-
PDF
- Split View
-
Views
-
Cite
Cite
Cheikh Sokhna, Oumar Gaye, Ogobara Doumbo, Developing Research in Infectious and Tropical Diseases in Africa: The Paradigm of Senegal, Clinical Infectious Diseases, Volume 65, Issue suppl_1, 15 August 2017, Pages S64–S69, https://doi.org/10.1093/cid/cix347
Close - Share Icon Share
Abstract
Infectious diseases represent one of the greatest potential barriers to achievement of the third Sustainable Development Goals in African countries and around the world because they continue to pose major public health challenges. The surveillance of infectious diseases has recently assumed greater importance in most African countries, both because of the emergence of infectious diseases and because strains of pathogens that cause tuberculosis, malaria, cholera, dysentery, and pneumonia have developed resistance to common and inexpensive antimicrobial drugs. However, data on the pathogen-specific causes of infectious diseases are limited. Developing research in infectious and tropical diseases in Africa is urgently needed to better describe the distribution of pathogen-borne diseases and to know which pathogens actually cause fever. This research is critical for guiding treatment and policies in Africa. More effective diagnostics are also needed for these diseases, which often are misdiagnosed or diagnosed too late. A comprehensive review of this type of research is presented here.
Infectious diseases continue to pose major public health challenges in African countries and around the world. Infectious diseases represent one of the greatest potential barriers to achievement of the third Sustainable Development Goals because they collectively account for 20% of mortality in all age groups (and 33% of mortality in the least developed countries) and 50% of child mortality [1]. The “big three” killer infectious diseases are malaria, human immunodeficiency virus (HIV), and tuberculosis, which together cause more than 6 million deaths per year [2]. In the past 20 years, major increases in funding have enabled significant progress to be made in the fight against these diseases, although optimism must be tempered [2]. Most frequently, this increased knowledge has resulted from large, worldwide, randomized trials [3–5]. In parallel to these multicentric studies, collaborative observational and technology-driven research has been developed, particularly to explore nonmalarial causes of fever in western Africa [6]. Here, we propose a comprehensive review of the type of research that is emerging on infections and tropical diseases in Africa.
MALARIA
Malaria remains the most prevalent vector-borne infectious disease and has the highest rates of morbidity and mortality. However, over the last decade, important changes have occurred in how malaria is managed in Africa. These changes include a shift from chloroquine- to artemisinin-based combination therapies as the first line of treatment and the mass distribution and use of insecticide-treated bed nets [7]. Together, these strategies have led to a dramatic reduction in the prevalence, morbidity, and mortality of malaria in most African countries [8]. More generally, disease-specific national plans for controlling disease have been implemented for malaria, tuberculosis, and HIV, which have succeeded in decreasing the incidence and the mortality of these diseases.
The reduction in morbidity due to malaria reveals a significant need to assess the considerable rate of other fever-causing agents, especially in dispensaries that provide front-line healthcare. In the absence of appropriate diagnostic tools, this situation raises challenges for physicians and nurses who lack the ability to treat these patients who are now the majority of febrile patients. The development and implementation of rapid diagnostic tests (RDTs) for malaria in dispensaries also revealed that the diagnosis of malaria could be excluded in a number of cases. However, it was also reported in Ghana in 2016 that 62% of febrile patients with negative RDTs still received antimalarial treatment contrary to guidelines [9]. Indeed, health workers still tend to rely on a clinically presumptive diagnosis of malaria rather than on RDTs results because of the fear of false-negative results [9]. This practice is dangerous because it may increase mortality due to infectious diseases in sub-Saharan Africa. It has already been demonstrated in Tanzania that mortality for malaria in smear-negative patients was higher (292/2412, 12.1%) than in the positive patients (142/2062, 6.9%, P < .001) (2). Only about 66% of the smear-negative patients had received antibiotic therapy [10]. Thus, the repertoire of other microorganisms involved in febrile episode must be characterized.
