspected or confirmed cases, and extended to all patient interactions from 1 April. Progressive visitor restrictions and training and support to all staff for measures, including hand hygiene, environmental and equipment cleaning, and PPE, were implemented before and throughout this period.
A, Numbers of community- and hospital-acquired cases admitted to hospital between 2 March and 6 April 2020, with associated timetable of local and national infection prevention and control measures implemented during this time period. “Hospital-acquired” cases include definite and probable cases. *Testing criteria prior to 11 March required an epidemiological link with a case of COVID-19 or a high-risk country; following 11 March they were extended to all admitted patients with acute respiratory distress syndrome, influenza-like illness, or pneumonia. B, Network representation of all definite and probable hospital-acquired cases and community-acquired cases identified as potential index cases between 2 March and 6 April 2020. Black links represent a possible transmission within the same bay; gray links represent transmission in the same ward, with the earliest compatible case on the ward identified as the index case. Direction of arrows represents possible direction of transmission based on dates of symptom onset, and the size of the node is proportionate to its number of identified possible secondary cases (out-degree). Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit; PPE, personal protective equipment.
Where hospital-acquired cases were identified, outbreak investigations were performed and IPC measures audited and reinforced. Cases were isolated and any exposed patients were cohorted and monitored for development of symptoms.
RESULTS
Of 435 cases of PCR-positive COVID-19 inpatients in this 6-week period, 47 (11%) met the definition for definite hospital acquisition, with a further 19 (4%) probable hospital-acquired. Symptom onset for these 66 hospital-acquired cases was a median of 26 days (interquartile range [IQR], 13–55 days) from admission.
The median age of hospital-acquired cases was 70 years (IQR, 60–80 years), compared with 65 years (IQR, 50–79 years) among community-acquired cases (P = .06). Between 2 March and 30 March, hospital-acquired cases constituted 21% of all cases; in the subsequent 2 weeks this fell to 7% (Figure 1A).
Possible Sources of Infection
Of 66 hospital-acquired cases, 36 (55%) were identified as having been in the same bay as a patient with PCR-confirmed COVID-19, in a timeframe compatible with possible transmission (Figure 1B). The median serial interval was 6 days (IQR, 3–9 days; range, 1–14 days). A further 9 (14%) had no identified contacts in the same bay, but had contacts on the same ward. For the remaining 21 (32%), no clear source of infection was apparent. This included 8 cases (12%) who had been accommodated in single-occupancy rooms for the majority of their admission.
Among the 36 cases with a possible index case in the same bay, 22 (61%) of the index infections were themselves hospital-acquired, with several possible chains of patient-to-patient in-hospital transmission. The remaining 14 (39%) were linked to 6 individual community-acquired putative index cases. Four index cases had no documented COVID-19 symptoms on admission but developed them following admission to a shared bay; the remaining 2 were admitted with symptoms, which were not immediately identified as suggestive of COVID-19 or did not meet contemporaneous testing criteria.
Forty-five (68%) hospital-acquired COVID-19 cases were not themselves associated with any linked secondary cases on the same bay or ward, partly due to prompt identification and isolation. However, there were several community- and hospital-acquired cases associated with 4 or more likely secondary infections.
Outcomes
At a minimum of 3 weeks of follow-up, 37 (56%) patients with hospital-acquired COVID-19 had been discharged, 5 (8%) remained inpatients, and 24 (36%) had died, a median of 8 days (IQR, 6–13 days) from symptom onset.
DISCUSSION
During the March–April 2020 peak of the London COVID-19 outbreak, around 15% of inpatient cases in our hospital were hospital-acquired. This is similar to reports from other hospital settings [12, 13], although lower than seen in outbreaks of severe acute respiratory syndrome and Middle East respiratory syndrome coronaviruses, in which nosocomial transmission may predominate [16, 17]. The case fatality rate in this vulnerable cohort was 36%.
There are multiple possible routes of in-hospital COVID-19 transmission. Evidence of patient-to-patient transmission through contact in the same bay was found in 55% of hospital-acquired cases, with a serial interval of 6 days (slightly higher than the 4–5 days reported in the literature [3, 9, 18]). For a further 14% of patients with no contact in the same bay but cases on the same ward, cross-infection may have occurred through use of shared facilities and equipment, or staff movement [4, 5, 19].
For the remaining 32% of infections, no source was identified. In particular, 12% had been in single-occupancy rooms for much of their stay, with minimal patient-to-patient contact. Likely sources include asymptomatic or undiagnosed patients, visitors, or staff members. Staff illness levels were high during this period, and while symptomatic staff were advised to self-isolate at home, surveillance testing of London healthcare workers found that 27% of those infected were asymptomatic [20].
The transmission analysis has several limitations. We only identified PCR-positive symptomatic inpatients, and not patients exposed in hospital who were discharged before developing symptoms, those exposed in outpatient or other healthcare settings, those with undiagnosed COVID-19, or staff. We were only able to identify contacts between patients based on their admission location, and not with staff members or contacts in other contexts (eg, emergency department). Presently these epidemiological data are not supported by sequencing, so potential transmissions are inferred.
Following a comprehensive IPC response, both the numbers and proportions of hospital-acquired cases fell considerably. Although it is challenging to attribute this to specific interventions, important contributors included expanded staff and patient testing, use of PPE for all patient contacts, enhanced IPC measures, and cohorting of suspected cases to increase capacity to isolate the most vulnerable [15], as well as the falling community incidence.
This high incidence and mortality of hospital-acquired COVID-19 demand urgent preventive actions. As lockdowns ease and community transmission may resurge, we would recommend a combination of measures including screening all patients on admission (to prevent transmission from unidentified or presymptomatic community-acquired cases), meticulous universal PPE and IPC precautions, and surveillance testing of staff and patients.
Note
Potential conflicts of interest. M. S. has received grants from the US National Institutes of Health, the Bill & Melinda Gates Foundation, the UK Medical Research Institute, the UK National Institute for Health Research, the Wellcome Trust, and Unitaid, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments