-
PDF
- Split View
-
Views
-
Cite
Cite
Jasper Fuk-Woo Chan, Anna Jinxia Zhang, Shuofeng Yuan, Vincent Kwok-Man Poon, Chris Chung-Sing Chan, Andrew Chak-Yiu Lee, Wan-Mui Chan, Zhimeng Fan, Hoi-Wah Tsoi, Lei Wen, Ronghui Liang, Jianli Cao, Yanxia Chen, Kaiming Tang, Cuiting Luo, Jian-Piao Cai, Kin-Hang Kok, Hin Chu, Kwok-Hung Chan, Siddharth Sridhar, Zhiwei Chen, Honglin Chen, Kelvin Kai-Wang To, Kwok-Yung Yuen, Simulation of the Clinical and Pathological Manifestations of Coronavirus Disease 2019 (COVID-19) in a Golden Syrian Hamster Model: Implications for Disease Pathogenesis and Transmissibility, Clinical Infectious Diseases, Volume 71, Issue 9, 1 November 2020, Pages 2428–2446, https://doi.org/10.1093/cid/ciaa325
Close - Share Icon Share
Abstract
A physiological small-animal model that resembles COVID-19 with low mortality is lacking.
Molecular docking on the binding between angiotensin-converting enzyme 2 (ACE2) of common laboratory mammals and the receptor-binding domain of the surface spike protein of SARS-CoV-2 suggested that the golden Syrian hamster is an option. Virus challenge, contact transmission, and passive immunoprophylaxis studies were performed. Serial organ tissues and blood were harvested for histopathology, viral load and titer, chemokine/cytokine level, and neutralizing antibody titer.
The Syrian hamster could be consistently infected by SARS-CoV-2. Maximal clinical signs of rapid breathing, weight loss, histopathological changes from the initial exudative phase of diffuse alveolar damage with extensive apoptosis to the later proliferative phase of tissue repair, airway and intestinal involvement with viral nucleocapsid protein expression, high lung viral load, and spleen and lymphoid atrophy associated with marked chemokine/cytokine activation were observed within the first week of virus challenge. The mean lung virus titer was between 105 and 107 TCID50/g. Challenged index hamsters consistently infected naive contact hamsters housed within the same cages, resulting in similar pathology but not weight loss. All infected hamsters recovered and developed mean serum neutralizing antibody titers ≥1:427 14 days postchallenge. Immunoprophylaxis with early convalescent serum achieved significant decrease in lung viral load but not in lung pathology. No consistent nonsynonymous adaptive mutation of the spike was found in viruses isolated from the infected hamsters.
Besides satisfying Koch’s postulates, this readily available hamster model is an important tool for studying transmission, pathogenesis, treatment, and vaccination against SARS-CoV-2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is causing a pandemic of coronavirus disease 2019 (COVID-19) [1]. This newly emerged coronavirus belongs to the genus Betacoronavirus, which also includes the highly pathogenic SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) [2]. Phylogenetic analysis suggested that all 3 coronaviruses likely originated from bats [3]. SARS-CoV-2 was first identified in patients with atypical pneumonia geographically linked to the Huanan seafood wholesale market with wild animal trade in Wuhan, China. The virus is highly transmissible among humans, with clusters occurring among close contacts in family, church, community, cruise ship, nursing home, and hospital settings [2, 4–7]. Within just 3 months, more than 100 000 cases of COVID-19 with more than 3000 deaths have been reported globally in more than 100 countries/territories [8]. As readily available and suitable experimental animal models are lacking, the major route of transmission is speculated to be through respiratory droplets, direct or indirect contact with other infectious bodily fluids, such as saliva, feces, tears, and blood.
Recent data showed that the clinical severity of COVID-19 ranges widely from asymptomatic infection to fatal disease. In children, COVID-19 is usually asymptomatic or mildly symptomatic [2, 9]. The majority of immunocompetent adult patients with COVID-19 present with fever, respiratory symptoms, and radiological ground-glass lung opacities [10]. In severe cases, especially among elderly and immunocompromised patients, besides respiratory failure, extrapulmonary manifestations such as diarrhea, confusion, hepatic and renal dysfunction, lymphopenia, thrombocytopenia, and elevated inflammatory biomarkers have also been reported [10].Small animal models that recapitulate the clinical and pathological features of COVID-19 in human are essential tools for studying the pathogenesis, transmission, antiviral treatments, and vaccines for this emerging global health threat. Based on the recent identification of human angiotensin-converting enzyme 2 (ACE2) as the cell entry receptor of SARS-CoV-2, a transgenic human ACE2 mouse model was preliminarily reported [11]. However, the expression of human ACE2 in these transgenic mice is not physiological and these mice are not readily available. A rhesus macaque model was also reported, but expertise and Biosafety Level-3 facilities to handle nonhuman primates are scarce [11]. In this study, we established a readily available small animal model for COVID-19 using the golden Syrian hamster (Mesocricetus auratus). Our in silico structural analysis predicted that the hamster ACE2 can bind with SARS-CoV-2 spike glycoprotein receptor-binding domain (RBD) at high binding affinity. The clinical features, viral kinetics, histopathological changes, and immune responses in SARS-CoV-2–infected Syrian hamsters closely mimic those described in many patients with COVID-19. Using this newly established model, we demonstrated the high transmissibility of SARS-CoV-2 among close contact animals.
METHODS
Amino Acid Sequences and Binding Energy Comparison Between Human and Other Mammalian ACE2
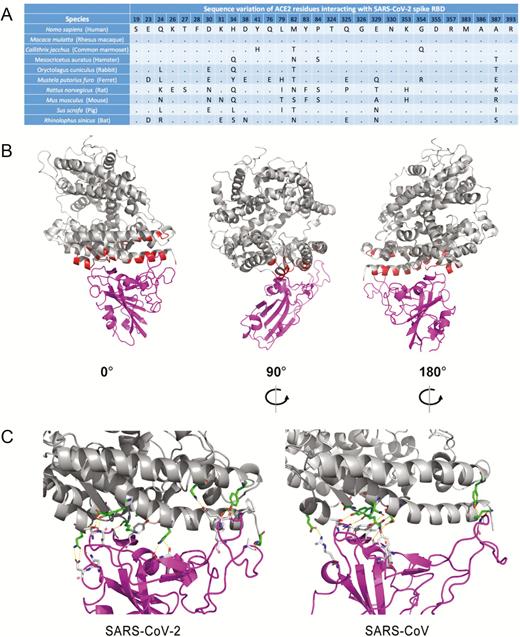
Multiple sequence alignment was generated using the human, rhesus macaque, common marmoset, hamster, rabbit, ferret, rat, mouse, pig, and bat ACE2 protein sequences (Uniprot accession numbers Q9BYF1, B6DUF3, F7CNJ6, G1TEF4, A0A1U7QTA1, Q2WG88, Q5EGZ1, Q8R0I0, K7GLM4, and E2DHI7, respectively) with MUSCLE [12]. Missing loop residues in the SARS-CoV-2 spike protein RBD (Protein Data Bank [PDB] ID: 6VSB) were modeled with I-TASSER [13]. The human ACE2 and SARS-CoV spike complex structure model (PDB ID: 2AJF) was used to model ACE2 and SARS-CoV-2 spike complex by superposition of SARS-CoV-2-RBD to SARS-CoV-RBD. ACE2 spike complexes of SARS-CoV-2 and SARS-CoV were then minimized with the Rosetta Relax application [14]. ACE2 spike interface binding energy was calculated by the InterfaceAnalyzer within Rosetta. Interface binding energies of other species were calculated by introducing mutations to the interface according to the amino acid differences with Rosetta protein-protein ΔΔG protocol. Amino acid residues of ACE2 within 4.0 Å of SARS-CoV-2 spike RBD were considered as interacting residues. Pymol was used to highlight the interacting sites in red in the ACE2 structure and to show the hydrogen bonds in yellow dashed lines in Figure 1.
