-
PDF
- Split View
-
Views
-
Cite
Cite
Prabhavathi Fernandes, David Pereira, Efforts to Support the Development of Fusidic Acid in the United States, Clinical Infectious Diseases, Volume 52, Issue suppl_7, June 2011, Pages S542–S546, https://doi.org/10.1093/cid/cir170
Close - Share Icon Share
Abstract
Fusidic acid (FA), though used widely throughout the world for decades, has never been approved in the United States. There is now a great need for an oral methicillin-resistant Staphylococcus aureus (MRSA) antibiotic with a long track record of safety. Cempra Pharmaceuticals successfully encouraged passage of a congressional amendment to allow for Hatch-Waxman market exclusivity when this antibiotic is approved in the United States. A new dosing regimen has been patented, allowing FA to be used as monotherapy, and decreased resistance selectivity has been shown. With almost no resistance to FA in the United States, the time is right for introduction into this market.
Noting the withdrawal of resources to discover and develop new antibiotics in parallel with the continuing evolution of virulent and drug-resistant bacteria, the Antimicrobial Availability Task Force of the Infectious Diseases Society of America (IDSA) published the now famous “Bad Bugs, No Drugs” article in which the most urgent needs were described [1, 2]. In this article, published in 2006, the same year Cempra Pharmaceuticals was founded, it was noted that multiple anti-MRSA compounds were in late-stage development, but the authors went on to add “However, the apparent plethora of available antibiotics for MRSA infection is somewhat misleading. A critical need is for effective antibiotics that can be taken orally, allowing for effective step-down therapy for nosocomial infection or initial therapy for infections acquired in the community.” Also in 2006, telithromycin (Ketek) was being subjected to intense scrutiny because of rare reports of serious and even fatal adverse events [3]. The events that accompanied the Food and Drug Administration (FDA) advisory committee hearings on telithromycin made it clear that future antibiotic development of new chemical entities would face new regulatory hurdles for approval, with a focus on risks of unexpected clinical effects. [4, 5] In June 2006, FA was initially considered for development by Cempra and its scientific advisory board as a marketed antibiotic that had proven to be safe and effective in the treatment of MRSA infections but had never been approved for use in the United States.
EVALUATION OF FUSIDIC ACID
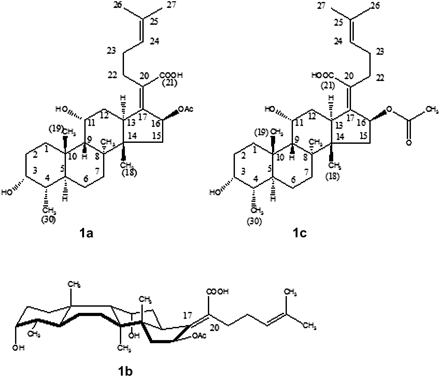
Cempra's initial evaluations of the chemical features of FA revealed a steroidlike structure (Figure 1a), but the stereochemistry at the ring junctions is different from that for steroids, such that the FA ring system assumes a unique boat configuration (Figure 1b). Early texts often described an incorrect structure for FA, which was in fact that of lumifusidic acid, an inactive isomer of FA formed by exposure to intense light, which results in a rearrangement around the C17–C20 double bond (Figure 1c).
(A), Fusidic acid (FA) has a steroidlike structure. (B), Actual configuration of FA, taking into account stereochemistry at the ring junctions; these are different from those for steroids, forcing the ring system into a boat configuration. (C), Earlier texts often showed an incorrect structure for FA, and in fact, the structure of lumifusidic acid.
Evaluations of FA's product profile revealed both positive and negative features. Among its positive attributes, FA is a unique member of the fusidane class of antibiotics with no known cross-resistance with any other class of antibiotic [6–8]. Reports of its clinical use outside the United States documented a track record of safety for >4 decades [9–14]. It is highly bioavailable orally, and has a long plasma half-life [15]. Its reported spectrum of antimicrobial activity was narrow but sufficient for targeting S. aureus, including MRSA, and β-hemolytic streptococci [16–20]. Streptococcus pyogenes, or group A β-hemolytic streptococci, are moderately susceptible to FA; minimum inhibitory concentrations (MICs) are reported in a narrow range of 4–8 μg/mL, and no resistance has been described. Although FA has moderate in vitro activity against β-hemolytic streptococci, it has demonstrated clinical efficacy in patients with β-hemolytic streptococcal infections, and also in coinfections with S. aureus [14]. These results can be explained by the relatively high and sustained blood concentrations achieved after FA dosing. The apparent clinical niche for this product included acute bacterial skin and skin structure infections, osteomyelitis, and other infections where staphylococci are the predominant pathogens [11, 14, 16, 21–25]. Importantly, it was also well known that large doses of FA could be given safely for prolonged periods of time [10, 11, 14, 22, 26].
