-
PDF
- Split View
-
Views
-
Cite
Cite
Breanna J Putman, Gregory B Pauly, Daniel T Blumstein, Urban invaders are not bold risk-takers: a study of 3 invasive lizards in Southern California, Current Zoology, Volume 66, Issue 6, December 2020, Pages 657–665, https://doi.org/10.1093/cz/zoaa015
Close - Share Icon Share
Abstract
Biological invasions threaten biodiversity worldwide, and therefore, understanding the traits of successful invaders could mitigate their spread. Many commonly invasive species do well in disturbed habitats, such as urban environments, and their abilities to effectively respond to disturbances could contribute to their invasiveness. Yet, there are noninvasive species that also do well in disturbed habitats. The question remains whether urban invaders behave differently in urban environments than noninvaders, which could suggest an “urban-exploiting” phenotype. In Southern California, the co-occurrence of invasive Italian wall lizards Podarcis siculus, brown anoles Anolis sagrei, and green anoles A. carolinensis, and native western fence lizards Sceloporus occidentalis offers an opportunity to test whether invasives exhibit consistent differences in risk-taking within human-altered habitats compared with a native species. We predicted that invasive lizards would exhibit more bold behavior by having shorter flight-initiation distances (FIDs) and by being found farther from a refuge (behaviors that would presumably maximize foraging in low-risk environments). Invasive populations had similar or longer FIDs, but were consistently found at distances closer to a refuge. Collectively, invasive lizards in urban habitats were not bolder than a native species. Reliance on nearby refuges might help species successfully invade urban habitats, and if a general pattern, may pose an added challenge in detecting or eliminating them.
Species that are not native to an area and cause ecological harm are considered invasive (Simberloff and Rejmanek 2011). Invasive species threaten global biodiversity (Novacek and Cleland 2001; Dudgeon et al. 2006; Orth et al. 2006); therefore, developing an understanding of mechanisms underlying successful invasions could help mitigate their impacts (Van Kleunen et al. 2010; Blackburn et al. 2011). Prior research has demonstrated the importance of propagule pressure (the number of individuals introduced and number of introduction events) for establishment success of non-native species (Lockwood et al. 2005; Colautti et al. 2006; Blackburn et al. 2015). Yet, an increasing body of research is focused on the behavioral correlates of invasion, which provide a mechanistic understanding of how animals survive and reproduce in areas outside of their native range (Chapple et al. 2012). The anthropogenically induced adaptation to invade hypothesis states that invasive species might exhibit “urban-exploiting” traits because they have had evolutionary histories with humans in disturbed habitats (Hufbauer et al. 2012). Invasives, therefore, could exhibit behaviors that favor success in urban habitats. For example, invasive house geckos Hemidactylus are more likely to occupy the vacant niches around artificial lights on buildings compared with native geckos (Yang et al. 2012; Zozaya et al. 2015). There are fundamental differences between native (noninvasive) and invasive species (Pauchard et al. 2018), and we need further studies to identify generalizations across taxonomic groups.
The trade-off between foraging and avoiding predation is pervasive among prey species (Lima and Dill 1990; Ydenberg 2010). Boldness refers to an animal’s willingness to take risks or expose itself to potential predators (Réale et al. 2007). Several studies demonstrate that invasive species are bold risk-takers (Short and Petren 2008; Myles-Gonzalez et al. 2015; Carthey and Banks 2018), meaning they may be more willing to remain in a patch to forage compared with native species. In predator-rich environments, this can be a costly response, but urbanization tends to reduce predation risk by lowering the abundance and diversity of natural predators (Eötvös et al. 2018). In addition, urban environments have more people than nonurban environments and frequent nonlethal interactions with people should lead to a reduction in antipredator responses over time (i.e., habituation; Geffroy et al. 2015; Blumstein 2016). Thus, boldness should be favored in urban environments, and several studies confirm increased boldness of urban animal populations compared with their nonurban counterparts (Møller 2008; Samia et al. 2015b; Battle et al. 2016).