Virus and Biosafety
SARS-CoV-2 was isolated from the nasopharyngeal aspirate specimen of a patient with laboratory-confirmed COVID-19 in Hong Kong [15]. The plaque purified viral isolate was amplified by 1 additional passage in VeroE6 cells to make working stocks of the virus as described previously [15]. All experiments involving live SARS-CoV-2 followed the approved standard operating procedures of The University of Hong Kong (HKU) Biosafety Level-3 facility.
Animal Model
Approval was obtained from the HKU Committee on the Use of Live Animals in Teaching and Research. Male and female Syrian hamsters, aged 6–10 weeks, were obtained from the Chinese University of Hong Kong Laboratory Animal Service Centre through the HKU Laboratory Animal Unit. The animals were kept in Biosafety Level-2 housing and given access to standard pellet feed and water ad libitum until virus challenge in our Biosafety Level-3 animal facility. Phosphate-buffered saline (PBS) was used to dilute virus stocks to the desired concentration, and inocula were back-titrated to verify the dose given. Dulbecco’s Modified Eagle Medium (DMEM) containing 105 plaque-forming units in 100 µL of SARS-CoV-2 was intranasally inoculated under intraperitoneal ketamine (200 mg/kg) and xylazine (10 mg/kg) anesthesia. Mock-infected animals were challenged with 100 µL of PBS. Animals were monitored twice daily for clinical signs of disease (Supplementary Figure 1). Their body weight and survival were monitored for 14 days postinoculation (dpi). Five animals in the virus-challenged group were sacrificed at 2, 4, and 7 dpi for virological and histopathological analyses. The remaining animals were sacrificed at 14 dpi. Blood and major organ tissues at necropsy were separated into 2 parts, one immediately fixed in 10% PBS-buffered formalin, the other immediately frozen at −80°C until use.
Histopathology, Immunohistochemical, Immunofluorescence, and TUNEL Staining
These staining and microscopy procedures on tissue sections were performed as described previously with modifications (Supplementary Methods) [16].
Viral Load and Median Tissue Culture Infectious Dose Assays
Viral load studies by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) assay were conducted on the blood and organ tissues. Quantitation of live infectious virus by median tissue culture infectious dose (TCID50) assay was performed on the nasal turbinate and lung tissues (Supplementary Methods) [15].
Chemokine/Cytokine Profiling
Chemokine/cytokine profiling was performed on the lung tissues of the virus-challenged and mock-infected animals by qRT-PCR (Supplementary Methods and Supplementary Table 1) [17].
Neutralizing Antibody Detection and Passive Immunization
Serum anti–SARS-CoV-2 neutralizing antibody detection by microneutralization assay and passive immunization were performed as described previously (Supplementary Methods) [18, 19].
Transmission Study Among Close Contact Animals
Eight hamsters were intranasally challenged with SARS-CoV-2 (0 dpi) (Supplementary Figure 2). Twenty-four hours later (1 dpi), each SARS-CoV-2–challenged hamster was transferred to a new cage, with each cage containing 1 naive hamster as a close contact. Five virus-challenged and 5 contact hamsters were sacrificed for viral load and histopathological studies at 4 dpi and 4 days postexposure, respectively. The remaining hamsters were kept for clinical observation until 14 dpi. The surface spike gene of viral isolates from the hamsters was Sanger sequenced as described previously [2].
Statistical Analysis
All data were analyzed with GraphPad Prism software (GraphPad Software, Inc). Weight losses were compared using 2-way ANOVA and Student’s t test was used to determine significant differences in virus titers between the different groups [16]. A P value less than .05 was considered statistically significant.
RESULTS
In Silico Prediction of Animal Species That Might Be Susceptible to SARS-CoV-2
As the binding affinity of ACE2 to SARS-CoV-2 surface spike glycoprotein may be dictated by the amino acid composition at the binding interface, structural analysis was performed and predicted 29 amino acid residues at the ACE2 interface that may interact with the SARS-CoV-2 spike glycoprotein RBD (Figure 1A, B). Comparison of the 29 amino acid residues’ alignment between human and other mammalian species revealed that the rhesus macaque ACE2 is 100% identical to human ACE2 at the interface region. Syrian hamster and common marmoset ACE2 proteins are also highly similar to human ACE2, each with 3–4 mutations at the interface. The binding energy prediction using ACE2–SARS-CoV-2 spike RBD docking and protein-protein interface mutation ΔΔG modeling showed that the hamster ACE2 exhibited the highest binding affinity to the spike of both SARS-CoV-2 (−49.96 Rosetta energy units [REU]) and SARS-CoV (−48.71 REU) among all the species other than humans and rhesus macaques (Supplementary Table 2). Notably, the SARS-CoV-2 spike exhibited higher binding affinity with ACE2 than did the SARS-CoV spike. This might be attributed to the more compact interacting interface with more hydrogen bonds formed between SARS-CoV-2 spike glycoprotein RBD and ACE2 (Figure 1C). A Syrian hamster model was therefore evaluated to simulate the clinical, virological, pathological, and immunological manifestations of SARS-CoV-2–induced COVID-19.
