-
PDF
- Split View
-
Views
-
Cite
Cite
Sudarshan Paramsothy, Ramesh Paramsothy, David T Rubin, Michael A. Kamm, Nadeem O. Kaakoush, Hazel M Mitchell, Natalia Castaño-Rodríguez, Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-analysis, Journal of Crohn's and Colitis, Volume 11, Issue 10, October 2017, Pages 1180–1199, https://doi.org/10.1093/ecco-jcc/jjx063
Close - Share Icon Share
Abstract
Faecal microbiota transplantation [FMT] has been investigated as a potential treatment for inflammatory bowel disease [IBD]. We thus performed a systematic review and meta-analysis assessing the effectiveness and safety of FMT in IBD.
A systematic review was conducted until January 2017. Studies were excluded if patients had co-infection or data were pooled across disease subtypes (ulcerative colitis [UC], Crohn’s disease [CD], pouchitis). Clinical remission was established as the primary outcome. Pooled effect sizes and 95% confidence intervals were obtained using the random effects model.
In all, 53 studies were included [41 in UC, 11 in CD, 4 in pouchitis]. Overall, 36% [201/555] of UC, 50.5% [42/83] of CD, and 21.5% [5/23] of pouchitis patients achieved clinical remission. Among cohort studies, the pooled proportion achieving clinical remission was 33% (95% confidence interval [CI] = 23%–43%] for UC and 52% [95% CI = 31%–72%] for CD, both with moderate risk of heterogeneity. For four RCTs in UC, significant benefit in clinical remission (pooled odds ratios [[P-OR] = 2.89, 95% CI = 1.36–6.13, p = 0.006) with moderate heterogeneity [Cochran’s Q, p = 0.188; I2 = 37%] was noted. Sub-analyses suggest remission in UC improved with increased number of FMT infusions and lower gastrointestinal tract administration. Most adverse events were transient gastrointestinal complaints. Microbiota analysis was performed in 24 studies, with many identifying increased diversity and a shift in recipient microbiota profile towards the donor post-FMT.
FMT appears effective in UC remission induction, but long-term durability and safety remain unclear. Additional well-designed controlled studies of FMT in IBD are needed, especially in CD and pouchitis.
1. Introduction
Faecal microbiota transplantation [FMT] has revolutionised the field of microbial therapeutics. It has proven extremely effective in the treatment of Clostridium difficile infection [CDI],1,2 and is considered to have potential in other conditions where disturbances in the enteric microbiota are implicated in disease pathogenesis, such as the inflammatory bowel diseases [IBD].3 Although FMT is a simple therapy in practice that was first described in Western medical literature over 50 years ago,4 and proposed as a treatment strategy for IBD over 25 years ago,5 it is only in recent years that there has been an exponential growth in patient, media, and research interest.6 The initial systematic review on the role of FMT in IBD published in 2012 consisted of only nine retrospective reports, deemed of insufficient quality to perform meta-analysis.7 Within 2 years, an updated systematic review identified 18 studies, including nine cohort studies of FMT in IBD on which a meta-analysis was performed.8 Since then, the number of available studies has again more than doubled, including the publication of the first four randomised controlled trials [RCTs] of FMT in ulcerative colitis [UC].9–12
In this latest systematic review and meta-analysis, we summarise the available literature and evaluate the efficacy of FMT in the various IBD subtypes of UC, Crohn’s disease [CD], and pouchitis, by performing meta-analyses on the associated prospective studies.
2. Methods
2.1. Search strategy
A systematic review was conducted in accordance with the PRISMA,13 Cochrane,14 and MOOSE15 guidelines. We searched five electronic databases [Pubmed, Medline, Cochrane, Biomed Central, and Embase] from inception to the January 4, 2017 using search terms as previously described7 [Table A1, available as Supplementary data at ECCO-JCC online]. No language limits or any other advance features were used. Major conference proceedings from 2011–2016 were searched to identify abstract publications, including: Digestive Diseases Week [DDW], European Crohn’s and Colitis Organisation [ECCO; including 2017], United European Gastroenterology Week [UEGW], American College of Gastroenterology [ACG], and Advances in IBD [AIBD]. References from previous review articles were also searched to identify studies that may have been missed by the above-mentioned searches. The clinicaltrials.gov registry was also searched.
2.2. Study selection criteria
2.2.1. Inclusion criteria
Articles were included in this systematic review if they reported on clinical efficacy and/or safety of FMT in inflammatory bowel disease in human subjects. FMT was defined as the infusion of faeces-derived matter and bacteria from a healthy individual[s] into a recipient. Case reports, case series, cohort studies, and RCTs were all included [full text or abstract publications]. For the meta-analyses, however, only cohort studies and RCTs were included.
2.2.2. Exclusion criteria
Studies were excluded if data for particular IBD subtypes [UC, CD, pouchitis] were pooled and not individually reported, due to inherent differences between these conditions. Studies were also excluded if they only included patients who had co-infection with Clostridium difficile or other pathogens, or if data on non-infected IBD patients were not individually reported or able to be extracted. In addition, studies reporting duplicate data were excluded.
2.2.3. Outcome measures
Efficacy of FMT in IBD was assessed as clinical remission [primary outcome] or clinical response as defined by the respective study authors [Tables 1–5]. Where possible, endoscopic [mucosal healing] and histologic data were also extracted. Safety was assessed using reported adverse event and serious adverse event data.
Case reports and case series of faecal microbiota transplantation [FMT] in ulcerative colitis [UC].
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Bennet et al., 19895 | 1 | Severe, steroid refractory | NR | Enema | NR | Multiple [not further specified] | NR | Yes [regimen not specified] | NR | 1 | - | NR | 1 | 6 months |
| Case report | Borody et al., 198919 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | - | 1 | 1 | 3 months |
| Case report | Borody et al., 201120 | 1 | Chronic relapsing UC | NR | NR | NR | NR | NR | NR | NR | 0 | 1 | NR | NR | 12 years |
| Case report | Hohmann et al., 201421 | 1 | Moderate | Wife & 10-month old child | NR | NR | 4 | Fresh | No | No | 0 | 0 | 0 | 0 | NR |
| Case report | Vandenplas et al., 201522 | 1 [paediatric] | Severe | Related [first 4 infusions: age-related niece, last 3 infusions: older brother] | Colonoscopy first 2 infusions, nasoduodenal. next 5 infusions | 100 g stool in 100 ml | 7 [interval not specified] | Fresh | No | NR | 1 | - | NR | 1 | 6 months |
| Case report | Seth et al., 201623 | 1 | Moderate [Mayo 9] | Unrelated [brother-in- law] | Colonoscopy | 200 g stool in 350 ml saline | 3 [every 2 weeks] | Fresh | No | Yes | 1 [Mayo 0, withdrawal of all medications] | - | 1 [Mayo 0, withdrawal of all medications] | 1 | 8 months |
| Case report | Kumagai et al., 201624 | 1 [paediatric] | Severe [PUCAI 85] | Related [mother] | Enema x 2, then nasoduodenal x 4 | 60 g stool in 250 ml saline | 6 [over 10 days] | Fresh | No | NR | 0 [required colectomy] | 0 | 0 | 0 | 3 months |
| Case report | Ni et al., 201625 | 1 | Moderately steroid- dependent [Mayo 9] | Related [father] | Percutaneous endoscopic caecostomy | 100 g stool in 250 ml saline | > 50 [daily for 1 month then 2 x week for 3 months] | Fresh | No | Yes | 1 [Mayo 0] | - | 1 [Mayo 0] | NR | 12 months |
| Case report | Shimzu et al., 201626 | 1 [paediatric] | Severe steroid dependent | Related [father] | Colonoscopy x 1 then enemas | Stool diluted in 250 ml saline | 16 [daily for first 5 days, then every 2–4 weeks over 10 months] | Fresh | No | Yes | 1 [PUCAI 0] | - | 0 | 0 | 10 months |
| Case series | Borody et al., 200127 | 3 | Active colitis, severe symptoms | NR | Enema | Stool diluted in 200 ml infusion | 5 [daily for 5 days] | Fresh | Vancomycin 500mg bd, metronidazole 400 mg bd, rifampicin 150 mg bd for 7–15 days | NR | 3/3 [100%] | - | 3/3 [100%] | NR | 8–16 months |
| Case series | Borody et al., 200328 | 6 | Active, not further specified | Recipient- identified [related & unrelated] | Enema | 200-300 g stool in 200-300 ml saline | 5, [daily for 5 days] | Fresh | Vancomycin 500 mg bd + metronidazole 400 mg bd + rifampicin 150 mg bd for 7–10 days | Yes | 6/6 [100%] | - | 6/6 [100%] | 6/6 [100%] | 1–13 years |
| Case series | Borody et al., 201229 | 62 | Active, not further specified | NR | NR | NR | NR | NR | NR | NR | 42/62 [68%] [0–1 on modified Powell- Tuck index] | 57/62 [92%] [> 2-point drop in Powell- Tuck index] | 12/21 [57%] | 12/21 [57%] | NR |
| Case series | Shah et al., 201230 | 16 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 8/16 [50%] [avoid surgery or medications] | NR | NR | NR |
| Case series | Brandt et al., 201331 | 11 | NR | NR | NR | NR | NR | NR | NR | NR | NR [safety study] | NR | NR | NR | Mean 14.7 months [range 7–31] |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Bennet et al., 19895 | 1 | Severe, steroid refractory | NR | Enema | NR | Multiple [not further specified] | NR | Yes [regimen not specified] | NR | 1 | - | NR | 1 | 6 months |
| Case report | Borody et al., 198919 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | - | 1 | 1 | 3 months |
