-
PDF
- Split View
-
Views
-
Cite
Cite
Myron M. Levine, Catterine Ferreccio, Robert E. Black, Rosanna Lagos, Oriana San Martin, William C. Blackwelder, Ty21a Live Oral Typhoid Vaccine and Prevention of Paratyphoid Fever Caused by Salmonella enterica Serovar Paratyphi B, Clinical Infectious Diseases, Volume 45, Issue Supplement_1, July 2007, Pages S24–S28, https://doi.org/10.1086/518141
Close - Share Icon Share
Abstract
In randomized, controlled field trials in Area Norte and Area Occidente of Santiago, Chile, 2 (Norte) or 3 (Occidente) doses of live oral typhoid vaccine Ty21a in enteric-coated capsules conferred protection against confirmed Salmonella enterica serovar Typhi disease (53% efficacy in Norte; 67% efficacy in Occidente) during 3 years of follow-up. There was also a trend in each trial showing protection against S. enterica serovar Paratyphi B disease (56% efficacy in Norte; 42% efficacy in Occidente). To enhance statistical power, an analysis was performed using pooled data from the 2 trials; this pooling of data was justified by the following facts: epidemiologic surveillance and microbiological methods were identical, the trials overlapped during 22 of the 36 months of follow-up in each trial, the estimates of efficacy against paratyphoid B fever in the 2 trials were roughly similar, and the ratio of follow-up of vaccine recipients to control subjects in both trials was ∼1 : 1. In the pooled analysis, Ty21a conferred significant protection against paratyphoid B fever (efficacy, 49%; 95% confidence interval, 8%–73%; P = .019).
Of the clinical enteric fevers that occur globally, ∼80% are caused by Salmonella enterica serovar Typhi (S. Typhi) and 20% by S. enterica serovars Paratyphi A and B (S. Paratyphi A and B) [1]. Killed whole-cell parenteral typhoid vaccines manufactured in the early twentieth century were often trivalent combinations that contained inactivated S. Typhi, S. Paratyphi A, and S. Paratyphi B [2]. During the period 1989–2004, S. Typhi strains carrying an R factor encoding resistance to several clinically relevant antibiotics (chloramphenicol, amoxicillin, and trimethoprim/sulfamethoxazole) emerged and disseminated throughout South and Southeast Asia [3]. As the Asian pandemic of multidrug-resistant typhoid diminished in the early twenty-first century, paratyphoid fever caused by S. Paratyphi A became increasingly common [4] and also emerged as a problem among travelers to Asia [5]. This changing epidemiologic profile has rekindled interest in vaccines to prevent paratyphoid fever.
Attenuated S. Typhi oral vaccine strain Ty21a was developed by Germanier and Furer [6] in the early 1970s. Phase 1 and 2 clinical trials in adult volunteers established the safety of Ty21a when administered in oral doses as high as 1011 cfu with buffer and demonstrated a high level of protection against experimental challenge with wild-type S. Typhi [7]. A randomized, controlled, double-blind field trial conducted in the late 1970s in ∼32,000 Egyptian schoolchildren 6–7 years of age established the biological efficacy of Ty21a under natural conditions of exposure; 22 cases of bacteriologically confirmed typhoid fever were observed in placebo recipients, versus only a single case in Ty21a recipients (efficacy, 96%; 95% CI, 77%–99%) [8]. However, that trial utilized a formulation that was not amenable to large-scale manufacture.
The manufacturer, Swiss Serum and Vaccine Institute (currently Berna Biotech, a Crucell Company), advanced the field by formulating Ty21a in enteric-coated capsules. Two randomized, placebo-controlled field trials of efficacy were initiated 1 year apart in Area Norte [9] and Area Occidente [10], adjacent administrative areas of Santiago, Chile, to evaluate the efficacy of different immunization regimens and 2 formulations of Ty21a in preventing typhoid fever. The occurrence of a substantial number of cases of paratyphoid B fever during those trials provides an opportunity to revisit the data to assess whether Ty21a can also confer protection against enteric fever caused by S. Paratyphi B. The stimulus to undertake a modern analysis of pooled data from these 2 field trials of Ty21a vaccine 2 decades after publication of the reports of the individual trials was stimulated by the decision of the editors of this supplement to prepare a Festschrift to honor Theodore E. Woodward. Among the dominant themes that permeate the long and productive career of Professor Woodward are his contributions to the fields of typhoid fever, rickettsioses, and military medicine. In the latter half of the twentieth century, Professor Woodward was the predominant US authority on clinical aspects of enteric fever and its treatment. Theodore Woodward was also an expert on typhoid vaccines and was particularly enthusiastic about the concept of using attenuated strains as live oral vaccines [11, 12]. Early in his career, the first author of the present article (M.M.L.) was mentored by Theodore Woodward in the area of typhoid vaccines, and, for many years thereafter, Professor Woodward served as an invaluable confidant. Accordingly, the present article, which focuses on a subject of keen interest to Theodore E. Woodward, was prepared as a tribute to the man and is included as part of the Festschrift supplement.
