-
PDF
- Split View
-
Views
-
Cite
Cite
Trond Amundsen, Sex roles and sexual selection: lessons from a dynamic model system, Current Zoology, Volume 64, Issue 3, June 2018, Pages 363–392, https://doi.org/10.1093/cz/zoy036
Close - Share Icon Share
Abstract
Our understanding of sexual selection has greatly improved during the last decades. The focus is no longer solely on males, but also on how female competition and male mate choice shape ornamentation and other sexually selected traits in females. At the same time, the focus has shifted from documenting sexual selection to exploring variation and spatiotemporal dynamics of sexual selection, and their evolutionary consequences. Here, I review insights from a model system with exceptionally dynamic sexual selection, the two-spotted goby fish Gobiusculus flavescens. The species displays a complete reversal of sex roles over a 3-month breeding season. The reversal is driven by a dramatic change in the operational sex ratio, which is heavily male-biased at the start of the season and heavily female-biased late in the season. Early in the season, breeding-ready males outnumber mature females, causing males to be highly competitive, and leading to sexual selection on males. Late in the season, mating-ready females are in excess, engage more in courtship and aggression than males, and rarely reject mating opportunities. With typically many females simultaneously courting available males late in the season, males become selective and prefer more colorful females. This variable sexual selection regime likely explains why both male and female G. flavescens have ornamental colors. The G. flavescens model system reveals that sexual behavior and sexual selection can be astonishingly dynamic in response to short-term fluctuations in mating competition. Future work should explore whether sexual selection is equally dynamic on a spatial scale, and related spatiotemporal dynamics.
Introduction
Model organisms have proven highly valuable in understanding fundamental questions in biology. This has particularly been the case in neurobiology, developmental biology, genetics, molecular biology, and to a certain extent evolution. Important model organisms include fruit flies Drosophila melanogaster, house mice Mus musculus, Norway rats Rattus norvegicus, zebra fish Danio rerio, and thale cress Arabidopsis thaliana. By contrast, model organisms have not been equally central to animal behavior and evolutionary ecology, due to the diversity of life histories, ecological adaptations, and social systems (e.g., Amundsen 2003). That being said, certain organisms have proven particularly useful for exploring fundamental principles of behavior, including sexual selection. Among fishes, influential models include guppies Poecilia reticulata and related poecilids, three-spined sticklebacks Gasterosteus aculeatus, pipefishes (Syngnathidae), and cichlids (Cichlidae) (Amundsen 2003), but several other taxa have also provided model organisms highly suitable for exploring specific research areas in behavior and evolution. If we are to understand nature’s diversity, we need to draw insights from a diversity of model organisms.
The aim of this article is to provide an overview of insights from a model system that has proven unusually dynamic, and hence exceptionally suitable for analyzing the regulation of sex roles and sexual selection: the small marine goby fish Gobiusculus flavescens (Figure 1). I place the G. flavescens work in a context of theoretical (and some empirical) work for each of the topics covered. These include animal sex roles, operational sex ratio (OSR) dynamics, sexual selection theory, ornamentation and signaling in males and females, mate choice, mating competition and mate search, environmental effects on sexual competition and sexual selection, and alternative reproductive tactics. Given the breadth of topics, however, it is beyond the scope of the article to provide a comprehensive discussion of the vast literature that exists on each topic.
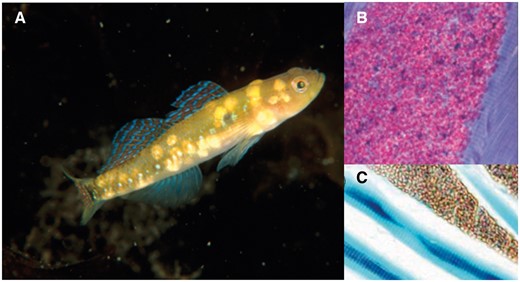
The model organism Gobiusculus flavescens (two-spotted goby) in mutual courtship display. The female in front. Photo: © Nils Aukan.
Gobies as Model Organisms
Gobies (Gobiidae) are mostly small, substrate-brooding fishes that occur in both marine and freshwater environments world-wide (Patzner et al. 2011). Gobiidae is one of the most speciose fish families, with about 2,000 species described (e.g., Agorreta et al. 2013). Recent molecular analyses have revealed that Gobiidae consists of 2 distinct sub-clades which separated about 54 million years ago, in the early Eocene (Thacker 2015). There is an ongoing discussion as to whether the sub-clades should be considered separate families or remain within Gobiidae (Thacker 2009, 2013; Pezold 2011; Thacker and Roje 2011; Agorreta et al. 2013; Tornabene et al. 2013). The “European sand gobies,” including the model organism of this article, cluster within the gobionelline-like gobies (sensu Agorreta et al. 2013) and would thus be part of a potential new Gobionellidae family (sensu Thacker 2009, 2013) representing the less speciose sub-clade (ca. 650 species, Thacker 2015).
Whether gobies constitute 1 or more phylogenetic families, they share many characteristics with respect to morphology and biology. Many species, including those of the “sand goby group” (Huyse et al. 2004; Thacker 2013), are small and occur at high densities in the wild. Gobies have paternal care of eggs, making them suitable models for testing theories regarding costs of reproduction, resource allocation, and parent–offspring conflict. The paternal care employed by gobies is the most common form of care in teleost fishes (Clutton-Brock 1991; Balshine 2012), having evolved independently in at least 22 evolutionary fish lineages (Mank et al. 2005). Thus, gobies, being often easy to study due to their small size and swift acclimation to laboratory conditions, allow analyses of male care dynamics of relevance to many other fish families (e.g., Blenniidae, Centrarchidae, Cichlidae, Gasterosteidae, and Pomacentridae).
In sexual selection research, the most widely used model organisms have historically been birds (Darwin 1871; Andersson 1994; Amundsen 2003). However, most birds (and mammals) do not acclimate easily to laboratory conditions, and only few birds mate and breed in captivity. By contrast, many gobies (and members of some other fish families) are easily kept in small aquaria and display their natural behavioral repertoire, including courtship, mating competition, mate choice, and breeding, in captivity. Such species are ideally suited for experimental tests of sexual behaviors and how these are affected by variation in the social and physical environment. Accordingly, work on several species of temperate gobies, many of them close relatives of G. flavescens, have provided insights of wide-ranging relevance on mate choice, mating competition, and sexual selection. The most extensively used models are sand gobies Pomatoschistus minutus (e.g., Forsgren et al. 1996b; Lindström 2001; Svensson and Kvarnemo 2003) and common gobies P. microps (e.g., Magnhagen 1994; Svensson et al. 1998; Heubel et al. 2008). Important contributions to mating dynamics and sexual selection have also been made on several other species, including the closely related painted gobies P. pictus (e.g., Amorim and Neves 2008; Amorim et al. 2013), marbled gobies P. marmoratus (Locatello et al. 2016) and lagoon gobies Knipowitschia panizzae (e.g., Mazzoldi et al. 2003; Pizzolon et al. 2008), all of which belong to the gobionelline Pomatoschistus lineage (Gobionellidae sensu Thacker 2009, 2015). These species all have a mainly European distribution (Thacker 2015). In Australia, the desert goby Chlamydogobius eremius, a member of the gobionelline Mugiogobius lineage, has recently become an important model for sexual selection research (e.g., Svensson et al. 2010; Lehtonen et al. 2016). Goby sexual selection models of the gobiine Gobius lineage (Gobiidae sensu Thacker 2009; 2015) include black gobies Gobius niger (e.g., Rasotto and Mazzoldi 2002; Scaggiante et al. 2005), grass gobies Zosterisessor ophiocephalus (e.g., Mazzoldi et al. 2000; Scaggiante et al. 2005), and round gobies Neogobius melanostomus (e.g., Marentette et al. 2009; Bleeker et al. 2017). In tropical environments, research on coral gobies (Gobiodon spp., e.g., Munday 2002, Paragobiodon xanthosomus, e.g., Wong et al. 2008) and blue-banded gobies Lythrypnus dalli (e.g., Lorenzi et al. 2009) have been instrumental in understanding mechanisms and function of sex change and social dynamics. Gobies are generally considered to have conventional sex roles, but sex role reversal occurs late in the breeding season in G. flavescens (Forsgren et al. 2004) and has also been reported in the American tidewater goby Eucyclogobius newberryi (Swenson 1997).
The two-spotted Goby G. flavescens: A Model for Sex Role Dynamics
The two-spotted goby G. flavescens belongs to the mostly European Pomatoschistus lineage of gobies (Agorreta et al. 2013) and is similar to the much-studied P. minutus and P. microps in many respects, including size, morphology, and breeding biology. Therefore, studies on G. flavescens can, together with work on these and related gobies, reveal joint patterns of reproductive dynamics. However, G. flavescens differs from these and most other extensively studied goby species in life-style and habitat. Most other members of the “sand goby group” (Huyse et al. 2004; Agorreta et al. 2013) are benthic, inhabiting shallow bays with substrates ranging from gravel to silt, and with species partly distributed in accordance with substrate characteristics. These species spend the majority of their time on, or partly immersed in, the substrate. By contrast, G. flavescens is semi-pelagic and inhabits kelp forests and seaweed beds (Figure 2) along the rocky shores of Western Europe (Figure 3). The preference for macro-algal habitats, which is unique to G. flavescens among European gobies, makes it extremely abundant over much of its distribution: for instance, it is by far the most abundant fish species of near-shore shallow waters in Norway. Being semi-pelagic means that individuals shift between residing among the macro-algal vegetation and foraging in the nearby water column (up to a few meters from shore), reflecting a trade-off between foraging and predator avoidance (Utne et al. 1993; Utne and Aksnes 1994). Individuals rarely rest on the substrate except during spawning and, in the case of males, during parental care. However, despite swimming, they usually “stay put” within a few meters range (usually less) most of the time. Unlike its close Pomatoschistus relatives, G. flavescens assembles in loose foraging shoals that range from less than ten to several hundred individuals, or even more in the case of juveniles (Svensson et al. 2000, personal observation). During the breeding season, however, most males defend territories in the kelp forest, and are thus often solitary (Forsgren et al. 2004). Males that do not breed usually join the female-dominated shoals, but sexual interactions are exceedingly rare in the shoals. During mate search and when ready to spawn, females occur in smaller unisexual shoals or sometimes solitarily (Myhre et al. 2012). The situation with solitary males and socially grouped females is unique among closely related gobies, and possibly among gobies in general.
Study sites, habitats, and nest substrates of G. flavescens in Scandinavia. (A–D) Study locations in West Sweden (A), West Norway (B), mid-Norway (C) and South Finland (D). (E–H) Diversity of kelp and seaweed habitats, dominated by Saccharina latissima (E), Laminaria hyperborea (F), Fucus serratus (G), and filamentous algae (H), respectively. (I–L) Diversity of nesting subtrates: blue mussel M. edulis (I), base of S. latissima (J), atop dead bryozoans on L. digitata (K), and acetate sheet inside artificial PVC nest (L). All photos: © Trond Amundsen.
Geographic distribution of G. flavescens. Reprinted from International Union for Conservation of Nature (IUCN) 2014. Gobiusculus flavescens. The IUCN Red List of Threatened Species. Version 2017-1.
The reason why G. flavescens is such a powerful model for understanding the dynamics of sex roles and sexual selection is the species’ exceptionally variable adult and operational sex ratio (OSR) (Forsgren et al. 2004). This variation has allowed extensive investigations on how mating competition regimes affect sexual behaviors and consequent sexual selection. It should, however, be pointed out that the G. flavescens model system is not the only fish (or other) model system that displays variation in OSR and mating competition. Such variation is widespread, not the least in fishes, but usually within the bounds of either conventional (male competition) or reversed (female competition) sex roles. What is near-unique about our study population of G. flavescens is the documented extent of variation, involving a complete shift from conventional to reversed sex roles within a single breeding season (Forsgren et al. 2004; Myhre et al. 2012). When we started exploring sex role dynamics in G. flavescens, no similarly dynamic system had been described in any vertebrate species (Forsgren et al. 2004). The conspicuous female ornamentation, different from that of the male, makes G. flavescens an especially suitable model for analyses of female ornamentation (Amundsen and Forsgren 2001). The female ornamentation of G. flavescens is unique among closely related members of the sand goby clade (Svensson et al. 2009a).
Besides its unusually dynamic breeding biology, G. flavescens also stands out as a uniquely suitable model system for logistic reasons. Because G. flavescens lives and breeds in shallow (mostly 0–3 m) and mostly clear coastal waters, the species’ social, sexual, and reproductive behaviors can be easily observed and quantified by snorkelers (Forsgren et al. 2004; Myhre et al. 2012). The species is unusually tolerant to disturbance, and can therefore be observed at close range (<1 m) while performing its natural repertoire of sexual and reproductive behaviors both in the field (e.g., Forsgren et al. 2004; Myhre et al. 2012) and in the laboratory (e.g., Amundsen and Forsgren 2003; Borg et al. 2006; Myhre et al. 2013; Wacker et al. 2013). Gobiusculus flavescens also readily breeds in captivity (e.g., Bjelvenmark and Forsgren 2003; Svensson et al. 2006). The species is extremely abundant along Scandinavian (and other East Atlantic) rocky shores (e.g., Fosså 1991), and easy to catch in large numbers for population studies (e.g., Wacker et al. 2014; Utne-Palm et al. 2015) or laboratory experiments. Population samples are typically collected by beach seine (Utne-Palm et al. 2015), whereas fish to be used in behavioral experiments are typically caught individually by dip nets while snorkeling (e.g., Wacker and Amundsen 2014). Due to its abundance and shallow breeding habitat, both natural and artificial nests in the field can be easily inspected (e.g., Forsgren et al. 2004) or collected (e.g., Mobley et al. 2009; Monroe et al. 2016), for instance for quantification of reproductive success, egg parameters, and parentage. Taken together, the species is ideally suited for analyses of sexual and reproductive dynamics.
The majority of published studies of mating dynamics in G. flavescens, including those discussed in the present article, have been conducted on a population on the West coast of Sweden. The work has been based at the Sven Lovén Centre for Marine Sciences in Fiskebäckskil, situated at the mouth of the Gullmar Fjord (58°14′60″ N, 11°26′44″ E, Figure 4). Field work has been conducted in the archipelago nearby the research station; experiments in aquaria or mesocosm tanks have been conducted at the station. Additionally, studies (especially on alternative reproductive tactics and parental care; e.g., Skolbekken and Utne-Palm 2001; Utne-Palm et al. 2015; Monroe et al. 2016) have been made on a population on the West coast of Norway, from a base at Espeland Marine Biological Station (60°16′11″ N, 5°13′19″ E, Figure 4).
Locations of study sites for G. flavescens. The majority of work referred to in this article was made in the archipelago around and at the Sven Lovén Centre for Marine Science at Kristineberg (research station; red circle in right panel), situated at the mouth of the Gullmar Fjord in West Sweden. Some studies were also carried out in West Norway (blue square in left panel). Red arrows: locations for studying sex role reversal (Forsgren et al. 2004), yellow arrows: locations for the mate sampling study (Myhre et al. 2012), green arrows: locations for studying sexual selection in the wild (Wacker et al. 2014), blue arrow: location for parentage study (Mobley et al. 2009). Remaining studies were made in laboratories at the Kristineberg Research Station.
Biology of the Model Organism
Male and female size
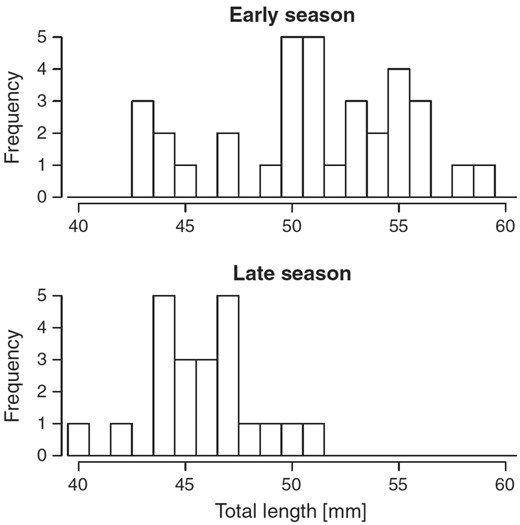
In the W Sweden study population, adults of both sexes are mostly 35–55 mm long (total length), with the majority of individuals being 40–50 mm (Wacker et al. 2014, T. Amundsen et al., unpublished data). In that and most other populations studied, the species is weakly sexually size dimorphic, with males being slightly larger than females (T. Amundsen et al., unpublished data). The W Norway population, however, has reversed sexual size dimorphism with females being on average larger than males, due to an abundance of very small males in this population (Utne-Palm et al. 2015).