| Case report | Borody et al., 201120 | 1 | Chronic relapsing UC | NR | NR | NR | NR | NR | NR | NR | 0 | 1 | NR | NR | 12 years |
| Case report | Hohmann et al., 201421 | 1 | Moderate | Wife & 10-month old child | NR | NR | 4 | Fresh | No | No | 0 | 0 | 0 | 0 | NR |
| Case report | Vandenplas et al., 201522 | 1 [paediatric] | Severe | Related [first 4 infusions: age-related niece, last 3 infusions: older brother] | Colonoscopy first 2 infusions, nasoduodenal. next 5 infusions | 100 g stool in 100 ml | 7 [interval not specified] | Fresh | No | NR | 1 | - | NR | 1 | 6 months |
| Case report | Seth et al., 201623 | 1 | Moderate [Mayo 9] | Unrelated [brother-in- law] | Colonoscopy | 200 g stool in 350 ml saline | 3 [every 2 weeks] | Fresh | No | Yes | 1 [Mayo 0, withdrawal of all medications] | - | 1 [Mayo 0, withdrawal of all medications] | 1 | 8 months |
| Case report | Kumagai et al., 201624 | 1 [paediatric] | Severe [PUCAI 85] | Related [mother] | Enema x 2, then nasoduodenal x 4 | 60 g stool in 250 ml saline | 6 [over 10 days] | Fresh | No | NR | 0 [required colectomy] | 0 | 0 | 0 | 3 months |
| Case report | Ni et al., 201625 | 1 | Moderately steroid- dependent [Mayo 9] | Related [father] | Percutaneous endoscopic caecostomy | 100 g stool in 250 ml saline | > 50 [daily for 1 month then 2 x week for 3 months] | Fresh | No | Yes | 1 [Mayo 0] | - | 1 [Mayo 0] | NR | 12 months |
| Case report | Shimzu et al., 201626 | 1 [paediatric] | Severe steroid dependent | Related [father] | Colonoscopy x 1 then enemas | Stool diluted in 250 ml saline | 16 [daily for first 5 days, then every 2–4 weeks over 10 months] | Fresh | No | Yes | 1 [PUCAI 0] | - | 0 | 0 | 10 months |
| Case series | Borody et al., 200127 | 3 | Active colitis, severe symptoms | NR | Enema | Stool diluted in 200 ml infusion | 5 [daily for 5 days] | Fresh | Vancomycin 500mg bd, metronidazole 400 mg bd, rifampicin 150 mg bd for 7–15 days | NR | 3/3 [100%] | - | 3/3 [100%] | NR | 8–16 months |
| Case series | Borody et al., 200328 | 6 | Active, not further specified | Recipient- identified [related & unrelated] | Enema | 200-300 g stool in 200-300 ml saline | 5, [daily for 5 days] | Fresh | Vancomycin 500 mg bd + metronidazole 400 mg bd + rifampicin 150 mg bd for 7–10 days | Yes | 6/6 [100%] | - | 6/6 [100%] | 6/6 [100%] | 1–13 years |
| Case series | Borody et al., 201229 | 62 | Active, not further specified | NR | NR | NR | NR | NR | NR | NR | 42/62 [68%] [0–1 on modified Powell- Tuck index] | 57/62 [92%] [> 2-point drop in Powell- Tuck index] | 12/21 [57%] | 12/21 [57%] | NR |
| Case series | Shah et al., 201230 | 16 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 8/16 [50%] [avoid surgery or medications] | NR | NR | NR |
| Case series | Brandt et al., 201331 | 11 | NR | NR | NR | NR | NR | NR | NR | NR | NR [safety study] | NR | NR | NR | Mean 14.7 months [range 7–31] |
NR, not recorded; bd, twice daily; PUCAI, paediatric ulcerative colitis activity index.
Case reports and case series of faecal microbiota transplantation [FMT] in ulcerative colitis [UC].
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Bennet et al., 19895 | 1 | Severe, steroid refractory | NR | Enema | NR | Multiple [not further specified] | NR | Yes [regimen not specified] | NR | 1 | - | NR | 1 | 6 months |
| Case report | Borody et al., 198919 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | - | 1 | 1 | 3 months |
| Case report | Borody et al., 201120 | 1 | Chronic relapsing UC | NR | NR | NR | NR | NR | NR | NR | 0 | 1 | NR | NR | 12 years |
| Case report | Hohmann et al., 201421 | 1 | Moderate | Wife & 10-month old child | NR | NR | 4 | Fresh | No | No | 0 | 0 | 0 | 0 | NR |
| Case report | Vandenplas et al., 201522 | 1 [paediatric] | Severe | Related [first 4 infusions: age-related niece, last 3 infusions: older brother] | Colonoscopy first 2 infusions, nasoduodenal. next 5 infusions | 100 g stool in 100 ml | 7 [interval not specified] | Fresh | No | NR | 1 | - | NR | 1 | 6 months |
| Case report | Seth et al., 201623 | 1 | Moderate [Mayo 9] | Unrelated [brother-in- law] | Colonoscopy | 200 g stool in 350 ml saline | 3 [every 2 weeks] | Fresh | No | Yes | 1 [Mayo 0, withdrawal of all medications] | - | 1 [Mayo 0, withdrawal of all medications] | 1 | 8 months |
| Case report | Kumagai et al., 201624 | 1 [paediatric] | Severe [PUCAI 85] | Related [mother] | Enema x 2, then nasoduodenal x 4 | 60 g stool in 250 ml saline | 6 [over 10 days] | Fresh | No | NR | 0 [required colectomy] | 0 | 0 | 0 | 3 months |
| Case report | Ni et al., 201625 | 1 | Moderately steroid- dependent [Mayo 9] | Related [father] | Percutaneous endoscopic caecostomy | 100 g stool in 250 ml saline | > 50 [daily for 1 month then 2 x week for 3 months] | Fresh | No | Yes | 1 [Mayo 0] | - | 1 [Mayo 0] | NR | 12 months |
| Case report | Shimzu et al., 201626 | 1 [paediatric] | Severe steroid dependent | Related [father] | Colonoscopy x 1 then enemas | Stool diluted in 250 ml saline | 16 [daily for first 5 days, then every 2–4 weeks over 10 months] | Fresh | No | Yes | 1 [PUCAI 0] | - | 0 | 0 | 10 months |
| Case series | Borody et al., 200127 | 3 | Active colitis, severe symptoms | NR | Enema | Stool diluted in 200 ml infusion | 5 [daily for 5 days] | Fresh | Vancomycin 500mg bd, metronidazole 400 mg bd, rifampicin 150 mg bd for 7–15 days | NR | 3/3 [100%] | - | 3/3 [100%] | NR | 8–16 months |
| Case series | Borody et al., 200328 | 6 | Active, not further specified | Recipient- identified [related & unrelated] | Enema | 200-300 g stool in 200-300 ml saline | 5, [daily for 5 days] | Fresh | Vancomycin 500 mg bd + metronidazole 400 mg bd + rifampicin 150 mg bd for 7–10 days | Yes | 6/6 [100%] | - | 6/6 [100%] | 6/6 [100%] | 1–13 years |
| Case series | Borody et al., 201229 | 62 | Active, not further specified | NR | NR | NR | NR | NR | NR | NR | 42/62 [68%] [0–1 on modified Powell- Tuck index] | 57/62 [92%] [> 2-point drop in Powell- Tuck index] | 12/21 [57%] | 12/21 [57%] | NR |
| Case series | Shah et al., 201230 | 16 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 8/16 [50%] [avoid surgery or medications] | NR | NR | NR |
| Case series | Brandt et al., 201331 | 11 | NR | NR | NR | NR | NR | NR | NR | NR | NR [safety study] | NR | NR | NR | Mean 14.7 months [range 7–31] |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Bennet et al., 19895 | 1 | Severe, steroid refractory | NR | Enema | NR | Multiple [not further specified] | NR | Yes [regimen not specified] | NR | 1 | - | NR | 1 | 6 months |
| Case report | Borody et al., 198919 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | - | 1 | 1 | 3 months |
| Case report | Borody et al., 201120 | 1 | Chronic relapsing UC | NR | NR | NR | NR | NR | NR | NR | 0 | 1 | NR | NR | 12 years |
| Case report | Hohmann et al., 201421 | 1 | Moderate | Wife & 10-month old child | NR | NR | 4 | Fresh | No | No | 0 | 0 | 0 | 0 | NR |
| Case report | Vandenplas et al., 201522 | 1 [paediatric] | Severe | Related [first 4 infusions: age-related niece, last 3 infusions: older brother] | Colonoscopy first 2 infusions, nasoduodenal. next 5 infusions | 100 g stool in 100 ml | 7 [interval not specified] | Fresh | No | NR | 1 | - | NR | 1 | 6 months |
| Case report | Seth et al., 201623 | 1 | Moderate [Mayo 9] | Unrelated [brother-in- law] | Colonoscopy | 200 g stool in 350 ml saline | 3 [every 2 weeks] | Fresh | No | Yes | 1 [Mayo 0, withdrawal of all medications] | - | 1 [Mayo 0, withdrawal of all medications] | 1 | 8 months |
| Case report | Kumagai et al., 201624 | 1 [paediatric] | Severe [PUCAI 85] | Related [mother] | Enema x 2, then nasoduodenal x 4 | 60 g stool in 250 ml saline | 6 [over 10 days] | Fresh | No | NR | 0 [required colectomy] | 0 | 0 | 0 | 3 months |
| Case report | Ni et al., 201625 | 1 | Moderately steroid- dependent [Mayo 9] | Related [father] | Percutaneous endoscopic caecostomy | 100 g stool in 250 ml saline | > 50 [daily for 1 month then 2 x week for 3 months] | Fresh | No | Yes | 1 [Mayo 0] | - | 1 [Mayo 0] | NR | 12 months |
| Case report | Shimzu et al., 201626 | 1 [paediatric] | Severe steroid dependent | Related [father] | Colonoscopy x 1 then enemas | Stool diluted in 250 ml saline | 16 [daily for first 5 days, then every 2–4 weeks over 10 months] | Fresh | No | Yes | 1 [PUCAI 0] | - | 0 | 0 | 10 months |
| Case series | Borody et al., 200127 | 3 | Active colitis, severe symptoms | NR | Enema | Stool diluted in 200 ml infusion | 5 [daily for 5 days] | Fresh | Vancomycin 500mg bd, metronidazole 400 mg bd, rifampicin 150 mg bd for 7–15 days | NR | 3/3 [100%] | - | 3/3 [100%] | NR | 8–16 months |
| Case series | Borody et al., 200328 | 6 | Active, not further specified | Recipient- identified [related & unrelated] | Enema | 200-300 g stool in 200-300 ml saline | 5, [daily for 5 days] | Fresh | Vancomycin 500 mg bd + metronidazole 400 mg bd + rifampicin 150 mg bd for 7–10 days | Yes | 6/6 [100%] | - | 6/6 [100%] | 6/6 [100%] | 1–13 years |
| Case series | Borody et al., 201229 | 62 | Active, not further specified | NR | NR | NR | NR | NR | NR | NR | 42/62 [68%] [0–1 on modified Powell- Tuck index] | 57/62 [92%] [> 2-point drop in Powell- Tuck index] | 12/21 [57%] | 12/21 [57%] | NR |
| Case series | Shah et al., 201230 | 16 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 8/16 [50%] [avoid surgery or medications] | NR | NR | NR |
| Case series | Brandt et al., 201331 | 11 | NR | NR | NR | NR | NR | NR | NR | NR | NR [safety study] | NR | NR | NR | Mean 14.7 months [range 7–31] |
NR, not recorded; bd, twice daily; PUCAI, paediatric ulcerative colitis activity index.