Interaction between the SARS-CoV-2 spike glycoprotein RBD and ACE2. A, Multiple alignment of the amino acid residues from ACE2 proteins of human and other mammalian species. The dots indicate amino acid residues that are identical between the human and mammalian ACE2. B, Interaction model of the SARS-CoV-2 spike glycoprotein S1 subunit RBD (magenta) and human ACE2 (gray) in front (left), side (middle), and back (right) views. The ACE2 amino acid residues within 4.0 Å of the SARS-CoV-2 spike glycoprotein S1 subunit RBD are highlighted in red. C, Hydrogen bonds between ACE2 and the spike glycoprotein S1 subunit RBDs of SARS-CoV-2 (left) and SARS-CoV (right). Hydrogen bonds are indicated by yellow dashed lines, and hydrogen bond donors and acceptors are represented by sticks (oxygen = red sticks; nitrogen = blue sticks; carbon = green sticks in ACE2 and gray sticks in RBDs). Abbreviations: ACE2, angiotensin-converting enzyme 2; RBD, receptor-binding domain; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Clinical Features, Tissue Tropism, Viral Load, and Virus Titer Changes
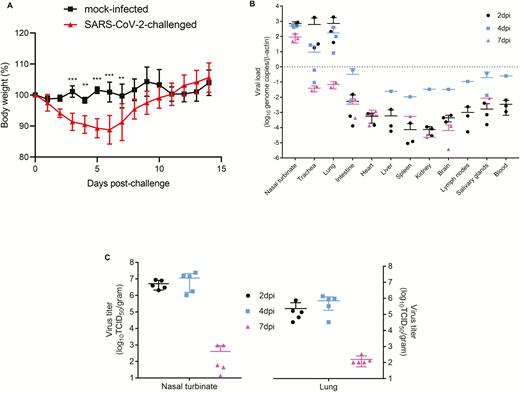
SARS-CoV-2–challenged but not mock-infected animals exhibited progressive mean body weight loss of up to approximately 11% from 1 dpi to 6 dpi, then gradually regained their weight by 14 dpi (Figure 2A). They developed lethargy, ruffled fur, hunched back posture, and rapid breathing since 2 dpi and started to recover at 7 dpi. None of the SARS-CoV-2–infected and mock-infected animals died.
Body weight changes and tissue tropism of SARS-CoV-2 infection in the Syrian hamster model. A, Body weight changes of SARS-CoV-2–challenged (n = 11 at 0 dpi to 7 dpi; n = 6 at 8 dpi to 14 dpi) and mock-infected (n = 3 at 0 dpi to 14 dpi) hamsters. B, Viral load by qRT-PCR assay in the respiratory tract tissues, extrapulmonary organ tissues, and blood of SARS-CoV-2–challenged hamsters at 2 dpi, 4 dpi, and 7 dpi (n = 3/day). C, Quantitation of virus titer by TCID50 assay in the nasal turbinate and lung of SARS-CoV-2–challenged hamsters at 2 dpi, 4 dpi, and 7 dpi (n = 5/day). **P < .01, ***P < .0001 by 2-way ANOVA. Error bars represent SDs of the mean. Abbreviations: ANOVA, analysis of variance; dpi, days postinoculation; qRT-PCR, quantitative reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TCID50, median tissue culture infectious dose.
To investigate the tissue tropism of SARS-CoV-2 in Syrian hamsters, we performed viral load studies on the upper respiratory tract (nasal turbinate), lower respiratory tract (trachea and lung), extrapulmonary organs (intestine, salivary glands, heart, liver, spleen, lymph nodes, kidneys, and brain), and blood harvested from virus-challenged animals at different time points, which represented the early (2 dpi), severe (4 dpi), and start of recovery (7 dpi) phases. The mean viral loads (Figure 2B) in the nasal turbinate, trachea, and lung (up to approximately 103 genome copies/β-actin) were consistently the highest among all tested organ tissues from 2 dpi to 7 dpi. The viral loads progressively decreased in airway tissues from 2 dpi to 7 dpi. Viral culture of lung tissues showed high titers of 105–107 TCID50/g at 2 dpi and 4 dpi (Figure 2C). With regard to extrapulmonary tissues, the mean viral load in the intestine was also relatively high, especially on 4 dpi. For other organs or blood, low mean viral loads (<1 genome copy/β-actin) were detected at 2 dpi and peaked at 4 dpi, then progressively decreased to undetectable levels at 7 dpi in most samples.
Histopathological Changes in the Respiratory Tract
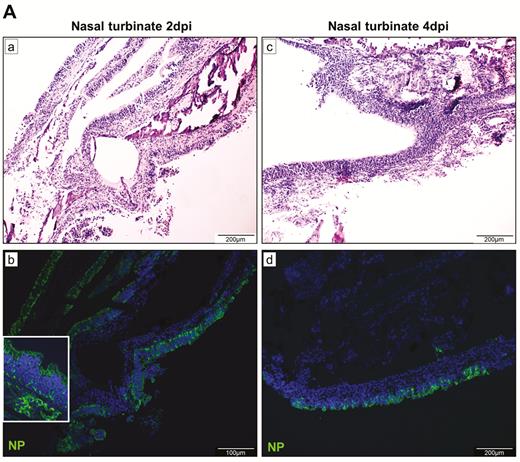
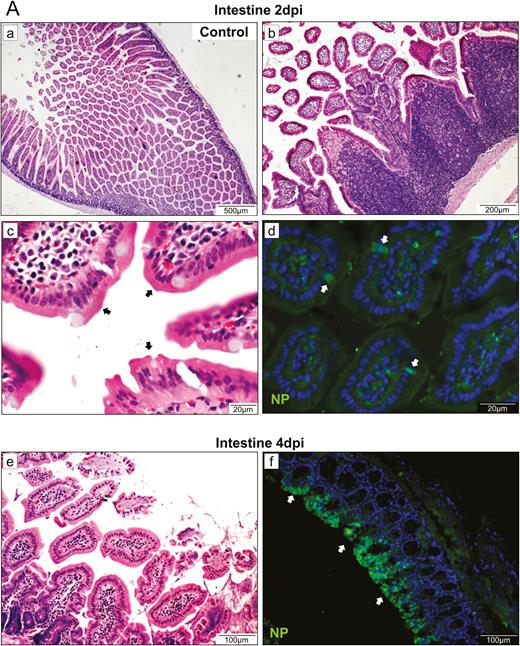
The nasal turbinate epithelium was intact with submucosal infiltration and blood vessel congestion at 2 dpi (Figure 3A). Abundant viral N protein expression was found throughout the epithelial layer and in some submucosal glands. At 4 dpi, epithelial cell desquamation with viral N protein expression and increased intraepithelial and submucosal infiltration were observed.