However, it was widely published that resistance to FA could occur during therapy and that this agent must be used in combination with rifampin or other agents to prevent the emergence of on-therapy resistance [17, 20]. High plasma protein binding of FA (up to 97%) was thought to limit free drug availability in infected tissues [15, 27]. FA was also known to be metabolized and excreted by the liver and to inhibit CYP3A4, suggesting possible drug interaction issues [16].
Cempra scientists examined published reports [28] indicating resistance rates on the order of 10−6–10−7 when FA was tested at twice the MIC, but much lower resistance frequencies, on the order of 10−11, when it was tested at 15–30 mg/L, concentrations of FA that could be safely achieved in plasma using dosing regimens approved in the European Union (EU) [15]. However, when the effects of avid protein binding were taken into consideration, it was evident that plasma levels of FA >15–30 mg/L would be required to provide sufficient free drug to limit the development of resistance in patients. A Cempra-sponsored survey of US and Canadian S. aureus isolates showed that in Canada, where FA had been approved for use for decades, the resistance rates were a manageable 8%, while in the United States virtually all S. aureus isolates were susceptible [8]. It was also noted that resistance was higher in countries where topical FA was used (T. Louie, personal communication). Accordingly, Cempra decided to develop only oral formulations of FA (CEM-102).
SOURCING FUSIDIC ACID
The first hurdle was to obtain a supplier of FA, a fermentation product that is difficult to synthesize commercially. Unable to obtain supplies from Leo Laboratories, the innovator company, Cempra established a business arrangement with Ercros (Ercros Industrial) to obtain a supply of FA and obtain exclusive access to this source for the US market.
APPROACHING THE FDA
Cempra, with hundreds of existing publications on FA, proceeded to consider a 505(b)(2) application strategy to obtain approval and 5 years of market exclusivity for this product in the United States. On meeting with the FDA, Cempra was advised that old publications, without access to the supporting data, could not serve as the basis for regulatory approval and that new data were required to meet substantial parts of the nonclinical and clinical requirements for a new drug application. After consideration of the significant expense required to obtain approval and market exclusivity in the United States for the relatively short period of 5 years, Cempra nonetheless decided that the commercial opportunity in the United States market was significant. Forecasts indicated that FA revenues would be in line with that expected from new patented antibiotic products, which typically achieve sales of >$500 million within the first 3–4 years. Nonclinical development was begun, including animal toxicology studies, mutagenicity studies, microbiologic surveillance, and other studies required for investigational new drugs in the United States.
EXCLUSIVITY
Early in this process, though after significant work had been performed to prove that FA could be a commercial success, Cempra was informed by its regulatory lawyer that FA was not eligible for Hatch-Waxman marketing exclusivity. The FDA Modernization Act (FDAMA), which took effect on 21 November 1997, repealed section 507 of the Federal Food, Drug, and Cosmetic (FD&C) Act, under which marketing applications for antibiotics were approved. With the enactment of FDAMA, marketing applications for antibiotics are submitted and approved under section 505 of the FD&C Act and are eligible for Hatch-Waxman exclusivity. However, FA had been the subject of a marketing application received by the FDA under section 507 of the FD&C Act, before passage of the FDAMA. Antibiotics that fell into this category, even those such as FA, which were never approved in the United States, were classified as “old” antibiotics and were not eligible for the exclusivity provisions afforded by section 505(c) [29]. Further discussions with lawyers and the FDA confirmed that a congressional amendment to the law would be required for FA to obtain market exclusivity in the United States.
AMENDING THE LAW
By February 2007, Cempra's FA program came to a temporary pause as the company considered its alternatives. Cempra's chemist (D.P.) challenged its president, Dr Fernandes, to speak with elected representatives to change the law. Cempra's data showed that FA was too valuable to not pursue further and decided to work toward amending US law to allow FA to obtain Hatch-Waxman exclusivity.
Cempra sought the help of investors’ experts in Washington DC and the IDSA to lobby for legislation to provide for Hatch-Waxman exclusivity for “old” antibiotics that had never received marketing approval in the United States. This amendment was first proposed by Senator Burr, termed the “Cempra Amendment,” and approved by the Senate and House of Representatives [30]. It was attached to a larger amendment being proposed by the IDSA and eventually added to the bill for the major Prescription Drug User Fee Act (PDUFA) [2]. This bill included many additional topics related to antibiotic development and marketing and was strongly endorsed by the IDSA. It was passed by the House and Senate in September 2007 [30], but the Cempra Amendment, allowing exclusivity for old antibiotics that had never been approved in the United States, was removed at the last minute from the PDUFA bill, and no changes were made to the law. The amendment was administratively removed from the PDUFA bill because the funding for the $14 million pay-as-you-go (PAYGO) amount (which compels new tax changes to not add to the federal deficit) had not been taken into account when the bill came up for a vote in the House.