Cohort studies of faecal microbiota transplantation [FMT] in ulcerative colitis.
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow Up . | NOS Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Angelberger et al., 201332 | 5 | Moderate- severe [Mayo ≥ 6] | Recipient- identified but first degree- relatives excluded | Nasojejunal & enema combined | Median 24 g stool in 250 ml saline for nasojejunal infusion, median 20 g stool in 100 ml saline for enema | 3 [daily for 3 days] | Fresh | Metronidazole 500 mg bd and probiotic [Yomogi or Omnibiotic] for 5–10 days before FMT | Yes | 0 [Mayo ≤ 2, no subscore > 1] | 1/5 [20%] [Mayo drop ≥ 3 and ≥ 30%, along with drop in bleeding subscore ≥ 1 or bleeding subscore ≤ 1] | NR | NR | 12 weeks | 5 |
| Cohort | Kump et al., 201333 | 6 | Moderate- severe [Mayo 8–11] | Unrelated | Colonoscopy [TI + colon] | 100-150 g stool in 200-350 ml | Single | Fresh | No | Yes | 0 [Mayo ≤ 2] | 2/6 [33%] [Mayo drop ≥ 3] | NR | NR | 3 months | 5 |
| Cohort | Kunde et al., 201334 | 10 [paediatric] | Mild- moderate [PUCAI 15–65] | Recipient- identified [related & unrelated] | Enema | Average 90 g stool [range 70-113 g] in 4 x 60 ml saline | 5 [daily for 5 days] | Fresh | No | No | 3/9 [33%] at 1 and 4 weeks [PUCAI < 10] | 7/9 [78%] at 1 Week 6/9 [67%] at 1 month [PUCAI drop > 15] | NR | NR | 4 weeks | 6 |
| Cohort | Cui et al., 201535 | 15 [data on 14] | Moderate- severe [Montreal] Steroid- dependent | Recipient- identified [related & unrelated] | Midgut through gastroscope | 150-200 ml infusion | 1–2, [1 week apart] | NR | No | NR | 4/14 [29%] [Montreal 0] | 8/14 [57%] [Montreal improvement ≥ 1] & discontinuation of steroids | NR | NR | > 3 months | 5 |
| Cohort | Damman et al., 201536 | 7 | Mild- moderate [UCDAI 3–10] | Recipient- identified [1 related, rest unrelated] | Colonoscopy | 175–290 ml of stool mixture [1 g stool:2-3 ml saline] | Single | Fresh | No | Yes | 1/7 [14%] at 4 weeks [UCDAI ≤ 2 & no subscore > 1] | 1/7 [14%] at 4 weeks [UCDAI drop ≥ 3] | NR | 1/7 [14%] at 4 weeks | 3 months | 6 |
| Cohort | Karolewska- Bochenek et al., 201537 | 4 [paediatric] | Moderate- severe | Unrelated | Gastroscopy | 50 ml infusion | 8 [daily first 5 days, alternate days in second week] | NR | No | NR | 0 | 4/4 [100%] | NR | NR | 4 weeks | 4 |
| Cohort | Kellermayer et al., 201538 | 3 [paediatric] | Immunotherapy-dependent but controlled mucosal disease at study commencement [Mayo 0–1] | Unrelated | Colonoscopy followed by enemas | 50 g stool in 250 ml saline; 60–250 ml delivered | 22–30 [daily for fortnight, thrice weekly for fortnight, then weekly for 6–12 weeks] | Frozen | No | Yes | 3/3 [100%] | - | 3/3 [100%] | 3/3 [100%] | 3 months | 3 |
| Cohort | Kump et al., 201539 | 17, 10 controls [triple antibiotic therapy] | Chronic active | NR | Colonoscopy [initially right colon, then left colon on subsequent infusions] | NR | 5 [fortnightly infusions] | NR | Triple therapy [not specified] for 10 days | Yes | FMT: 4/17 [24%], control: 0 [Mayo ≤ 2] | FMT: 10/17 [59%], control: 2/10 [20%] [Mayo drop ≥ 3] | NR | NR | 90 days | 6 |
| Cohort | Scaldaferri et al., 201540 | 8, 7 controls | Mild-moderate [partial Mayo ≥ 4, endoscopic Mayo ≥ 1] | Recipient- identified | NR | 200 ml of faecal slurry | 3 [interval not specified] | NR | Not specified | Yes | FMT: 3/8 [38%], control: 2/7 [29%] [partial Mayo ≤ 2, all subscores ≤ 1] | FMT: 4/8 [50%], control: 2/7 [29%] [partial Mayo drop ≥ 2] | 33% [Mayo 0 at Week 6] | NR | 12 weeks | 7 |
| Cohort | Suskind et al., 201541 | 4 [paediatric] | Mild-moderate [PUCAI] | NR | Nasogastric | 30 g stool in 100 ml saline | Single | Fresh | Rifaximin 200 mg tds for 3 days, omeprazole day before and day of FMT | Yes | 0 [PUCAI < 10] | 0 | NR | NR | 12 weeks | 6 |
| Cohort | Vermeire et al., 201642 | 8 | Moderate- severe; refractory, failed immunotherapy and anti-TNF | Unrelated & related | Nasogastric 3, rectal tube 5 | 200 g stool in 400 ml | 2 [daily for 2 days] | Fresh | No | Yes | 2/8 [25%] | NR | 2/8 [25%] [Mayo endoscopy subscore ≤ 1] | NR | 8 weeks | 5 |
| Cohort | Wei et al., 201543 | 11 | Mild- moderate [Mayo 2–10] | Unrelated | Colonoscopy | 60 g stool in 350 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days before FMT | Yes | 8/11 [73%] [Mayo < 2] | NR | NR | NR | 4 weeks | 4 |
| Cohort | Ren et al., 201544 | 7 | Severe [Mayo ≥ 10] | Relatives or healthy volunteers | Gastroscopy or colonoscopy or combined gastroscopy & colonoscopy | Gastroscopy, 100-200 ml; colonoscopy, 200-300 ml | 1–3 infusions [5 pts x 1, 1 pt x 2, 1 pt x 3] | Fresh | No | No | 5/7 [71%] [Day 30] [partial Mayo ≤ 2, subscores ≤ 1] | 7/7 [100%] [partial Mayo drop ≥ 3 or 30% drop] | NR | NR | median 90 days, range 30–210 days | 5 |
| Cohort | Karakan et al., 201645 | 14 | Steroid- dependent or non-responsive | NR | Colonoscopy | NR | 1–6 [interval not specified] | NR | NR | Yes | 6/14 [43%] | 11/14 [78.5%] | NR | NR | 3–18 months | 4 |
| Cohort | Goyal et al., 201646 | 12 [paediatric] | Mild- moderate [PUCAI < 65] | Recipient- identified [related & unrelated] | Both gastroscopy/ jejunoscopy [20-30 ml] and colonoscopy [200-250 ml] in TI/caecum | 150 g stool in 250 ml saline | Single | Fresh | Metronidazole/ vancomycin for 5 days, ceasing 48 before FMT | Yes | 0 [PUCAI < 10] | 2/12 [17%] [PUCAI drop ≥ 15] | NR | NR | 6 months | 6 |
| Cohort | Laszlo et al., 201647 | 4 | Moderate- severe | Related [family member] | Colonoscopy | 150 ml faecal suspension diluted in 400-425 ml saline | Single | Fresh | No | Yes | 4/4 [100%] | - | 2/4 [50%] | NR | 5 months | 5 |
| Cohort [RCT for pectin, FMT in both arms] | Wei et al., 201648 | 20 [10 FMT alone; 10 FMT + 5 days oral pectin] | Mild-moderate [Mayo 2–10] | Unrelated | Colonoscopy | 60 g stool in 500 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days before FMT | Yes | 7/20 [35%] [3/10 FMT, 4/10 FMT + pectin] [Mayo ≤ 2] | 13/20 [65%] [7/10 FMT, 6/10 FMT + pectin] [Mayo drop > 30%, 1 point drop in tarry stools or increase > 16 points in IBDQ] | NR | NR | 12 weeks | 5 |
| Cohort [data from ongoing RCT] | Pai et al., 201649 | 2 [paediatric] | Active | Unrelated | Enemas | NR | 12 [biweekly for 6 weeks] | Frozen | NR | NR | 0 | 0 | NR | NR | NR | 7 |
| Cohort | Jacob et al., 201650 | 20 | Active [Mayo ≥ 3, endoscopic subscore ≥ 1] | Unrelated multidonor [2-donor concentrate] | Colonoscopy [TI + right colon] | 120 ml infusion | Single | Frozen | No | Yes | 3/20 [15%] [Mayo ≤ 2, no subscore > 1] | 7/20 [35%] [Mayo drop ≥ 3 and bleeding subscore ≤ 1] | 2/20 [10%] [Mayo endoscopy subscore 0] | NR | 4 weeks | 6 |
| Cohort | Nishida et al., 201651 | 41 | Mild- moderate [Mayo 3–9, endoscopic subscore ≥ 1] | Related [family member] | Colonoscopy [caecum] | 150-200 g stool in 500 ml saline | Single | Fresh | No | Yes | 0 [Mayo ≤ 2, no subscore > 1] | 11/41 [27%] [Mayo drop ≥ 3 and/or Mayo clinical score drop ≥ 2 with rectal bleeding subscore decrease ≥ 1] | NR | NR | 8 weeks | 6 |