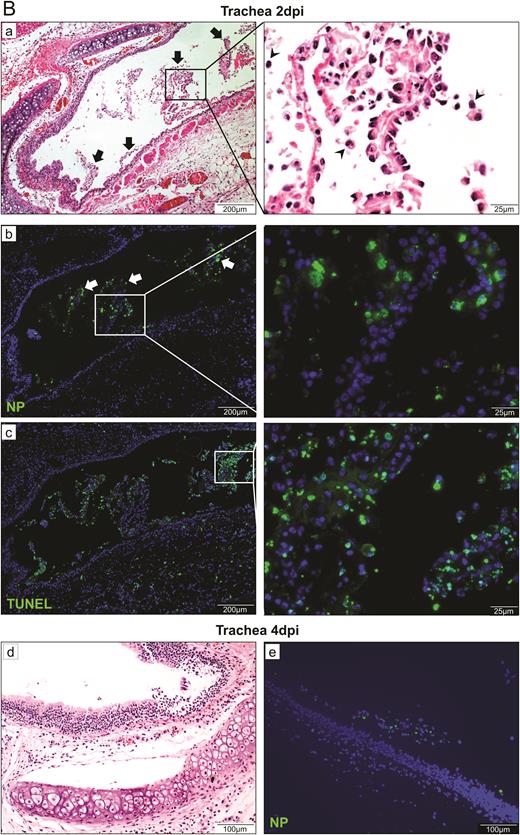
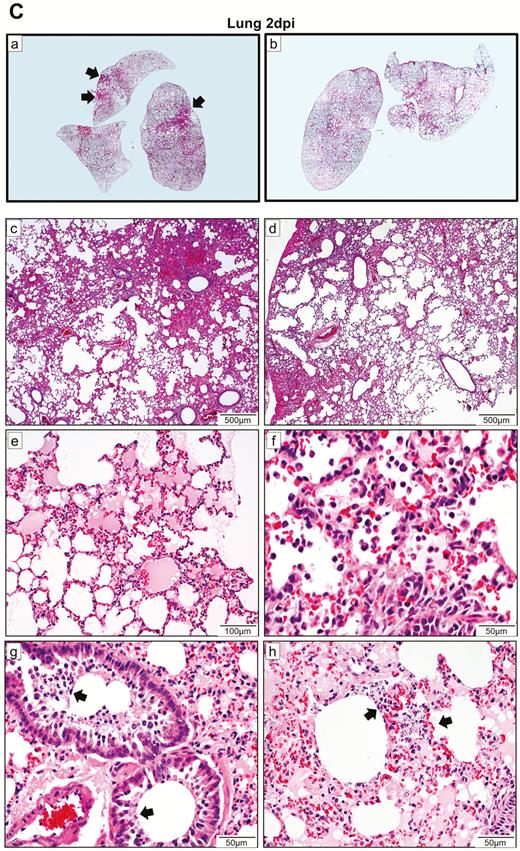
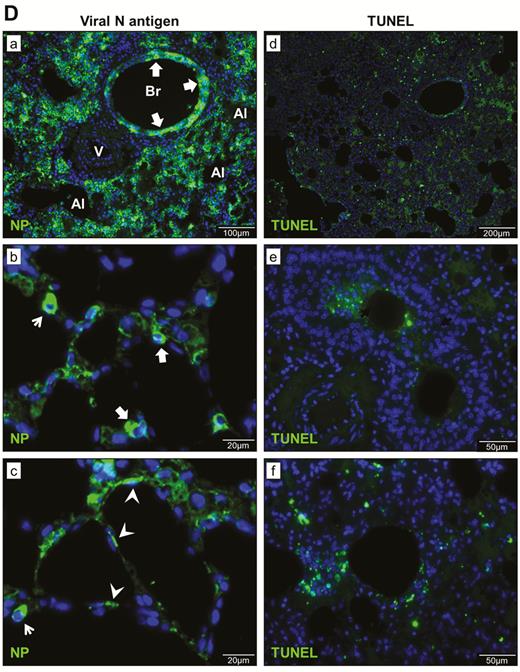
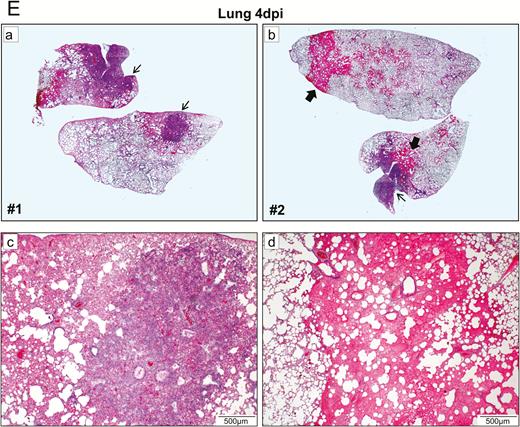
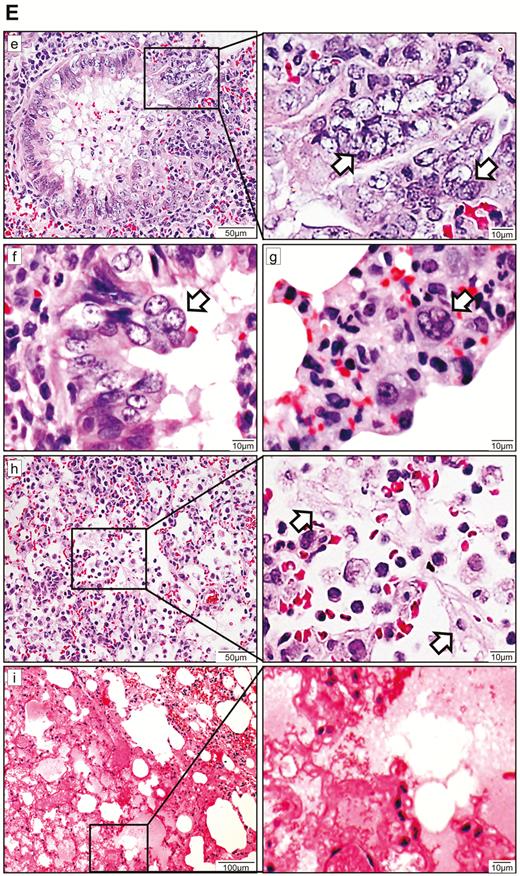
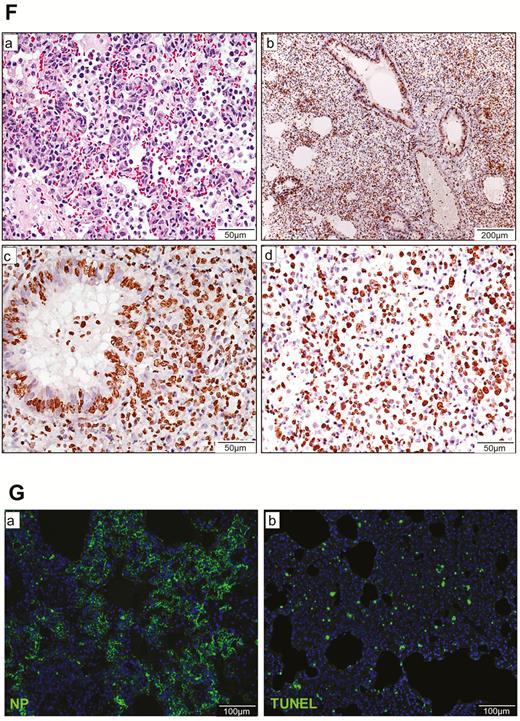
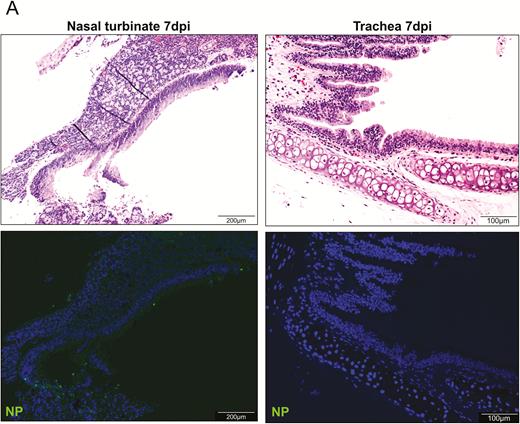
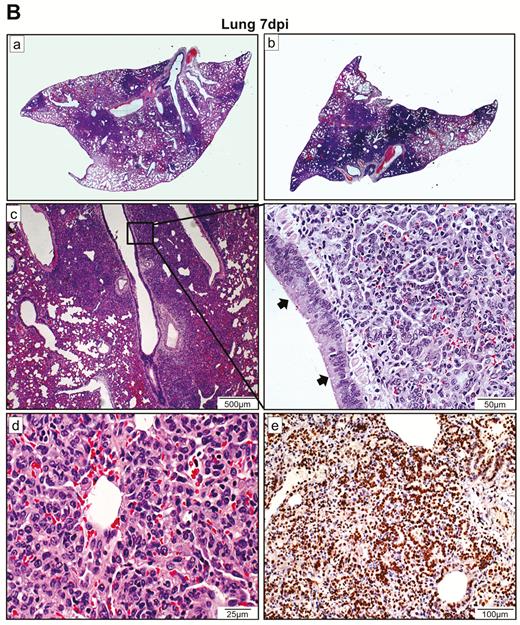
Histopathological, immunohistochemical, immunofluorescence, and TUNEL staining findings of respiratory tract tissues at days 2 and 4 after SARS-CoV-2 infection. A, Images of H&E- and immunofluorescence-stained nasal turbinate sections at 2 dpi and 4 dpi. (a) At 2 dpi, the epithelial layer was relatively intact with submucosal infiltration. (b) SARS-CoV-2 N protein was present almost throughout the entire epithelial layer and in some submucosal glands (insert). Cell nuclei were stained by DAPI. (c) At 4 dpi, epithelial cell death and desquamation were mild, but the intraepithelial and submucosal infiltration increased. (d) Viral N protein was expressed in the epithelial cells and cell debris. B, Trachea. (a) At 2 dpi, epithelial desquamation and loss of mucosal integrity with plugs of cell debris in the tracheal lumen were observed (black arrows) along with lamina propria connective tissue infiltration. The black square area was magnified showing detached epithelial cells mixed with mononuclear cells (arrowheads). (b) Viral N protein (white arrows and magnified images) was mostly found in intraluminal cell debris. (c) The same section of trachea was positive by TUNEL staining indicating apoptotic cell death. At 4 dpi, (d) tracheal epithelial desquamation and submucosal infiltration are mild and (e) viral N protein was only found in intraluminal cell debris. C, Histopathological changes in the lungs at 2 dpi. Images (a) and (b) represent lung tissues of two virus-challenged hamsters at low magnification (4x), showing patches of focal inflammation with intense color and pleural invaginations (arrows) were seen. (c and d) Varying severities of diffuse lung damage, including (e) protein-rich exudates and hyaline membrane filled alveolar space; (f) large amount of mononuclear cells infiltrating alveolar sac; (g) cell debris in bronchiolar lumen (arrows); and (h) alveolar collapse with cell death and acute hemorrhage (arrows). D, Viral N protein expression and TUNEL staining in the