SENEGALESE NETWORK FOR THE EXPLORATION OF NONMALARIAL CAUSES OF FEVER
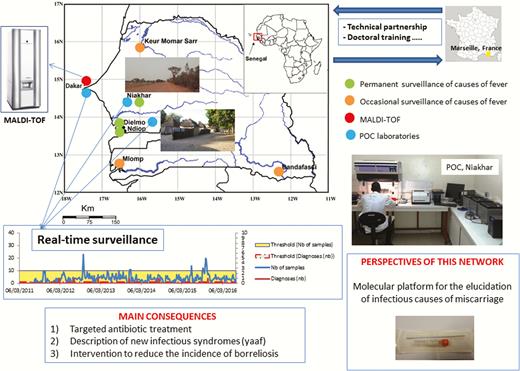
In Senegal, the proportion of morbidity due to malaria fell from 33.57% in 2006 to 3.1% in 2009. The surveillance network that was developed a few years ago includes various geographic study areas (Figure 1). Dielmo and Ndiop are 2 villages located approximately 280 km southeast of Dakar, near the Gambian border in an area of Sudan-type savanna. The Niakhar study area covers 30 villages, 120 km east of Dakar, with a population of 45000 and a density of approximately 152 inhabitants/km2. Mlomp includes a group of 11 villages located in Casamance, southwestern Senegal, within the Guinea savanna and mangrove ecological zone. The Bandafassi study area (Sudano-Guinean savanna ecological zone) is located in eastern Senegal, near the border between Senegal, Mali, and Guinea. Finally, Keur Momar Sarr is a village situated at the southern end of Lake Guiers (Figure 1).
Network of exploration of infectious diseases in Senegal, western Africa.
Repertoire of Nonmalarial Causes of Fever
The first step was to define the prevalence of the different nonmalarial causes of fever in these 5 study regions and to establish a repertoire, which revealed the following results. Every year in the rural village of Dielmo, between 5% and 25% of inhabitants present with tick-borne borreliosis caused by Borrelia crocidurae without acquiring specific immunity [11]. In the Niakhar area, the prevalence of borreliosis is currently the leading cause of fever before malaria and has been estimated by some healthcare dispensaries (unpublished data) as causing up to 20% of fevers. These results support the claim that borreliosis is one of the most common causes of healthcare consultations for fever in all age groups in rural western Africa [12, 13]. Q fever is frequently encountered in Senegal, and the seroprevalence of Coxiella burnetii is up to 24% in Dielmo [14]. Tropheryma whipplei has been identified in 6.4% of blood samples of febrile patients who tested negative for malaria by RDT, with most patients having a concomitant cough [15]. Tropheryma whipplei is also a cause of epidemic fever in Senegal [16]. Bartonella quintana DNA was detected in the blood of 2% of patients with fever and head lice [17]. At least 5 named Rickettsia species have been identified in Senegal, including Rickettsia conorii, Rickettsia africae, Rickettsia aeschlimannii, Rickettsia massiliae, and Rickettsia felis [18, 19]. After this had been identified, a quick and simple diagnostic platform was installed locally.
Point-of-Care Laboratories
The point-of-care (POC) laboratories operate 24 hours a day and 7 days a week to provide rapid diagnoses, largely based on immunochromatography and real-time polymerase chain reaction (PCR) tests [20]. Based on the previous analysis of the local repertoire, a POC laboratory was established in Dielmo in 2010 [21] and, in addition to testing for dengue and malaria, it tested the pathogens highlighted during the repertoire establishment phase (Table 1). In the first year after the POC laboratory was established, 440 blood samples were collected from febrile patients, and a pathogen was identified in 127 cases (28.9%), including malaria in 54 cases, B. crocidurae in 35 cases, R. felis in 30 cases, Bartonella spp. in 23 cases, C. burnetii in 1 cases, and T. whipplei in 1 case, with some patients presenting with a coinfection. No cases of dengue were diagnosed. Various technical difficulties have since been overcome and due to the fact that they are thermostable, easy to transport, and protected against contamination, lyophilized, ready-to-use mixes for individual tests have been introduced to POC laboratories in Dielmo [21]. A second POC laboratory was established in January 2016 in the Niakhar area.