| Cohort | Zhang et al., 201652 | 19 | Moderate- severe [Mayo ≥ 6] | NR | Midgut through gastroscope | 150-200 ml infusion | Single | Fresh | No | NR | 2/19 [11%] [Mayo ≤2, no subscore > 1] | 11/19 [58%] [Mayo drop ≥ 3 or ≥ 30%, along with drop in bleeding subscore ≥ 1 or bleeding subscore ≤ 1] | NR | NR | ≥ 3 months | 5 |
| Cohort | Grewal et al., 201653 | 17 | Moderate- severe, steroid- dependent | NR | NR | NR | 7 [2 infusions 2 weeks apart, then 5 infusions every 4 weeks] | NR | NR | NR | 15/17 [88%] [Week 4] 10/17 [59%] at 1 year with steroid cessation | NR | NR | NR | 12 months | 5 |
| Cohort [open- label extension cohort of RCT placebo arm] | Paramsothy et al., 201711 | 37 | Mild-moderate [Mayo 4–10] | Unrelated multidonor [3–7 donors/ infusion] | Colonoscopy followed by enemas | 37.5 g stool in 150 ml saline | 40 [5/week for 8 weeks] | Frozen | No | No | 17/37 [46%] [steroid- free Mayo subscore ≤ 1 for bleeding & stool frequency combined] | NR | 8/37 [22%] [steroid- free Mayo endoscopy subscore 0] | NR | 8 weeks post FMT [total 16 weeks] | 5 |
| Cohort | Ishikawa et al., 201754 | 17, 19 controls [triple antibiotic therapy] | Active [Lichtiger Clinical Activity Index ≥ 5 or endoscopic Mayo subscore ≥ 1] | Recipient- identified [related & unrelated] | Colonoscopy | 150-250 g stool in 350-500 ml saline | Single | Fresh | Amoxycillin 1500 mg, fosfomycin 3000 mg, metronidazole 750 mg daily for 2 weeks till 2 days before FMT | Yes | FMT: 9/17 [53%], control: 3/19 [16%] [CAI ≤ 3] | FMT: 14/17 [82%], control: 13/19 [68%] [CAI < 10 & drop ≥ 3] | NR | NR | 4 weeks | 9 |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow Up . | NOS Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Angelberger et al., 201332 | 5 | Moderate- severe [Mayo ≥ 6] | Recipient- identified but first degree- relatives excluded | Nasojejunal & enema combined | Median 24 g stool in 250 ml saline for nasojejunal infusion, median 20 g stool in 100 ml saline for enema | 3 [daily for 3 days] | Fresh | Metronidazole 500 mg bd and probiotic [Yomogi or Omnibiotic] for 5–10 days before FMT | Yes | 0 [Mayo ≤ 2, no subscore > 1] | 1/5 [20%] [Mayo drop ≥ 3 and ≥ 30%, along with drop in bleeding subscore ≥ 1 or bleeding subscore ≤ 1] | NR | NR | 12 weeks | 5 |
| Cohort | Kump et al., 201333 | 6 | Moderate- severe [Mayo 8–11] | Unrelated | Colonoscopy [TI + colon] | 100-150 g stool in 200-350 ml | Single | Fresh | No | Yes | 0 [Mayo ≤ 2] | 2/6 [33%] [Mayo drop ≥ 3] | NR | NR | 3 months | 5 |
| Cohort | Kunde et al., 201334 | 10 [paediatric] | Mild- moderate [PUCAI 15–65] | Recipient- identified [related & unrelated] | Enema | Average 90 g stool [range 70-113 g] in 4 x 60 ml saline | 5 [daily for 5 days] | Fresh | No | No | 3/9 [33%] at 1 and 4 weeks [PUCAI < 10] | 7/9 [78%] at 1 Week 6/9 [67%] at 1 month [PUCAI drop > 15] | NR | NR | 4 weeks | 6 |
| Cohort | Cui et al., 201535 | 15 [data on 14] | Moderate- severe [Montreal] Steroid- dependent | Recipient- identified [related & unrelated] | Midgut through gastroscope | 150-200 ml infusion | 1–2, [1 week apart] | NR | No | NR | 4/14 [29%] [Montreal 0] | 8/14 [57%] [Montreal improvement ≥ 1] & discontinuation of steroids | NR | NR | > 3 months | 5 |
| Cohort | Damman et al., 201536 | 7 | Mild- moderate [UCDAI 3–10] | Recipient- identified [1 related, rest unrelated] | Colonoscopy | 175–290 ml of stool mixture [1 g stool:2-3 ml saline] | Single | Fresh | No | Yes | 1/7 [14%] at 4 weeks [UCDAI ≤ 2 & no subscore > 1] | 1/7 [14%] at 4 weeks [UCDAI drop ≥ 3] | NR | 1/7 [14%] at 4 weeks | 3 months | 6 |
| Cohort | Karolewska- Bochenek et al., 201537 | 4 [paediatric] | Moderate- severe | Unrelated | Gastroscopy | 50 ml infusion | 8 [daily first 5 days, alternate days in second week] | NR | No | NR | 0 | 4/4 [100%] | NR | NR | 4 weeks | 4 |
| Cohort | Kellermayer et al., 201538 | 3 [paediatric] | Immunotherapy-dependent but controlled mucosal disease at study commencement [Mayo 0–1] | Unrelated | Colonoscopy followed by enemas | 50 g stool in 250 ml saline; 60–250 ml delivered | 22–30 [daily for fortnight, thrice weekly for fortnight, then weekly for 6–12 weeks] | Frozen | No | Yes | 3/3 [100%] | - | 3/3 [100%] | 3/3 [100%] | 3 months | 3 |
| Cohort | Kump et al., 201539 | 17, 10 controls [triple antibiotic therapy] | Chronic active | NR | Colonoscopy [initially right colon, then left colon on subsequent infusions] | NR | 5 [fortnightly infusions] | NR | Triple therapy [not specified] for 10 days | Yes | FMT: 4/17 [24%], control: 0 [Mayo ≤ 2] | FMT: 10/17 [59%], control: 2/10 [20%] [Mayo drop ≥ 3] | NR | NR | 90 days | 6 |
| Cohort | Scaldaferri et al., 201540 | 8, 7 controls | Mild-moderate [partial Mayo ≥ 4, endoscopic Mayo ≥ 1] | Recipient- identified | NR | 200 ml of faecal slurry | 3 [interval not specified] | NR | Not specified | Yes | FMT: 3/8 [38%], control: 2/7 [29%] [partial Mayo ≤ 2, all subscores ≤ 1] | FMT: 4/8 [50%], control: 2/7 [29%] [partial Mayo drop ≥ 2] | 33% [Mayo 0 at Week 6] | NR | 12 weeks | 7 |
| Cohort | Suskind et al., 201541 | 4 [paediatric] | Mild-moderate [PUCAI] | NR | Nasogastric | 30 g stool in 100 ml saline | Single | Fresh | Rifaximin 200 mg tds for 3 days, omeprazole day before and day of FMT | Yes | 0 [PUCAI < 10] | 0 | NR | NR | 12 weeks | 6 |
| Cohort | Vermeire et al., 201642 | 8 | Moderate- severe; refractory, failed immunotherapy and anti-TNF | Unrelated & related | Nasogastric 3, rectal tube 5 | 200 g stool in 400 ml | 2 [daily for 2 days] | Fresh | No | Yes | 2/8 [25%] | NR | 2/8 [25%] [Mayo endoscopy subscore ≤ 1] | NR | 8 weeks | 5 |
| Cohort | Wei et al., 201543 | 11 | Mild- moderate [Mayo 2–10] | Unrelated | Colonoscopy | 60 g stool in 350 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days before FMT | Yes | 8/11 [73%] [Mayo < 2] | NR | NR | NR | 4 weeks | 4 |
| Cohort | Ren et al., 201544 | 7 | Severe [Mayo ≥ 10] | Relatives or healthy volunteers | Gastroscopy or colonoscopy or combined gastroscopy & colonoscopy | Gastroscopy, 100-200 ml; colonoscopy, 200-300 ml | 1–3 infusions [5 pts x 1, 1 pt x 2, 1 pt x 3] | Fresh | No | No | 5/7 [71%] [Day 30] [partial Mayo ≤ 2, subscores ≤ 1] | 7/7 [100%] [partial Mayo drop ≥ 3 or 30% drop] | NR | NR | median 90 days, range 30–210 days | 5 |
| Cohort | Karakan et al., 201645 | 14 | Steroid- dependent or non-responsive | NR | Colonoscopy | NR | 1–6 [interval not specified] | NR | NR | Yes | 6/14 [43%] | 11/14 [78.5%] | NR | NR | 3–18 months | 4 |
| Cohort | Goyal et al., 201646 | 12 [paediatric] | Mild- moderate [PUCAI < 65] | Recipient- identified [related & unrelated] | Both gastroscopy/ jejunoscopy [20-30 ml] and colonoscopy [200-250 ml] in TI/caecum | 150 g stool in 250 ml saline | Single | Fresh | Metronidazole/ vancomycin for 5 days, ceasing 48 before FMT | Yes | 0 [PUCAI < 10] | 2/12 [17%] [PUCAI drop ≥ 15] | NR | NR | 6 months | 6 |