Body size varies significantly between years (Wacker et al. 2014), and geographically (T. Amundsen et al., unpublished data). In all years and all populations studied, variation in size is greater in males than in females (T. Amundsen et al., unpublished data).
Ecology
Gobiusculus flavescens occurs along rocky shores from N Norway to Portugal (Miller 1986; Borges et al. 2007), including parts of the Baltic Sea (Figure 3). In the Nordic study populations, it mainly occurs at 0–5 m depth during the breeding season, with nests often just 1–2 m below the low tide mark. The species inhabits both sheltered and semi-exposed shores, but appears to be absent or less abundant at the most exposed locations. Due to its very high abundance in rocky shores kelp forests (Fosså 1991; Utne-Palm et al. 2015), and because most of the Nordic coastlines are rocky shores (Figure 2), G. flavescens is a keystone species in coastal ecosystems (Fosså 1991; Giske et al. 1991; Nordeide and Salvanes 1991; Hop et al. 1992). In Norway, G. flavescens has been reported to be the main prey of first- and second-year codfish in studied fjord systems (Fosså 1991; Nordeide and Salvanes 1991) and has been central in models of fjord ecosystem productivity (Giske et al. 1991; Salvanes et al. 1992).
Breeding
The species is mostly annual, with both males and females usually having only 1 reproductive season (Johnsen 1945). In the Nordic countries, breeding commences in April–May and usually ends in late July (Forsgren et al. 2004; Myhre et al. 2012; Wacker et al. 2014), yet with some variation seemingly related to latitude and climate (personal observation). In more southerly locations, breeding may start earlier and/or end later (Collins 1981; Miller 1986, A.M.S. Faria, personal communication). Gobiusculus flavescens is a substrate brooder, with males defending nests in which one or usually more females deposit clutches of eggs (Mobley et al. 2009; Wacker et al. 2014; Monroe et al. 2016). Breeding occurs in natural crevices, with no nest building or modification of the nesting substrate (as is common in benthic gobies inhabiting more sheltered locations) (Figure 2). Common nest substrates include empty mussels (e.g., Mytilus edulis, Mobley et al. 2009; Wacker et al. 2014), which appear to be a favored substrate, natural crevices in the algal vegetation (e.g., at the base of kelp leaves and in their holdfasts, Gordon 1983, personal observation), and under stones. Gobiusculus flavescens appears opportunistic in choice of breeding substrate, with nests found on a range of kelp and seaweed species and in several species of mussel (Wacker et al. 2014, personal observation). A typical male territory includes many potential nesting sites, especially because G. flavescens often breeds on algae. It is not always obvious whether a male primarily defends an area (with several nesting opportunities), or a specific nesting structure (e.g., a cavity on a kelp), prior to mating. The species readily breed in artificial nests made of PVC tubing (Figure 2), both in the laboratory and in the field (e.g., Forsgren et al. 2004; Wacker et al. 2013; Monroe et al. 2016).
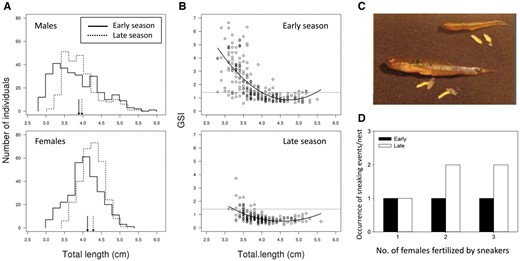
Male G. flavescens compete for ownership of favorable nest substrates by visual displays and physical aggression, and attract females to their nests with elaborate courtship, involving lateral displays with erected fins (Figure 1) and undulating lead swims toward the nest (Amundsen and Forsgren 2001; Forsgren et al. 2004). Males also produce sounds close to the nest just prior to mating, and in the nest during spawning (de Jong et al. 2018). Spawning females attach each individual egg to the substrate, which may take 1–2 h for a clutch of usually 500–2,000 eggs (Pélabon et al. 2003; Svensson et al. 2006; Forsgren et al. 2013). Males are typically either unsuccessful in mating, or mate with several females in succession. Thus, successful males in Norwegian and Swedish study populations mate with a median of 4–5 females (Figure 5a; Mobley et al. 2009; Monroe et al. 2016). The total brood size in a male’s nest can therefore be very large (Figure 5), at the extreme >10,000 eggs (Gordon 1983, personal observation). Consecutive clutches are often of similar age, suggesting that they are spawned in quick succession, but significant age differences among clutches within a brood may occur (personal observation). Once the nest is full, the male is “out of mating competition” until the brood hatches. The eggs are usually laid in a single layer (Figure 2i–l), and hatch after a period of 1–3 weeks, depending on sea temperature (Skolbekken and Utne-Palm 2001; Bjelvenmark and Forsgren 2003; Svensson 2006). During this period, the brood is cared for by the male, by fanning and cleaning the eggs (Skolbekken and Utne-Palm 2001; Bjelvenmark and Forsgren 2003), and by defending them against predators (e.g., conspecific or hetero-specific fishes or small shore crabs Carcinus maenas). Once the brood hatches, the male may engage in attracting females for a new brood. In the laboratory, the recess time between hatching and engagement in courtship can be negligible (Eriksen 2007). Unless disturbed, caring males usually spend >50% of their time in the nest, during which they cannot forage (Skolbekken and Utne-Palm 2001; Bjelvenmark and Forsgren 2003). Assuming a recess time of a few days between successive broods, and a normal climatic succession, a male can theoretically care for about 6 broods over the course of a southern Nordic breeding season. Further north, lower sea temperatures allow for fewer breeding cycles; for instance, a maximum of about 3 successive broods in mid-Norway (T. Amundsen, unpublished data). Mortality of males is high during the breeding season in the W Sweden main study population (Forsgren et al. 2004), and costs of reproduction may prevent males that are still alive from realizing their potential number of breeding events. Like males, females can reproduce repeatedly over the course of the breeding season, with reproductive rate affected by temperature. In P. minutus, temperature affects the reproductive rate more in males than in females (Kvarnemo 1994); this is likely also the case for G. flavescens.
Relationships between mating success and reproductive success (A), and between nest size and reproductive success (B), in G. flavescens. Mating and reproductive success were quantified from parentage analyses using microsatellites. Mussels in (B) are all blue mussels Mytilus edulis. Reproduced from (A) Figure 2 and (B) Figure 1 in Mobley et al. (2009), BMC Evolutionary Biology 9:6.
Male and female ornamentation
Both male and female G. flavescens are extravagantly ornamented (Figure 1; Amundsen and Forsgren 2001). Males have a series of iridescent blue lateral spots and two larger dark spots, one at the base of the tail and one at the base of the pectoral fin. They also sport an enlarged and colorful dorsal fin, with alternating lines of iridescent blue and orange–red coloration. The anal fin of males is uniformly gray in color, and is displayed during exaggerated aggressive encounters, during which the whole body may turn darker. Females have only traces of iridescent spots along the sides, and lack significant fin pigmentation (Figures 1 and 8). However, gravid females display conspicuously orange-colored bellies, which they actively display to males during courtship (Figure 1), by bending their bodies for maximal exposure (Amundsen and Forsgren 2001; Sköld et al. 2008). Female belly coloration is mainly caused by variably yellow to orange eggs that are visible through the semi-transparent skin, but also by red pigment cells (erythrophores) in the belly skin (Figure 6a–c; Svensson et al. 2005; Sköld et al. 2008).
Variation in OSR and mating competition over the breeding season. The figure shows within breeding-season trajectories of operational sex ratio (OSR) (A), propensity for aggressive behavior (B), and propensity to court (C) in a study of sex role dynamics in G. flavescens. The open circles in (A) and white-to-black bars in (B) and (C) represent 4 recording sessions distributed over the course of the breeding season. The OSR changes from male- to female-biased (A), with a concerted decrease in male mating competition (by male–male aggression and courtship efforts), and a simultaneous increase in female mating competition by the same means (B, C). Propensities to behave by aggression or courtship represent the likelihood that the actual behavior takes place at a given encounter between same- or opposite-sex individuals. Reproduced (A) from Figure 1 and (B–C) from Figure 2 in Forsgren et al. (2004), Nature 429:551–554.
General procedures
Our work on G. flavescens is based on a range of approaches, including (1) observational and experimental work in the field, (2) experimental work in aquaria and mesocosm tanks in the laboratory, (3) analyses of egg quality, color, and its chemical basis and regulation in whole-fish, gonads, and skin, and (4) population sampling. Body length (total length, to the nearest 0.5 mm) is recorded using a measuring board, body mass to the accuracy of 0.01 g on a digital scale, and coloration of fish, gonads, eggs, and biopsies by standardized photographic methods. Condition is usually quantified as residuals from length–mass correlations, except when small and large males are compared, in which case condition factor is used. Fish size (body mass and total length) is usually recorded at a research station laboratory, but occasionally using portable devices in the field in cases where fish are to be returned swiftly to their natural habitat (and nests).
Unusually Dynamic Sex Roles
Sex roles and sex ratios: definitions, dynamics, and theories of regulation
In many animal species, male reproductive success is limited by access to mates (females), whereas female reproductive success is limited by resources required to produce and care for offspring (Bateman 1948; Trivers 1972). In such species, mating competition and consequent sexual selection are expected to be stronger in males (Darwin 1871; Andersson 1994). The icon of such conventional (or traditional) sex roles is the peafowl (e.g., Petrie et al. 1991). However, already Darwin (1871) was aware that species exist in which females are the more mate-limited sex, resulting in sexual selection for large body size and conspicuous coloration in females. Examples include several waterbirds (Colwell and Oring 1988; Emlen and Wrege 2004) and pipefishes (Berglund and Rosenqvist 2003). Such cases are today described to have reversed sex roles (Berglund et al. 1986b; reviewed in Eens and Pinxten 2000). In such species, females compete for access to males.
The term “sex roles” is used with a multitude of meanings in human society, and is, unfortunately, also used in several meanings in evolutionary science (see, e.g., Vincent et al. 1992; Forsgren et al. 2004; Ah-King and Ahnesjö 2013). This warrants a clear definition of the term as used in the present article. It also serves as a warning that the scientific discourse about “sex roles” is sometimes muddled by different uses of the term. Building on seminal works by Williams (1975) and Emlen and Oring (1977), Vincent et al. (1992) and later Kvarnemo and Ahnesjö (1996) defined sex roles to solely describe which sex faces the strongest mating competition: conventional when strongest in males, reversed when strongest in females. This is the meaning of sex roles employed in this article – and in all our work on G. flavescens (e.g., Forsgren et al. 2004). However, the term sex roles has also been used (i) to encompass competition by courtship only, describing which sex is most active in courtship while adopting other terms for agonistic mating competition (male–male or female–female) (Saraiva et al. 2012). We suggest that variation in courtship only is better termed courtship roles, as sometimes done (e.g., Gwynne and Simmons 1990; Borg et al. 2002). Moreover, and more commonly, the sex role term has been used (ii) in the broader meaning of encompassing both mating competition and mate choice. The basis for such a broader concept is the theory that the two are usually inversely related: when one sex is the more competitive, the other sex will be the more-choosy (Trivers 1972). That need not always be the case, however, for instance if quality variation is much greater in the less-competitive sex (Owens and Thompson 1994) or if competition and choice interact (Berglund et al. 2005). Another and more commonplace practice is to (iii) include parental care when defining sex roles: conventional sex roles then encompasses predominant male–male competition and female care; reversed sex roles predominant female–female competition and male care (e.g., Liker et al. 2013; Janicke et al. 2016). This is in line with Darwin’s (1871) bird-based reasoning: in several avian taxa, the extent of care and competition are inversely related and the broader definition therefore largely “works” (Liker et al. 2013). However, as emphasized by Vincent et al. (1992), predominant male mating competition occurs in many species with male parental care. This is particularly often the case in fishes, in which uniparental male care is the more common form of care (Gross and Sargent 1985; Clutton-Brock 1991). In the majority of fishes with paternal care, including three-spined sticklebacks G. aculeatus (Bakker 1994) and several species of gobies (e.g., Lindström 1988; Borg et al. 2002), mating competition is clearly stronger in males than in females under most circumstances. For these reasons, we (e.g., Forsgren et al. 2004; Myhre et al. 2012, this article) follow Vincent et al. (1992) and Kvarnemo and Ahnesjö (1996) in using the sex role term in its simplest and most fundamental form: to describe which sex experiences the strongest mating competition. This definition is applicable to all sexual species.
Mating competition as a driver of sexual selection
Mating competition is one of the major processes that drive sexual selection, and the “direction” and strength of mating competition is therefore expected to affect sexual selection (Kokko and Monaghan 2001). This may be the reason why OSR effects on mating competition are often taken to imply effects on sexual selection. However, mating competition should not be equated with sexual selection. Apart from mating competition, sexual selection is driven by mate choice, post-mating sperm competition and cryptic choice, and several other processes (Andersson 1994; Eberhard 1996; Birkhead and Møller 1998). These processes may work in concert (i.e., be additive). However, they could also select for different traits, for instance if traits that make males superior in competition are not the same as those important in female mate choice (Qvarnström and Forsgren 1998; Wong and Candolin 2005). They could also work in opposite directions on the same traits (Hunt et al. 2009), for instance if large males are more successful in competition but females prefer small males (Petrie 1983). In such instances of conflicting selection pressures from competition, choice, and other mechanisms, increasingly strong mating competition in a sex need not necessarily imply stronger overall sexual selection on that sex. In most cases, however, traits promoting success in competition are likely to be selected.
The OSR describes the relative abundance of males and females “on the mating market”—whether there are more individuals of one or the other sex that are ready to mate at any point of time (Emlen and Oring 1977; Kvarnemo and Ahnesjö 1996). The OSR can either be male-biased (more mating-ready males than females, the more commonplace situation among animals), or female-biased (more mating-ready females than males). It can also be relatively even, as in socially monogamous seabirds with limited extra-pair sex. OSR theory predicts that the sex facing a shortage of potential mates (i.e., toward which the OSR is biased) should show stronger mating competition (Vincent et al. 1992; Kvarnemo and Ahnesjö 1996, 2002). Such competition could be manifested in agonistic interactions with same-sex competitors (by displays or physical aggression), in efforts to attract the other sex by courtship, or both.
Fundamentally, the OSR is determined by variation in adult sex ratio (ASR) and potential reproductive rate (Parker and Simmons 1996; Kvarnemo and Ahnesjö 2002). When the ASR varies little from unity, the potential reproductive rate is the main factor determining the OSR. However, strong ASR biases toward males or females can override the effect of sex differences in potential reproductive rate.
Recently, Szekely et al. (2014b) have argued that the ASR regulates recource competition whereas the OSR regulates mating competition. It has also been suggested that sex differences in cost of reproduction rather than OSR are the ultimate determinants of mating competition (sex roles) (Kokko and Monaghan 2001; Kokko and Johnstone 2002). There is an ongoing theoretical debate about the role of OSR in shaping mating competition, sex roles, and sexual selection (e.g., Kokko and Jennions 2008; Klug et al. 2010a; Kokko et al. 2012; Fromhage and Jennions 2016; Clutton-Brock 2017; Jennions and Fromhage 2017). However, neither our empirical work with the G. flavescens model system nor this article aim to address all issues raised in that debate. Instead, the aim of our work has been to empirically explore the role of the OSR as a driver of mating competition and sexual selection.
Prior to our work, empirical studies on other model systems had established that variation in OSR was associated with variation in the strength of mating competition in several species (see Weir et al. 2011; de Jong et al. 2012), yet mostly within the bounds of either conventional (e.g., Kvarnemo et al. 1995) or reversed sex roles (e.g., Vincent et al. 1994). Complete sex role reversals in response to OSR variation had, previous to our work, only been found in 2 species of katydid insects, Anabrus simplex (Gwynne 1993) and Kawanaphila nartee (Gwynne and Simmons 1990; Simmons and Bailey 1990; Gwynne et al. 1998), regulated by food supply. In these species, the change is mainly in courtship roles (which sex is most actively courting). In sticklebacks G. aculeatus, female courtship had been found to increase dramatically over the breeding season (Kynard 1978).