lungs. (a) SARS-CoV-2 N protein distribution in the lung (“Br” = bronchiole; “Al” = alveoli; “V” = blood vessel); (b) and (c) are magnified images illustrating viral N protein–positive staining in alveolar macrophages (thin arrows in b and c) and type I (arrowheads in c) and type II pneumocytes (thick arrows in b); (d) to (f) show TUNEL labeling in representative fields of the lung tissues. E, Histopathological changes in the lungs at 4 dpi. (a and b) Low-magnification images (4×) showing diffuse inflammation, large areas of consolidation, pleural invaginations (thin arrows), and severe hemorrhage (thick arrows). (c) Representative images of patchy consolidation and (d) pulmonary hemorrhage. High magnification showing (e) intense peribronchiolar infiltration, epithelial cell swelling, intraepithelial layer infiltration, mixture of cell debris and exudates filling in the bronchiolar lumen, and a gigantic multinucleated syncytial body in the magnified image (arrows); (f) and (g) show various sizes and locations of syncytial bodies (arrows); (h) alveolar collapse with mononuclear cell infiltrate and dead epithelial cells in alveolar space (arrows); and (i) marked hemorrhagic exudates with tissue destruction. F, (a) Extensive pulmonary cellular proliferation forming multiple layers of thickened alveolar wall. (b) to (d) Immunohistochemical staining showed extensive expression of proliferation marker Ki67 in bronchiolar epithelium and alveoli. G, Diffuse distribution of (a) viral N protein and (b) TUNEL staining showed diffuse apoptotic cell death in the lungs. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; dpi, days postinoculation; H&E, hematoxylin and eosin; NP, nucleocapsid protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TUNEL, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling.
The trachea demonstrated epithelial cell swelling, focal cilia loss, and mononuclear cell infiltration into the epithelium and lamina propria at 2 dpi (Figure 3B). Desquamation of large areas of the epithelium mixed with infiltrating mononuclear cells and cell debris was observed. Viral N protein was abundantly expressed in detached epithelial cells. Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) staining indicated severe apoptosis. At 4 dpi, viral N protein was only associated with detached cell debris.