| Cohort | Laszlo et al., 201647 | 4 | Moderate- severe | Related [family member] | Colonoscopy | 150 ml faecal suspension diluted in 400-425 ml saline | Single | Fresh | No | Yes | 4/4 [100%] | - | 2/4 [50%] | NR | 5 months | 5 |
| Cohort [RCT for pectin, FMT in both arms] | Wei et al., 201648 | 20 [10 FMT alone; 10 FMT + 5 days oral pectin] | Mild-moderate [Mayo 2–10] | Unrelated | Colonoscopy | 60 g stool in 500 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days before FMT | Yes | 7/20 [35%] [3/10 FMT, 4/10 FMT + pectin] [Mayo ≤ 2] | 13/20 [65%] [7/10 FMT, 6/10 FMT + pectin] [Mayo drop > 30%, 1 point drop in tarry stools or increase > 16 points in IBDQ] | NR | NR | 12 weeks | 5 |
| Cohort [data from ongoing RCT] | Pai et al., 201649 | 2 [paediatric] | Active | Unrelated | Enemas | NR | 12 [biweekly for 6 weeks] | Frozen | NR | NR | 0 | 0 | NR | NR | NR | 7 |
| Cohort | Jacob et al., 201650 | 20 | Active [Mayo ≥ 3, endoscopic subscore ≥ 1] | Unrelated multidonor [2-donor concentrate] | Colonoscopy [TI + right colon] | 120 ml infusion | Single | Frozen | No | Yes | 3/20 [15%] [Mayo ≤ 2, no subscore > 1] | 7/20 [35%] [Mayo drop ≥ 3 and bleeding subscore ≤ 1] | 2/20 [10%] [Mayo endoscopy subscore 0] | NR | 4 weeks | 6 |
| Cohort | Nishida et al., 201651 | 41 | Mild- moderate [Mayo 3–9, endoscopic subscore ≥ 1] | Related [family member] | Colonoscopy [caecum] | 150-200 g stool in 500 ml saline | Single | Fresh | No | Yes | 0 [Mayo ≤ 2, no subscore > 1] | 11/41 [27%] [Mayo drop ≥ 3 and/or Mayo clinical score drop ≥ 2 with rectal bleeding subscore decrease ≥ 1] | NR | NR | 8 weeks | 6 |
| Cohort | Zhang et al., 201652 | 19 | Moderate- severe [Mayo ≥ 6] | NR | Midgut through gastroscope | 150-200 ml infusion | Single | Fresh | No | NR | 2/19 [11%] [Mayo ≤2, no subscore > 1] | 11/19 [58%] [Mayo drop ≥ 3 or ≥ 30%, along with drop in bleeding subscore ≥ 1 or bleeding subscore ≤ 1] | NR | NR | ≥ 3 months | 5 |
| Cohort | Grewal et al., 201653 | 17 | Moderate- severe, steroid- dependent | NR | NR | NR | 7 [2 infusions 2 weeks apart, then 5 infusions every 4 weeks] | NR | NR | NR | 15/17 [88%] [Week 4] 10/17 [59%] at 1 year with steroid cessation | NR | NR | NR | 12 months | 5 |
| Cohort [open- label extension cohort of RCT placebo arm] | Paramsothy et al., 201711 | 37 | Mild-moderate [Mayo 4–10] | Unrelated multidonor [3–7 donors/ infusion] | Colonoscopy followed by enemas | 37.5 g stool in 150 ml saline | 40 [5/week for 8 weeks] | Frozen | No | No | 17/37 [46%] [steroid- free Mayo subscore ≤ 1 for bleeding & stool frequency combined] | NR | 8/37 [22%] [steroid- free Mayo endoscopy subscore 0] | NR | 8 weeks post FMT [total 16 weeks] | 5 |
| Cohort | Ishikawa et al., 201754 | 17, 19 controls [triple antibiotic therapy] | Active [Lichtiger Clinical Activity Index ≥ 5 or endoscopic Mayo subscore ≥ 1] | Recipient- identified [related & unrelated] | Colonoscopy | 150-250 g stool in 350-500 ml saline | Single | Fresh | Amoxycillin 1500 mg, fosfomycin 3000 mg, metronidazole 750 mg daily for 2 weeks till 2 days before FMT | Yes | FMT: 9/17 [53%], control: 3/19 [16%] [CAI ≤ 3] | FMT: 14/17 [82%], control: 13/19 [68%] [CAI < 10 & drop ≥ 3] | NR | NR | 4 weeks | 9 |
NOS, Newcastle-Ottawa Scale; NR, not recorded; bd, twice daily; PUCAI, Paediatric Ulcerative Colitis Activity Index; RCT, randomised controlled trials; tds, three times daily; TNF, tumour necrosis factor; IBDQ, Inflammatory Bowel Disease Questionnaire.
Cohort studies of faecal microbiota transplantation [FMT] in ulcerative colitis.
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow Up . | NOS Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Angelberger et al., 201332 | 5 | Moderate- severe [Mayo ≥ 6] | Recipient- identified but first degree- relatives excluded | Nasojejunal & enema combined | Median 24 g stool in 250 ml saline for nasojejunal infusion, median 20 g stool in 100 ml saline for enema | 3 [daily for 3 days] | Fresh | Metronidazole 500 mg bd and probiotic [Yomogi or Omnibiotic] for 5–10 days before FMT | Yes | 0 [Mayo ≤ 2, no subscore > 1] | 1/5 [20%] [Mayo drop ≥ 3 and ≥ 30%, along with drop in bleeding subscore ≥ 1 or bleeding subscore ≤ 1] | NR | NR | 12 weeks | 5 |
| Cohort | Kump et al., 201333 | 6 | Moderate- severe [Mayo 8–11] | Unrelated | Colonoscopy [TI + colon] | 100-150 g stool in 200-350 ml | Single | Fresh | No | Yes | 0 [Mayo ≤ 2] | 2/6 [33%] [Mayo drop ≥ 3] | NR | NR | 3 months | 5 |
| Cohort | Kunde et al., 201334 | 10 [paediatric] | Mild- moderate [PUCAI 15–65] | Recipient- identified [related & unrelated] | Enema | Average 90 g stool [range 70-113 g] in 4 x 60 ml saline | 5 [daily for 5 days] | Fresh | No | No | 3/9 [33%] at 1 and 4 weeks [PUCAI < 10] | 7/9 [78%] at 1 Week 6/9 [67%] at 1 month [PUCAI drop > 15] | NR | NR | 4 weeks | 6 |
| Cohort | Cui et al., 201535 | 15 [data on 14] | Moderate- severe [Montreal] Steroid- dependent | Recipient- identified [related & unrelated] | Midgut through gastroscope | 150-200 ml infusion | 1–2, [1 week apart] | NR | No | NR | 4/14 [29%] [Montreal 0] | 8/14 [57%] [Montreal improvement ≥ 1] & discontinuation of steroids | NR | NR | > 3 months | 5 |
| Cohort | Damman et al., 201536 | 7 | Mild- moderate [UCDAI 3–10] | Recipient- identified [1 related, rest unrelated] | Colonoscopy | 175–290 ml of stool mixture [1 g stool:2-3 ml saline] | Single | Fresh | No | Yes | 1/7 [14%] at 4 weeks [UCDAI ≤ 2 & no subscore > 1] | 1/7 [14%] at 4 weeks [UCDAI drop ≥ 3] | NR | 1/7 [14%] at 4 weeks | 3 months | 6 |
| Cohort | Karolewska- Bochenek et al., 201537 | 4 [paediatric] | Moderate- severe | Unrelated | Gastroscopy | 50 ml infusion | 8 [daily first 5 days, alternate days in second week] | NR | No | NR | 0 | 4/4 [100%] | NR | NR | 4 weeks | 4 |
| Cohort | Kellermayer et al., 201538 | 3 [paediatric] | Immunotherapy-dependent but controlled mucosal disease at study commencement [Mayo 0–1] | Unrelated | Colonoscopy followed by enemas | 50 g stool in 250 ml saline; 60–250 ml delivered | 22–30 [daily for fortnight, thrice weekly for fortnight, then weekly for 6–12 weeks] | Frozen | No | Yes | 3/3 [100%] | - | 3/3 [100%] | 3/3 [100%] | 3 months | 3 |
| Cohort | Kump et al., 201539 | 17, 10 controls [triple antibiotic therapy] | Chronic active | NR | Colonoscopy [initially right colon, then left colon on subsequent infusions] | NR | 5 [fortnightly infusions] | NR | Triple therapy [not specified] for 10 days | Yes | FMT: 4/17 [24%], control: 0 [Mayo ≤ 2] | FMT: 10/17 [59%], control: 2/10 [20%] [Mayo drop ≥ 3] | NR | NR | 90 days | 6 |
| Cohort | Scaldaferri et al., 201540 | 8, 7 controls | Mild-moderate [partial Mayo ≥ 4, endoscopic Mayo ≥ 1] | Recipient- identified | NR | 200 ml of faecal slurry | 3 [interval not specified] | NR | Not specified | Yes | FMT: 3/8 [38%], control: 2/7 [29%] [partial Mayo ≤ 2, all subscores ≤ 1] | FMT: 4/8 [50%], control: 2/7 [29%] [partial Mayo drop ≥ 2] | 33% [Mayo 0 at Week 6] | NR | 12 weeks | 7 |