Prevalence of Pathogens Responsible of Fever Diagnosed in 2 Point-of-Care Laboratories Established in Dielmo (2010–2016) and Niakhar (2016), Rural Senegal
| . | Year . | Rickettsia felis . | Other Rickettsia . | Borrelia spp. . | Tropheryma whipplei . | Bartonella spp. . | Bartonella quintana . | Coxiella burnetii . | Streptococcus pneumoniae . | Staphylococcus aureus . | Salmonella sp. . | Plasmodium falciparum . | Total Positives . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dielmo | ||||||||||||||
| Total POC | 2010–2016 | 70 | 5 | 185 | 17 | 29 | 16 | 7 | 6 | 0 | 0 | 95 | 430 | 3307 |
| Prevalences (%) | 2010–2016 | 2.12 | 0.15 | 5.59 | 0.51 | 0.88 | 0.48 | 0.21 | 0.18 | 0.00 | 0.00 | 2.87 | 13.00 | … |
| Niakhar | ||||||||||||||
| Total POC | 2016 | 0 | 0 | 91 | 0 | 0 | 0 | 0 | 26 | 6 | 0 | 18 | 141 | 810 |
| Prevalences (%) | 2016 | 0.00 | 0.00 | 11.23 | 0.00 | 0.00 | 0.00 | 0.00 | 3.21 | 0.74 | 0.00 | 2.22 | 17.41 | … |
| . | Year . | Rickettsia felis . | Other Rickettsia . | Borrelia spp. . | Tropheryma whipplei . | Bartonella spp. . | Bartonella quintana . | Coxiella burnetii . | Streptococcus pneumoniae . | Staphylococcus aureus . | Salmonella sp. . | Plasmodium falciparum . | Total Positives . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dielmo | ||||||||||||||
| Total POC | 2010–2016 | 70 | 5 | 185 | 17 | 29 | 16 | 7 | 6 | 0 | 0 | 95 | 430 | 3307 |
| Prevalences (%) | 2010–2016 | 2.12 | 0.15 | 5.59 | 0.51 | 0.88 | 0.48 | 0.21 | 0.18 | 0.00 | 0.00 | 2.87 | 13.00 | … |
| Niakhar | ||||||||||||||
| Total POC | 2016 | 0 | 0 | 91 | 0 | 0 | 0 | 0 | 26 | 6 | 0 | 18 | 141 | 810 |
| Prevalences (%) | 2016 | 0.00 | 0.00 | 11.23 | 0.00 | 0.00 | 0.00 | 0.00 | 3.21 | 0.74 | 0.00 | 2.22 | 17.41 | … |
Abbreviation: POC, point-of-care.
Prevalence of Pathogens Responsible of Fever Diagnosed in 2 Point-of-Care Laboratories Established in Dielmo (2010–2016) and Niakhar (2016), Rural Senegal
| . | Year . | Rickettsia felis . | Other Rickettsia . | Borrelia spp. . | Tropheryma whipplei . | Bartonella spp. . | Bartonella quintana . | Coxiella burnetii . | Streptococcus pneumoniae . | Staphylococcus aureus . | Salmonella sp. . | Plasmodium falciparum . | Total Positives . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dielmo | ||||||||||||||
| Total POC | 2010–2016 | 70 | 5 | 185 | 17 | 29 | 16 | 7 | 6 | 0 | 0 | 95 | 430 | 3307 |
| Prevalences (%) | 2010–2016 | 2.12 | 0.15 | 5.59 | 0.51 | 0.88 | 0.48 | 0.21 | 0.18 | 0.00 | 0.00 | 2.87 | 13.00 | … |
| Niakhar | ||||||||||||||
| Total POC | 2016 | 0 | 0 | 91 | 0 | 0 | 0 | 0 | 26 | 6 | 0 | 18 | 141 | 810 |
| Prevalences (%) | 2016 | 0.00 | 0.00 | 11.23 | 0.00 | 0.00 | 0.00 | 0.00 | 3.21 | 0.74 | 0.00 | 2.22 | 17.41 | … |
| . | Year . | Rickettsia felis . | Other Rickettsia . | Borrelia spp. . | Tropheryma whipplei . | Bartonella spp. . | Bartonella quintana . | Coxiella burnetii . | Streptococcus pneumoniae . | Staphylococcus aureus . | Salmonella sp. . | Plasmodium falciparum . | Total Positives . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dielmo | ||||||||||||||
| Total POC | 2010–2016 | 70 | 5 | 185 | 17 | 29 | 16 | 7 | 6 | 0 | 0 | 95 | 430 | 3307 |
| Prevalences (%) | 2010–2016 | 2.12 | 0.15 | 5.59 | 0.51 | 0.88 | 0.48 | 0.21 | 0.18 | 0.00 | 0.00 | 2.87 | 13.00 | … |
| Niakhar | ||||||||||||||
| Total POC | 2016 | 0 | 0 | 91 | 0 | 0 | 0 | 0 | 26 | 6 | 0 | 18 | 141 | 810 |
| Prevalences (%) | 2016 | 0.00 | 0.00 | 11.23 | 0.00 | 0.00 | 0.00 | 0.00 | 3.21 | 0.74 | 0.00 | 2.22 | 17.41 | … |
Abbreviation: POC, point-of-care.