Adult sex ratio
Our work on G. flavescens has investigated ASR because it, together with variation in potential reproductive rate (Clutton-Brock and Parker 1992), drives OSR variation (e.g., Ahnesjö et al. 2008). ASRs can vary substantially in animals, both naturally and as a consequence of sex-biased harvesting regimes (e.g., Adams et al. 2000; Forsgren et al. 2002; Szekely et al. 2014a, 2014b). Strongly biased sex ratios are particularly prevalent in species without chromosomal sex determination, like in many fishes (Charnov and Bull 1989). For example, sex-changing fishes almost always have strongly female-biased sex ratios (e.g., Wacker et al. 2016), with extreme cases including haremic species like anthiases (Fam. Serranidae) having only 10–20% males (Molloy et al. 2007). By contrast, the XY and ZW chromosomal sex determination of mammals and birds constrains sex ratio variation even if significant deviations from unity are still common (Liker et al. 2013). Such deviations could result from minor biases in primary sex ratio, but more commonly from sex differences in mortality (Trivers 1972; Szekely et al. 2014a). In humans, modestly biased ASRs are common, either female-biased as a result of high male early-life mortality (Pouget 2017), male-biased as a consequence of infanticide and sex-differential care (Brooks 2012), or locally fluctuating (Kramer et al. 2017). Such biases may have significant impacts on human society, behavior, and well-being (e.g., Brooks 2012; Schacht and Smith 2017; Zhou and Hesketh 2017).
Measuring adult (and operational) sex ratios in the wild is difficult in many organisms (e.g., Ancona et al. 2017; Kappeler 2017). If G. flavescens, however, recording ASR is relatively straightforward, as males and females inhabit the same shallow-water habitat and because the species occurs at very high densities, is relatively stationary, easy to observe at close range, and easy to sex. Males and females are usually easily distinguishable in the species, based on coloration, body form, and behavior (Figure 1).
Gobiusculus flavescens is not a sex-changer but a strongly biased ASR, often with more females than males, is commonplace (T. Amundsen et al., unpublished data). During our initial work on male mate choice in the model system (Amundsen and Forsgren 2001, 2003), we experienced increasing difficulties in finding males for our experiments in mid-July, toward the end of the breeding season. By contrast, gravid females appeared to be highly abundant, often actively courting the few males present. This situation appeared very different from that experienced early in the breeding season. Realizing that we might be faced with a rather unique system of sex role reversal over the breeding season, during the following breeding season we conducted an extensive field study to test whether our impressions reflected reality. The aims of the study were to test whether the OSR changed from male-biased to female-biased over the course of the season and, if so, whether mating competition changed accordingly, from conventional sex roles early in the season to reversed sex roles later on. Swimming transects (18–33 m) along 10 stretches of coastline by a total of 6 different islands, we quantified numbers of males and females in each transect 4 times over the breeding season, from late May until mid-July. In line with our hypothesis, we found a drastic decline in the number of males observed, with only about 10% as many males in mid-July as in May. For females, the reduction in numbers over the season was far less pronounced (Figure 1 in Forsgren et al. 2004). Thus, G. flavescens experienced a more dramatic change in the ASR (including individuals ready and not ready to breed) than reported in any vertebrate species before, as far as we know. The cause of this change is almost certainly male mortality, as a result of increased predation on solitary and displaying males, costs from repeated cycles of care (Smith and Wootton 1995), or both (Forsgren et al. 2004; Wacker et al. 2013). A higher male than female mortality by the end of the breeding has also been found in sticklebacks G. aculeatus (Kynard 1978). In G. flavescens, the temporal change in ASR was of a magnitude clearly overriding any change in male and female reproductive rates over the season. Temporal changes in ASR are not uncommon (e.g., Ancona et al. 2017) but were exceptionally extreme in G. flavescens.
Operational sex ratio
The OSR of an organism is usually different from the ASR because only a fraction of males and females are ready to mate at any point of time (e.g., Kvarnemo and Ahnesjö 2002). The degree of difference between the 2 measures is variable among and within species (e.g., Szekely et al. 2014b). At the extreme, ASR and OSR may show opposite temporal dynamics (Carmona-Isunza et al. 2017).
One way to express the distinction between adult and OSR is to estimate which individuals are “out” of mating competition (caring or maturing eggs, or excluded from breeding due to competition) and which are “in” (ready to mate, Parker and Simmons 1996; Ahnesjö et al. 2001; Kvarnemo and Ahnesjö 2002). In repeated spawners like G. flavescens, females need time to mature a new clutch after spawning, and only those with mature eggs are part of the “mating pool” (Parker and Simmons 1996). Recently spawned females are slim whereas maturing egg-batches cause females to display more or less distended bellies (e.g., Svensson et al. 2009b). Only those that are ready or near-ready to spawn should, by definition, be included in OSR estimates. In G. flavescens, female maturity can be judged visually from belly extension and coloration (spent females are mostly drab). We recorded female “roundness” on a 3-graded scale, and included the upper 2 roundness classes in our OSR estimate.
Males of G. flavescens are usually either stationary or roaming. Males with a nest, or else ready to breed, are territorial and stationary. Thus, we excluded roaming males when estimating OSR (Forsgren et al. 2004). In G. flavescens, like in many other substrate-brooding fishes, successful males can simultaneously care for multiple clutches sequentially spawned by several females. With spawning often lasting 1–2 h for each female, a male can theoretically mate with >10 females during a single day. Thus, at a given point in time, a male represents a breeding opportunity for >1 female, provided that he has space for >1 additional clutch in his nest. How many females can spawn in his nest is thus a function of nest size and the amount of eggs already in the nest (the percent nest area already occupied). In G. flavescens, typically 4–5 females spawn in a male’s nest (Figure 5a). Mussel nests are often full or near-full with such numbers of clutches (Mobley et al. 2009; Monroe et al. 2016). Knowing typical clutch size (Pélabon et al. 2003; Svensson et al. 2006; Forsgren et al. 2013) and area covered by such a clutch (Bjelvenmark and Forsgren 2003) from laboratory studies, we could calculate, for each nest, how many more clutches could be accommodated in the nest. For instance, a male with an empty mussel nest of average size would represent a mating opportunity for about 4 females (Forsgren et al. 2004). If the nest was half-full, he could accommodate 2 more clutches. In estimating OSR of the population, we included recorded nest fullness in the calculation, multiplying the number of territorial (stationary) males with the average number of further clutches a nest could accommodate at that time (Table 1 in Forsgren et al. 2004). When males can care for clutches from multiple females, it is not the number of males per se that defines mating opportunities for females, but the number of further clutches these males can accommodate and care for at any point of time (Parker and Simmons 1996).
Seasonal trajectory of OSR
Based on the criteria described above, we estimated the trajectory of OSR over the better part of the breeding season, from late May until late July (Figure 6a). In May, the OSR was heavily male-biased (OSR > 0.8) whereas it was even more heavily female-biased in late July (OSR < 0.1), with a near-linear change over the 2 intermediate recording periods (Figure 6a; Forsgren et al. 2004). A similarly extreme change in OSR over a single breeding season has, to our knowledge, not been reported in any other vertebrate. However, that is not to say that such changes do not occur in other animals, as temporal changes in OSR have been relatively little studied (but see Kynard 1978; Wootton et al. 1995).
A reversal of sex roles
With OSR changing from strongly male- to strongly female-biased over the course of the breeding season, we predicted stronger mating competition in males early in the season and stronger mating competition in females late in the season (Forsgren et al. 2004), expressed by efforts to entice opposite-sex individuals to mate (courtship) or by intra-sexual aggression (visual displays or physical aggression toward competitors). Thus, we recorded courtship and intra-sexual aggression by males and females at each stage of the season (details of behaviors: see Forsgren et al. 2004). This is most easily done by counting the number of occurrences of each behavior for each sex and time of season (the frequency of competitive behaviors; de Jong et al. 2012). However, such frequencies are essentially the products of (i) the number of encounters between opposite- or same-sex individuals and (ii) their propensity to compete by courtship and aggression at a given encounter (Figure 7; de Jong et al. 2012). Because a change in OSR entails a change in density of one or both sexes, it inevitably affects the frequency of encounters (e.g., Vincent et al. 1994) and could lead to OSR effects on the frequency of courtship or aggression without any true change in the propensity to court or to be aggressive at encounters (Figure 7; de Jong et al. 2012). However, it is the propensity to act by courtship or aggression (Figure 7) that is predicted to change with a changing OSR, and which reflects mating competition. We therefore analyzed data on courtship and aggression by calculating the likelihood of these behaviors to happen at a given observed encounter (Forsgren et al. 2004). Such a propensity-based approach to mating competition has obvious strengths but had only rarely before been used in analyses of OSR effects (but see Berglund 1994; Borg et al. 2002).
How to measure mating competition: by frequencies of behaviors or propensities to behave? If OSR effects on courtship or aggression are measured by how often an act happens under various sex ratios, changes in encounter rate with opposite or same sex individuals will cause changes in numbers of courtship or agonistic acts even in the absence of any effect of sex ratio on individual behavior (the propensity for courtship and aggression at encounters). In this figure, the term competitor-to-resource ratio (CRR) is used instead of OSR in order to make the logic independent of sex of the actor [see de Jong et al. (2012) for further detail]. (A–D) Effects on courtship. With an increasing CRR (i.e., fewer potential mates), there will be fewer mate encounters (thin dashed lines). Even if this causes an increased propensity to court (A–C, thin lines), the frequency of courtship (bold lines) will decrease over the whole (A) or part (B, C) of the CRR range. In (D), we assume no effect of CRR on the propensity to court, in which case courtship frequency will decrease due to fewer encounters. (E–H) Effects of CRR on aggression (agonistic behavior). With increasing CRR (i.e., more competitors), frequencies of same-sex encounters (thin dashed lines) will increase. Depending on how this affects the propensity to behave aggressively upon encounters, the result will be smaller or greater differences between trajectories for aggression propensity (thin lines) and frequency of aggression (bold lines). Trajectories could be qualitatively different over certain ranges of CCR (E–G), or over the full CRR range (H). Reprinted (A–D) from Figure 1 and (E–H) from Figure 2 in de Jong et al. (2012), Behavioral Ecology 23:1170–1177, by permission of Oxford University Press.
In both sexes, we found a dramatic change in the propensity to compete over the breeding season, in accordance with predictions from OSR theory (Figure 6b, c). The change was, as predicted, opposite in the 2 sexes, for both courtship and aggression. Males showed a strong propensity to behave aggressively to other males, and to court females, early in the season, but with a dramatic decline for both behaviors as the OSR became more female-biased over the season (Figure 6b, c). Females, on the other hand, were very little engaged in courtship and very rarely aggressive at the start of the season, but were eager to court and often behaved agonistically to other females when the OSR was female-biased toward the end of the breeding season (Figure 6b, c). In result, males were much more eager to court and compete than females early in the season, whereas females were much more eager to court and compete than males late in the season. Thus, as predicted from OSR theory (Emlen and Oring 1977; Kvarnemo and Ahnesjö 1996), our study of G. flavescens revealed a complete change in sex roles during a 3-month breeding season. The change was from conventional sex roles (predominant male mating competition) when the OSR was male-biased at the start of the season, to reversed (predominant female mating competition) as the OSR became female-biased later in the season (Figure 6; Forsgren et al. 2004).
Analyzing personality traits of G. flavescens in a later study (Magnhagen et al. 2014), we found that males studied late in the season behaved less boldly (in standardized personality tests) than those tested earlier in the season (Magnhagen et al. 2014). The reduction in male boldness matches the near-absence of male–male competition late in the season. In substrate brooders like G. flavescens, territory and nest defence may render males more vulnerable to predators, and parental care may entail costs (energetically or by compromised immune-competence) that are either fatal or leave them out of the mating pool due to poor condition (Forsgren et al. 2004; Wacker et al. 2014).
Setting the stage for the model system: OSR and dynamics of sexual selection
The documented temporal dynamics of sex roles linked to OSR variation entailed a unique potential of the model system for exploring sexual dynamics. This set the stage for much of our later work with G. flavescens, in the field and in the laboratory. As it is becoming increasingly clear that sexual selection varies in time and space in many species, understanding the underlying dynamics becomes increasingly important. With mating competition today found to vary in relation to OSR in a range of species and taxa (Weir et al. 2011; de Jong et al. 2012), it is not unlikely that other vertebrates (and other animals) may have similarly dynamic sex roles as G. flavescens even if documented examples are few. Any system where, for one or another reason, the OSR changes dramatically over the season—or between breeding seasons—may potentially display similar dynamics. Reversals of courtship or sex roles have now been documented in at least 2 more fish species, both of the Blenniidae family. In Petroscirtes breviceps, Shibata and Kohda (2006) found sex roles to change from conventional at the start of the season to reversed at peak season and then back again to conventional in late season, which they interpreted as a response to nest site limitation. In peacock blennies Salaria pavo, Saraiva et al. (2012) found spatial variation in courtship roles, with males more active in courtship than females in the Gulf of Trieste, and females more active in Southern Portugal. Male aggression toward females showed the same spatial pattern, whereas intra-sexual aggression was far more prevalent in males than females in both populations (Saraiva et al. 2012). Male–female aggression can be interpreted as a terminal form of courtship when females are not responsive, as is commonly observed among fishes. Saraiva et al. (2012) attributed the different courtship roles to population differences in nest density, nest competition, and in particular to a higher prevalence of sneaker males in S Portugal. These findings suggest that insights gained from the G. flavescens model system may have wide-ranging relevance. We believe that this relevance extends well beyond fishes, and may apply widely across animal taxa.
Sex Role Reversal: Just Season or a Causal Relationship with OSR?
By their very nature, field studies of OSR variation and related changes in mating competition (Forsgren et al. 2004; Myhre et al. 2012) are correlational: what these studies established was a concerted change in OSR and mating competition over the breeding season. Exploring natural variation in the wild, such studies cannot strictly establish conclusive causation even if observed patterns are highly suggestive of a causal relationship. Hypothetically, the change in mating competition (courtship and aggression) over the season could result from some other factor co-varying with time of season even if there are no obvious candidates for co-variates causing such effects.
An aquarium experiment that failed and what to learn from it
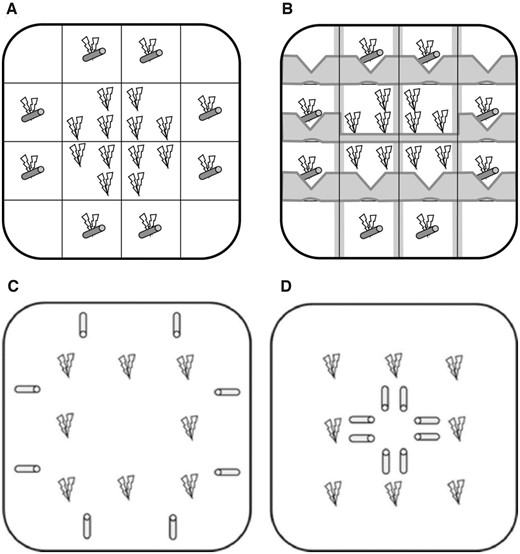
We performed 2 different experiments to test whether OSR per se affects courtship and competition, first in small aquaria (de Jong et al. 2009; Wacker et al. 2012) and later in bigger mesocosm tanks (Wacker et al. 2013). Both experiments focused on effects of sex ratio on competition behavior, as expressed by courtship and intra-sexual aggression.
In the aquarium experiment, we compared a male-biased and a female-biased OSR, at 2 densities (Table 1 in de Jong et al. 2009), by providing all males with a nest and using only ready-to-spawn females. The experiment was conducted in relatively small (60 L) aquaria with the males housed in the larger (40 L) part toward one end and females in the smaller (20 L) part at the other end, separated by a transparent acrylic divider. Recording the frequency of courtship by males to females, by females to males, and all instances of intra-sexual interactions, we found overall little effect of sex ratio (or density) on frequencies of courtship or aggression by males or females (Figures 1 and 3 in de Jong et al. 2009). However, the male courtship frequency was significantly higher when the sex ratio was female-biased, seemingly opposite to expectation from OSR theory (Figure 1b in de Jong et al. 2009).