| Cohort | Suskind et al., 201541 | 4 [paediatric] | Mild-moderate [PUCAI] | NR | Nasogastric | 30 g stool in 100 ml saline | Single | Fresh | Rifaximin 200 mg tds for 3 days, omeprazole day before and day of FMT | Yes | 0 [PUCAI < 10] | 0 | NR | NR | 12 weeks | 6 |
| Cohort | Vermeire et al., 201642 | 8 | Moderate- severe; refractory, failed immunotherapy and anti-TNF | Unrelated & related | Nasogastric 3, rectal tube 5 | 200 g stool in 400 ml | 2 [daily for 2 days] | Fresh | No | Yes | 2/8 [25%] | NR | 2/8 [25%] [Mayo endoscopy subscore ≤ 1] | NR | 8 weeks | 5 |
| Cohort | Wei et al., 201543 | 11 | Mild- moderate [Mayo 2–10] | Unrelated | Colonoscopy | 60 g stool in 350 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days before FMT | Yes | 8/11 [73%] [Mayo < 2] | NR | NR | NR | 4 weeks | 4 |
| Cohort | Ren et al., 201544 | 7 | Severe [Mayo ≥ 10] | Relatives or healthy volunteers | Gastroscopy or colonoscopy or combined gastroscopy & colonoscopy | Gastroscopy, 100-200 ml; colonoscopy, 200-300 ml | 1–3 infusions [5 pts x 1, 1 pt x 2, 1 pt x 3] | Fresh | No | No | 5/7 [71%] [Day 30] [partial Mayo ≤ 2, subscores ≤ 1] | 7/7 [100%] [partial Mayo drop ≥ 3 or 30% drop] | NR | NR | median 90 days, range 30–210 days | 5 |
| Cohort | Karakan et al., 201645 | 14 | Steroid- dependent or non-responsive | NR | Colonoscopy | NR | 1–6 [interval not specified] | NR | NR | Yes | 6/14 [43%] | 11/14 [78.5%] | NR | NR | 3–18 months | 4 |
| Cohort | Goyal et al., 201646 | 12 [paediatric] | Mild- moderate [PUCAI < 65] | Recipient- identified [related & unrelated] | Both gastroscopy/ jejunoscopy [20-30 ml] and colonoscopy [200-250 ml] in TI/caecum | 150 g stool in 250 ml saline | Single | Fresh | Metronidazole/ vancomycin for 5 days, ceasing 48 before FMT | Yes | 0 [PUCAI < 10] | 2/12 [17%] [PUCAI drop ≥ 15] | NR | NR | 6 months | 6 |
| Cohort | Laszlo et al., 201647 | 4 | Moderate- severe | Related [family member] | Colonoscopy | 150 ml faecal suspension diluted in 400-425 ml saline | Single | Fresh | No | Yes | 4/4 [100%] | - | 2/4 [50%] | NR | 5 months | 5 |
| Cohort [RCT for pectin, FMT in both arms] | Wei et al., 201648 | 20 [10 FMT alone; 10 FMT + 5 days oral pectin] | Mild-moderate [Mayo 2–10] | Unrelated | Colonoscopy | 60 g stool in 500 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days before FMT | Yes | 7/20 [35%] [3/10 FMT, 4/10 FMT + pectin] [Mayo ≤ 2] | 13/20 [65%] [7/10 FMT, 6/10 FMT + pectin] [Mayo drop > 30%, 1 point drop in tarry stools or increase > 16 points in IBDQ] | NR | NR | 12 weeks | 5 |
| Cohort [data from ongoing RCT] | Pai et al., 201649 | 2 [paediatric] | Active | Unrelated | Enemas | NR | 12 [biweekly for 6 weeks] | Frozen | NR | NR | 0 | 0 | NR | NR | NR | 7 |
| Cohort | Jacob et al., 201650 | 20 | Active [Mayo ≥ 3, endoscopic subscore ≥ 1] | Unrelated multidonor [2-donor concentrate] | Colonoscopy [TI + right colon] | 120 ml infusion | Single | Frozen | No | Yes | 3/20 [15%] [Mayo ≤ 2, no subscore > 1] | 7/20 [35%] [Mayo drop ≥ 3 and bleeding subscore ≤ 1] | 2/20 [10%] [Mayo endoscopy subscore 0] | NR | 4 weeks | 6 |
| Cohort | Nishida et al., 201651 | 41 | Mild- moderate [Mayo 3–9, endoscopic subscore ≥ 1] | Related [family member] | Colonoscopy [caecum] | 150-200 g stool in 500 ml saline | Single | Fresh | No | Yes | 0 [Mayo ≤ 2, no subscore > 1] | 11/41 [27%] [Mayo drop ≥ 3 and/or Mayo clinical score drop ≥ 2 with rectal bleeding subscore decrease ≥ 1] | NR | NR | 8 weeks | 6 |
| Cohort | Zhang et al., 201652 | 19 | Moderate- severe [Mayo ≥ 6] | NR | Midgut through gastroscope | 150-200 ml infusion | Single | Fresh | No | NR | 2/19 [11%] [Mayo ≤2, no subscore > 1] | 11/19 [58%] [Mayo drop ≥ 3 or ≥ 30%, along with drop in bleeding subscore ≥ 1 or bleeding subscore ≤ 1] | NR | NR | ≥ 3 months | 5 |
| Cohort | Grewal et al., 201653 | 17 | Moderate- severe, steroid- dependent | NR | NR | NR | 7 [2 infusions 2 weeks apart, then 5 infusions every 4 weeks] | NR | NR | NR | 15/17 [88%] [Week 4] 10/17 [59%] at 1 year with steroid cessation | NR | NR | NR | 12 months | 5 |
| Cohort [open- label extension cohort of RCT placebo arm] | Paramsothy et al., 201711 | 37 | Mild-moderate [Mayo 4–10] | Unrelated multidonor [3–7 donors/ infusion] | Colonoscopy followed by enemas | 37.5 g stool in 150 ml saline | 40 [5/week for 8 weeks] | Frozen | No | No | 17/37 [46%] [steroid- free Mayo subscore ≤ 1 for bleeding & stool frequency combined] | NR | 8/37 [22%] [steroid- free Mayo endoscopy subscore 0] | NR | 8 weeks post FMT [total 16 weeks] | 5 |
| Cohort | Ishikawa et al., 201754 | 17, 19 controls [triple antibiotic therapy] | Active [Lichtiger Clinical Activity Index ≥ 5 or endoscopic Mayo subscore ≥ 1] | Recipient- identified [related & unrelated] | Colonoscopy | 150-250 g stool in 350-500 ml saline | Single | Fresh | Amoxycillin 1500 mg, fosfomycin 3000 mg, metronidazole 750 mg daily for 2 weeks till 2 days before FMT | Yes | FMT: 9/17 [53%], control: 3/19 [16%] [CAI ≤ 3] | FMT: 14/17 [82%], control: 13/19 [68%] [CAI < 10 & drop ≥ 3] | NR | NR | 4 weeks | 9 |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow Up . | NOS Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Angelberger et al., 201332 | 5 | Moderate- severe [Mayo ≥ 6] | Recipient- identified but first degree- relatives excluded | Nasojejunal & enema combined | Median 24 g stool in 250 ml saline for nasojejunal infusion, median 20 g stool in 100 ml saline for enema | 3 [daily for 3 days] | Fresh | Metronidazole 500 mg bd and probiotic [Yomogi or Omnibiotic] for 5–10 days before FMT | Yes | 0 [Mayo ≤ 2, no subscore > 1] | 1/5 [20%] [Mayo drop ≥ 3 and ≥ 30%, along with drop in bleeding subscore ≥ 1 or bleeding subscore ≤ 1] | NR | NR | 12 weeks | 5 |
| Cohort | Kump et al., 201333 | 6 | Moderate- severe [Mayo 8–11] | Unrelated | Colonoscopy [TI + colon] | 100-150 g stool in 200-350 ml | Single | Fresh | No | Yes | 0 [Mayo ≤ 2] | 2/6 [33%] [Mayo drop ≥ 3] | NR | NR | 3 months | 5 |
| Cohort | Kunde et al., 201334 | 10 [paediatric] | Mild- moderate [PUCAI 15–65] | Recipient- identified [related & unrelated] | Enema | Average 90 g stool [range 70-113 g] in 4 x 60 ml saline | 5 [daily for 5 days] | Fresh | No | No | 3/9 [33%] at 1 and 4 weeks [PUCAI < 10] | 7/9 [78%] at 1 Week 6/9 [67%] at 1 month [PUCAI drop > 15] | NR | NR | 4 weeks | 6 |
| Cohort | Cui et al., 201535 | 15 [data on 14] | Moderate- severe [Montreal] Steroid- dependent | Recipient- identified [related & unrelated] | Midgut through gastroscope | 150-200 ml infusion | 1–2, [1 week apart] | NR | No | NR | 4/14 [29%] [Montreal 0] | 8/14 [57%] [Montreal improvement ≥ 1] & discontinuation of steroids | NR | NR | > 3 months | 5 |
| Cohort | Damman et al., 201536 | 7 | Mild- moderate [UCDAI 3–10] | Recipient- identified [1 related, rest unrelated] | Colonoscopy | 175–290 ml of stool mixture [1 g stool:2-3 ml saline] | Single | Fresh | No | Yes | 1/7 [14%] at 4 weeks [UCDAI ≤ 2 & no subscore > 1] | 1/7 [14%] at 4 weeks [UCDAI drop ≥ 3] | NR | 1/7 [14%] at 4 weeks | 3 months | 6 |