The flight initiation distance (FID) is the distance an animal flees from an approaching threat (Cooper et al. 2015). Studies across taxa consistently show that FID varies with risk (Stankowich and Blumstein 2005; Cooper and Avalos 2010; Samia et al. 2015a). Hence, FID is considered a robust estimate of risk-taking. In general, FID is long when the risk of capture is high (Cooper et al. 2015). A related variable is the distance to the closest refuge or hiding place. If animals are reliant on refuge to avoid predation, being far from a refuge is risky and should positively correlate with FID (Cooper 2016). However, this relationship may be altered in urban environments where predation risk is low (Eötvös et al. 2018). In a low-risk environment, an animal that maximizes foraging would be one that is not afraid to wander far from a hiding place and has a short FID. Successful urban exploiters, such as many invasive species, could exhibit these traits compared with a native species that has not been successfully introduced outside of its native range and is less urban tolerant (Møller 2008).
The successful introduction and establishment of invasive Italian wall lizards Podarcis siculus, brown anoles Anolis sagrei, and green anoles A. carolinensis in Southern California provide an ideal system to compare risk-taking behaviors in urban environments between invasive species and a native species. These lizards were introduced into urban habitats across Southern California within the last 25–35 years. We investigated whether the 3 invasive species differed in FID and distance to refuge compared with the native western fence lizard Sceloporus occidentalis, a species that occurs in urban habitats in Southern California, but has not been successfully introduced outside of its native range. Conversely, all 3 invasive species are well known to occur in urban areas around the globe outside of their native ranges (Kraus 2009). With increasing urbanization, fence lizards have either failed to establish or been extirpated from many urbanized sites where invasive lizard species have been established and are expanding. Multiyear surveys of these lizard populations show that as the invasive populations expand, they displace the native fence lizards (Fisher et al., unpublished data; Pauly and Putman, unpublished data), suggesting that the invasives exhibit traits better suited for these urban habitats.
For this study, we used a pairwise approach (see Van Kleunen et al. 2010), comparing the behaviors of an invasive population at 1 site to those of a native fence lizard population at the same site. We used this approach across 5 urban sites in Southern California (Figure 1, Table 1). Our study species have been shown to modify risk-taking behaviors in response to urbanization, predation risk, and/or human activity levels (Irschick et al. 2005; Vervust et al. 2007; Grolle et al. 2014; Chejanovski et al. 2017). Here, we tested for differences in FID and distance from the closest refuge. We predicted that urban invasive lizards would exhibit more risky behaviors than urban native fence lizards by having shorter FIDs and being found at longer distances from refuge.
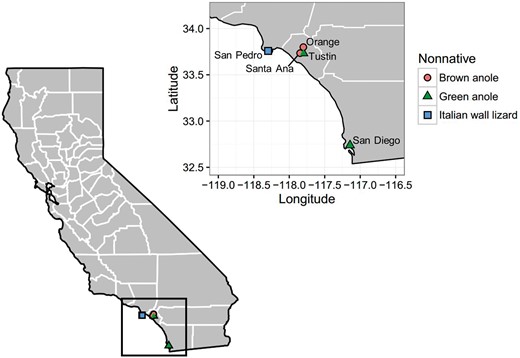
Map of California, USA, showing the sites sampled for this study. Counties are outlined in white (our research took place in Los Angeles, Orange, and San Diego Counties); the black box highlights the enlarged area. The locations of the invasive lizard populations examined in this study are indicated by the different colored symbols on the map. Native western fence lizards are present at all sites.