While these results at first sight contradict sex ratio regulation of mating competition, in retrospect we realized limitations to the set-up that we believe contributed to the negative results. First, the density of fish was clearly higher than occurring in mating situations in the wild (personal observation), with unknown consequences for mating behavior. This is not unique to our study but is often the case in laboratory studies of fish behavior. The high density may be why only about 60% of the males took up a nest, despite there being equally many nests as males (Wacker et al. 2012). Moreover, at very high densities, male aggression may be reduced, due to the cost of frequent aggressive encounters (Emlen and Oring 1977; Grant et al. 1995; Weir et al. 2011). In the high-density situation of the test aquaria, both males and females were continuously and simultaneously exposed to multiple opposite-sex individuals (potential mates) and same-sex competitors, at both sex ratios and densities. Second, by the nature of the set-up, males and females were not allowed to complete interactive courtship and mate. This was intentional, but may have been experienced as constant mating rejection, with unknown effects on the propensity to court. Finally, and perhaps most importantly (de Jong et al. 2009,, 2012), the set-up prevented us from recording propensities to court and compete at a given encounter. This was technically because we were unable to separate individual encounters as multiple individuals of each sex were always in visual contact across the acrylic divider, and is a problem shared with most other aquarium tests of mating competition (de Jong et al. 2012). More fundamentally, it was because separate encounters with individual other fish do not occur in this type of set-up: the fish fundamentally experienced one continuous encounter with multiple con- and hetero-sexuals. Thus, the set-up only allowed recording of frequencies of behaviors. As outlined above, frequencies of competitive behaviors (courtship, aggression) by individuals may not always reflect their propensity to behave competitively when encountering a potential mate (courtship) or intra-sexual competitor (aggression) (Figure 7; de Jong et al. 2012). This is particularly the case for courtship, for which frequencies of behavior and propensities to behave would have different, and often opposite, trajectories of response to OSR variation (Figure 7a–d; de Jong et al. 2012). Reviewing experiments on OSR effects on courtship in various species, we indeed found a marked difference between frequency-based and propensity-based studies: frequency-based studies tend to produce results that at first sight appear opposite to expectations from OSR theory; propensity-based studies tend to support OSR theory (Figure 3 in de Jong et al. 2012).
While one should always be cautious in discarding findings that do not fit expectation, we retrospectively believe the set-up of this experiment illustrates that seemingly well-designed experiments may fail to match in-the-wild-reality to an extent that leaves them less informative.
Support for a causal effect of OSR on sex roles
In order to perform a more realistic test for a causal relationship between OSR and mating competition, we manipulated sex ratio (and density) in a mesocosm experiment, using large tanks of 500 and 2000 L (Wacker et al. 2013). The density treatment was achieved by having equally many fish in tanks of the 2 sizes; the sex ratio treatment by keeping the number of males constant and varying the number of females, resulting in a 2 densities × 3 sex ratios design. The main benefits of the design were that densities better mimicked the situation in the wild, and that, like in the wild, males and females could engage in unconstrained mating behavior and could also mate. This experiment confirmed a causal effect of sex ratio (but not of density) on mating competition behavior: male engagement in courtship and aggression was higher with an even sex ratio than when the sex ratio was female-biased (moderately or strongly) (Figure 2 in Wacker et al. 2013). Notably, an even sex ratio of ready-to-mate individuals in this set-up implied a male-biased OSR, because each male could accommodate clutches from multiple females (as outlined above, see Forsgren et al. 2004).
However, even this set-up precluded a clear distinction between individual encounters, as the environment was relatively open in order to facilitate observation, with the consequence that fishes could often see more than one other fish at a time. We were therefore limited to recording frequencies of behaviors also for this design. However, as the number of males was kept constant across treatments, with OSR variation caused by varying the number of females, male–male encounter rates would not be affected by OSR. In consequence, frequencies of male–male aggression would reflect propensities to behave aggressively in this specific design. As predicted from OSR theory, we found a strong effect of OSR on the male propensity to behave aggressively. Thus, the mesocosm experiment provided support for a causal effect of OSR on sex role variation.
Female Ornamentation: Male Preference, Causes, and Dynamics
Until about 20 years ago, ornamentation (including coloration) in females had been little studied and largely overlooked, with the predominant view being that conspicuous female traits were due to genetic correlation (see Amundsen 2000a, 2000b). During recent decades, however, there has been an increasing recognition that female extravaganza can be a result of male mate choice, female–female competition, or other selection pressures (reviewed in, e.g., Amundsen and Pärn 2006; Kraaijeveld et al. 2007; Clutton-Brock 2009). Critical studies have, however, been hampered by female ornamentation often being identical to or a “paler version” of male ornamentation (e.g., Hill 1993; Amundsen et al. 1997). This has rendered it impossible to entirely rule out genetic correlation which has historically been the dominant interpretation of conspicuous traits in females (Darwin 1871; Lande 1981). However, female ornamentation that differs from that of males of the same species occurs in several taxa. For example, sex-changing fishes often display conspicuous coloration both as females and males, yet in very different ways (e.g., Michael 2001). Ornamental traits that are unique to females offer the best test cases for sexual (and other) functions of showy female traits but have, unfortunately, been very little studied. Thus, the ornamentation of G. flavescens, with both sexes showy yet in very different ways (Figure 1, and Figure 1 in Amundsen and Forsgren 2001), offers an excellent opportunity for testing female ornament function (Amundsen and Forsgren 2001).
Male choice for female coloration
Today it is well established that male mate choice can occur under a range of social and ecological conditions (Edward and Chapman 2011). Prior to our work, several studies of birds had demonstrated a male preference for female ornamentation (Amundsen 2000b). In fishes, male mate choice for more fecund females has been documented in a range of species (Sargent et al. 1986). Males of several fishes also display strong preferences for conspecifics over heterospecifics, which contributes in maintaining reproductive isolation (e.g., Schlupp 2010; West and Kodric-Brown 2015; Roberts and Mendelson 2017). In sex-role reversed pipefishes, several studies have found males to be choosier than females, preferring more ornamented females (e.g., Berglund et al. 1986a; Berglund and Rosenqvist 2001). In fishes with conventional sex roles, a male preference for female temporary colors that signal readiness to spawn have been found in some species (e.g., Rowland et al. 1991; McLennan 1995) whereas little has been known about whether such preferences exist when coloration varies among mature females (but see Beeching et al. 1998).
In order to test if female coloration was subject to selection by males, we conducted a dyadic aquarium test in which males were offered a choice between 2 females that differed in belly coloration (more or less brightly orange) but that were closely matched in size (Amundsen and Forsgren 2001, 2003). As is usual in such tests, the respondent male could see the females but not get into physical contact, in order to avoid a bias caused by the stimulus fish. The test was performed late in the breeding season, but only for logistical reasons, as we at that point were unaware of the seasonal dynamics of sex roles and sexual selection in the species. We found a very strong preference for the more colorful female, both in terms of time in association and courtship displays (Figure 8f; Amundsen and Forsgren 2001). The same strong effect was found when giving males a choice between 2 females that differed in natural coloration, and when letting them choose between 2 females that differed in coloration by experimental manipulation (Figure 8f; Amundsen and Forsgren 2001). The latter experiment was important because it ruled out any confounding covariates and thus provided conclusive evidence that males responded to female coloration as such. The results indicated that the bright orange belly of G. flavescens could, at least in part, be due to sexual selection by male choice.
Female ornamentation in G. flavescens. (A) Female in “normal body coloration,” with colorful gonads (insert) visible through the skin. (B) Female aggregating dorsal and lateral melanophores to become near-transparent during courtship. (C) Belly skin biopsies (lateral to lateral) showing melanophore (brown–black) and erythrophore (orange–red) pigment cells maximally dilated (left) and maximally aggregated (right). (D) Belly coloration correlates strongly with gonad carotenoid content. Solid circles indicate females visually judged as “colorful”; open circles indicate females judged as “drab.” (E) Gonads have higher carotenoid content late than early in the season. (F) Male preference for the more colorful female when given a choice between 2 females that differed in experimentally manipulated belly coloration, in terms of percent of time spent near the more colorful female (upper) and percent of displays directed at the more colorful female (lower). (A, B) Photos by T. Amundsen, gonad insert by P. A. Svensson. (C) Adapted, with permission, from Figure 2 in Svensson et al. (2005), Journal of Experimental Biology 208:4391–4397. (D) Reproduced from Figure 2 in Svensson et al. (2006), Functional Ecology 20:689–698, by permission of John Wiley and Sons. (E) Reproduced from Figure 3C in Svensson et al. (2009), Behavioral Ecology 20:346–353, by permission of Oxford University Press. (F) Reproduced from Figure 4 in Amundsen and Forsgren (2001), PNAS 98:13155–13160, copyright National Academy of Sciences.
In the years following our study, there has been an increased, if not extensive, interest in male choice in relation to female coloration in fishes. Extant studies have mostly revealed a male preference for female coloration (sockeye salmon Onchorhynchus nerka, Foote et al. 2004; lagoon gobies K. panizzae, Pizzolon et al. 2008; the cichlid Pelvicachromis taeniatus, Baldauf et al. 2011). However, a study of female coloration (red pelvic spines) in three-spined sticklebacks G. aculeatus (Nordeide 2002) found males to prefer drab rather than colorful females. In a population of the same species in which females display red throat coloration, Wright et al. (2015) found no male preference for female throat coloration. Clearly, more studies are needed before we can conclude whether female coloration in fishes is generally subject to selection by male choice. Across taxa, however, there is increasing evidence that male choice plays a part in female ornament evolution (Clutton-Brock 2007, 2009).
Causes of female coloration
The orange belly coloration of G. flavescens females results from 2 sources, pigmented eggs and epidermal red pigment cells (Figure 8a–c; Svensson et al. 2009a). The external visibility of the gonads is regulated by modulation of melanophore pigment cells: when the pigment is aggregated, the skin becomes more transparent and the gonads highly conspicuous. The fact that gonads are part of the signal makes the dynamics and signaling potential different from “ordinary” ornaments that are usually external, without any physiological or reproductive function. In G. flavescens, like in other animals, the obvious candidate to cause egg pigmentation was carotenoids. Using HPLC, we documented a high content of astaxanthin, a carotenoid typical of marine fishes, in the eggs (Svensson 2006). We also found significant concentrations of idoxanthin and adonixanthin, which are both metabolites of astaxanthin (Svensson 2006). The total egg carotenoid concentration correlated strongly with female belly coloration as quantified from digital images, using the CIE L*a*b* color system (Figure 8d; Svensson et al. 2006). Importantly, image analyses confirmed that females visually judged to be colorful and drab, respectively, differed very significantly in measured belly coloration (Table 1 in Svensson et al. 2006) and also in carotenoid content (Table 2 in Svensson et al. 2006). Thus, these analyses validated the visual judgment of coloration applied in the mate choice experiments (Figure 8f; Amundsen and Forsgren 2001, 2003).
Gobiusculus flavescens, like other animals, cannot synthesize carotenoids but get them from prey organisms, in the case of G. flavescens mostly from calanoid copepods (Berg 1979). Astaxanthin is an antioxidant of potential value during the fragile phase of pre- and post-hatching larval development (e.g., Blount et al. 2000). Carotenoids may also positively affect immune function and thus health [Blount et al. (2003), see Blount (2004) and Svensson and Wong (2011) for reviews on carotenoid function]. At the same time, astaxanthin could act as an antioxidant in the adult female, and be used to form red pigment cells in the skin. This makes for a complex trade-off in the allocation of carotenoids, between own use as an antioxidant, deposition in developing eggs for antioxidant or immune function, or deposition in skin pigment cells (Svensson 2006; Svensson and Wong 2011). Compared with other species studied for male choice in relation to female coloration, G. flavescens is unique in directly displaying its egg quality during courtship, while at the same time modulating belly coloration by means of skin pigmentation and chromatophore regulation (Svensson et al. 2005; Sköld et al. 2008).
Benefits of female coloration
From a male perspective, mating with a “more orange” female may provide direct benefits in terms of fertilizing high-quality eggs (Blount et al. 2000) and indirect benefits if egg and skin carotenoids signal a high genetic quality. Because of the inability of animals to synthesize carotenoids, carotenoid-based ornaments have been suggested to act as honest quality indicators (Hill 1991; Lozano 1994). This idea has gained considerable empirical support, but the evidence is not unequivocal and the functions and dynamics of carotenoids in animals are clearly more complex than initially suggested (Olson and Owens 1998; Svensson and Wong 2011; Royle et al. 2015).
Analyzing for relationships between natural belly coloration, carotenoid content, and measures of reproductive success in G. flavescens, we found that colorful females produced significantly larger clutches than drab ones, but did not find any significant effect of belly color or egg carotenoids on egg development and hatching success. We found, however, near-significant effects of belly coloration and egg carotenoid content on the length of newly hatched larva (Tables 2 and 3 in Svensson et al. 2006). Carotenoid content showed a marginally significant negative relationship with time until spawning for the females (Svensson et al. 2006). Overall, these results suggest some positive effect of belly color and egg carotenoid content on reproduction, but the results should be treated with caution due to the many tests performed. At the same time, the overall high developmental and hatching success of eggs (Svensson et al. 2006), with consequent limited individual variation, may have given tests on these 2 parameters low power in detecting effects with the sample sizes of the study.
Across species, the evidence for female ornaments to signal offspring quality is equivocal (Amundsen and Pärn 2006; Nordeide et al. 2013), and suggest complex and variable relationships between female ornamentation and offspring production. In G. aculeatus, females with redder pelvic spines had less carotenoids in their eggs, suggesting a trade-off between ornaments and offspring (Nordeide et al. 2006). A similar negative correlation between skin redness and egg carotenoid content has also been found in trout Salmo trutta (Wilkins et al. 2017), and female ornamentation has been found to decrease offspring viability in Arctic charr Salvelinus alpinus (Janhunen et al. 2011). Nordeide et al. (2013) have suggested that red female spines in G. aculeatus are due to genetic correlation, with antagonistic selection (Arnqvist and Rowe 2002) on the use of carotenoids by the 2 sexes. Indeed, a recent genetic study has revealed that loci coding for red coloration in G. aculeatus are located at the same place in the genome in males and females (Yong et al. 2016). Such conflicting selection on males and females is, however, not relevant for G. flavescens, where carotenoid-based ornamentation is a uniquely female trait.
Dynamics and regulation of female coloration
In animals where coloration stems from dermal pigment cells (e.g., fishes, amphibians, and decapods), individuals can modify their color by dilation or aggregation of chromatophore pigments (e.g., Aspengren et al. 2009; Stuart-Fox and Moussalli 2008, 2009; Sköld et al. 2013, 2016). Such color change can have signaling as well as protective functions (e.g., Stuart-Fox and Moussalli 2008; Stuart-Fox et al. 2008; Olsson et al. 2017). Gobiusculus flavescens females have high densities of dorsal and lateral melanophores that account for their baseline brownish body coloration. Melanophore density is, however, much less in the belly region, which is therefore more transparent (Figure 8c). By contrast, the belly has red erythrophore pigment cells that are absent from other body parts (Figure 8c). Thus, G. flavescens has the potential to modulate the “darkness” and transparency of their body as well as their degree of redness by means of pigment cell regulation (Svensson et al. 2005; Sköld et al. 2008).
Given the strong female mating competition late in the breeding season (Figure 6; Forsgren et al. 2004) and the male preference for colorful females when female competition is at its strongest (Figure 8f; Amundsen and Forsgren 2001), one would expect female visual signaling to be particularly important late in the season. Svensson et al. (2009a) found belly coloration (as expressed by a* in the CIE L*a*b* color space) to be dynamically regulated by gonad pigmentation (fixed) and 2 dynamic aspects of skin coloration: the redness of the belly, and the degree of skin transparency (causing variation in the degree to which the colorful gonads are visible through the skin) (Figure 4 in Svensson et al. 2009a). Comparing coloration of mature females between early and late season, we found that females sampled late in the season had indeed more colorful bellies. This was partly due to more colorful gonads with a higher carotenoid content (Figure 8e), but also to more red-pigmented, and at the same time more transparent, bellies late in the season (Figure 3 in Svensson et al. 2009a). Analyzing belly coloration across stages of egg maturation (“roundness” of females), we confirmed that belly coloration increased as the females matured, due to a combination of more colorful gonads and higher skin transparency. At the same time, female belly coloration was highly variable among fully mature females (Figure 2 in Svensson et al. 2009b), showing that female coloration in G. flavescens is not merely a signal of readiness to spawn.