Methods
Rationale for analyzing pooled data from the Area Norte and Area Occidente field trials. The primary aims of the Norte and Occidente trials addressed the ability of different formulations (Occidente) and immunization regimens (Norte and Occidente) of Ty21a to prevent bacteriologically confirmed typhoid fever. The trials were powered on the basis of the expected incidence of typhoid fever in the control group and an estimation of the efficacy of the vaccine. Although assessment of the ability of Ty21a to prevent paratyphoid B fever was a secondary aim, neither study was powered to detect a significant difference between vaccine and placebo, because of the much lower expected incidence of S. Paratyphi B disease. Analysis of pooled data from the Norte and Occidente trials would markedly increase statistical power to detect a significant difference between vaccine and placebo groups.
Area Norte trial. Parents of 91,954 of 137,697 schoolchildren 5–19 years of age, the target age range, consented for their children to participate [9]. During May and June 1982, the participants were randomly allocated to receive, 1 week apart, either 2 doses of Ty21a in enteric-coated capsules (containing 2 × 109–5 × 109 cfu), 1 dose of Ty21a and 1 dose of placebo, or 2 doses of placebo. The code was broken and the data analyzed after 36 months of follow-up (July 1982–June 1985).
Area Occidente trial. Parents of 135,482 (96%) of the 141,127 schoolchildren 6–19 years of age consented for their children to participate [10]. During July and August 1983, participating children were randomly allocated to receive 3 doses of vaccine in 1 of 2 different formulations or placebo, administered according to an immunization schedule with either a short interval (every other day) or a long interval (21 days) between doses. One formulation of Ty21a was enteric-coated capsules, and the other was a triad of gelatin capsules, with 1 containing lyophilized vaccine and the other 2 containing NaHCO3 buffer. Surveillance was performed for 3 years (September 1983–August 1986) before the code was broken and data analyzed [10].
Surveillance for suspected enteric fever cases and bacteriologic confirmation. Identical surveillance methods were used in Norte and Occidente to identify suspected cases of enteric fever and to confirm the diagnosis by bacteriologic isolation of S. Typhi from cultures of blood, bone marrow, or bile-stained duodenal fluid [9, 10]. During the period of the trials, ∼85%–90% of health care visits in Norte and Occidente occurred in government facilities where intensive surveillance could be maintained. Notices were sent to the parents of all schoolchildren to inform them of the symptoms of typhoid fever and of the availability of free diagnostic services at area clinics for children with suspected cases. Physicians and nurses were kept aware of the importance of obtaining cultures from children with suspected cases by clinical conferences, letters, and visits to health centers and hospitals. Outpatients with suspected enteric fever had 2 blood samples drawn 30 min apart for culture, whereas children with suspected cases who were admitted to the hospital had 3 blood samples and a bone marrow sample obtained for culture; some hospitalized children also had cultures of duodenal fluid performed [13]. Only cases for which culture yielded S. Typhi were considered to be confirmed typhoid. A by-product of these epidemiologic and bacteriologic methods was detection and confirmation of cases of paratyphoid fever by isolation of S. Paratyphi A or B.
Efficacy of Ty21a against typhoid and paratyphoid B fevers. The efficacy of Ty21a in preventing typhoid fever in the Norte and Occidente trials has been published elsewhere [9, 10]. To examine the efficacy of Ty21a in preventing paratyphoid B fever, we limited the analysis to the formulation (enteric-coated capsules) of Ty21a that conferred significantly better protection against typhoid fever in the Occidente trial and that was widely commercialized thereafter [10]. We also confined the analysis to immunization regimens wherein enteric-coated capsules of Ty21a were administered within a period of 8 days, including 3 doses given at an interval of every other day in Occidente and 2 doses given 1 week apart in Norte (the maximum number of doses given in that trial). The 3-dose, every-other-day regimen, which conferred better protection than 3 doses given 21 days apart in Occidente [10], is the most commonly recommended schedule worldwide for Ty21a [14]. The enteric-coated capsule formulation of Ty21a was licensed in most countries on the basis of data from the first 3 years of surveillance in the Occidente trial [10].
Statistical analysis. For each trial separately, as well as for the combined data from both trials, incidence rates of typhoid and paratyphoid B fever in vaccine and placebo recipients were estimated as the number of cases confirmed over 36 months of follow-up divided by the number of subjects who received the assigned regimen. The statistical significance of the difference between incidence rates (and, hence, of the observed vaccine efficacy) was assessed by an exact binomial test on the proportion, PV, of total cases that occurred among vaccinated subjects; for this test, the expected proportion was assumed to be the proportion of total subjects who were vaccinated. Vaccine efficacy (VE) was estimated as 1 - R.R = h × PV/(1 - PV), where h is the ratio of the numbers of placebo and vaccine recipients, respectively (taken as an estimate of the ratio of total follow-up times). R is, then, the ratio of disease incidences in vaccine recipients compared with placebo recipients. The same relationships among VE, R, and PV were used to obtain a 95% CI for VE from a 95% CI for PV.