Characteristics of each site
| Site . | Land use classification . | Mean impervious surface cover (%) . | Years urbanized . | Invasive species present . | Year introduced . |
|---|---|---|---|---|---|
| Orange | Developed, medium intensity | 50 | 1970–1980 | Anolis sagrei | Early 2000s |
| Santa Ana | Developed, medium intensity | 70 | 1980–1990 | Anolis sagrei | Unknown |
| San Diego | Developed, low intensity | 25 | before 1950 | Anolis carolinensis | Late 1990s |
| Tustin | Developed, medium intensity | 55 | 1980–1990 | Anolis carolinensis | Late 1980s |
| San Pedro | Developed, low intensity | 44 | before 1950 | Podarcis siculus | 1994 |
| Site . | Land use classification . | Mean impervious surface cover (%) . | Years urbanized . | Invasive species present . | Year introduced . |
|---|---|---|---|---|---|
| Orange | Developed, medium intensity | 50 | 1970–1980 | Anolis sagrei | Early 2000s |
| Santa Ana | Developed, medium intensity | 70 | 1980–1990 | Anolis sagrei | Unknown |
| San Diego | Developed, low intensity | 25 | before 1950 | Anolis carolinensis | Late 1990s |
| Tustin | Developed, medium intensity | 55 | 1980–1990 | Anolis carolinensis | Late 1980s |
| San Pedro | Developed, low intensity | 44 | before 1950 | Podarcis siculus | 1994 |
Land use classification is defined by the National Land Cover Database (2016). The mean impervious surface cover is an estimate of urbanization intensity and was determined by taking the average of the percent surface covered by water-resistant materials (e.g., concrete) within a 100-m radius of each lizard observation in this study. Years urbanized refers to the range of dates when the site was developed, and this was assessed through historical aerial images. Year introduced refers to when the invasive lizards were introduced to the site based on interviews with people at each site.
Characteristics of each site
| Site . | Land use classification . | Mean impervious surface cover (%) . | Years urbanized . | Invasive species present . | Year introduced . |
|---|---|---|---|---|---|
| Orange | Developed, medium intensity | 50 | 1970–1980 | Anolis sagrei | Early 2000s |
| Santa Ana | Developed, medium intensity | 70 | 1980–1990 | Anolis sagrei | Unknown |
| San Diego | Developed, low intensity | 25 | before 1950 | Anolis carolinensis | Late 1990s |
| Tustin | Developed, medium intensity | 55 | 1980–1990 | Anolis carolinensis | Late 1980s |
| San Pedro | Developed, low intensity | 44 | before 1950 | Podarcis siculus | 1994 |
| Site . | Land use classification . | Mean impervious surface cover (%) . | Years urbanized . | Invasive species present . | Year introduced . |
|---|---|---|---|---|---|
| Orange | Developed, medium intensity | 50 | 1970–1980 | Anolis sagrei | Early 2000s |
| Santa Ana | Developed, medium intensity | 70 | 1980–1990 | Anolis sagrei | Unknown |
| San Diego | Developed, low intensity | 25 | before 1950 | Anolis carolinensis | Late 1990s |
| Tustin | Developed, medium intensity | 55 | 1980–1990 | Anolis carolinensis | Late 1980s |
| San Pedro | Developed, low intensity | 44 | before 1950 | Podarcis siculus | 1994 |
Land use classification is defined by the National Land Cover Database (2016). The mean impervious surface cover is an estimate of urbanization intensity and was determined by taking the average of the percent surface covered by water-resistant materials (e.g., concrete) within a 100-m radius of each lizard observation in this study. Years urbanized refers to the range of dates when the site was developed, and this was assessed through historical aerial images. Year introduced refers to when the invasive lizards were introduced to the site based on interviews with people at each site.
Materials and Methods
Study species
We used native western fence lizards as an ecologically relevant species for comparisons with the invasive lizards in Southern California. Fence lizards are one of the most common native lizards in the urban habitats where the invasives have established. Similar to all 3 invasive species, fence lizards are diurnal, they conspicuously bask in the open, males are territorial, and they exhibit male-biased sexual-size dimorphism (see Supplementary Table S1). It is likely that native fence lizards and the 3 invasive species have recent evolutionary histories with urbanization; however, fence lizards have not been successfully introduced outside of their native range (i.e., they differ in invasiveness) and they are being displaced by the invasive lizards in these urban habitats. Therefore, fence lizards might exhibit behaviors that are not as successful in foreign or urban environments than those of the invasives.
Brown anoles are native to Cuba and have been successfully introduced to the Southeastern United States, Hawaii, and elsewhere, with the earliest records of introduction to the United States dating to the late 1800s (Kraus 2009). This species was likely introduced to California as stowaways on nursery plant imports from Florida and Hawaii; thus, these populations have likely been in urban settings and experienced multiple introduction events over the course of their history in the United States. Brown anoles are traditionally considered a trunk-ground ecomorph (Elstrott and Irschick 2004), occupying the lower portions of tree trunks or rocks under tree canopies.