When females court males, as occurs frequently late in the season (Forsgren et al. 2004), and often involve multiple females (Myhre et al. 2012), they typically become more transparent and enter a state which we informally denote “the glow” (Figure 8b, and Figure 1d in Sköld et al. 2008). This effect is due to aggregation of dermal pigment cells (Svensson et al. 2005). We explored the hormonal regulation of pigment cells by exposing skin biopsies to hormonal treatments. When all pigment cells in skin biopsies were aggregated by noradrenaline treatment, the skin got more transparent and at the same time less colorful (Figure 2 in Svensson et al. 2005). However, during the glow, it appears that the basal-body dark-pigmented melanophore cells are “turned off” (pigment aggregated) whereas the red-pigmented erythrophore cells in the belly are at the same time “turned on” (pigment dilated). By exposing skin biopsies to a range of hormones (and hormone blends) present in fish, we found no single hormone to cause simultaneous aggregation of melanophores and dilation of erythrophores. However, a combination of melatonin and melanocyte-stimulating hormone (MSH) caused the skin to get more transparent (melanophore aggregation) while at the same time becoming more red (erythrophore dilation) (Figures 2 and 3 in Sköld et al. 2008). This is the effect observed during “the glow,” suggesting that female belly coloration during display is modulated by a blend of hormones. Thus, pigment cell modulation affects the degree to which the colorful gonads are visible through the skin, but may also add “extra redness” to the effect of gonads on belly coloration. Notably, we found no effect of sex steroids (T, 11kT, E2) on pigment regulation (Table 1 in Sköld et al. 2008).
The complex and dynamic interaction between skin transparency and egg pigmentation in producing a colorful belly appears unique to G. flavescens among its Nordic goby relatives (Svensson et al. 2009a). Comparing belly coloration, egg carotenoids, and skin transparency among 6 goby species (including G. flavescens) that occur in the same area (Figure 1 in Svensson et al. 2009a), we found G. flavescens to be the only to have a strongly colored belly, despite that 2 other species (P. microps and G. niger) had significant (yet more variable) concentrations of gonad carotenoids. These 2 species, however, had much less transparent skin (Figure 2 in Svensson et al. 2009a). What made G. flavescens stand out as conspicuously colorful was the combination of highly transparent skin and consistently high gonad carotenoid concentrations (Figure 3 in Svensson et al. 2009a). By contrast, females of other goby species inhabiting the same waters were largely camouflaged, either by gray–brown patterns that blend in with the substrate (benthic species) or by transparency in the case of the pelagic Aphia minuta (Svensson et al. 2009a).
The closely related goby K. panizzae displays a female ornament that may shed light on the evolution of the belly color signaling system in G. flavescens. As gonads mature, K. panizzae females develop an increasingly colorful yellow belly that is also variable among mature females (Massironi et al. 2005). The colorful belly is actively displayed to males during courtship, appears to be a signal of female quality (Massironi et al. 2005), and males prefer more colorful females, like in G. flavescens (Pizzolon et al. 2008). However, contrary to G. flavescens, the yellow belly of K. panizzae is solely caused by skin pigmentation, with gonads basically unpigmented (Massironi et al. 2005). The apparent similarity of the 2 systems raises the intriguing question of what evolved first in G. flavescens: the male preference for colorful females, or the belly transparency that makes gonad coloration externally visible to males. The “sand goby group,” including K. panizzae and G. flavescens, share common ancestry about 5 million years ago (Huyse et al. 2004).
To our knowledge, the work on G. flavescens is the only detailed exploration of a female ornament that is, at least partly, a display of pigmented gonads. However, gonads are visible through the skin in a range of fishes including several gobies and wrasses (Fam. Labridae, Baird 1988), potentially with similar functions and dynamics as revealed in G. flavescens.
Taken together, our studies of female ornamentation in G. flavescens have documented a strong male preference for more colorful females, a complex causation and regulation of the female ornament which clearly reveals a signaling function, and potential, yet so far tentative, benefits from coloration. However, more work is clearly needed to fully understand this complex type of female sexual signaling.
Mate Sampling, Mate Competition, and Mate Choice
While mate preferences and mate choice by females have been extensively explored in animals, we know surprisingly little about how female animals behave during mate search, and how many potential mates they visit before mating (Amundsen 2003). Much of extant work, theoretically and empirically, have focused on sampling tactics and decision rules. An initial focus was whether females would employ a best-of-n or threshold criterion tactic (Janetos 1980; Real 1990; Gibson and Langen 1996). Empirical work on birds have largely supported best-of-n models, as females have often been found to revisit and mate with previously visited males. By contrast, fishes may appear to more often employ a threshold tactic, mating with the last male visited (Amundsen 2003). Later, more sophisticated, models that take information theory into account have painted a more complex yet probably more realistic picture (Luttbeg 2002; Wiegmann et al. 2010a, 2010b; Castellano and Cermelli 2011). Most of what we know empirically about mate sampling stems from a relatively small number of studies of birds (e.g., Dale et al. 1990; Fiske and Kålås 1995; Dakin and Montgomerie 2014) and fishes (e.g., Gronell 1989; Forsgren 1997b; Fagundes et al. 2007). A general insight from these studies is that females typically sample quite few males (median numbers often ≤ 5), but with extensive individual variation.
The strength of sexual selection by mate choice can be affected by the number of potential partners that are sampled before a mating decision is made (Jennions and Petrie 1997; Benton and Evans 1998). From the mate-searching individual’s perspective, the likelihood of encountering a high-quality mate increases as more potential mates are sampled, yet in a deceleration function (Real 1990). However, rejecting a potential partner to continue searching may entail costs, in terms of time and energy, but also in lost mating opportunities (Real 1990). The latter cost is because the highest quality male in a sampled set is increasingly likely to get mated with time spent searching. If potential mates are in short supply, a searching individual may remain unmated if it rejects one or more mating options. Mate search and mating decisions become increasingly complex and dynamic when both sexes may execute mate choice (Bergstrom and Real 2000), as in G. flavescens (Myhre et al. 2012).
Number of males sampled
In species with male territoriality, like G. flavescens, mate search is primarily conducted by females, who may visit a number of males before mating. In G. flavescens, the opportunity cost of extensive sampling is likely small early in the season, due to the male-biased OSR, with an abundance of mating-ready males. By contrast, the opportunity cost may be significantly late in the season, when a strongly female-biased OSR implies that mating-ready males are scarce. A female G. flavescens that rejects a mating opportunity in late season could easily find herself without a nest in which she could spawn her eggs and have them cared for. Thus, we predicted females to sample fewer males late than early in the season. This was exactly the pattern found: females visited on average about 3 times as many males early as they did late in the season (Figure 9a; Myhre et al. 2012). A negative effect of female competition on the extent of mate sampling has also been found in the pied flycatcher Ficedula hypoleuca (Dale et al. 1992). Moreover, a recent experiment on sand gobies P. minutus, simulating female mate sampling in the laboratory, found that a low male density and the presence of potential female competitors reduced the time until mating for females (Lindström and Lehtonen 2013). It may be noteworthy that the median number of males sampled by G. flavescens (Myhre et al. 2012) was higher than in most, if not all, other studies of mate search in fishes or other taxa.
Effects of time of season on female mate search behavior in G. flavescens. “Early” refers to late April and May (male-biased OSR), “Late” to mid-June to mid-July (female-biased OSR). Females from one locality were captured, individually marked, released at another locality, and followed during mate search [see Myhre et al. (2012) for details]. (A) Number of males visited (sampled) during 30 min observations of mate-searching females, (B) female propensity to initiate courtship, (C) female propensity to terminate courtship, (D) frequency of multi-female courtship, and (E) likelihood of male courtship in relation to the number of simultaneously courting females. About 30% of the females mated during 30 min of observation. (A–D) Open boxes represent females that did not mate during observation, shaded boxes those that mated during observation. Reproduced (A) from Figure 2, (B–C) from Figure 1 and (D–E) from Figure 2 in Myhre et al. (2012), American Naturalist 179:741–755, with permission of University of Chicago Press.
Competition and choice during mate sampling
During a G. flavescens female visit to a male, some sort of courtship interaction would usually occur, even if most visits do not lead to mating. These interactions could be initiated by either the female or the male, and likewise be terminated by either the male or the female if mating does not commence. The potential for males, and not only females, to assess and reject potential partners during mate sampling had, to our knowledge, not previously been empirically investigated. Instead, extant work on mate sampling has generally made the implicit assumption that visits without mating would be due to rejection on the part of the female. This is likely to be mostly true in many species, but is less likely in mutual choice systems.
The sex experiencing the strongest mating competition would be expected to initiate courtship more often. In G. flavescens, this would be males early in the season and females late in the season (Forsgren et al. 2004). As predicted, we found males to initiate the majority of courtship interactions early in the season, whereas almost all courtship interactions were initiated by females late in the season (Figure 9b; Myhre et al. 2012).
Termination of courtship, on the other hand, would represent rejection of a potential mate. The more-choosy sex should therefore terminate courtship interactions more often. As for courtship initiation, we found a strong effect of time of season on which sex terminated courtship most often. Late in the season, females almost never terminated courtship, whereas females were more likely than males to terminate courtship early in the season (Figure 9c; Myhre et al. 2012). These patterns indicate that females become indiscriminate with whom to mate when female competition is strong late in the season. At this time, about 75% of courtship events included 2 or more competing females (Figure 9d). A similar seasonal pattern has been found in the stickleback G. aculeatus, where “by the end of the breeding season, any male with a nest was seldom found without several females courting him” (Kynard 1978). Female–female competition during courtship may negatively impact the likelihood of mating to a point where the male refrains from courting the females, as invariably happened when >5 females simultaneously courted a male (Figure 9e; Myhre et al. 2012). Taken together, female mate search behavior changed over the season as predicted from sex role theory, with much fewer males sampled, a strong increase in eagerness to court, and very infrequent mate rejection, late in the season.
Mate Choice and Body Size in Males and Females
Body size is closely related to fitness across the animal kingdom (e.g., Blanckenhorn 2000). A large body size may reflect both genetic and phenotypic quality in both sexes. In the sex experiencing the strongest mating competition, a large body size (either skeletally or in terms of condition) would usually imply a high resource-holding potential (RHP, Parker 1974), giving large-bodied individuals an advantage in gaining and maintaining resources including territories, nests, and mates (e.g., Magnhagen and Kvarnemo 1989). Such an advantage would have significant fitness consequences in the sex experiencing the strongest mating competition. As outlined above, this is usually but not always males. In females, a large body size is often indicative of high fecundity (e.g., Honek 1993; Koops et al. 2004; Harding et al. 2007). Variation in RHP and fecundity is particularly extensive in taxa with indeterminate growth. In such organisms, including fishes (e.g., Fleming 1996; Marteinsdottir and Begg 2002), the largest adult individuals may be several times larger than the smallest ones, with the greatest variation displayed by long-lived species. This creates large contrasts in size among intra-sexual competitors as well as among potential mates, for both sexes. In males of many species, intra-sexual competition and female mate choice reinforce each other in causing positive selection on body size (Hunt et al. 2009).
Effects of partner size
As predicted from the extensive size variation in fishes, larger-bodied males are often preferred by females (e.g., Bisazza and Marconato 1988) and males (e.g., Côté and Hunte 1989; Wong and Jennions 2003). In monogamous fishes, mutual mate choice for size can lead to assortative mating (Rueger et al. 2016). However, actual mate choice may be affected by factors (e.g., parental quality, Forsgren 1997a) that could interact with or override size (e.g., Qvarnström and Forsgren 1998; Wong and Candolin 2005). It is far from given that males or females would always express a preference for large-bodied partners, either because other factors are more important or because of a cost to choosiness (e.g., Wong and Jennions 2003), for instance when mating competition is strong.
The dynamics of the G. flavescens system makes it possible to analyze how mate choice relates to mating competition regime. Interestingly, we found females but not males to display a preference for larger mates. Using a standard 2-stimulus mate choice set-up, with a response (choosing) female seeing a small male at one end of the aquarium and a large male at the other end, we found females to express a clear preference for larger males early in the breeding season. Late in the season, however, females appeared indiscriminate with respect to male size (Figure 10; Borg et al. 2006). This finding is as predicted from OSR theory, as mature females in the wild have an abundance of males to choose from early in the season, but a shortage of potential mates late in the season (Forsgren et al. 2004). Moreover, mating with a large male may confer greater benefits to females when competition is strong early in the season, because a large male is less likely to have his nest overtaken and the brood lost. Like in many other species, larger-bodied G. flavescens males are more likely to keep a nest under competition (Figure 2 in Wacker et al. 2012). It is noteworthy that test females experienced no sex ratio treatment in the laboratory. Thus, their change in preference with season must either reflect an ontogenetic change in preferences or “memorized” recent experience of mating competition from the wild (Borg et al. 2006).
Female preference for large males in relation to time of season, in G. flavescens. The figure shows the proportions of females that responded more strongly to large and small males, respectively, in a 2-choice aquarium set-up. Reprinted, with permission from Elsevier, from Figure 1 in Borg et al. (2006), Animal Behaviour 72:763–771.
In contrast to females, we found no or at most a weak preference for female size among males, using the same type of experimental set-up (Pélabon et al. 2003). These results were obtained studying apparently healthy males (Pélabon et al. 2003); an absence of preference for larger females was also found among males infected by microsporidian parasites (Pélabon et al. 2005, the nature of infection shown in Figure 14). Both these studies were conducted during the latter half of the breeding season (late June to mid-July), at a time when gravid females would be present in excess and males thus have an opportunity to be choosy (Forsgren et al. 2004). While the lack of a clear male preference for female size may appear in contrast to theory, a further investigation suggests that fitness benefits to a male from choosing larger females (i.e., rejecting smaller ones) would be small, for a number of reasons. First, variation in female size is relatively modest, reflecting the fact that G. flavescens is an annual species for which individual age would vary by only a few months (all having hatched during May–July the previous year). Thus, in G. flavescens, a male that rejects a relatively small female would be unlikely to soon be visited by a much larger one (Pélabon et al. 2003). Second, variation in fecundity was less strongly related to female size than in many other fishes (female body length explaining only 37% of variation in fecundity, Figure 1 in Pélabon et al. 2003). This would further reduce the likelihood that rejecting a small female to later mate with a larger one would provide a significant fecundity benefit. Finally, while late-season males have the opportunity to exert choice of partners, it is not obvious that the benefit from doing so would always outweigh its costs. With an abundance of gravid females willing to mate late in the season, a male first accepting to mate with a less-than-average fecund females could likely mate again with another female shortly afterward, and so on until his nest was full, which might take less than a day. Thus, the benefit of choosing larger females for mating (i.e., rejecting small ones) would likely have been negligible even if there had been greater variation in female size and a more consistent relationship between female size and fecundity than found in our study (Pélabon et al. 2003). This situation may be different from that of choice for colorful females, where the male could gain an egg quality benefit from choice, and not just more eggs.
Effects of own size
The size of the choosing individual may affect its choosiness. This could for instance happen when there is mutual mate choice (e.g., Jones and Hunter 1993) for size. In such a case, smaller males may face fewer mating options and have less scope for choice (e.g., Foote 1988). When competition is strong, low-quality individuals may benefit from either relaxing their choosiness or reversing their preference in favor of less-sought-after mates (Fawcett and Johnstone 2003).
Having established that male G. flavescens overall prefer more ornamented females late in the season (Amundsen and Forsgren 2001), we explored whether variation in preference was related to the choosing male’s own size. That turned out to be the case, with the smallest males seemingly indiscriminate with respect to female coloration despite showing a clear eagerness to mate (Figure 3 in Amundsen and Forsgren 2003). We interpreted this to suggest that small males could not afford to be choosy, as they would be less attractive to females and/or loose out in contest competition over nests (Amundsen and Forsgren 2003). This argument likely applies in the first part of the breeding season, as large males are generally competitively superior in G. flavescens (Figure 2 in Wacker et al. 2012). It is less likely that small males would be discriminated against (Borg et al. 2006) or face strong nest competition (Wacker et al. 2014) late in the season, when the study was conducted. Taken at face value, the result may suggest that small males are selected to be indiscriminate in response to strong male–male competition early in the season, and that this preference is not plastic and hence maintained also when it entails no benefit late in the season. However, the study included relatively few small (indiscriminate) males (Amundsen and Forsgren 2003), which call for caution in interpretations.
OSR, Mating Competition, and Sexual Selection
Mating competition and mate choice are the major processes causing sexual selection (Andersson 1994). However, the 2 processes only lead to sexual selection if success is related to heritable phenotypic traits. Sexual selection on a trait requires the trait to explain a significant component of fitness variation among individuals, with certain trait values associated with higher fitness. Phenotypic sexual selection can be analyzed at 2 levels: as potential selection or as realized selection. The “potential for selection” approach originated from Bateman’s (1948) classical analysis of variance in reproductive success, showing that variation in number of mates had a stronger effect on reproductive success in males than in females. This insight, which is broadly applicable (Trivers 1972), was the basis for the “Bateman gradient” () (Andersson and Iwasa 1996) and the “opportunity for selection (I),” and “opportunity for sexual selection (Is)” indices (Wade and Arnold 1980). The Bateman gradient () quantifies the degree to which increased mating success is associated with reproductive success. The opportunity for selection (I) is simply an index reflecting variation in reproductive success; the opportunity for sexual selection (Is) is the equivalent index reflecting variation in mating success. The opportunity for selection indices express the maximum potential strength of selection given a certain variance in mating and reproductive success. These indices say nothing about which traits may be sexually selected but quantifies the potential for phenotypic selection.