Results
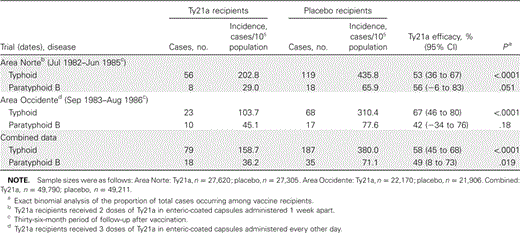
Efficacy of Ty21a in preventing typhoid fever. The efficacy of the described regimens of Ty21a in enteric-coated capsules in preventing typhoid fever in the individual Norte and Occidente trials is summarized in table 1 [9, 10]; also shown is an estimate of vaccine efficacy based on pooled data from the 2 trials.
Efficacy of Ty21a in preventing typhoid fever and paratyphoid B fever in randomized, placebo-controlled field trials in schoolchildren in Area Norte and Area Occidente, Santiago, Chile.
Efficacy of Ty21a in preventing paratyphoid B fever. The efficacy of Ty21a in preventing paratyphoid fever caused by S. Paratyphi B during the 3-year periods of surveillance in the individual trials in Norte and Occidente is shown in table 1. In the individual trials, there was a clear trend suggesting a protective effect of vaccine, but the number of cases was insufficient to reach statistical significance (χ2 with Yates correction).
Efficacy of Ty21a in preventing paratyphoid B fever, based on pooled data from the Area Norte and Area Occidente trials. We deem it statistically valid to pool the data from these 2 trials for the following reasons: (1) The ages of participants in the 2 trials were very similar; (2) Area Norte and Area Occidente, which are contiguous, had very similar socioeconomic levels and demographic profiles; (3) the numbers of subjects in each group in the trials were very similar; (4) identical surveillance methods were utilized to detect suspected cases, and identical bacteriologic methods were used to confirm cases; (5) the trials were initiated only 1 year apart, so 22 of the 36 months of follow-up in each trial occurred during the same calendar period; (6) in each trial, the ratio of the period of follow-up for the cases and controls was ∼1 : 1; and (7) the levels of efficacy of Ty21a in preventing both paratyphoid B fever and typhoid fever were roughly similar in the 2 trials. Table 1 demonstrates that Ty21a conferred an estimated vaccine efficacy of 49% in preventing paratyphoid B fever when pooled data from the 2 trials are analyzed. In this pooled data analysis, the difference in incidence of paratyphoid B fever between vaccine and placebo groups is statistically significant (P = .019), and the lower level of the 95% CI exceeds 0%.
Efficacy of Ty21a in preventing paratyphoid A fever, based on pooled data from the Area Norte and Area Occidente trials. In total, only 7 cases of paratyphoid A fever were observed (3 in Ty21a vaccine recipients and 4 in placebo recipients), making it impossible to draw conclusions about whether Ty21a provides partial protection against S. Paratyphi A.
Discussion
Typhoid fever and paratyphoid fever are clinically indistinguishable [15]. Whereas parenteral Vi polysaccharide and oral Ty21a typhoid vaccines are currently recommended for use in travelers and for control of endemic disease [14], there are no modern licensed paratyphoid vaccines. This raises the question of whether Vi and Ty21a can cross-protect against paratyphoid fever. Purified Vi vaccine protects by eliciting serum antibodies against Vi, an antigen virtually ubiquitous among S. Typhi strains. However, because Vi antigen is absent in S. Paratyphi A and B, Vi vaccine cannot protect against paratyphoid A or B fever. In contrast, attenuated S. Typhi Ty21a, which does not express Vi, mediates protection by eliciting serum and mucosal antibodies to S. Typhi O and H and other antigens and by stimulating an array of cell-mediated immune responses (including cytotoxic T cells) [16]. Data from efficacy trials of Ty21a conducted in Area Norte and Area Occidente suggest that Ty21a confers moderate protection against paratyphoid B fever, as well as against typhoid fever. Although the number of confirmed paratyphoid B cases in the individual trials was insufficient to reach statistical significance, analysis of pooled data from the 2 trials, which increased statistical power (table 1), indicates that Ty21a does, indeed, provide moderate protection against paratyphoid B fever.