| Cohort | Karolewska- Bochenek et al., 201537 | 4 [paediatric] | Moderate- severe | Unrelated | Gastroscopy | 50 ml infusion | 8 [daily first 5 days, alternate days in second week] | NR | No | NR | 0 | 4/4 [100%] | NR | NR | 4 weeks | 4 |
| Cohort | Kellermayer et al., 201538 | 3 [paediatric] | Immunotherapy-dependent but controlled mucosal disease at study commencement [Mayo 0–1] | Unrelated | Colonoscopy followed by enemas | 50 g stool in 250 ml saline; 60–250 ml delivered | 22–30 [daily for fortnight, thrice weekly for fortnight, then weekly for 6–12 weeks] | Frozen | No | Yes | 3/3 [100%] | - | 3/3 [100%] | 3/3 [100%] | 3 months | 3 |
| Cohort | Kump et al., 201539 | 17, 10 controls [triple antibiotic therapy] | Chronic active | NR | Colonoscopy [initially right colon, then left colon on subsequent infusions] | NR | 5 [fortnightly infusions] | NR | Triple therapy [not specified] for 10 days | Yes | FMT: 4/17 [24%], control: 0 [Mayo ≤ 2] | FMT: 10/17 [59%], control: 2/10 [20%] [Mayo drop ≥ 3] | NR | NR | 90 days | 6 |
| Cohort | Scaldaferri et al., 201540 | 8, 7 controls | Mild-moderate [partial Mayo ≥ 4, endoscopic Mayo ≥ 1] | Recipient- identified | NR | 200 ml of faecal slurry | 3 [interval not specified] | NR | Not specified | Yes | FMT: 3/8 [38%], control: 2/7 [29%] [partial Mayo ≤ 2, all subscores ≤ 1] | FMT: 4/8 [50%], control: 2/7 [29%] [partial Mayo drop ≥ 2] | 33% [Mayo 0 at Week 6] | NR | 12 weeks | 7 |
| Cohort | Suskind et al., 201541 | 4 [paediatric] | Mild-moderate [PUCAI] | NR | Nasogastric | 30 g stool in 100 ml saline | Single | Fresh | Rifaximin 200 mg tds for 3 days, omeprazole day before and day of FMT | Yes | 0 [PUCAI < 10] | 0 | NR | NR | 12 weeks | 6 |
| Cohort | Vermeire et al., 201642 | 8 | Moderate- severe; refractory, failed immunotherapy and anti-TNF | Unrelated & related | Nasogastric 3, rectal tube 5 | 200 g stool in 400 ml | 2 [daily for 2 days] | Fresh | No | Yes | 2/8 [25%] | NR | 2/8 [25%] [Mayo endoscopy subscore ≤ 1] | NR | 8 weeks | 5 |
| Cohort | Wei et al., 201543 | 11 | Mild- moderate [Mayo 2–10] | Unrelated | Colonoscopy | 60 g stool in 350 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days before FMT | Yes | 8/11 [73%] [Mayo < 2] | NR | NR | NR | 4 weeks | 4 |
| Cohort | Ren et al., 201544 | 7 | Severe [Mayo ≥ 10] | Relatives or healthy volunteers | Gastroscopy or colonoscopy or combined gastroscopy & colonoscopy | Gastroscopy, 100-200 ml; colonoscopy, 200-300 ml | 1–3 infusions [5 pts x 1, 1 pt x 2, 1 pt x 3] | Fresh | No | No | 5/7 [71%] [Day 30] [partial Mayo ≤ 2, subscores ≤ 1] | 7/7 [100%] [partial Mayo drop ≥ 3 or 30% drop] | NR | NR | median 90 days, range 30–210 days | 5 |
| Cohort | Karakan et al., 201645 | 14 | Steroid- dependent or non-responsive | NR | Colonoscopy | NR | 1–6 [interval not specified] | NR | NR | Yes | 6/14 [43%] | 11/14 [78.5%] | NR | NR | 3–18 months | 4 |
| Cohort | Goyal et al., 201646 | 12 [paediatric] | Mild- moderate [PUCAI < 65] | Recipient- identified [related & unrelated] | Both gastroscopy/ jejunoscopy [20-30 ml] and colonoscopy [200-250 ml] in TI/caecum | 150 g stool in 250 ml saline | Single | Fresh | Metronidazole/ vancomycin for 5 days, ceasing 48 before FMT | Yes | 0 [PUCAI < 10] | 2/12 [17%] [PUCAI drop ≥ 15] | NR | NR | 6 months | 6 |
| Cohort | Laszlo et al., 201647 | 4 | Moderate- severe | Related [family member] | Colonoscopy | 150 ml faecal suspension diluted in 400-425 ml saline | Single | Fresh | No | Yes | 4/4 [100%] | - | 2/4 [50%] | NR | 5 months | 5 |
| Cohort [RCT for pectin, FMT in both arms] | Wei et al., 201648 | 20 [10 FMT alone; 10 FMT + 5 days oral pectin] | Mild-moderate [Mayo 2–10] | Unrelated | Colonoscopy | 60 g stool in 500 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days before FMT | Yes | 7/20 [35%] [3/10 FMT, 4/10 FMT + pectin] [Mayo ≤ 2] | 13/20 [65%] [7/10 FMT, 6/10 FMT + pectin] [Mayo drop > 30%, 1 point drop in tarry stools or increase > 16 points in IBDQ] | NR | NR | 12 weeks | 5 |
| Cohort [data from ongoing RCT] | Pai et al., 201649 | 2 [paediatric] | Active | Unrelated | Enemas | NR | 12 [biweekly for 6 weeks] | Frozen | NR | NR | 0 | 0 | NR | NR | NR | 7 |
| Cohort | Jacob et al., 201650 | 20 | Active [Mayo ≥ 3, endoscopic subscore ≥ 1] | Unrelated multidonor [2-donor concentrate] | Colonoscopy [TI + right colon] | 120 ml infusion | Single | Frozen | No | Yes | 3/20 [15%] [Mayo ≤ 2, no subscore > 1] | 7/20 [35%] [Mayo drop ≥ 3 and bleeding subscore ≤ 1] | 2/20 [10%] [Mayo endoscopy subscore 0] | NR | 4 weeks | 6 |
| Cohort | Nishida et al., 201651 | 41 | Mild- moderate [Mayo 3–9, endoscopic subscore ≥ 1] | Related [family member] | Colonoscopy [caecum] | 150-200 g stool in 500 ml saline | Single | Fresh | No | Yes | 0 [Mayo ≤ 2, no subscore > 1] | 11/41 [27%] [Mayo drop ≥ 3 and/or Mayo clinical score drop ≥ 2 with rectal bleeding subscore decrease ≥ 1] | NR | NR | 8 weeks | 6 |
| Cohort | Zhang et al., 201652 | 19 | Moderate- severe [Mayo ≥ 6] | NR | Midgut through gastroscope | 150-200 ml infusion | Single | Fresh | No | NR | 2/19 [11%] [Mayo ≤2, no subscore > 1] | 11/19 [58%] [Mayo drop ≥ 3 or ≥ 30%, along with drop in bleeding subscore ≥ 1 or bleeding subscore ≤ 1] | NR | NR | ≥ 3 months | 5 |
| Cohort | Grewal et al., 201653 | 17 | Moderate- severe, steroid- dependent | NR | NR | NR | 7 [2 infusions 2 weeks apart, then 5 infusions every 4 weeks] | NR | NR | NR | 15/17 [88%] [Week 4] 10/17 [59%] at 1 year with steroid cessation | NR | NR | NR | 12 months | 5 |
| Cohort [open- label extension cohort of RCT placebo arm] | Paramsothy et al., 201711 | 37 | Mild-moderate [Mayo 4–10] | Unrelated multidonor [3–7 donors/ infusion] | Colonoscopy followed by enemas | 37.5 g stool in 150 ml saline | 40 [5/week for 8 weeks] | Frozen | No | No | 17/37 [46%] [steroid- free Mayo subscore ≤ 1 for bleeding & stool frequency combined] | NR | 8/37 [22%] [steroid- free Mayo endoscopy subscore 0] | NR | 8 weeks post FMT [total 16 weeks] | 5 |
| Cohort | Ishikawa et al., 201754 | 17, 19 controls [triple antibiotic therapy] | Active [Lichtiger Clinical Activity Index ≥ 5 or endoscopic Mayo subscore ≥ 1] | Recipient- identified [related & unrelated] | Colonoscopy | 150-250 g stool in 350-500 ml saline | Single | Fresh | Amoxycillin 1500 mg, fosfomycin 3000 mg, metronidazole 750 mg daily for 2 weeks till 2 days before FMT | Yes | FMT: 9/17 [53%], control: 3/19 [16%] [CAI ≤ 3] | FMT: 14/17 [82%], control: 13/19 [68%] [CAI < 10 & drop ≥ 3] | NR | NR | 4 weeks | 9 |
NOS, Newcastle-Ottawa Scale; NR, not recorded; bd, twice daily; PUCAI, Paediatric Ulcerative Colitis Activity Index; RCT, randomised controlled trials; tds, three times daily; TNF, tumour necrosis factor; IBDQ, Inflammatory Bowel Disease Questionnaire.
Randomised controlled trials of faecal microbiota transplant [FMT] in ulcerative colitis.