By contrast, realized sexual selection expresses the degree to which specific traits (e.g., body size or ornaments) are subject to sexual selection in a given system. Such selection is typically analyzed by standardized selection differentials (s′) and selection gradients (β′) (Lande and Arnold 1983), describing the strength of selection on the phenotypic trait in question. This approach relies on the identification and measurement of phenotypic traits potentially subject to sexual selection.
There has been extensive debate on how best to measure sexual selection during the last decade, in parallel with our work on the G. flavescens system. The debate has centered on a number of topics, including: (i) the value of indices of potential selection vs. realized selection for understanding sexual selection (Jones 2009; Krakauer et al. 2011; Jennions et al. 2012; Henshaw et al. 2016; Janicke and Morrow 2018), (ii) the effects of mate monopolization and random mating on the relationship between OSR and sexual selection indices (Sutherland 1985; Klug et al. 2010a; Jennions et al. 2012), (iii) which index of selection best predicts total sexual selection in nature (Mills et al. 2007; Jones 2009; Fitze and Le Galliard 2011; Henshaw et al. 2016), and (iv) which individuals to include in real-world measures of sexual selection (Klug et al. 2010b). Elaborating on this complex yet important debate is beyond the scope of the present paper.
However, in our work on sexual selection in G. flavescens, we have adopted a complementary approach, analyzing both the potential for selection and realized phenotypic selection on specific traits. In one of the studies, we also analyzed how I and Is related to 2 other indices of sexual selection: the Bateman gradient () (Andersson and Iwasa 1996) and the maximum standardized sexual selection differential () (Jones 2009). An advantage of the potential for selection approach (e.g., I and Is) is that it neither requires measurement nor knowledge of the particular traits targeted by sexual selection, which makes it relatively easy to measure in many systems (including ones where realized selection on particular traits is hard to measure). However, variance-based approaches only provide an upper limit to the strength of sexual selection (potential selection), but do not answer how much of the potential selection is realized in selection on phenotypic traits. Ultimately, understanding how animal phenotypes (morphology and behavior) are shaped by sexual selection requires knowledge of which traits are targeted by selection, and the nature of selection on these traits. Such knowledge either requires successful a priori assumptions on which traits are under selection, or systematic exploration of candidate traits. When animals have multiple traits that could be under sexual selection, as is usually the case (e.g., in G. flavescens), such exploration is required. Thus, it may be harder to detect significant realized sexual selection on a specific trait than to document a significant potential for sexual selection. This practical limitation to analyses of realized selection is exacerbated by the fact that measures of sexually selected traits often have significant measurement error, and more so than measures of mating or reproductive success. Thus, the 2 approaches to measuring sexual selection have different strengths and limitations and cannot replace each other (Wacker 2013). We therefore recommend a combination of the 2 approaches whenever feasible.
Measuring sexual selection by experiment
In the mesocosm sex ratio experiment outlined above (“Support for a causal effect of OSR on sex roles,” Wacker et al. 2013), we investigated both the potential for selection and realized (actual) selection on a range of male traits. We analyzed the potential for sexual selection by estimating the opportunity for selection (I) based on reproductive success measured as number of eggs in the nest (Wacker et al. 2013). The breeding success (and consequent fitness) of a male is the product of number of mates, fecundity of these mates, and survival of eggs and later larvae. While larval survival is generally intractable in G. flavescens, egg survival is somewhat affected by filial cannibalism (Bjelvenmark and Forsgren 2003) but hatching success of eggs present at hatching time is very high (>90%; Table 2 in Svensson et al. 2006). The number of eggs in a nest correlated strongly with number of mates (Figure 2 in Mobley et al. 2009) in a field study, implying that the number of eggs in a male’s nest largely reflects his success in attracting many and fecund partners under the constraint of limited nest size (Table 2 in Mobley et al. 2009). Thus, we interpreted the opportunity for selection I, as measured from numbers of eggs in the nests, to reflect sexual selection. We found I to be significantly greater with an even than with a female-biased sex ratio (Wacker et al. 2013). Notably, an even sex ratio in this experiment implied a male-biased OSR, as each male could cater for eggs from several females in their nest. As nests were gradually filled up over the 6-day course of the experiment, the male-bias was somewhat reduced but far from eliminated.
Notably, variation in mating success inevitably increases with a change from a female-biased to a male-biased sex ratio even in the absence of any selection (i.e., with random mating), for purely numerical reasons (Jennions et al. 2012). This has been overlooked in much work on OSR in relation to selection. When nest size puts a limit to mating success, as in G. flavescens, this limitation will affect variation in mating success both under random mating and strong selection. Thus, we simulated the lower bound of I (which we termed Imin) under random mating, taking nest size into account, and assuming that a maximum of 4 females could spawn in an average nest (Forsgren et al. 2004). However, sexual selection may also have an upper bound, either because of nest-space limitations, or because care is mildly depreciable (Clutton-Brock 1991) at large brood sizes and leads to an optimal brood size beyond which males should refrain from attracting additional mates. Care of excessively large broods can be depreciable, if increases in brood size above some level constrains efficient ventilation (by fanning), cleaning, and defence against predators. In G. flavescens, nest size may considerably affect the upper bound to potential sexual selection, as no male could have more mates than required to fill up his nest. The upper bound to potential selection (Imax) was simulated based on maximal mate monopolization (i.e., when no female would spawn in an empty nest until all nests with eggs were full). We found the opportunity for selection (I) in our experiment to be significantly greater than it would be under random mating (Imin), whereas it was close to and not significantly different from the upper theoretical bound to selection (Imax) in the study system (Figure 11b; Wacker et al. 2013). These results reveal a strong potential for sexual selection.
Sexual selection in male G. flavescens. (A) Male morphological (upper) and ornamental (lower) traits potentially subject to sexual selection. (B) Measured opportunity (I, open circles and line) for selection in relation to theoretical minimum (filled triangles) and maximum (filled circles) opportunity for selection under different sex ratios. Measured opportunity is closer to the upper than the lower theoretical bound. Note that a change in sex ratio by itself increases the opportunity for selection. The shaded areas are outside the theoretical bound for sex ratio effects on opportunity for selection in the model system [see Wacker et al. (2013) for further explanation]. Reproduced, by permission of John Wiley and Sons, from (A) Figure 1 and (B) Figure 3 in Wacker et al. (2013), Evolution 67:1937–1949.
The most parsimonious interpretation of the results would be that there is phenotypic selection for male traits that promote high mating success (e.g., body size, ornamentation, and courtship). However, in substrate brooders like G. flavescens, mate monopolization (and consequent high I) can theoretically occur even in the absence of phenotypic selection (Wacker et al. 2013). This could happen because females of several substrate-brooding species (e.g., Unger and Sargent 1988), including the goby P. minutus (Forsgren et al. 1996a), seemingly prefer to spawn in nests where there are eggs from before. This could be a form of mate choice copying (“parasitizing” mate assessment by previous mates), or in order to reduce the risk of egg cannibalism by the male. An experiment on P. minutus provided support for the cannibalism hypothesis but not for mate choice copying (Forsgren et al. 1996a). It is unknown whether female G. flavescens have a preference for males with eggs in their nest. However, it is not unlikely that such a preference exists and may explain part of the variation in mating success.
More importantly, the study showed that the opportunity for selection changed more steeply in response to OSR than it would have done with random mating (significantly different slopes, Figure 11b) (Wacker et al. 2013). This conclusively documents a true effect of OSR on the opportunity for sexual selection. Our approach to analyzing how OSR affects sexual selection is novel, and overcomes the “problem” that estimates of I change with OSR even in the absence of selection (Jennions et al. 2012). We encourage future studies of OSR and sexual selection to similarly test whether the slope of measured variation in success (I) is greater than the slope simulated from random mating. Doing so would provide stronger tests of OSR effects on the potential for sexual selection than in most extant studies, and could significantly improve our understanding. Notably, OSR effects on the slope of I cannot be explained by a preference for males with eggs.
In G. flavescens, obvious candidates for sexual selection in males are the iridescent-blue lateral spots, and also the colorful dorsal fins (Figures 1 and 11a). These extravagant traits are conspicuously displayed both in courtship and in male–male interactions (Amundsen and Forsgren 2001; Forsgren et al. 2004). Another obvious candidate is body size and related bodily traits (Figure 11a). Male size is more variable than that of females and the largest males weigh 4–5 times that of the smallest ones of the same population (T. Amundsen et al., unpublished data). Body size has been shown to affect nest occupation both in G. flavescens (Figure 2 in Wacker et al. 2012) and other gobies. In line with this, we found significant positive selection for torso area and size of lateral blue spots (Table 2 in Wacker et al. 2013), but only in the even sex ratio treatment where males had to compete for females. Torso area and blue spot area were corrected for standard length in analyses, meaning that the selected individuals were more heavily built and more colorful than the average fish of their size. In this study, there was no selection for fish length per se. Torso area (Figure 11a; Wacker et al. 2013) is a “non-standard” measure in fishes, possibly because it requires standardized photographs of individuals and not only the usual weight and length measures. It is a compound measure of skeletal size, body musculature, fat deposits, and stomach fullness. The positive selection for torso area (controlled for fish length) suggests that “body build” matters in mating competition.
We could not reliably measure the size and coloration of the dorsal fins because we were unable to sufficiently standardize fin extension while photographing live fish. Likewise, we did not measure color qualities (e.g., spectral reflectance) of the iridescent blue spots. It is possible but yet to be demonstrated that these aspects of male ornamentation were also subject to sexual selection.
The most important finding from the experiment was the conclusive evidence for a causal effect of OSR on mating competition behaviors, on the potential for sexual selection, and on realized sexual selection on phenotypic traits (ornamental coloration and morphology). These experimentally documented effects mirror the covariation of mating competition with OSR over the course of the breeding season (Forsgren et al. 2004; Myhre et al. 2012; Wacker et al. 2014). Thus, they validate our claim that the seasonal dynamics of mating competition are causally related to the change in OSR, and not caused by some unknown factor that happens to vary in concert with OSR over the the breeding season.
Methodologically, the study provided a novel and strong way to test for true OSR effects on the potential for sexual selection, by comparing the slope of the opportunity for selection (I) in response to OSR variation, with the slope simulated from random mating. It also represents a new way of exploring the opportunity for selection, by simulating its upper and lower bounds and testing how experimental results relate to these. The study is likely unique in testing effects of OSR variation at 3 different levels in the same experiment: (i) mating competition behavior, (ii) the potential for selection, and (iii) realized selection on phenotypic traits. This comprehensive approach is obviously labor-intensive, but provides a coherent and integrated picture of how OSR affects sexual selection that could not otherwise be achieved.
In the wild, our findings would suggest that male ornamentation and body build is subject to selection early but not late in the season, in line with a male-biased OSR and a stronger potential for sexual selection early in the season. Such temporal variation in selection regimes would weaken overall sexual selection on males, and could even contribute in maintaining variation in male traits, for instance as a result of opposing selection on male size between early and late season.
Sexual selection in the wild
Sexual selection is harder to detect in the field than in experimental laboratory set-ups, due to the many factors that affect mating success in natural environments. While some obvious confounding variables (e.g., nest size) can be taken into account, there may be important ones that are not even known. Nonetheless, ongoing sexual selection in the wild can be investigated by comparing individuals that breed and those that do not breed (by selection differentials) and variation in individual success (analyzed by selection gradients). The G. flavescens model system has both strengths and weaknesses for such analyses. The strengths include the fact that nests can be fairly easily found and reasonable numbers of nests and males easily collected. A limitation is that it is very hard to distinguish breeders from non-breeders except when breeders defend a nest. Our analyses of sexual selection in the wild are therefore based on males found to hold a nest (mostly in a mussel, Figure 2) (Mobley et al. 2009; Wacker et al. 2014). Given the strongly female-biased OSR late in the season (Forsgren et al. 2004), we predicted males to have higher mating and reproductive success late than early in the season. This turned out to be true, with on average about twice as many eggs (ca. 2700 vs. 1400) in the nests late than early in the season. The difference was to a large extent due to fewer unmated nest-holders late in the season, but also to broods being larger among those that were mated (Table 1 in Wacker et al. 2014). This resulted in a significantly higher opportunity for selection (I, based on number of eggs) and sexual selection (Is, based on number of mates) early than late in the season (Wacker et al. 2014), as would be expected from OSR theory.
As argued above, not only Is, but also I, reflects sexual selection in this system: Is reflects the number of females that has spawned in a nest whereas I is a composite measure of number of mates and the fecundity of these mates. We could measure both in this study because, unlike in the mesocosm experiment of the previous section, all broods were genotyped for parentage analyses (Wacker et al. 2014). We similarly found the maximum standardized sexual selection differential (s′max) (Jones 2009) to be greater early than late in the season, whereas the Bateman gradient () (Andersson and Iwasa 1996) was similar early and late. This was because, in our study, the reduced potential for sexual selection was due to reduced variation in mating success and reproductive success, rather than a change in the degree to which mating success translated into reproductive success (Wacker et al. 2014). The results revealed that the Bateman gradient failed to detect important changes in sexual selection in our study, despite that it has proven successful in predicting sexual selection in other model systems (e.g., Jones et al. 2000; Fritzsche and Arnqvist 2013). This calls for caution in drawing inferences about sexual selection from one index alone (Wacker et al. 2014), as the nature of selection may vary between systems and may be more or less well captured by each index.
The patterns emerging when analyzing for selection on specific male traits (Table 2 in Wacker et al. 2014) were less clear and partly contradictory to results found in the laboratory (Table 2 in Wacker et al. 2013). However, small sample sizes for these analyses along with the males having been collected at various stages of breeding render these results less conclusive (Wacker et al. 2014).
With a male-biased OSR early in the breeding season, one would expect competitively superior males to exclude small ones from breeding, especially if high-quality nest sites are limited. This seems to be the case, as artificial PVC nest tubes introduced to the breeding habitat are usually quickly occupied (K. de Jong, unpublished data). Artificial nests have also had high occupancy rates in other populations (W Norway, Monroe et al. 2016, Mid-Norway: T. Amundsen and S. Wacker, unpublished data). Thus, small males may be forced to or strategically postpone breeding until competition over nests and females is relaxed later in the season, when male density is lower and gravid females abundant. By contrast, one would expect large males to be the ones successful in getting a nest when male density is high and competition strong, as it is early in the breeding season. One would therefore expect nest-holding males to be larger than the population average early in the season, and also larger than those breeding later (Wacker et al. 2014). Late in the season, male density is much lower (Figure 1 in Forsgren et al. 2004) and male competition almost absent (Figure 6b, c). At this time, one might expect any male still alive to be able to gain a nest, and thus no difference in size between nest-holding males and the population average. As predicted, we found early season breeders to be clearly larger than the population mean, resulting in significant positive selection for male size. However, late season breeders were clearly smaller than the population mean (Figure 1 in Wacker et al. 2014) and thus also smaller than early-season breeders (Figure 12), with consequent negative selection on male body size (Wacker et al. 2014). This was a result that we did not predict, and which we therefore can only interpret post hoc. We suggest that large males, having started to breed early in the season and having cared for several consecutive broods, have paid a greater cost of care than smaller ones that have either employed a sneaker tactic or bred fewer times, if at all. This may render small males more fit for costly care than large ones late in the season, and thus more attractive to females at that time. Whether or not this hypothesis is true, the results indicate opposing selection on male body size between early and late season (Wacker et al. 2014).
Gobiusculus flavescens males that breed late in the season are smaller than those breeding early in the season. Reproduced, by permission of John Wiley and Sons, from Figure 2 in Wacker et al. (2014), Molecular Ecology 23:3587–3599.