Green anoles A. carolinensis are native to the Southeastern United States and have become established in Hawaii, other Pacific islands, and several locations in California (Kraus 2009; Pauly and Borthwick 2015). This anole was also likely introduced as stowaways on nursery plant imports and perhaps as pet trade releases, and invasive populations have been appearing in the United States since at least the mid-1900s (Kraus 2009). Green anoles are considered trunk-crown ecomorphs (Elstrott and Irschick 2004), occupying the higher portions of tree canopies.
Italian wall lizards are native to Italy and the Adriatic coast and have established populations in other parts of Europe and the United States (Kraus 2009). Four males and 3 females were collected in Sicily and deliberately introduced in 1994 to a San Pedro, California backyard by a homeowner (Deichsel et al. 2010). This single source has been confirmed via genetic analysis (Kolbe et al. 2013). Although wall lizards may be considered to use a more active foraging mode than fence lizards (Capula and Aloise 2011), our personal observations of their movement patterns and previous literature on home range sizes of introduced Podarcis in urban neighborhoods suggest they do not roam widely from a core use area (Brown et al. 1995).
Study sites
We selected 5 urban sites that had a population of native fence lizards and an established population of an invasive lizard species (Figure 1). Three of our sites, Orange, San Pedro, and Tustin, were residential neighborhoods with impervious surfaces (water-resistant materials such as pavements) accounting for 44–55% of land cover (Table 1). The other 2 sites were an urban park, Balboa Park, in San Diego and a highly urbanized nonresidential area in Santa Ana (Figure 1, Table 1). The invasive populations have relatively small distributions at each site (<1 km2). At all sites except for Santa Ana, fence lizards overlap with the invasive lizards along the distribution edge of the invasive population (i.e., natives are absent from the core of the invasives’ distribution). At the Santa Ana site, the invasive lizards live among commercial buildings and businesses and the western fence lizards are ∼2.25 km away in an area with similar urban development (National Land Cover Database classification of developed, medium intensity land with 50–79% impervious surface cover). Because the invasive and native lizard populations are within large homogenous urban developments, they should have access to similar microhabitats.
Behavioral quantification
Behavioral assays were conducted from 18 May to 17 June 2016 between 10:00 h and 15:00 h. Sites were visited haphazardly to avoid sampling order effects. Two people conducted behavioral trials; they were of similar build and height, always wore dull-colored clothing, and walked at the same walking pace (0.5 m/s), which was standardized beforehand. Once a lizard was spotted, an observer walked directly toward the lizard and noted when it fled (any movement away from its initial location), and the total distance between the lizard and the observer when the trial was started (termed the start distance). FID was measured using transect tape after the trial. While conducting trials, we continually walked in one direction to ensure that we did not sample the same location, and thus the same lizard, twice. Although these visual encounter surveys could bias observations toward bold individuals within a species, it should not affect our ability to detect relative differences in behaviors among species.
Only lizards that were large adults were sampled and females that appeared gravid were not included. Gravid females allowed close approaches and appeared swollen. We included lizards with original and regrowing tails because previous studies suggest that tail autotomy does not strongly affect FID (Samia et al. 2015a). We conducted trials on cloudless days and a mean ± standard deviation (SD) daytime air temperature of 21°C ± 2°C (range: 19°C–25°C); however, neither ambient air nor substrate temperatures strongly affect variation in lizard FID (Samia et al. 2015a), and because native fence lizards and the invasives were sampled simultaneously at each site, temperature should not impose a systematic bias on our results.
After each trial, we measured the height at which the lizard was found. We also recorded the substrate type as 1 of 6 microhabitat categories: ground, wood (downed branches and logs), rock, vegetation (including trees, shrubs, or leaves), human-made wall (including fences, ledges, planter beds, or buildings), or human-made objects other than a wall. We also measured the distance each lizard was from the closest refuge (±5 cm). If the lizard sought refuge during the trial, we measured the distance from its initial location to its hiding place. If the lizard fled, but not to a refuge, we measured the distance from its initial location to the nearest crevice/hole that a lizard could hide in or vegetation that could conceal the lizard. We determined appropriate hiding locations based on behavioral observations of the lizards at each site. All methods were approved by the CSU Northridge Institutional Animal Care and Use Committee (1516-002b) and meet the Animal Behavior Society guidelines for ethical treatment of animals.
