-
PDF
- Split View
-
Views
-
Cite
Cite
B. R. Goldin, S. L. Gorbach, Clinical Indications for Probiotics: An Overview, Clinical Infectious Diseases, Volume 46, Issue Supplement_2, February 2008, Pages S96–S100, https://doi.org/10.1086/523333
Close - Share Icon Share
Abstract
Probiotic bacteria are used to treat or prevent a broad range of human diseases, conditions, and syndromes. In addition, there are areas of medical use that have been proposed for future probiotic applications. Randomized double-blind studies have provided evidence of probiotic effectiveness for the treatment and prevention of acute diarrhea and antibiotic-induced diarrhea, as well as for the prevention of cow milk–induced food allergy in infants and young children. Research studies have also provided evidence of effectiveness for the prevention of traveler's diarrhea, relapsing Clostridium difficile–induced colitis, and urinary tract infections. There are also studies indicating that probiotics may be useful for prevention of respiratory infections in children, dental caries, irritable bowel syndrome, and inflammatory bowel disease. Areas of future interest for the application of probiotics include colon and bladder cancers, diabetes, and rheumatoid arthritis. The probiotics with the greatest number of proven benefits are Lactobacillus rhamnosus strain GG and Saccharomyces boulardii.
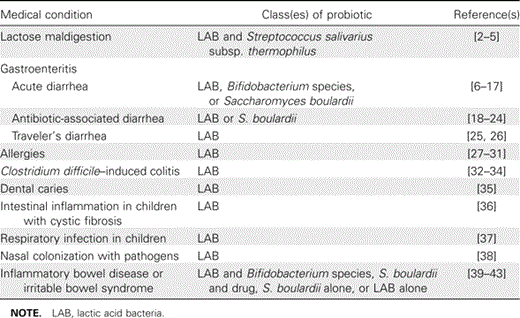
Probiotics have been defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [1]. Probiotics have been used to treat a wide range of diseases, ailments, and conditions that affect humans and animals. Additional medical applications have been proposed for potential future uses, depending on the outcomes of future experimental studies. The clinical uses of probiotics are broad; however, the clinical indications based on evidence-based studies are much narrower and are open to continuing evaluation. Table 1 contains a partial list of human diseases and conditions that probiotics have been used to prevent and/or treat.
Medical applications in humans for different classes of probiotics.
Documentation of the Health Effects of Probiotics for Human Diseases and Disorders
Lactose malabsorption. A large number of people, as they age, experience a decline in the level of lactase (β-galactosidase) in the intestinal brush border mucosa. This decline causes lactose to be incompletely absorbed, resulting in flatus, bloating, abdominal cramps, and moderate-to-severe (watery) diarrhea. This results in a severe limitation in consumption of dairy products among the elderly population. There have been several studies that have demonstrated that, during the fermentative process involved in the production of yogurt, lactase is produced, which can exert its influence in the intestinal tract [2–5]. The organisms commonly used for the production of yogurt are Lactobacillus bulgaricus and Streptococcus salivarius subsp. thermophilus. Kim and Gilliland [4] found that feeding lactose-intolerant individuals yogurt caused a significant reduction in the level of breath hydrogen compared with that in subjects who were fed milk. The level of hydrogen in the breath is an indication of the extent of lactose metabolism in the large bowel. Kolars et al. [5] observed that the ingestion of 18 g of lactose in yogurt caused the production of 67% less hydrogen in the breath compared with that produced by a similar dose of lactose delivered in milk. Analysis of aspirates obtained from the duodenum 1 h after the consumption of yogurt showed significant levels of lactase [5]. These studies indicate that the delivery of lactase to the intestine via the consumption of lactase-producing probiotics is a practical approach for treatment of lactose malabsorption.
Acute diarrhea. There are at least 12 studies that have reported the use of probiotics to either treat or prevent acute diarrhea [6–17]. The majority of these studies were done with infants or children, the etiologic agent was either rotavirus or unknown, and the probiotic used was Lactobacillus rhamnosus strain GG (Lactobacillus GG) (ATCC 53103) [7–14]. Other probiotics that have shown positive results for the treatment of acute gastroenteritis include Lactobacillus reuteri and Saccharomyces boulardii [15–17]. The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition conducted the most extensive trial using Lactobacillus GG for the treatment of moderate-to-severe diarrhea in children [7]. The study included 287 children aged 1–36 months from 10 countries. The patients were randomized to be given either placebo or Lactobacillus GG along with the standard treatment, oral rehydration solution. Patients who received Lactobacillus GG had decreased severity and shorter duration of illness and a shorter hospital stay and were found to have a decreased likelihood of persistent diarrheal illness [7]. A similar study was conducted with 137 children aged 1–36 months who were admitted to the hospital with diarrhea and were randomized to receive placebo or Lactobacillus GG plus oral rehydration solution. Children given Lactobacillus GG had a significantly shorter duration of illness [8]. A study of 26 children in Thailand with watery diarrhea showed a significantly shorter duration of symptoms for those who received treatment with Lactobacillus GG [9]. A similar investigation involving 40 children that was conducted in Pakistan found that those who received treatment with Lactobacillus GG were less likely to have persistent diarrhea and had fewer episodes of vomiting, compared with the placebo group [10]. In a preventive study of 81 children aged 1–36 months who were hospitalized for illnesses other than diarrhea, symptoms of hospital-acquired rotavirus gastroenteritis were prevented by administration of Lactobacillus GG [12]. In another prevention study conducted in Peru, 204 children aged 6–24 months who were undernourished were randomized to receive placebo or Lactobacillus GG. There was a significant decrease in the rate of incidence of diarrhea among the children who received Lactobacillus GG who were not being breast-fed [14]. In one study, Lactobacillus reuteri was shown to shorten the duration of diarrhea in children [15]. In a clinical trial involving 130 children, S. boulardii was found to be effective for the treatment of acute diarrhea in children [16], and, in another study of 92 adults, a similar finding was reported [17].