In the meantime, Cempra had conducted phase 1 studies in Canada, where the drug is marketed, and had completed significant preclinical studies to include in an investigational new drug application in the United States. Moreover, Cempra's macrolide program had also produced a lead compound that was clearly differentiated from telithromycin. There was declining interest among investors to push forward with the development of FA because of difficulties in securing a viable period of market exclusivity upon approval. Cempra's macrolide candidate had entered phase 1 trials, requiring significant funding, and FA was becoming a drag on the limited resources of the company. Termination of the FA program was again considered.
REVISITING AND PASSING OF THE US AMENDMENT
Although work on the FA product was slowed and almost halted, Cempra decided not to abandon the program, and continued to pursue passage of a Congressional amendment. The “old” antibiotics exclusivity topic had to be reintroduced into the Senate and House, and new champions in both houses of Congress had to be found. Coincidently, Senator Edward Kennedy was admitted to Duke Hospital at that time, and his office was alerted to a publication in Clinical Infectious Diseases reporting the high probability of suffering a postsurgical infection due to MRSA [31]. This publication was duly sent to the Kennedy offices asking for support in reintroducing an amendment. This time, funding for the $14 million PAYGO amount was secured with the help of government consultants and the Congressional Budget Office to help pay for the amendment. In the summer of 2008, it was added to a supplemental Medicare bill, which was unanimously passed by both houses of Congress [32]. In October 2008, President Bush signed this bill into law (Public Law 110–379), reenergizing Cempra's further development of FA.
FUNDING TRIALS AND MOVING FORWARD
Despite this major legislative success, Cempra was still faced with the significant challenge of raising money to fund the clinical development of CEM-102 in the worst downturn in the US economy since the Great Depression. Nonetheless, FA was considered by its investors to be a safe and effective product and relatively low risk compared with new chemical entities in development. These investors were persuaded to stand fast and support Cempra's CEM-102 program based on FA's known mode of action, its extensive track record of clinical safety, and its excellent activity profile against MRSA and other pathogens. Investors wanted a safe bet on an important project, and FA fit the bill. Cempra successfully closed a substantial financing round in April 2009 and moved forward through phase 2 trials [33].
A NEW DOSING REGIMEN: GIVING AN OLD DRUG NEW TRICKS
Through the expertise of Drs Ronald N. Jones and Paul G. Ambrose and their colleagues at JMI laboratories and the Institute for Clinical Pharmacodynamics, respectively, Cempra realized that dosing regimens used in Europe had not been optimized. Pharmacokinetic-pharmacodynamic (PK-PD) models were designed and executed to identify a new front-loaded dosing regimen that took advantage of FA's nonlinear PK-PD profile [34]. Some were concerned that high doses would result in unacceptable nausea, as reported in some clinical publications [23]. However, during Cempra's phase 1 trials, in which escalating doses of CEM-102 were tested in healthy subjects, it was discovered that very high blood concentrations of FA could be rapidly achieved without eliciting gastrointestinal intolerance (nausea) using a novel front-loaded dosing regimen [35]. By design, and as shown in phase 1 trials, initial loading doses provided trough levels of FA near-steady state levels within 24 h and subsequent maintenance doses sustained these levels of free drug at more than 10–20 times the MIC of target pathogens [35]. This dosing strategy was also intended to minimize FA resistance selection and obviate negative effects of protein binding on FA activity. A patent application was filed to protect this novel dosing regimen, and a phase 2 clinical trial was designed to evaluate this regimen along with the dosing regimen that is approved outside of the United States. Linezolid was chosen as the comparator for this trial, as it is the only oral antibiotic approved for treating MRSA infections in the United States [33].
VALUE CREATION FOR PATIENTS AND INVESTORS
Cempra's FA development saga may yet create real commercial value for its investors, who have taken considerable risks under uncertain and challenging circumstances. Unquestionably, this potential value is due to the hard-earned opportunity to develop an “old,” yet dose-optimized, oral antibiotic with 5 years of exclusivity on approval of a new drug application as well as protection by a novel loading dose patent for at least 18 years. Beyond commercial value objectives, Cempra has overcome many scientific, regulatory, and legal hurdles to meet one of the most important current antibiotic needs within the United States, as emphasized by the IDSA, public health care agencies, and the medical community [1, 36]. Future studies will determine whether a novel FA formulation and dosing regimen will perform as well as predicted by PK-PD modeling and early clinical trial results. The results to date predict that it could be a safe and effective oral alternative for the treatment of acute bacterial skin and skin structure infections if approved and marketed in the United States [33, 35].
The authors especially thank and acknowledge Dr Don Cox for suggesting that a change in the law was possible, Ms Nancy Buc, for Regulatory Law guidance, Mr Stephen Conafay and Mr Joel Johnson for helping to “show the way” in Washington DC, and Ms Erin Fry for her tireless support and resolve to never give up.
Supplement sponsorship. This article was published as part of a supplement entitled “Fusidic Acid Enters the United States,” sponsored by Cempra Pharmaceuticals.
Potential conflicts of interest. P. F. is president and chief executive officer and D.P. is vice president of chemistry at Cempra Pharmaceuticals.





Comments