The main bacterial organisms that are identified can be successfully treated with doxycycline, but many of them are not sensitive to amoxicillin and/or cotrimoxazole, which are usually the empirically recommended antibiotic treatment. These observations may change the treatment strategy for acute unexplained fevers in West Africa, in the context of a decline in malaria in many parts of sub-Saharan Africa.
Description of New Syndromes
Observational research coupled with technology may also enable new clinical involvements to be described. In Ndiop, in November 2011, an 8-month-old girl suffered from fever accompanied by severe cutaneous eruptions that evolved from small vesicles to ulcers up to 5 cm in diameter. Blood samples tested in the POC laboratory for the pathogens described above were negative, but quantitative PCR specific for R. felis was positive for the cutaneous lesion swab. The patient recovered after 5 days of treatment with doxycycline. The Wolof word “yaaf” was proposed to identify this clinical entity, which corresponded to a primary infection with R. felis [22]. Only the ability to diagnose a spectrum of infectious diseases in a rural front-line dispensary directly after the admission of the patient enabled this clinical picture to be described.
The Role of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry
Culture remains essential in clinical microbiology, particularly for patients hospitalized in teaching hospitals [23], notably in order to determine antibiotic susceptibility [24]. However, identification methods are often costly. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) has become the gold standard for bacterial identification in laboratories in developing countries [25]. Alongside our molecular platform, in 2012, a MALDI-TOF MS was introduced to the clinical microbiology laboratory in the Hôpital Principal de Dakar (Senegal) and was used for the routine identification of infectious agents, making it possible to identify 2082 bacteria of the 2429 bacteria (85.7%) tested [26] at the species level. The robustness of the identification results performed using MALDI-TOF MS in Dakar was confirmed by comparison with results obtained in Marseille, France [27]. The 10 most common bacteria represented 94.2% of all bacteria routinely identified in the laboratory in Dakar (Escherichia coli, Klebsiella pneumoniae, Streptococcus agalactiae, Acinetobacter baumannii, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus haemolyticus, Enterobacter cloacae, Enterococcus faecalis, and Staphylococcus epidermidis) [25]. The correct identification of the Candida species was also demonstrated for more than 98% of the strains tested [28], as well as other applications such as the identification of biting midges of the genus Culicoides [29].
From Diagnosis to Intervention and Prevention
As tick-borne relapsing fevers were a neglected public health problem and a major cause of fever in rural Senegal, as demonstrated by our results [11–13], we implemented a borreliosis preventive control that included awareness-raising among local residents and cementing floors in bedrooms and outbuildings in order to avoid contact between the inhabitants and tick vectors. This led to a significant reduction in the incidence of borreliosis, from 10.55 to 2.63 cases per 100 person-years in Dielmo and from 3.79 to 1.39 per 100 person-years in Ndiop [28]. Public health authorities should adopt this effective tool for promoting rural health through national prevention programs.
PARASITOLOGY
Schistosomiasis
Schistosomiasis continues to be a public health problem in Africa. Urinary schistosomiasis is the most common form in West Africa. In Senegal, transmission takes place continuously along the Senegal River Basin [30] and occurs seasonally in most parts of the country. Several studies have been conducted to understand the epidemiology of urinary schistosomiasis in the district of Niakhar, an area of seasonal transmission in Senegal, and to show whether, with repeated mass treatment, it is possible to eliminate urinary schistosomiasis. The studies show that the district of Niakhar is endemic for urinary schistosomiasis, and overall prevalence has been significantly reduced from 57.7% to 10.1%. Repeated annual treatments are suggested to have had a considerable impact on the transmission dynamics of Schistosoma haematobium in Niakhar due to the nature of the epidemiological system with seasonal transmission [31, 32].