Statistical analyses
Because we were interested in testing behavioral differences between invasive lizards and a native lizard within urban sites, we considered each invasive species independently, comparing them to co-occurring fence lizard population(s). This approach (separating statistical tests by site or by species) is common when species occurrences are unbalanced among sites (see Edwards and Lailvaux 2012; Husak and Lovern 2014). By testing species pairings at multiple sites, we could qualitatively assess similarity of patterns observed across sites (i.e., do invasive populations consistently respond differently than native populations at each site).
We fitted general linear models (GLMs) to explain square root transformed FID for each invasive lizard/native lizard comparison (3 separate models). We included invasion status (invasive vs. native) and site (when appropriate) as factors in the models. We included site merely to account for site differences, but this was not the main focus of our study. Previous studies show that starting distance, distance to refuge, and the height at which the animal is found explain significant variation in FID (Blumstein 2010; Samia et al. 2015a), so these were included as additional covariates. Prior to analyses, we looked for interactions between factors in these models and if none was significant, they were excluded.
We fitted 2-way ANOVAs to explain square root transformed distance to refuge for each invasive/native lizard comparison. We included invasion status, site (when appropriate), and their interaction as factors in these models. To avoid violating the assumption of homoscedasticity, we fitted each ANOVA with a heteroscedasticity-consistent coefficient covariance matrix using the white.adjust option in the car package (Fox and Weisberg 2019). For the model comparing brown anoles to western fence lizards, we removed 2 outlying data points that had studentized residuals larger than 4, and that strongly affected the normality of the data. Both observations were from western fence lizards that were found several meters from a refuge and the removal of these points did not change the overall results of the model (Supplementary Table S2).
We also used data collected on microhabitat use to determine whether any observed behavioral differences were associated with differences in microhabitat use at each site. We used Fisher’s exact tests (1 for each site) to test whether the proportion of substrates where lizards were found was the same between the native and invasive species. We also calculated Levins’ measure of niche breadth to estimate the diversity of microhabitats used by lizards at each site (Levins 1968; Pianka 1986). We calculated Levin’s B as we did in Putman et al. (2019) and standardized the values on a scale of 0–1 so that 0 indicates the sole use of a single microhabitat by individuals and 1 indicates a uniform distribution of individuals among the microhabitat categories.
We did not include sex as a factor in any models. Although sex differences could be present in our behaviors of interest, this was not the main focus of our research question and should, if anything, increase variation in a nonsystematic way. All tests were performed in R (R v. 3.2.1) with alpha set to 0.05. We used the phia package to run post hoc analyses on interaction contrasts.
Results
Flight Initiation Distance
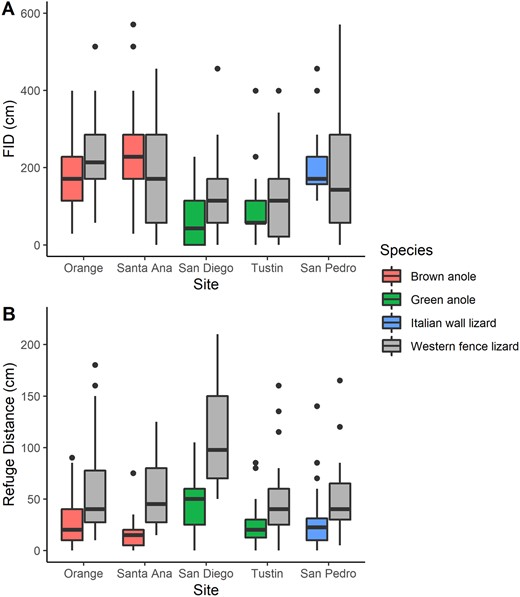