Antibiotic-associated diarrhea. There have been a number of studies of the ability of probiotics to reduce the frequently observed intestinal adverse effects and diarrhea associated with the clinical use of antibiotics [18–24]. In a study of 119 children who received antibiotics for respiratory infections, during the first 2 weeks after antibiotic treatment began, the group receiving Lactobacillus GG had an ∼70% reduction in diarrheal symptoms, compared with the group receiving placebo [18]. In another study, in which 202 children receiving oral antibiotics were followed, 8% of the children who received Lactobacillus GG concurrently with antibiotics experienced diarrheal symptoms, compared with 26% of the placebo group [19]. In 2 studies involving 60 and 120 adult patients who received antibiotic treatment to eliminate Helicobacter pylori, a significantly lower number of patients experienced nausea and diarrhea when they simultaneously received Lactobacillus GG versus placebo [20, 22]. There have been a number of studies that used other bacterial probiotics to treat antibiotic-associated diarrhea in which the treatment was not successful [24]. There are, however, at least 3 published studies that demonstrate the ability of S. boulardii to reduce antibiotic-associated diarrhea [24].
Traveler's diarrhea. People traveling to warmer climates and less-developed countries experience a high incidence of diarrhea, often in the 50% range. A published study that tracked Finnish travelers to Turkey found that, at 1 of 2 resorts, oral ingestion of Lactobacillus GG conferred a significant protection rate, of 39.5% and 27.9%, in weeks 1 and 2 of the study, respectively. In the other resort area, no protection from consumption of Lactobacillus GG was noted [25]. A possible explanation for the discrepancy between the 2 resort sites is the availability of adequate refrigeration facilities, which is particularly relevant for probiotic preparations in warm climate situations. Also studied were 245 travelers from New York who went to various developing countries for periods of 1–3 weeks [26]. The travelers were provided Lactobacillus GG or a placebo, and Lactobacillus GG afforded a protection rate of 47%.
Prevention and treatment of allergic reactions. The most extensive studies of the modification of allergic reactions have been reported for atopic eczema with Lactobacillus GG as the probiotic [27–31]. There has also been a study that reported the use of Bifidobacterium animalis Bb12 to reduce the severity of atopic dermatitis [30]. In one study, 159 pregnant women with a family history of atopic disease were given either Lactobacillus GG capsules or a placebo for 2–4 weeks before their expected delivery date [27]. Mothers who chose to breast-feed their newborns continued to receive Lactobacillus GG or placebo for 6 months, and women who did not breast-feed gave the Lactobacillus GG or placebo to their infants. There was a 50% reduction in the frequency of atopic eczema in the first 2 years of the children's lives for the group given Lactobacillus GG. The breast milk of the mothers in the Lactobacillus GG group had higher levels of transforming growth factor β>2. In a follow-up study [28], the group that received Lactobacillus GG still had a significantly lower percentage of atopic eczema 4 years after birth, compared with the placebo group. In another study, 27 infants with atopic eczema were randomized into 3 groups, given Lactobacillus GG, Bifidobacterium lactis Bb12, or placebo. After 2 months, the SCORAD score, reflecting the extent and severity of atopic eczema, indicated a significant improvement in the skin condition of patients given probiotic-supplemented formulas (P=.002) [30]. A similar study involving 31 infants with atopic eczema who were removed from exposure to cow milk and were given either Lactobacillus GG or a placebo showed that treatment with Lactobacillus GG resulted in a significant improvement in their conditions that was not observed in the placebo group [31].
Treatment of relapsing gastoenteritis induced byClostridium difficiletoxin. Secondary to antibiotic treatment, disturbance of the intestinal flora can result in C. difficile growth and toxin production in the intestinal tract [32]. There have been several studies that showed that treatment with Lactobacillus GG prevents relapse of gastroenteritis after use of antibiotics. Clinical experience has shown a 60% relapse rate after therapy with metronidozole or vancomycin. Only 16% of patients who received Lactobacillus GG experienced a relapse, and, after a second course of Lactobacillus GG, there was a 94% overall cure rate [33, 34].