Leishmaniases
Leishmaniases are a group of diseases caused by protozoan parasites from more than 20 Leishmania species that are transmitted to humans through the bites of infected female phlebotomine sandflies. There are 3 main forms of the disease: cutaneous leishmaniasis, visceral leishmaniasis, or kala-azar, and mucocutaneous leishmaniasis. Recurrent epidemics of visceral leishmaniasis in east Africa (Ethiopia, Kenya, South Sudan, and Sudan) have caused high morbidity and mortality in affected communities [33].
Mansonellosis
Large parts of African countries are colonized by Mansonella, a very common but poorly described filarial nematode. The prevalence of this nematode is often very high in endemic areas, even in children, and increases with age. Mansonella perstans, Mansonella streptocerca, Mansonella ozzardi, and Mansonella rodhaini are the 3 parasites responsible for human mansonellosis in Africa. Mansonella perstans is a human filarial nematode that is highly prevalent in some areas across sub-Saharan Africa and South America. It is a little known but widespread human filarial parasite, more than 100 million people may be infected, and approximately 600 million people living in the 33 countries are at high risk for M. perstans infection in Africa alone.
Some studies have been carried out on its epidemiology and the associated health consequences in endemic populations, and no simple and effective drug therapy for treatment and control of the infection has been identified. Data recently obtained by our team from Senegal [34] showed virtual absence of microfilariae in 1159 Culicoides collected in a region highly endemic for mansonellosis. This may mean the presence of another vector, at least in Senegal. In equatorial Africa (in Gabon, particularly) another species of Mansonella may cause human mansonellosis [35].
CONCLUSIONS AND PERSPECTIVES
Knowledge of the local repertoire of pathogens responsible for fever is critical for the appropriate treatment and prevention of infectious diseases. The establishment of this sentinel network system for the molecular detection of emerging pathogens in patients with fever in rural dispensaries in Senegal adds significant value to our epidemiological understanding of the causes of fever in rural Senegalese populations. The network also improves the quality of diagnosis and treatment for the research program that is working to improve quality of life in the study population and may serve as a catalyst for further research in this community.
The continuous evolution of microorganisms and changes in the environment and in human habits cannot be modeled [36]. The recent example of the Ebola virus outbreak has demonstrated the need for molecular biology platforms, sometimes even mobile platforms [37], in low-income countries. For studies on infectious diseases, technology-driven research is preferable to hypothesis-driven research [6], as recently highlighted by Quick et al. who demonstrated the value of real-time portable genome sequencing for Ebola surveillance. The authors sequenced 142 Ebola virus samples in Guinea, generating results less than 24 hours after receipt of the positive samples [38]. We believe that the rapid and effective identification of microorganisms as performed by POC laboratories and the ability to easily and quickly diagnose most emerging infections [20], including in rural Africa [21], remains the key to understanding infectious diseases and to improving the management of pathogens that are currently underdiagnosed.
In addition, the development of real-time surveillance systems that enable rapid and flexible responses to be made will be a significant improvement for the future of low-income countries [39]. Finally, after the elucidation of nonmalarial causes of fever, a future challenge will be to understand maternal and perinatal fever and the burden of stillbirth, which remain considerable challenges for public health in low-income countries [40]. We are convinced that, having elucidated the causes of nonmalarial fever, such molecular platforms could help to prevent death and complications during pregnancy, childbirth, and in newborns (Figure 1).
Notes
Financial support. This work was supported by the Fondation Méditerranée Infection.
Supplement sponsorship. This article appears as part of the supplement “Emerging Concepts and Strategies in Clinical Microbiology and Infectious Diseases,” sponsored by IHU Méditerranée Infection.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Correspondence: C. Sokhna, Aix-Marseille Univ, URMITE, IHU Méditerranée-Infection, UM63, CNRS 7278, IRD 198, Inserm U1095, Campus International UCAD-IRD, BP 1386, CP 18524, Dakar, Sénégal (cheikh.sokhna@ird.fr).





Comments