Invasive lizards either had longer or similar FIDs as the native lizard at the same site (Figure 2A). Italian wall lizards in San Pedro had longer mean FIDs than native western fence lizards (Estimate ± standard error [SE]: −2.38 ± 0.99, t = −2.41, P = 0.019; Supplementary Table S5). The model comparing FIDs of brown anoles to fence lizards showed a significant interaction between invasion status and site (Estimate ± SE: −4.21 ± 1.72, t = −2.44, P = 0.016; Supplementary Table S3), but neither of the interaction contrasts was significant (brown anoles vs. fence lizards at Orange: Χ2 = 3.46, P = 0.126; brown anoles vs. fence lizards at Santa Ana: Χ2 = 1.94, P = 0.164). We also found that brown anoles were less sensitive to starting distance than fence lizards (start distance × invasion status: Estimate ± SE: 0.007 ± 0.003, t = 2.50, P = 0.014, Supplementary Figure S1; Supplementary Table S3). Green anoles at both sites had similar FIDs as fence lizards (Estimate ± SE: 2.27 ± 1.95, t = 1.16, P = 0.247), but perch height negatively affected FID in fence lizards (e.g., those found on higher perches had lower FIDs) whereas height did not affect FID in green anoles (height × invasion status: Estimate ± SE: −0.04 ± 0.02, t = −2.08, P = 0.040, Supplementary Figure S2; Supplementary Table S4). Full results from the models can be found in Supplementary Tables S3–S5.
Boxplots comparing behaviors of invasive lizards to a native lizard at 5 Southern California sites. (A) FID; (B) the distance to the closest refuge when the lizard was found. Boxplots show the median, interquartile ranges, and outlying data points.
Distance to refuge
Compared with native western fence lizards, invasive Italian wall lizards (F1,63 = 10.13, P = 0.002), brown anoles (F1,99 = 41.93, P < 0.001), and green anoles (F1,104 = 29.92, P < 0.001) were consistently found closer to a refuge (Figure 2B). For the model comparing green anoles and fence lizards, there was an invasion status by site interaction (F1,104 = 7.61, P = 0.007). Post hoc comparisons showed that green anoles in San Diego exhibited a greater difference in mean refuge distance from fence lizards (F1,104 = 41.06, P < 0.001) than those in Tustin (F1,104 = 4.58, P = 0.035), but both comparisons still showed that green anoles were found closer to refuge than fence lizards.
Microhabitat use
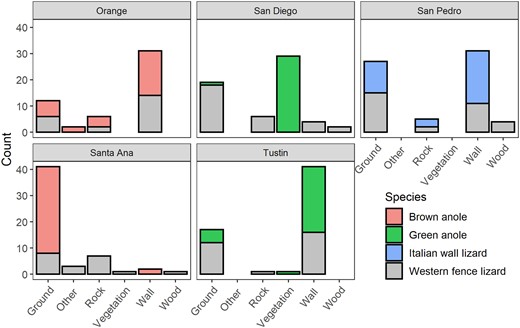
Depending on the site, invasive lizards either used the same microhabitats (brown anoles in Orange: P = 0.734), marginally differed in microhabitat use (Italian wall lizards in San Pedro: P = 0.066; green anoles in Tustin: P = 0.064), or almost exclusively used a different microhabitat (brown anoles in Santa Ana: P < 0.001; green anoles in San Diego: P < 0.001) compared with western fence lizards (Figure 3). Invasive lizard populations tended to have a lower breadth of microhabitats used than the native lizard populations based on standardized Levin’s B values (native fence lizards: Orange = 0.210, Santa Ana = 0.445, San Diego = 0.274, Tustin = 0.219, San Pedro = 0.360; invasive brown anoles: Orange = 0.288, Santa Ana = 0.024; invasive green anoles: San Diego = 0.014, Tustin = 0.095; invasive Italian wall lizards: San Pedro = 0.243). Orange was the only site where an invasive lizard had slightly higher niche breadth than the native fence lizard with standardized values of 0.288 and 0.210, respectively.
Microhabitat use of invasives and a native lizard, as number of individuals found on each substrate category. Invasive brown anole populations occur in Orange and Santa Ana, invasive green anole populations occur in San Diego and Tustin, and an invasive Italian wall lizard population occurs in San Pedro. Invasive populations were compared with a native western fence lizard population at each site.