There are several plausible immunological explanations for such cross-protection. One is based on shared epitopes among the O antigens. S. Typhi O antigen exhibits epitopes 9 and 12 (9 is dominant), whereas S. Paratyphi B has epitopes 1, 4, 5, and 12 (4 is dominant); S. Paratyphi A O antigen contains epitopes 1, 2, and 12 (2 is dominant). The oligosaccharide repeat units of these O antigens that provide their immunologic specificity each consist of 4 sugar residues. All 3 serovars share a common trisaccharide backbone (α-D-mannose-1→2-α-L-rhamnose-1→3-α-D-galactose), to which a fourth dideoxyhexose sugar (tyvelose in S. Typhi, abequose in S. Paratyphi B, and paratose in S. Paratyphi A) that is α1,3 linked to the D-mannose confers the immunodominant specificity, giving rise to immunodominant epitopes 9, 4, and 2, respectively [17]. Thus, serum and mucosal antibodies stimulated by Ty21a against shared epitope 12 may contribute to the observed cross-protection against S. Paratyphi B. If so, one might expect Ty21a to protect also against S. Paratyphi A disease.
Tagliabue et al. [18] reported that 3 doses of Ty21a in enteric-coated capsules (2 days between doses) stimulates a form of antibody-dependent cellular cytotoxicity in which IgA antibodies provide the specificity for mononuclear cells (from either vaccine recipients or naive subjects) to kill Salmonella organisms. Interestingly, they showed that postimmunization serum samples from Ty21a vaccine recipients and mononuclear cells were able to kill S. Paratyphi A and S. Paratyphi B, as well as S. Typhi. In contrast, no antibacterial killing effect was demonstrable against S. Paratyphi C or S. enterica serovar Tel Aviv, which do not share O antigen epitopes with S. Typhi.
Ty21a also stimulates strong classical cell-mediated immune responses involving specific T cells that are believed to function through several effector mechanisms, including production of IFN-γ and other cytokines when stimulated by inactivated whole bacilli and purified S. Typhi flagella and the appearance of cytotoxic T cells that kill target cells infected with S. Typhi [16]. Thus, an alternative explanation for cross-protection is that these cell-mediated immune responses may, in part, be directed against protein antigens common to S. Typhi and S. Paratyphi B.
There were so few cases of S. Paratyphi A disease during the surveillance in Norte and Occidente that it was not possible to ascertain whether Ty21a also protects against S. Paratyphi A. However, observations from a randomized controlled field trial in Plaju, Indonesia, suggest that, under those epidemiologic conditions, Ty21a did not protect against paratyphoid A [19]. In Plaju, where 3 doses of Ty21a in enteric-coated capsules (or in a “liquid” formulation) were administered at an interval of 7 days between doses, there was a high incidence of S. Paratyphi A disease and of S. Typhi disease in the placebo group. Whereas both formulations significantly protected against typhoid fever, there was no hint of a protective effect against paratyphoid A fever [19]. The lack of cross-protection against S. Paratyphi A in Plaju may have been due to a particularly high force of infection present in that trial venue [19]. Volunteer studies conducted by Hornick, Woodward, and others with inactivated whole-cell parenteral typhoid vaccines showed that the protection conferred by these vaccines could be overcome if the challenge inoculum ingested by vaccinated subjects was sufficiently high [20]. Thus, there is precedent for a high force of infection overcoming antibacterial immunity.
Ty21a appears to offer moderate protection against S. Paratyphi B under epidemiologic conditions such as those that were prevalent in Santiago, Chile, in the 1980s. In contrast, protection against S. Paratyphi A, at least in situations with a high force of infection, may have to await the development of a future paratyphoid A vaccine.
Acknowledgments
Financial support. The Area Norte and Area Occidente field trials of Ty21a performed in the 1980s were supported by grants from the World Health Organization, the Pan American Health Organization, and the US Department of Defense (M.M.L. was principal investigator on these grants). R.L. has received research funding from the Ty21A manufacturer, Berna Biotech, for clinical studies with their oral cholera vaccine. No external support supported the recent reanalysis of the pooled data undertaken for this supplement.
Supplement sponsorship. This article was published as part of a supplement entitled “Tribute to Ted Woodward,” sponsored by an unrestricted grant from Cubist Pharmaceuticals and a donation from John G. McCormick of McCormick & Company, Hunt Valley, Maryland.
Potential conflicts of interest. Berna Biotech, a Crucell Company and manufacturer of live oral typhoid vaccine Ty21a, holds the commercial license to live oral cholera vaccine strain CVD 103-HgR, of which M.M.L. is coinventor and co–patent holder. In 2007, M.M.L. will be a speaker on typhoid vaccines, including Ty21a, in a satellite symposium on typhoid fever supported by Berna Biotech at an international meeting; his travel to the meeting will be supported by Berna Biotech. M.M.L. has also been a consultant to Crucell on matters other than typhoid vaccines. All other authors: no conflicts.





Comments