Prevention of dental caries. Children in a day care center who were given Lactobacillus GG for 7 months were examined for dental caries, and the children in the 3–4-year-old age group had significantly lower rates of dental caries and a reduced oral count of Streptococcus mutans compared with before the treatment [35].
Elimination of nasal pathogens. In a study of 209 healthy subjects, the consumption of a fermented milk product containing probiotics resulted in a significantly higher proportion of subjects with pathogenic bacteria eliminated from the nasal cavity, compared with consumption of a yogurt drink in the placebo group [38]. The pathogens removed included Staphylococcus aureus, Streptococcus pneumoniae, and β-hemolytic streptococci.
Treatment and prevention of relapses of inflammatory bowel disease. One of the major potential applications of probiotics is for the treatment and prevention of relapses of Crohn disease, ulcerative colitis, and irritable bowel syndrome. There have been reports of beneficial effects for inflammatory bowel disease that resulted from the administration of Lactobacillus salivarius [39], Escherichia coli strain Nissle [40], S. boulardii [41], and VSL#3 (VSL Pharmaceuticals), a mixture of probiotics [42]. These studies found fewer relapses and reduced steroid use among patients who received these probiotics. However, the studies were small, and the results were equivocal. There has been a report that VSL#3 reduced symptoms in patients with irritable bowel syndrome [43].
Potential Medical Indications for Probiotics in the Future
Several diseases and conditions have been proposed to be treatable with probiotics on the basis of animal studies, preliminary human studies, uncontrolled studies, anecdotal observations, or simply speculation. These uses can be classified as potential applications of probiotics in the future or that require ongoing research. There have been animal studies and one small human trial that indicate that Lactobacillus GG may be useful for alleviating joint symptoms among patients with rheumatoid arthritis [44, 45]. There are several animal studies that show that probiotics inhibit initiation or progression of colon and bladder cancers [46, 47]. In vitro, cell culture, and animal studies have indicated that probiotics bind and prevent the absorption of aflotoxins, which have been implicated in the etiology of liver cancer in humans [48, 49]. A rat model of ethanol-induced liver damage has been used to demonstrate the protective effects of probiotics [50]. An animal model of diabetes showed that Lactobacillus GG could lower levels of blood hemoglobin A1c and could improve glucose tolerance [51]. Probiotics studied in a mouse model have demonstrated a possible role for these agents in the prevention or treatment of graft-versus-host disease in transplant recipients [52].
Conclusions
The current and proposed uses of probiotics cover a wide range of diseases and ailments. An attempt has been made to classify the quality of evidence that supports these various applications [53]. These classifications are based on existing studies, most of which are cited in this article, and not on an exhaustive review of the entire literature on probiotics. The broad classifications include (table 2) applications with proven benefits, applications with substantial evidence that require additional support, promising applications that need substantial additional evidence, and proposed future applications. Proven benefits of probiotics include the treatment of acute and antibiotic-associated diarrhea; applications with substantial evidence include the prevention of atopic eczema and traveler's diarrhea; promising applications include the prevention of respiratory infections in children, prevention of dental caries, elimination of nasal pathogen carriage, prevention of relapsing C. difficile–induced gastroenteritis, and treatment of inflammatory bowel disease; and proposed future applications include the treatment of rheumatoid arthritis, treatment of irritable bowel syndrome, cancer prevention, prevention of ethanol-induced liver disease, treatment of diabetes, and prevention or treatment of graft-versus-host disease. The use of probiotics in medical practice is rapidly increasing, as are studies that demonstrate the efficacy of probiotics. A note of caution should be applied: negative findings are being reported, as would be expected as more studies are being performed and as more applications are being sought for the use of probiotics. Overall, probiotics appear to be here to stay as part of the physician's armamentarium for the prevention and treatment of disease; however, more evidence-based research is required to firmly establish medical areas of use and areas in which probiotics are not applicable.
Present and future clinical applications of probiotics, by level of evidence of efficacy.
Acknowledgments
S.L.G., the journal's editor, was not involved in the editorial review or decision to publish this article.
Supplement sponsorship. This article was published as part of a supplement entitled “Developing Probiotics as Foods and Drugs: Scientific and Regulatory Challenges,” sponsored by the Drug Information Association, the National Institutes of Health National Center for Complementary and Alternative Medicine (1R13AT003805-01 to Patricia L. Hibberd), the California Dairy Research Foundation, Chr. Hansen, the Dannon Company, General Mills, Institut Rosell, and Yakult International.
Potential conflicts of interest. B.R.G. and S.L.G. had a patent and license agreement for Lactobacillus GG. The patent expired in June 2006, and the license in January 2007. There are currently no conflicts of interest.






Comments