Resource competition vs. mating competition
In animals that require a territory or a particular nesting structure to breed, competition for mates is often preceded by competition for such breeding resources. A typical example is migratory birds, where males often compete for territories even before the females have arrived to the breeding grounds. Similarly, a goby male needs a nest (mussel or other) to breed. Accordingly, male G. flavescens (Wacker et al. 2012), as well as other gobies (e.g., Lindström 1988; Magnhagen and Kvarnemo 1989), may compete for ownership of nests. When nests are in short supply, only competitively superior goby males may be able to obtain a nest (e.g., Forsgren et al. 1996b). Assuming that competition for nests precedes competition for mates, Ahnesjö et al. (2001) argued that only males that have succeeded in nest competition are “qualified to mate” and thus involved in mating competition (see also Kvarnemo and Ahnesjö 2002). In G. flavescens, however, anecdotal observations in the wild suggest that males sometimes engage in courtship without having previously defended a single, well-defined, nest. With the species being highly opportunistic in choice of breeding substrate, and with more-or-less good nesting substrates available in excess in most territories, a male may be able to find a suitable substrate to which he can lead an interested female for spawning, even if he has not resided there on beforehand. Males are also much more prone to compete for and spend time in nests when there is a female present (personal observation). Such observations suggest that resource competition and mating competition may not be as clearly separated as often assumed. This may render the distinction between males qualified and unqualified to mate less clear.
In order to test for potential interactions between mate competition and resource competition, we experimentally varied OSR and nest abundance. Males were either faced with a shortage of nests but not of females, a shortage of females but not of nests, or no shortage of either (Wacker and Amundsen 2014). Notably, nest shortage (fewer nests than males) did not increase competition behavior (aggression and courtship) above the baseline level when neither mates nor nests were in short supply. Thus, nest shortage per se did not seem to significantly affect competition. By contrast, a shortage of females induced a strong increase in competition behavior relative to the “no-shortage baseline” (Figures 1 and 2 in Wacker and Amundsen 2014). Fewer nests were occupied by males when there was a shortage of females than when there was no female shortage (Figure 3 in Wacker and Amundsen 2014). With a shortage of nests but not of females, males behaved less aggressively and courted less than when females were in short supply, and in fact no more than in a treatment with ample access to both nests and females. Thus, nest shortage seems not to elicit increased male competition, unless there is a shortage of females with whom to mate.
These results call into question the traditional assumption that resource (nest) competition comes before mating competition and, more fundamentally, that these are truly distinguishable processes. While this may often be the case, as in many hole-nesting birds, our results suggest that it need not always be so. Instead, the findings suggest that, in G. flavescens, the 2 processes are inter-related, and maybe impossible to fully separate, both empirically and theoretically (Wacker and Amundsen 2014). If this is true and to a greater or smaller extent also true for other species, it calls into question whether one can distinguish between males that are “qualified to mate” (nest-holders) and those that are not (non-nestholders) (Ahnesjö et al. 2001), in G. flavescens and any species where male nest-related behaviors are affected by presence or absence of females. The general argument by Ahnesjö et al. (2001) is that only those males that hold a nest take part in mating competition (see also Szekely et al. 2014b). Hence, Ahnesjö et al. (2001) suggest that this subset (which they term Q, for Qualified), instead of ASR, is what should be combined with potential reproductive rate to determine the OSR and thus predict mating competition. We fully endorse this argument on general grounds: provided nest (resource) competition precedes mating competition, Q should be both quantifiable and more relevant than ASR. If, however, males do not always establish in a nest before engaging in mate attraction (i.e., resource and mating competition does not occur in sequence), it becomes less clear which males are qualified to mate. When this is the case, quantifying Q from numbers of males residing in nests may underestimate the true number of males in the mating pool, and thus bias OSR estimates “in the female direction” (i.e., overestimating any female bias or underestimating any male bias). We encourage more studies to test, in similar systems, whether resource competition and mating competition are truly sequential and independent, as is generally assumed (e.g., Ahnesjö et al. 2001), or whether they are instead simultaneous and inter-related, as suggested by our study. It should be noted that these 2 alternatives are the extremes of a continuum: the most commonplace situation may be one where resource (e.g., nest) shortage by itself elicits competition, but where resource competition significantly increases when there is a shortage of, and thus competition for, mates.
In systems like that of G. flavescens, it is conceivable that the concept of individuals qualified to mate (Q) is relevant, but not necessarily reflected in nest occupation. As outlined at the start of this article, males are either relatively stationary (i.e., mostly hovering over a small area, often <1 m2) or roaming. They will often but not always behave aggressively to other males getting near. We have interpreted such stationary males to be territorial, and to be available for mating, even when never observed in a nest. A territory in the kelp forest is likely to include a number of potential nest sites to which a male can lead an interested female. He may be aware of and have inspected several such potential nest sites, without spending much time there, as we have occasionally observed in the wild (T. Amundsen, personal observation). Alternatively, he may be able to instantaneously locate suitable nest sites if visited by a female, even if he has not inspected these sites before. Under such scenarios, Q < ASR (Ahnesjö et al. 2001) would be a reality (roaming males are not “in”) but would represent the number of territorial (stationary) males rather than the number of males occupying a nest. This is essentially the approach we have taken when calculating OSR in G. flavescens in the field: we have included stationary males whether or not we have seen any nest, but excluded roaming ones (Forsgren et al. 2004). In any species or population where potential nest sites are present in excess within territories, a scenario may apply in which males “control” potential nest sites by territoriality without spending much (or any) time in the nests before engaging in courtship. Hence, the relevance of “strict sense Q” (males occupying a nest) for estimating mating competition, and the degree to which resource and mating competition are separated, may vary not only between species, but also within species.
Notably, we found significant selection on male size in the mate-limited, but not in the nest-limited, treatment (Figure 4 in Wacker and Amundsen 2014): nest-holding males were larger than non-nest-holders with female shortage, but not with nest shortage. The finding that males engage in nest competition mainly when there is a shortage of females makes sense from a cost–benefit perspective. Given that nest defence is costly, the cost may only be balanced by sufficient benefits when the likelihood of mating is high. The strength of sexual selection under mate limitation decreased over the breeding season (Wacker and Amundsen 2014).
Environmental variation, mating behavior, and sexual selection
In the wild, most animals live in complex environments, with habitat type and structure varying among and within populations. Similarly, critical breeding resources (for instance suitable nest sites) may be abundant or scarce (e.g., Forsgren et al. 1996b; Borg et al. 2002), and they may be anything from uniformly distributed to highly clumped. Environmental heterogeneity may affect male reproductive behavior (e.g., aggression) directly, or via effects on inter-nest distances (Bakker 1994). The nature of the habitat, and also the availability of suitable nest sites, is today commonly affected by anthropogenic disturbances. For example, the wide-spread eutrophication of freshwater and marine environments has affected sexual selection in a range of fish species including gobies, via effects on turbidity or habitat structure (e.g., Seehausen et al. 1997; Järvenpää and Lindström 2004; Wong et al. 2007; Candolin and Wong 2012; Sundin et al. 2016).
The natural habitat structure of G. flavescens is highly variable and often complex (Figure 2), and is also temporally dynamic, between and especially within years. For instance, kelp forests (often dominated by Saccharina spp., Laminaria spp., or a combination) typically have high structural complexity, whereas seaweed beds (e.g., Fucus serratus-dominated) have less “3D-complexity.” The type and degree of structural complexity can vary considerably among nearby islands, between windward and leeward sides of the same island, and between sheltered bays and exposed rock-faces. It may also vary temporally, as perennial macro-algae become increasingly overgrown by annual filamentous algae as the season progresses (Figure 2h). Such filamentous algae can both reduce and increase structural complexity, depending on the initial species composition and habitat structure. When it comes to breeding substrate, G. flavescens appears to favor breeding in empty mussels, but such breeding sites are in short supply in most of the habitat and their distribution can be highly variable. In some cases, available breeding sites can be highly clumped, as in a cluster of live and dead blue mussels attached to a rock-face.
While there is reason to believe that G. flavescens is faced with an unusually complex and variable habitat, both when it comes to structure and nest substrates (Figure 2), structural and other within-population habitat variation is almost ubiquitous among animals. Thus, analyses of how environmental complexity and variation affects processes related to mating and breeding should be of great relevance for understanding natural systems. For instance, a less structured environment may facilitate mate detection and comparison of potential mates but may at the same time increase the risk of mating interference, because behavioral interactions including mating are more often visible to nearby conspecifics and to heterospecific predators. Similarly, a highly clumped distribution of nests may facilitate mate assessment by females, but also lead to more male–male aggression and potentially preclusion of less competitive males from breeding. Despite this, most experimental work on mating competition in the laboratory (including that on G. flavescens) has been conducted in structurally simple environments, motivated by logistic tractability and, for behavioral work, also observability.
Effects of structural complexity
In G. flavescens, 2 studies have addressed the role of environmental characteristics for mating competition and consequent reproduction. Creating spatial complexity by means of opaque acrylic barriers in 2 × 2 m mesocosm tanks, and comparing this with a non-structured environment in the same sort of tanks (Figure 13a, b), Myhre et al. (2013) analyzed how environmental structure affected courtship, competition, and breeding. They found strong effects of structure on the behavior of both sexes. For females, a structured environment caused less movement and less frequent encounters with males but, despite the latter, a shorter time until mating (Figures 2 and 3 in Myhre et al. 2013). This may have been because fewer courtship interactions were interrupted by other males in the structured environment, as was also found, likely for the simple reason that ongoing courtship interactions were not detected by other males. The same reason may explain less frequent multi-male courtship in the structured environment (Figure 4 in Myhre et al. 2013). Taken together, the results indicate that habitat structure negatively impacts both female mate choice (fewer encounters) and male–male competition (less direct mating competition). This is consistent with the finding of positive selection on male size in the open but not in the structured environment. The results indicate that a more complex habitat may relax sexual selection (Myhre et al. 2013). Given the often highly variable environments of many species, including G. flavescens, this insight may be of importance for understanding variation in sexual selection in the wild. The study suggests that the structurally simple environments often employed in laboratory experiments, including several of those on G. flavescens, runs a risk of overestimating sexual selection compared with a natural situation in the wild. This emphasizes the value of complementing laboratory experiments with systematic field studies, in order to evaluate the “real-life” relevance of laboratory findings to natural ecosystems.
Experimental designs for testing effects of habitat structure (A, B) and nest spacing (C, D) in G. flavescens. Tubes (8 in each treatment) indicate artificial PVC nests, the branched structures artificial algae placed in the aquaria. The upper 2 panels illustrate a study comparing mating behavior and sexual selection between an open (A) and a physically structured (B) environment (Myhre et al. 2013). In (B), spatial structuring is achieved by opaque Plexiglas dividers formed to allow fish to move across but significantly preventing visual contact between the compartments containing nests. The lower 2 panels illustrate a study comparing mating behavior and sexual selection between environments with a dispersed (C) or clumped (D) nest distribution (Mück et al. 2013). Reprinted, by permission of Oxford University Press, from Figure 1 in Myhre et al. (2013), Behavioral Ecology 24:553–563 (A, B), and, by permission from Springer, from Figure 1 in Mück et al. (2013), Behavioral Ecology and Sociobiology 67:609–617 (C, D).
Effects of nest distribution
Mück et al. (2013) manipulated nest distribution (artificial PVC tubes) to be either highly clumped (aggregated nests) or maximally dispersed, in tanks of the same size (Figure 13c, d). When nests were aggregated, a larger proportion of the males behaved aggressively (Figure 2 in Mück et al. 2013) but fewer of them succeeded in occupying a nest and becoming mated. Moreover, those males that mated had smaller broods in their nests (Figure 3 in Mück et al. 2013). These effects resulted in a higher variance in reproductive success and, hence, a higher opportunity for selection (I), in the aggregated treatment (Figure 5 in Mück et al. 2013). In environments where nest sites are usually clumped, we may therefore expect strong sexual selection by male–male competition. Clumped nests may also facilitate female comparison of potential partners, but may at the same time increase the risk of mating interference, limit the range of males available for mating (as many males are unable to defend a nest in aggregations), and increase the risk of sneaking by competitively inferior males. Thus, clumping of breeding resources may severely constrain female choice and could lead to a system where male–male competition overrides female choice. As females do not always prefer to mate with the more dominant males (e.g., Forsgren 1997a; Qvarnström and Forsgren 1998; Candolin 2004; Wong and Candolin 2005), nest site distribution may therefore have a significant impact on realized sexual selection. Notably, in G. flavescens and many other animals, including substrate brooding fishes, the same population can include both clumped nest substrates (e.g., mussel clusters) and individual nest substrates (e.g., solitary mussels) that are widely dispersed. In such cases, sexual selection may vary on a micro-scale, with strong selection on male competitive ability in nest-structure aggregations and less competition but more scope for female choice where there is a greater distance between favored substrates (and thus males). This situation may promote a dynamic in which less competitive males aim to avoid nest aggregations and rather try to take up nests further away from competitors. Taken together, variation in structural complexity and distribution of favored nesting resources may have significant impacts on the type and strength of sexual selection processes. If traits selected for in competition are not the same as those favored in mate choice, such environmental variation may contribute in maintaining variation in male traits.
Effects of parasite infection
Parasites often have a severe impact on body condition and performance of animals (Poulin 2007), and significantly impact animal behavior (Moore 2002). Parasites are central to sexual selection, both because of their commonplace negative effect on performance and sexual signaling, and because sexual ornaments may evolve as signals of parasite resistance and immuno-competence (Hamilton and Zuk 1982; Folstad and Karter 1992).
Studies of G. flavescens in Germany have revealed that the species can be host to a wide range of parasites (Zander 2003, 2004, 2005). In the W Sweden G. flavescens study population, a proportion of both males and females is infected by a unicellular Kabatana sp. microsporidian parasite (Barber et al. 2009). The microsporidians multiply in colonies in the musculature, destroying the muscle fibers of infected tissue and being visible as ulcerous whitish spots on the exterior of the fish (Figure 14; Barber et al. 2009). Heavily infected individuals appear to have difficulties swimming properly (personal observation) but most individuals are less heavily infected. While there has been much research on effects of parasites on morphological traits including ornaments, less knowledge exists on how parasites affect mating behaviors of males and females. The goby-microsporidian infection system is well suited to explore such effects, both because the parasite directly affects the musculature and because infections are visible externally. The external visibility allows quantification of infection in live animals, significantly facilitating study designs for behavioral work. Equally importantly, it also means that the fishes can visually assess the parasite status of conspecific males and females, which is not the case for internal parasites. Comparing courtship behavior between parasitized and non-parasitized males, we found a 30% reduction in courtship among parasitized males (Figure 2 in Pélabon et al. 2005). As high-courtship males are often preferred by females in gobies (e.g., Forsgren 1997b) and other animals (e.g., Grant and Green 1996), such a reduction may significantly decrease mating success. Notably, the negative effect on courtship was present despite no detectable negative effect of parasite infection on body condition. Hence, the study suggests that courtship may be a sensitive index of sub-lethal effects of parasites and other stressors.
Microsporidian Kabatana sp. infection of male G. flavescens. (A) Heavily infected male. Each spot is a colony of microsporidians. (B) Longitudinal section through infected flank muscle, with Masson’s trichrome staining indicating the spore mass (red) and surrounding, intact myofibrils (blue). (C) VOF-stained longitudinal section of non-infected (blue) and enlarged infected muscle fibers (brown) in the mandible. (A) Photo: © Anders Salesjö, (B, C) modified from Figure 2 in Barber et al. (2009), Diseases of Aquatic Organisms 83:145–152. When the fish gets infected, microsporidian spores migrate to the striated muscles of the fish (B, C), where they multiply to replace the muscle fibers, essentially leaving infected musculature gelatinous and non-functional. Muscles in any part of the body can be infected. Skeletal musculature is often infected and, when extensive like in (A), the swimming ability of the fish is impaired.
Alternative Reproductive Tactics
In many fishes, individual males employ alternative reproductive tactics, with some being territorial (bourgeois), aiming to monopolize breeding resources and females, others displaying a “sneaker” tactic of parasitic spawning, and others again mimicking females to gain access to spawning male–female pairs (Taborsky 1997; Oliveira et al. 2008). Territorial and sneaker tactics are co-occurring in populations of very many species, whereas female mimicry tactics seem less widespread (e.g., Taborsky 1998). Sometimes, sneaking and female mimicry tactics are hard to distinguish, because small males often resemble females and because both tactics are parasitic. Alternative reproductive tactics can be obligate or facultative. If facultative, males can either variably act as sneakers or territorials depending on competitive situation and own resource-holding potential (RHP) (Parker 1974), display an ontogenetic change from sneaker to territorial as they grow (Magnhagen 1992), or a combination (Oliveira et al. 2008).