In the lungs, patches of focal inflammation and pleural invaginations were seen at 2 dpi (Figure 3C). Diffuse alveolar destruction, protein-rich fluid exudate, hyaline membrane formation, marked mononuclear cell infiltration, cell debris–filled bronchiolar lumen, and alveolar collapse with hemorrhage were observed. Viral N protein was abundantly expressed in bronchiolar epithelial cells, alveolar macrophages, and type I and II pneumocytes (Figure 3D). TUNEL staining showed diffuse signals in the lung, bronchiolar lumen cell debris, and collapsed alveolar walls. At 4 dpi, increasing lung consolidation and severe pulmonary hemorrhage were seen (Figure 3E). Large syncytial bodies with multiple nuclei were seen in the epithelia of bronchioles and alveoli. Marked cellular proliferation with extensive expression of the proliferation marker Ki67 protein in both bronchiolar and alveolar cells was detected (Figure 3F). More abundant viral N protein expression and TUNEL staining signals were found all over the alveolar wall (Figure 3G).
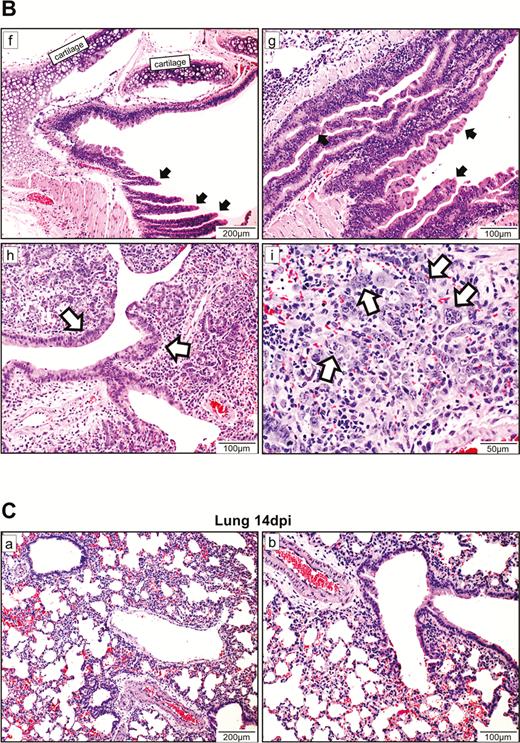
At 7 dpi, in the nasal turbinate mucosa, the intraepithelial and submucosal infiltration persisted, but viral N protein expression in the epithelial layer was much reduced and mainly located in the cell debris (Figure 4A). Tracheal epithelium recovered to an intact layer with cell proliferation forming projections into the lumen with scarce viral N protein expression. A marked increase in pulmonary cellularity with the peaking of lung consolidation was seen (Figure 4B). The mononuclear cell infiltrate and fluid exudate were largely replaced by massive pneumocyte proliferation with intensified Ki67 expression. The proliferative changes involved the epithelia of the trachea, small bronchi, bronchioles, and alveoli. In the trachea and small bronchi, the hyperplastic cells formed papillary projections into the lumen. The bronchioles and terminal bronchioles, which are normally lined by simple cuboidal epithelium, were replaced by multiple layers of irregularly arranged epithelial layers due to hyperplastic regeneration. Similarly, regenerative hyperplasia in the alveoli formed irregular-sized and -shaped structures. During this proliferative phase, viral N protein was no longer easily detectable. At 14 dpi, only mild pulmonary congestion and inflammatory cell infiltration were still detectable (Figure 4C). The air-exchange structures were remodeled and restored to normal.
Histopathological changes of upper and lower respiratory tract tissues at days 7 and 14 after SARS-CoV-2 infection. A, At 7 dpi, nasal turbinate tissue (left panels) showed mild submucosal infiltration. The epithelium was intact with a few mononuclear cells and cell debris seen in the luminal side. Viral N protein was only seen in the debris. Tracheal epithelial layer (right panels) was restored with cell proliferation forming projections into the lumen. No viral N protein expression was detected. B, (a) and (b) Lung sections of virus-challenged hamsters showing consolidation involving nearly half of the cutting area. (c) Lung consolidation around 2 bronchi and a pulmonary blood vessel with proliferative bronchial epithelial (arrows) and alveolar cells; (d) proliferation of alveolar cells formed granular structures, which were (e) stained strongly positive by Ki67 antibody; epithelial cell proliferation and hyperplasia in (f) trachea and (g) small bronchi with the hyperplastic cells forming papillary projections into the lumen (arrows); (h) bronchioles and terminal bronchioles and (i) alveoli showing irregular-sized and -shaped hyperplastic cells (arrows). C, Histopathological changes of the lung at 14 dpi. (a) and (b) The lungs showed mild blood vessel congestion and mononuclear cell infiltration, bronchiolar and alveolar epithelium proliferation, and restored tissue structures with largely resolved inflammation. Abbreviations: dpi, days postinoculation; NP, nucleocapsid protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Histopathological Changes in the Extrapulmonary Organ Tissues
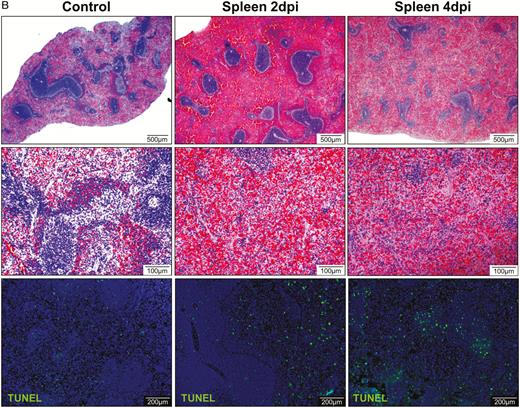
Although low levels of viral RNA were detected in multiple extrapulmonary organ tissues by qRT-PCR, viral N protein was detected only in the intestine (Figure 5A). At 2 dpi, intestinal mucosal epithelial cells were intact. The lamina propria exhibited more mononuclear cell infiltration. The Peyer’s patches were enlarged without forming germinal centers. Few mucosal epithelial cells and interstitial histiocytes were weakly positive for viral N protein. At 4 dpi, severe epithelial cell necrosis, damaged and deformed intestinal villi, and increased lamina propria mononuclear cell infiltration were observed. A large number of intestinal epithelial enterocytes were positive for viral N protein. The spleen size was markedly reduced at 2 and 4 dpi. Histopathology showed depletion of white and red pulps with reduced number and size of follicles (Figure 5B). TUNEL staining showed increased apoptosis at 2 and 4 dpi. At 14 dpi, increased follicular size and lymphocytes in red pulp were observed (Supplementary Figure 3A). The bronchial lymph nodes and mesenteric lymph nodes showed subcapsular and medullary lymphatic sinus ectasia with pale eosinophilic lymph (Supplementary Figure 3B). Viral N protein was not detected, but TUNEL-positive cells were abundantly found. The heart showed mild focal myocardial degeneration and interstitial edema at 2 and 4 dpi (Supplementary Figure 3C), but viral N protein was not detected. No histopathological changes were observed in other organs.