Discussion
We compared 2 measures of risk-taking between invasive and native urban lizards with the hypothesis that the former would exhibit behaviors better suited for exploiting urban habitats. We found that 3 species of invasive lizards were not more bold in their responses than a native species. Invasives tended to have greater or similar FIDs and they were found consistently closer to refuge than the native western fence lizards. These patterns were generally consistent across 3 invasive species that differed in aspects of their ecology and at sites that differed in habitat, implying that successful urban introductions of commonly invasive lizards might be associated with certain behavioral types.
Our results suggest that successful urban exploiters do not wander far from a hiding place. Although urban habitats are generally considered to have lower predation risk than nonurban habitats, there are other risks to consider such as those associated with human activity (e.g., motorized vehicles, bicycles) and the presence of human companion animals (e.g., cats and dogs). Indeed, domestic and feral cats kill an estimated 258–822 million reptiles each year in the United States (Loss et al. 2013). Southern alligator lizards Elgaria multicarinata in Southern California also show increased frequencies of tail loss with increasing urbanization, suggesting that predation pressure is higher in these urban areas (Putman et al. 2020). Thus, remaining near a refuge could be beneficial in avoiding urban risks. Other studies have shown that urban individuals are found at distances closer to refuge than nonurban individuals (or individuals less exposed to humans) suggesting that this might be an adaptive response in urban habitats (Engelhardt and Weladji 2011; Batabyal et al. 2017). It is also possible that the differences in distance to refuge between native and invasive lizards are dictated by differences in thermal preferences and/or performance. For instance, fence lizards might need to seek high-temperature microhabitats away from refuge to enhance physiological performance (Michelangeli et al. 2018), or they might be better at escaping from predators (e.g., have higher maximal sprint speed) than the invasive species (Lind and Cresswell 2005; Husak 2006). However, these explanations seem unlikely given that fence lizard populations are not thriving in urbanized Southern California habitats. Regardless of whether distance to refuge results from differences in perceived predation risk or physiological limitations, invasive lizards are still not using sites far from a hiding place, thereby limiting their use of the overall habitat in urban environments. Importantly, invasive lizards’ tendency to stay near a hiding place likely decreases detectability during the invasion process.
Contrary to expectations, urban invasive lizards did not have lower FIDs than a native lizard. The anthropogenically induced adaptation to invade hypothesis states that successful invaders might have longstanding affiliations with humans (Hufbauer et al. 2012), and therefore, they might be more tolerant of human disturbance (i.e., more bold) than a species that has not been successfully introduced outside of its native range. Yet, other studies have similarly found invasives to be more cautious than native congeners by freezing in place more often, having a higher propensity to hide, and responding more to predator cues (Weis 2010; Chapple et al. 2011; Bezzina et al. 2014; Cisterne et al. 2014). In addition, lizards living in areas with humans, dogs, cats, and chickens have been shown to flee at greater distances than those living in less disturbed habitats (Williams et al. 2019). Italian wall lizards were the only invasive species to have significantly higher FIDs compared with co-occurring fence lizards. Italian wall lizards might flee more readily because their large body size and bright green coloration make them more conspicuous than native western fence lizards (Stiller and McBrayer 2013; Samia et al. 2015a). Cryptic species tend to have shorter FIDs than conspicuous species (Møller et al. 2019). Increased caution might be a characteristic of successful invasion, and additional studies of diverse taxa will allow a more general understanding of urban-exploiting traits.