Low level of sneaking
Sneaking is widespread among substrate-brooding fishes (Coleman and Jones 2011), and has been reported to frequently occur both in the gobies P. minutus (e.g., Jones et al. 2001a, 2001b) and P. microps (e.g., Magnhagen 1994). However, the frequency of sneaking and proportion of clutches that are parasitized vary greatly among studies and species (Coleman and Jones 2011). In 2 populations of P. minutus, the proportion of broods with parasitic eggs was similar at about 35–50% (Jones et al. 2001a, 2001b). In the West Swedish study population of G. flavescens, however, we found that the proportion of eggs fertilized by parasitic (non-nest-holding) males was generally very small (<1%, Mobley et al. 2009; Wacker et al. 2014). In particular, sneaking was almost absent late in the season (Table 2 in Mobley et al. 2009), when male competition is minimal or non-existent (e.g., Forsgren et al. 2004). At this time, there would be little if any competition for nest sites, so that even competitively inferior (small) males could establish as territorials. As described above, we indeed found nest-holding (bourgeois) males to be mostly small at this time (Wacker et al. 2014). Evidence from a W Norwegian population has documented that the same male can act as a sneaker early in the season and as a nest-holder later on (Monroe et al. 2016).
Geographic variation in tactics
Notably, the dynamics of alternative reproductive tactics in G. flavescens appear to be very different in the population studied in W Norway (Utne-Palm et al. 2015; Monroe et al. 2016). In this population, a major proportion of the males are very small, and typically smaller than females (Figure 15a; Utne-Palm et al. 2015), unlike the situation in W Sweden where very small males are rare (T. Amundsen et al., unpublished data). The small males in the W Norway population tend to have very large testes for their size (Figure 15b, c), and to store sperm in the seminal vesicles (Utne-Palm et al. 2015) that would produce mucus for lining the nest before spawning among territorial males. Thereby, sneaker males, who do not need to produce any mucus, likely maximizes their fertilizing success. Consistent with the prevalence of an apparent sneaker morph, nearly a third of male nests both early and late in the breeding season contained eggs sired by one or more parasitic males (Figure 15d; Monroe et al. 2016). The differences in mating tactics between populations in W Sweden and W Norway raise the intriguing question whether sex roles and sexual selection in G. flavescens are dynamic not only on a temporal, but also on a spatial, scale.
Alternative reproductive tactics are prevalent in a West Norwegian population of G. flavescens. In this population, males are generally smaller than females and there is an abundance of very small males (A), the smallest males have much larger testes (gonado-somatic index GSI) for their size (B, C), and sneaking occurs both early and late in the season (D). Reproduced from (A) Figure 1, (B) Figure 2 and (C) Figure 6 in Utne-Palm et al. (2015), PLoS One 10:e0143487, and from (D) Figure 2d in Monroe et al. (2016), Journal of Evolutionary Biology 29:2362–2372, by permission of John Wiley and Sons.
Conclusions and Prospects
I hope to have shown that the G. flavescens model system has great potential in analyzing and understanding temporal dynamics of sex roles and sexual selection. The work presented here is only a start for future studies to build upon. A main quality of the system is its extremely dynamical nature, to my knowledge so far unparalleled in any other vertebrate model. The system is also exceptional in the ease by which large-scale field and laboratory studies can be combined. In the field, the species is easy to observe in its shallow habitat with typically clear oceanic water, is highly abundant yet mostly relatively stationary, and is basically unaffected by close-range presence of an observer. Gobiusculus flavescens is also easy to collect in large numbers for laboratory experiments and population analyses, and behaves naturally and breeds readily in captivity. None of these characteristics are unique to the G. flavescens system, but only few systems have all these qualities present at the same time, and to the same degree as G. flavescens.
Taken together, the G. flavescens studies indicate a central role of the OSR in regulating the strength and “direction” of mating competition. Insights gained from this model system should be of relevance to any animal that experiences natural fluctuations in adult and operational sex ratios. Such fluctuations appear to be common but have not been extensively studied. This is particularly the case for within-breeding season changes in competition regime. I encourage scientists to explore model systems with similarly extensive variation in OSR (including both male-biased and female-biased situations), in order to establish to what extent insights from studies of G. flavescens are broadly applicable. The most suitable model systems would be ones where behaviors and breeding can be easily recorded both in the field and in the laboratory, like in G. flavescens. I encourage a search for further species with extensive OSR variation, and the use of such species to test for relationships between OSR, mating competition, and sexual selection. Only when the relationships between OSR and mating competition have been explored in a number of model organisms can we robustly generalize about how OSR variation affects variation in sex roles and sexual selection.
Mating competition and sexual selection may vary not only temporally, but also spatially. While most work on G. flavescens has been carried out in West Sweden, recent published and unpublished evidence suggest that the dynamics of sex ratios and sexual selection vary geographically. Future work on this and other model systems should explore whether spatial variation in OSRs have similar effects on mating competition as have temporal dynamics, and in particular how spatial and temporal variation interact in shaping sexual selection.
Acknowledgments
I thank all my collaborators in research on two-spotted gobies over nearly 2 decades. In particular, I would like to thank Elisabet Forsgren who introduced me to gobies and co-managed the two-spotted goby project with me during many years. I also thank Elisabet Forsgren, Sebastian Wacker, Karen de Jong, Jarle Tryti Nordeide, Ingo Schlupp, and 3 anonymous reviewers for comments on the manuscript. I thank Ingo Schlupp for the invitation to Behaviour 2017 that spurred this paper. Any insights on sex roles and sexual selection emanating from work on the G. flavescens model system are due to joint efforts, empirically and intellectually, by all the authors on the various papers. I am deeply grateful to collaborators Iain Barber, Jon Blount, Bjørn Bjerkeng, the late Angela Davies, Sam Dupont, Elizabet Espy, Joe Ironside, Adam Jones, Fredrik Jutfelt, Carin Magnhagen, Ian Mayer, Melanie Monroe, Hanno Sandvik, Helen Sköld, Peter Surai, and Anne Christine Utne Palm; to former post-docs Kenyon B. Mobley and Christophe Pélabon; to former Ph.D. students Jens Bjelvenmark, Åsa A. Borg, Karen de Jong, Lise Cats Myhre, P. Andreas Svensson, and Sebastian Wacker; to former M.Sc. students Uwe Berger, Camilla Brevik, Jorunn M. Eriksen, Aleksandra I. Johansen, Isabel Mück, and Gry Sagebakken, and not the least to the countless field assistants (none named, none forgotten) who made the intensive field and laboratory work possible. I have been greatly inspired by collaborations and discussions with colleagues and friends in the Nordic Goby Network, including Katja Heubel, Lotta Kvarnemo, Kai Lindström, Carin Magnhagen, Ola Svensson, and Anne Christine Utne Palm. The work would not have been possible without generous scientific advice from many scientific colleagues, in particular those at the Sven Lovén Centre for Marine Sciences at Kristineberg, and similarly generous help from technical staff at Kristineberg and at the Espeland Marine Biological Station of the University of Bergen. I would finally like to thank those who have recently worked with me to establish a Mid-Norway study system for G. flavescens, first of all Sebastian Wacker to whom I am greatly indebted, but also NTNU technical staff and the many volunteers who have made great efforts in the field. This work has greatly inspired my current thinking about sexual dynamics and the potential of the G. flavescens model system in sexual selection research.
Funding
The work on which this review article is based has been funded by grants from the Research Council of Norway [Grant Nos. 133553, 146744, 166596, and 178444], the Norwegian University of Science and Technology, the Norwegian Academy of Science and Letters, the Royal Swedish Academy of Sciences, the Nordic Marine Academy, the EU Transnational Access to Research Infrastructures Scheme, the Nordic Council program NORDFORSK, and the National Science Foundation [USA, Grant No. OISE/0701086].




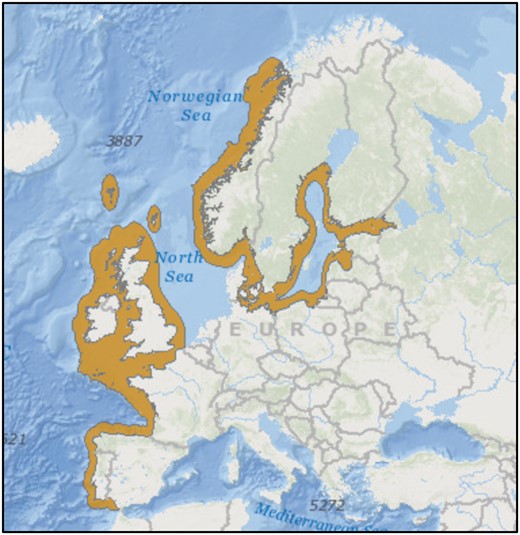
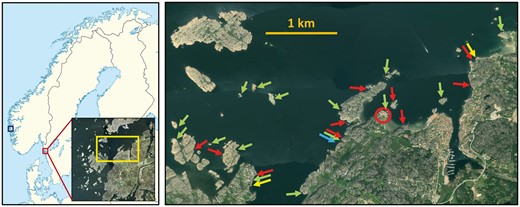
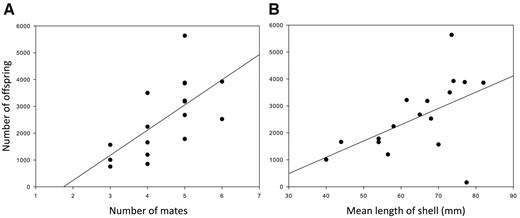
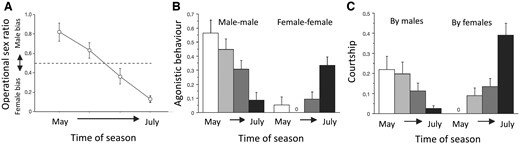
![How to measure mating competition: by frequencies of behaviors or propensities to behave? If OSR effects on courtship or aggression are measured by how often an act happens under various sex ratios, changes in encounter rate with opposite or same sex individuals will cause changes in numbers of courtship or agonistic acts even in the absence of any effect of sex ratio on individual behavior (the propensity for courtship and aggression at encounters). In this figure, the term competitor-to-resource ratio (CRR) is used instead of OSR in order to make the logic independent of sex of the actor [see de Jong et al. (2012) for further detail]. (A–D) Effects on courtship. With an increasing CRR (i.e., fewer potential mates), there will be fewer mate encounters (thin dashed lines). Even if this causes an increased propensity to court (A–C, thin lines), the frequency of courtship (bold lines) will decrease over the whole (A) or part (B, C) of the CRR range. In (D), we assume no effect of CRR on the propensity to court, in which case courtship frequency will decrease due to fewer encounters. (E–H) Effects of CRR on aggression (agonistic behavior). With increasing CRR (i.e., more competitors), frequencies of same-sex encounters (thin dashed lines) will increase. Depending on how this affects the propensity to behave aggressively upon encounters, the result will be smaller or greater differences between trajectories for aggression propensity (thin lines) and frequency of aggression (bold lines). Trajectories could be qualitatively different over certain ranges of CCR (E–G), or over the full CRR range (H). Reprinted (A–D) from Figure 1 and (E–H) from Figure 2 in de Jong et al. (2012), Behavioral Ecology 23:1170–1177, by permission of Oxford University Press.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cz/64/3/10.1093_cz_zoy036/3/m_zoy036f7.jpeg?Expires=1716312361&Signature=eoXmhUurXKmnSQETTNxh25h3UOjeXSM1vdszFAr5XI2Nxdu-sgXRnj2H9zOvuY-GG23KZS73PFVSwvrBE6PR8CVLwtlk0KMYtkI6fjomcbkHlbZjyfCrCUQH6LHOl0orVPBrpY80DROqtE7f9EwDYBXCprJrzWH6o9p6bef05fBcoakMq0SE00Ewj30pDSuOLk-fwak5GvIcAB7cDyUCCLhND3lOksM5pOPQLsI-chNw8BvqWAu-ppu9UgrhPkWtcJDOQT5aIMcYWob~GetA~mgmiPMYCm7Pi1G0zj0C9l3wEoVQNBU5NExVjFvMoyNLvsbde9yP3KOWzu3UEo4LTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
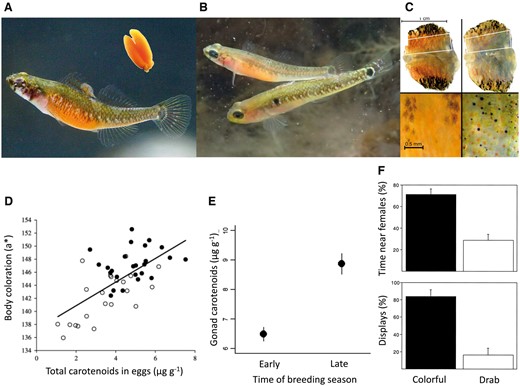
![Effects of time of season on female mate search behavior in G. flavescens. “Early” refers to late April and May (male-biased OSR), “Late” to mid-June to mid-July (female-biased OSR). Females from one locality were captured, individually marked, released at another locality, and followed during mate search [see Myhre et al. (2012) for details]. (A) Number of males visited (sampled) during 30 min observations of mate-searching females, (B) female propensity to initiate courtship, (C) female propensity to terminate courtship, (D) frequency of multi-female courtship, and (E) likelihood of male courtship in relation to the number of simultaneously courting females. About 30% of the females mated during 30 min of observation. (A–D) Open boxes represent females that did not mate during observation, shaded boxes those that mated during observation. Reproduced (A) from Figure 2, (B–C) from Figure 1 and (D–E) from Figure 2 in Myhre et al. (2012), American Naturalist 179:741–755, with permission of University of Chicago Press.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cz/64/3/10.1093_cz_zoy036/3/m_zoy036f9.jpeg?Expires=1716312361&Signature=FuDD3Doxrfiv-fAuMZxy8dYsGhUgUJ-P4iVWVwFynQK-~frRKPby6nxrCyQWXJV0zcxq8RT5cwl~stzLk07mknIrWDeHpBYkbek3LL8LfQC-H6JLFYvsfFzjYy6uvkFjLjPqC3aSeU45FBG-1ozcS0HRDMVpsXJSa7avWMPhSA~K00c6wpaQPeUOS1znKpe4UdR0HLJJHYSxlLzeq94dBJlUdDdZpx14dvrwC62sk68PuMRvpwy7kJkLzuFtSrKpkegQbmTKSBmAXeEuxPt84YNUec~ET-yQItXhM9FCpQXf4ts0RfjTcZEStcANCV2OQvcRxgvizqeHPFSIo~P7Uw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
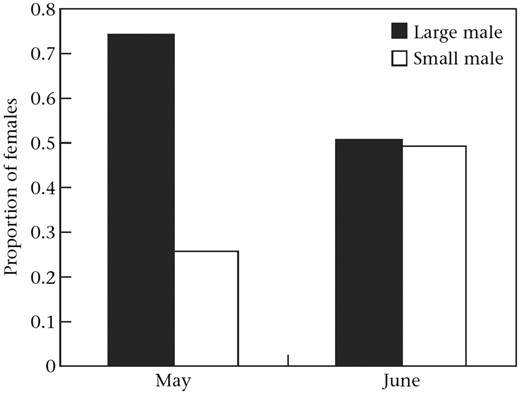
![Sexual selection in male G. flavescens. (A) Male morphological (upper) and ornamental (lower) traits potentially subject to sexual selection. (B) Measured opportunity (I, open circles and line) for selection in relation to theoretical minimum (filled triangles) and maximum (filled circles) opportunity for selection under different sex ratios. Measured opportunity is closer to the upper than the lower theoretical bound. Note that a change in sex ratio by itself increases the opportunity for selection. The shaded areas are outside the theoretical bound for sex ratio effects on opportunity for selection in the model system [see Wacker et al. (2013) for further explanation]. Reproduced, by permission of John Wiley and Sons, from (A) Figure 1 and (B) Figure 3 in Wacker et al. (2013), Evolution 67:1937–1949.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cz/64/3/10.1093_cz_zoy036/3/m_zoy036f11.jpeg?Expires=1716312361&Signature=OdlJr2t77efSMG1FoCg1UPHO6vKmmReVsQE7ZHJe7bRq9eI8w50nw3GiDHe550SjDu0pnF0ysczuQ5eoI~AP7gmUeD~V3CJ-k8g8jH7vPO8dTXMJIWsHl6oNmJNAxsxGSq4UGGW50ylCoT~YEbDWuncwBvAekcwPUFW4Olfi1zlogJ30B~isXveWOBRcyITWp~ZPabQmyduT4EpRBdkcauGewB73eBsckmtlxdMmSxVr~JuuurAqRlmG3saSONwfWCXkAG~Ia5C~So73wZsyurnlTi42dqD6GZyXt25mHM9wXmZDtPd6S0HvY5ekA1-p~WLQor25vWzNDsHd~-Tteg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)