Representative histopathological changes of the intestine and spleen. A, H&E and immunofluorescence staining of intestinal tissue at 2 dpi. (a) Mock-infected hamster's intestine; (b) and (c) intestinal mucosal epithelia and Peyer’s patches of virus-challenged hamsters at 2 dpi; (d) a few mucosal epithelial cells and interstitial histiocytes were found weakly positive for viral NP (arrows); (e) epithelial cell necrosis and (f) viral NP expression (arrows) in the intestine at 4 dpi. B, Images of H&E- and TUNEL-stained spleen sections at 2 dpi and 4 dpi showing depletion of white and red pulps with reduced number and size of follicles and increased apoptosis. Abbreviations: dpi, days postinoculation; H&E, hematoxylin and eosin; NP, nucleocapsid protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TUNEL, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling.
Chemokine/Cytokine Profile, Neutralizing Antibody Response, and Passive Immunization
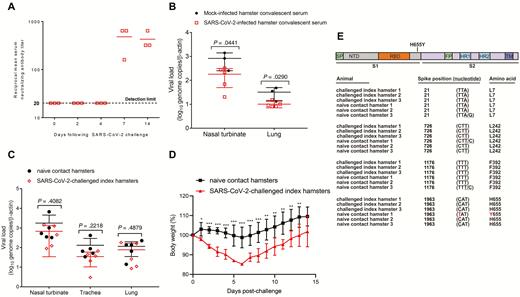
In line with the viral replication kinetics, the chemokine/cytokine profile in the lungs of the SARS-CoV-2–infected animals also exhibited a time-dependent trend of gene expression, with triggering at 2 dpi, peaking at 4 dpi, and resolving at 7 dpi (Supplementary Figure 4). Interferon-γ was potently induced at 2 dpi, implying that SARS-CoV-2 triggered the innate immune response mediated by natural killer cells and T cells. Consistent with interferon-γ induction, proinflammatory chemokines/cytokines were induced and peaked at 4 dpi, which represented the potent activation of inflammation and virus-induced cell death. At 7 dpi, type II interferon and proinflammatory cytokines such as interleukin-6 dropped to the basal level and increased transforming growth factor β (TGF-β) indicated the resolution of acute inflammation. Serum neutralizing antibodies against SARS-CoV-2 were detected in challenged animals as early as 7 dpi. The mean serum neutralizing antibody titers at 7 and 14 dpi were ≥1:480 and ≥1:427 (1:160 to ≥1:640), respectively (Figure 6A). Passive immunization resulted in significantly reduced nasal turbinate and lung viral loads (P < .05) (Figure 6B), but there was no observable improvement in clinical signs and histopathological changes.
Application of the hamster model for studying the effects of immunoprophylaxis and transmission of SARS-CoV-2. Passive immunization study: A, Reciprocal serum SARS-CoV-2–specific neutralizing antibody titers in SARS-CoV-2–infected hamsters on the indicated days following intranasal challenge. The mean antibody titers from 3 hamsters per group are shown on a logarithmic scale. The dotted line indicates the lower limit of detection (<1:20). B, Viral load in the nasal turbinate and lung tissues of the hamsters that received SARS-CoV-2–infected hamsters' convalescent sera collected at 14 dpi (n = 5) and those that received mock-infected hamster convalescent sera (n = 3). Error bars represent SDs of the mean. Student t test was used to calculate statistical significance. Transmission study of SARS-CoV-2 among close contact hamsters: C, Viral load by qRT-PCR assay in the respiratory tract tissues of SARS-CoV-2–challenged and naive contact hamsters at 4 dpi and 4 days after exposure, respectively (n = 5/group). D, Body weight changes in SARS-CoV-2–challenged index (n = 8 at 0–4 dpi; n = 3 at 5–14 dpi as 5 animals were sacrificed at 4 dpi) and naive contact (n = 8 at 0–4 dpi; n = 3 at 5–14 dpi) hamsters. E, Amino acid substitution in spike-coding sequences. Samples obtained from the SARS-CoV-2–challenged index hamsters and naive contact hamsters were subjected to Sanger sequencing. Spike-coding sequences of each sample were compared, and the positions corresponding to the spike gene were listed. The nucleotide substitutions were underlined. *P < .05, **P < .01, ***P < .0001 by 2-way ANOVA. Error bars represent SDs of the mean. Abbreviations: ANOVA, analysis of variance; dpi, days postinoculation; FP, fusion peptide; HR1, heptad repeat 1; HR2, heptad repeat 2; NTD, N-terminal domain; qRT-PCR, quantitative reverse transcription–polymerase chain reaction; RBD, receptor-binding domain; S1, spike S1 subunit; S2, spike S2 subunit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SP, signal peptide; TM, transmembrane domain.
Transmission of SARS-CoV-2 Among Close Contact Animals
To study the transmissibility among close contacts, we housed SARS-CoV-2–challenged index hamsters and naive contact hamsters in the same cage together in a 1:1 ratio. The upper and lower respiratory tract specimens of both the index virus-challenged and naive contact animals at 4 days after exposure were RT-PCR-positive for SARS-CoV-2 RNA (Figure 6C). The mean viral loads in the respiratory tract tissues of the challenged animals were not significantly different. Interestingly, the index animals exhibited significantly more weight loss (P < .05) than the contact animals between 2 and 8 dpi (Figure 6D). The histopathological changes and viral N protein expression in the contact animals’ nasal turbinate, trachea, lung, and extrapulmonary tissues were similar to those of the challenged animals (Supplementary Figure 5). These findings confirmed that SARS-CoV-2 had spread by close contact from the challenged index animals to the naive contact animals. Importantly, all contact animals were infected in this transmission study. Sequencing of the surface spike gene from the index and contact animals showed no changes except for 1 contact animal with an H655Y mutation (Figure 6E).
DISCUSSION
The clinical and histopathological findings from this SARS-CoV-2 hamster model closely resemble the manifestations of upper and lower respiratory tract infection in humans. The airway involvement is evident from nasal turbinate to trachea and pulmonary alveoli associated with changes of inflammation, cellular viral N protein expression, and high viral load during the first week. The disease progressed with increasing respiratory rate, decreasing activity, and progressive weight loss, and was most severe by 6 dpi, which is similar to the disease course of patients with COVID-19 [20]. Histopathology showed progression from the initial phase of exudative inflammation to diffuse alveolar damage with hemorrhage and necrosis, and finally the proliferative phase after 1 week. Our findings were in agreement with the human autopsy findings, except that our model also demonstrated extensive tissue apoptosis [21]. None of the challenged or contact hamsters died as less than 5% of human infections are lethal [10].