Compared with native fence lizards, the invasive anoles were also less sensitive to factors that influence FID such as starting distance and height. Starting distance refers to the distance at which the threat (human) starts to approach the target animal (Cooper et al. 2015). Generally, there is a strong positive relationship between starting distance and FID because once the animal detects an oncoming threat, it should flee to reduce costs associated with monitoring (Blumstein 2003). Brown anoles appear more likely to remain even when humans start approaching them from far away (Supplementary Table S3). Cooper (2010) also found that FID for another trunk-grown anole ecomorph, A. lineatopus, was insensitive to starting distance, suggesting that these species’ ecologies might influence escape behaviors. Green anoles, on the contrary, did not modify FID based on perch height whereas fence lizards did (Supplementary Table S4). Our results suggest that fence lizards in San Diego and Tustin perceive higher perches as relatively safe positions because their FIDs decreased with height, and this same response has been shown in other lizard species, including arboreal anoles (Schneider et al. 2000; Cooper 2006). We likely did not find this same pattern in the other fence lizard populations because they were less often found on high perches. Green anoles did not perceive higher positions as safer than lower positions. Cooper (2006) suggests that anoles that rely on crypsis may be less sensitive to height, but it remains to be tested whether green anoles rely more on crypsis as an antipredator defense than western fence lizards. Overall, the lack of sensitivity of the 2 anole species to certain risk factors could contribute to their invasiveness if they are maximizing fitness-relevant activities, such as foraging, during nonlethal human approaches.
Although we did not find strong or consistent differences in FID between invasives and a native species, this does not necessarily indicate that invasives are generally less bold. FID is just a single measure of boldness (Réale et al. 2007) and invasives could differ in other ways including time spent hiding after a human approach (i.e., refuge use and latency to emerge) or time spent foraging. For instance, successful urban lizards have lower latencies to emerge from a refuge after predatory attacks than their nonurban counterparts (Lapiedra et al. 2017; Pellitteri-Rosa et al. 2017), and invasive species (P. siculus) take less time to emerge from a thermally unfavorable refuge compared with native species (Damas-Moreira et al. 2019). Such behaviors by both parties could enhance the competitive ability of the invasives if they are better able to exploit resources (Lapiedra et al. 2017).
The amount of niche overlap, and hence, interspecific interactions, between the invasive lizards and the native lizard is still not entirely known. Our observations suggest that the invasive lizards are displacing the native fence lizards at each site because the fence lizards are absent from the core of the invasives’ distributions (where they once occurred), a pattern consistent with other observations of native-invasive lizard interactions (Ribeiro and Sá-Sousa 2018). However, because of the little overlap between the 2 species’ distributions, it is unlikely that the differences in behaviors we recorded for this study are the direct result of interspecific competition/aggression. Yet, we found interesting differences (or lack thereof) in microhabitat use between the 2 species at each site (Figure 3), with greater overlap between the species in residential neighborhoods relative to nonresidential sites. Successful urban invaders might be more flexible in microhabitat use. For instance, green anoles have been shown to modify microhabitat use in the presence of interspecific competitors (Edwards and Lailvaux 2012; Stuart et al. 2014), and urban invasive geckos are more likely to take advantage of vacant niches compared with native geckos (Yang et al. 2012). Although we found lower niche breadth for the invasive lizards on a per site basis, the preferred substrates varied across our anole populations (e.g., brown anoles primarily used walls in Orange and ground in Santa Ana) suggesting flexibility in microhabitat use in the invasive lizards. If fence lizards are less flexible than the invasives, this could partially explain why they have disappeared from the cores of the invasive lizards’ distributions.
Acknowledgments
We thank S. Kennedy-Gold for help in collecting field data, K. Kaiser for thoughtful discussions on study design, the Blumstein Lab group and R. Espinoza for helpful comments on manuscript drafts, R. Fisher and K. Lovich for information on the history of the San Diego A. carolinensis population, and G. Nafis for information on the Santa Ana A. sagrei population. We also thank J. Alvarado, C. Caverly, and the other community scientists who helped document the invasive lizard populations and the many more who continue to document introductions by submitting observations to the Natural History Museum’s Reptiles and Amphibians of Southern California (RASCals) project.
Funding
This work was supported by the Urban Nature Research Center at the Natural History Museum of Los Angeles County, and the National Science Foundation through a Postdoctoral Research Fellowship in Biology (DBI-1611562 to B.J.P.).
Authors’ Contributions
B.J.P., G.B.P., and D.T.B. designed the study; B.J.P. collected and analyzed data, and wrote the manuscript; G.B.P. and D.T.B. provided resources and edits to manuscript drafts.
Conflict of Interest
The authors declare that they have no conflicts of interest to this work.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
References
Author notes
Present address: Department of Biology, California State University, San Bernardino, CA 92407, USA.