With regard to extrapulmonary manifestations, although diarrhea is not clinically evident in challenged hamsters, histopathology showed intestinal mucosal inflammation, epithelial necrosis, and viral N protein expression in enterocytes. This finding corroborates with the clinical manifestation of diarrhea in only 2.0–10.1% of patients with COVID-19, although up to 53% of their stool samples are RT-PCR positive [7, 22, 23]. Myocardial degenerative changes were noted both in our infected hamsters and patient autopsies despite the absence of viral antigen detection in the tissues, which is also consistent with the occasional report of heart failure in patients with COVID-19 [22]. Lymphoid atrophy and apoptosis in spleen were observed, which correlate with the frequent clinical finding of lymphopenia (83.2%) [10]. The lymphoid necrosis is largely related to the marked activation of innate immune response in infected hamsters with high levels of chemokines/cytokines induced by SARS-CoV-2 infection. A cytokine storm was observed in severe COVID-19, SARS, MERS, and influenza in humans [24–27]. The mounting of an adaptive immune response as evidenced by increasing titers of neutralizing antibody was associated with the control of the viral load. Passive immunization with early convalescent serum SARS-CoV-2 resulted in significantly lower viral loads in the respiratory tract without apparent differences in clinical signs and histopathological changes. Further studies with serum samples containing higher neutralizing antibody titers should reveal the possible benefit or immunopathology associated with convalescent plasma therapy [28].
Besides fulfilling the Koch’s postulates by reproducing the clinical and pathological changes of pneumonia by virus challenge, recovery of pure virus from infected tissues, and detecting the rise of SARS-CoV-2–specific neutralizing antibody, we also demonstrated virus transmission by close contact between the challenged index hamsters to the naive contact hamsters housed in the same cages. It is notable that all 5 naive contact animals in 5 individual cages were infected. The minimal amount of weight loss in the naive contact animals could be related to a lower virus inoculum when compared with the high single intranasal virus dose received by the challenged index animals. Although it was likely that the challenged animals were continuously shedding a high viral load in respiratory droplets, which served as the most important portal of transmission, they might have also shed virus in their feces as demonstrated by the viral load study and N protein expression in the intestine. Hamsters are hindgut fermenters that eat their own feces, a feeding behavior called coprophagy, to recover nutrients digested in the hindgut but unabsorbed [29]. Thus, a fecal–oral route of transmission could not be excluded in this model as well as in humans.
Readily available small animal models that resemble the clinical and pathological features of human COVID-19 are urgently needed to study the pathogenesis, antiviral treatment, and vaccination. The external subdomain of the spike glycoprotein RBD of SARS-CoV-2 shares only approximately 40% amino acid identity with SARS-CoV, but this new virus still utilizes ACE2 as the cell entry receptor, which are abundantly found in the epithelial lining of human and animals [2, 3, 30]. Our molecular docking and computation showed that the RBD of SARS-CoV-2 binds well with not only the ACE2 of humans and macaques but also with that of Syrian hamsters. Moreover, the hamster was demonstrated to be a good model for studying respiratory viruses, including SARS-CoV, influenza virus, and adenovirus [31–34]. Unlike our hamster model for SARS-CoV-2, it is also notable that weight loss or intestinal involvement was absent in the hamster model infected by the 2003 SARS-CoV [31]. More importantly, the viral load in the nasal turbinate (7-log10 TCID50/g) of SARS-CoV-2–infected hamsters was consistently approximately 1-log higher than that in their lung (~5.5–6-log10 TCID50/g) at 2, 4, and 7 dpi, whereas the viral load in the nasal turbinate of the 2003 SARS-CoV–infected hamsters was generally similar or lower than that in the lung [31]. This might help explain why SARS-CoV-2 is more transmissible, as the upper respiratory tract involvement by the virus is no less than that of the lower respiratory tract, whereas SARS-CoV predominantly infects the lower respiratory tract.
Our study had a number of limitations. First, the virus inocula used in this study and the previous report of a 2003 SARS-CoV hamster model were different, which might contribute to the differences in findings. Second, we tested the mRNA but not protein expression of the hamsters’ chemokine/cytokine profiles as enzyme immunoassays for hamster chemokines/cytokines were unavailable. Finally, we focused our sequencing on the surface spike gene, which is essential for virus–host cell entry. Complete genome sequencing may reveal additional adaptive mutations in other genome regions. Nevertheless, unlike nonhuman primates and human ACE2-transgenic mice, this hamster model is readily available, physiological, and highly resembles COVID-19. Thus, it would be an important platform for studying the pathogenesis, transmission, treatments, and vaccines for COVID-19.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors sincerely thank the staff of the Laboratory Animal Unit of The University of Hong Kong and the Laboratory Animal Service Centre of The Chinese University of Hong Kong for their facilitation of the study.
Author contributions. J. F.-W. C., A. J. Z., S. Y., and K.-Y. Y. had roles in the study design, data collection, data analysis, data interpretation, and writing of the manuscript. V. K.-M. P. and C. C.-S. C. had roles in the study design, experiments, data collection, data analysis, and data interpretation. A. C.-Y. L., W.-M. C., Z. F., H.-W. T., L. W., R. L., J. C., Y. C., K. T., C. L., J.-P. C., K.-H. K., H. Chu, K.-H. C., S. S., Z. C., H. Che, and K. K.-W. T. had roles in the experiments, data collection, data analysis, and/or data interpretation. All authors reviewed and approved the final version of the manuscript.
Disclaimer. The funding sources had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Financial support. This study was partly supported by the donations of May Tam Mak Mei Yin, Richard Yu, Carol Yu, Michael Seak-Kan Tong, the Shaw Foundation Hong Kong, Respiratory Viral Research Foundation Limited, Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited, Chan Yin Chuen Memorial Charitable Foundation, Marina Man-Wai Lee, the Hong Kong Hainan Commercial Association South China Microbiology Research Fund, and the Jessie and George Ho Charitable Foundation, and by funding from the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for Department of Health of the Hong Kong Special Administrative Region Government; the Theme-Based Research Scheme (grant number T11/707/15) of the Research Grants Council, Hong Kong Special Administrative Region; the Sanming Project of Medicine in Shenzhen, China (grant number SZSM201911014); and the High Level-Hospital Program, Health Commission of Guangdong Province, China.
Potential conflicts of interests. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
J.F-W.C., A.J.Z., and S.Y. contributed equally to this work.





Comments