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre- antibiotic . | Bowel lavage . | Primary endpoint . | Clinical remission . | Clinical response . | Endoscopic remission . | Endoscopic response . | Histologic remission . | Follow- up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBRCT | Moayeddi et al., 20159 | 75: 38 FMT, 37 controls | Mild- severe, [Mayo 4–12] | Unrelated | Enema | 50g stool in 50 ml infusion | 6 [weekly] | Frozen 21, fresh 15, combination fresh & frozen 1 | No | No | Clinical and endoscopic remission Mayo < 3 with endoscopic Mayo 0 9/38 [24%] vs 2/37 [5%], p = 0.03 | 9/38 [24%] vs 2/37 [5%], p = 0.03 [Mayo < 3] | 15/38 [39%] vs 9/37 [24%], p = 0.16 [Mayo drop ≥ 3] | 9/38 [24%] vs 2/37 [5%], p = 0.03 [Mayo endoscopy subscore 0] | NR | 7 FMT, 1 placebo | 7 weeks |
| DBRCT | Rossen et al., 201510 | 48: 23 FMT, 25 control autologous stool | Mild- moderate [SCCAI 4–11] | Unrelated & related | Nasoduodenal | Minimum 60 g stool in 500 ml | 2 [3 weeks apart] | Fresh | No | Yes | Clinical remission and endoscopic improvement SCCAI ≤ 2 in combination with ≥ 1 point drop in combined Mayo endoscopic score [rectum & sigmoid], 7/23 [30%] vs 5/25 [20%], p = 0.51 | 7/23 [30%] vs 8/25 [32%], p = NS [SCCAI ≤ 2] | 11/23 [48%] vs 13/25 [52%], p = NS [SCCAI drop ≥ 1.5] | NR | 8/23 [35%] vs 9/25 [36%], p = NS (≥ 1 point drop in combined Mayo endoscopic score [rectum & sigmoid]) | NR | 12 weeks |
| DBRCT | Paramsothy et al., 201711 | 81: 41 FMT, 40 controls | Mild- moderate [Mayo 4–10] | Unrelated multi- donor [3–7 donors/ infusion] | Colonoscopy followed by enemas | 37.5 g stool in 150 ml saline infusion | 40 [5/week for 8 weeks] | Frozen | No | Yes | Steroid-free clinical remission and endoscopic improvement, Mayo ≤ 2, all subscores ≤ 1, ≥ 1 point drop in endoscopy subscore, off steroids 11/41 [27%] vs 3/40 [8%], p = 0.02 | 18/41 [44%] vs 8/40 [20%], p = 0.02 [steroid- free Mayo subscore ≤ 1 for bleeding & stool frequency combined] | 22/41 [54%] vs 9/40 [23%], p = 0.01 [steroid- free drop in combined Mayo subscore for bleeding & stool frequency of ≥ 3 or 50%] | 5/41 [12%] vs 3/40 [8%], p = NS [steroid- free Mayo endoscopy subscore 0] | 13/41 [32%] vs 4/40 [10%], p = 0.02 [steroid- free Mayo endoscopy subscore ≤ 1 with drop ≥ 1] | NR | 8 weeks post FMT [total 16 weeks] |
| DBRCT | Costello et al., 201712 | 73: 38 FMT, 35 control autologous stools | Mild- moderate [Mayo 3–10] | Unrelated multi- donor [3–4 donors/ infusion] | Colonoscopy followed by enemas | NR | 3 [3/week] | Frozen | No | Yes | Steroid-free remission, Mayo ≤ 2, endoscopic subscore ≤ 1, off steroids 12/38 [32%] vs 3/35 [9%], p = 0.02 | 19/38 [50%] vs 6/35 [17%], p < 0.01 [steroid-free SCCAI ≤ 2] | 21/38 [55%] vs 7/35 [20%], p < 0.01 [steroid- free Mayo drop ≥ 3] | 21/38 [55%] vs 6/35 [17%], p < 0.01 [steroid- free Mayo endoscopy subscore ≤ 1] | NR | NR | 8 weeks |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre- antibiotic . | Bowel lavage . | Primary endpoint . | Clinical remission . | Clinical response . | Endoscopic remission . | Endoscopic response . | Histologic remission . | Follow- up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBRCT | Moayeddi et al., 20159 | 75: 38 FMT, 37 controls | Mild- severe, [Mayo 4–12] | Unrelated | Enema | 50g stool in 50 ml infusion | 6 [weekly] | Frozen 21, fresh 15, combination fresh & frozen 1 | No | No | Clinical and endoscopic remission Mayo < 3 with endoscopic Mayo 0 9/38 [24%] vs 2/37 [5%], p = 0.03 | 9/38 [24%] vs 2/37 [5%], p = 0.03 [Mayo < 3] | 15/38 [39%] vs 9/37 [24%], p = 0.16 [Mayo drop ≥ 3] | 9/38 [24%] vs 2/37 [5%], p = 0.03 [Mayo endoscopy subscore 0] | NR | 7 FMT, 1 placebo | 7 weeks |
| DBRCT | Rossen et al., 201510 | 48: 23 FMT, 25 control autologous stool | Mild- moderate [SCCAI 4–11] | Unrelated & related | Nasoduodenal | Minimum 60 g stool in 500 ml | 2 [3 weeks apart] | Fresh | No | Yes | Clinical remission and endoscopic improvement SCCAI ≤ 2 in combination with ≥ 1 point drop in combined Mayo endoscopic score [rectum & sigmoid], 7/23 [30%] vs 5/25 [20%], p = 0.51 | 7/23 [30%] vs 8/25 [32%], p = NS [SCCAI ≤ 2] | 11/23 [48%] vs 13/25 [52%], p = NS [SCCAI drop ≥ 1.5] | NR | 8/23 [35%] vs 9/25 [36%], p = NS (≥ 1 point drop in combined Mayo endoscopic score [rectum & sigmoid]) | NR | 12 weeks |
| DBRCT | Paramsothy et al., 201711 | 81: 41 FMT, 40 controls | Mild- moderate [Mayo 4–10] | Unrelated multi- donor [3–7 donors/ infusion] | Colonoscopy followed by enemas | 37.5 g stool in 150 ml saline infusion | 40 [5/week for 8 weeks] | Frozen | No | Yes | Steroid-free clinical remission and endoscopic improvement, Mayo ≤ 2, all subscores ≤ 1, ≥ 1 point drop in endoscopy subscore, off steroids 11/41 [27%] vs 3/40 [8%], p = 0.02 | 18/41 [44%] vs 8/40 [20%], p = 0.02 [steroid- free Mayo subscore ≤ 1 for bleeding & stool frequency combined] | 22/41 [54%] vs 9/40 [23%], p = 0.01 [steroid- free drop in combined Mayo subscore for bleeding & stool frequency of ≥ 3 or 50%] | 5/41 [12%] vs 3/40 [8%], p = NS [steroid- free Mayo endoscopy subscore 0] | 13/41 [32%] vs 4/40 [10%], p = 0.02 [steroid- free Mayo endoscopy subscore ≤ 1 with drop ≥ 1] | NR | 8 weeks post FMT [total 16 weeks] |
| DBRCT | Costello et al., 201712 | 73: 38 FMT, 35 control autologous stools | Mild- moderate [Mayo 3–10] | Unrelated multi- donor [3–4 donors/ infusion] | Colonoscopy followed by enemas | NR | 3 [3/week] | Frozen | No | Yes | Steroid-free remission, Mayo ≤ 2, endoscopic subscore ≤ 1, off steroids 12/38 [32%] vs 3/35 [9%], p = 0.02 | 19/38 [50%] vs 6/35 [17%], p < 0.01 [steroid-free SCCAI ≤ 2] | 21/38 [55%] vs 7/35 [20%], p < 0.01 [steroid- free Mayo drop ≥ 3] | 21/38 [55%] vs 6/35 [17%], p < 0.01 [steroid- free Mayo endoscopy subscore ≤ 1] | NR | NR | 8 weeks |
DBRCT, double-blind ramdomised placebo-controlled trial; NR, not recorded; SCCAI, Simple Clinical Colitis Activity Index; NS, not significant.
Randomised controlled trials of faecal microbiota transplant [FMT] in ulcerative colitis.
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre- antibiotic . | Bowel lavage . | Primary endpoint . | Clinical remission . | Clinical response . | Endoscopic remission . | Endoscopic response . | Histologic remission . | Follow- up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBRCT | Moayeddi et al., 20159 | 75: 38 FMT, 37 controls | Mild- severe, [Mayo 4–12] | Unrelated | Enema | 50g stool in 50 ml infusion | 6 [weekly] | Frozen 21, fresh 15, combination fresh & frozen 1 | No | No | Clinical and endoscopic remission Mayo < 3 with endoscopic Mayo 0 9/38 [24%] vs 2/37 [5%], p = 0.03 | 9/38 [24%] vs 2/37 [5%], p = 0.03 [Mayo < 3] | 15/38 [39%] vs 9/37 [24%], p = 0.16 [Mayo drop ≥ 3] | 9/38 [24%] vs 2/37 [5%], p = 0.03 [Mayo endoscopy subscore 0] | NR | 7 FMT, 1 placebo | 7 weeks |
| DBRCT | Rossen et al., 201510 | 48: 23 FMT, 25 control autologous stool | Mild- moderate [SCCAI 4–11] | Unrelated & related | Nasoduodenal | Minimum 60 g stool in 500 ml | 2 [3 weeks apart] | Fresh | No | Yes | Clinical remission and endoscopic improvement SCCAI ≤ 2 in combination with ≥ 1 point drop in combined Mayo endoscopic score [rectum & sigmoid], 7/23 [30%] vs 5/25 [20%], p = 0.51 | 7/23 [30%] vs 8/25 [32%], p = NS [SCCAI ≤ 2] | 11/23 [48%] vs 13/25 [52%], p = NS [SCCAI drop ≥ 1.5] | NR | 8/23 [35%] vs 9/25 [36%], p = NS (≥ 1 point drop in combined Mayo endoscopic score [rectum & sigmoid]) | NR | 12 weeks |
| DBRCT | Paramsothy et al., 201711 | 81: 41 FMT, 40 controls | Mild- moderate [Mayo 4–10] | Unrelated multi- donor [3–7 donors/ infusion] | Colonoscopy followed by enemas | 37.5 g stool in 150 ml saline infusion | 40 [5/week for 8 weeks] | Frozen | No | Yes | Steroid-free clinical remission and endoscopic improvement, Mayo ≤ 2, all subscores ≤ 1, ≥ 1 point drop in endoscopy subscore, off steroids 11/41 [27%] vs 3/40 [8%], p = 0.02 | 18/41 [44%] vs 8/40 [20%], p = 0.02 [steroid- free Mayo subscore ≤ 1 for bleeding & stool frequency combined] | 22/41 [54%] vs 9/40 [23%], p = 0.01 [steroid- free drop in combined Mayo subscore for bleeding & stool frequency of ≥ 3 or 50%] | 5/41 [12%] vs 3/40 [8%], p = NS [steroid- free Mayo endoscopy subscore 0] | 13/41 [32%] vs 4/40 [10%], p = 0.02 [steroid- free Mayo endoscopy subscore ≤ 1 with drop ≥ 1] | NR | 8 weeks post FMT [total 16 weeks] |
| DBRCT | Costello et al., 201712 | 73: 38 FMT, 35 control autologous stools | Mild- moderate [Mayo 3–10] | Unrelated multi- donor [3–4 donors/ infusion] | Colonoscopy followed by enemas | NR | 3 [3/week] | Frozen | No | Yes | Steroid-free remission, Mayo ≤ 2, endoscopic subscore ≤ 1, off steroids 12/38 [32%] vs 3/35 [9%], p = 0.02 | 19/38 [50%] vs 6/35 [17%], p < 0.01 [steroid-free SCCAI ≤ 2] | 21/38 [55%] vs 7/35 [20%], p < 0.01 [steroid- free Mayo drop ≥ 3] | 21/38 [55%] vs 6/35 [17%], p < 0.01 [steroid- free Mayo endoscopy subscore ≤ 1] | NR | NR | 8 weeks |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre- antibiotic . | Bowel lavage . | Primary endpoint . | Clinical remission . | Clinical response . | Endoscopic remission . | Endoscopic response . | Histologic remission . | Follow- up . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBRCT | Moayeddi et al., 20159 | 75: 38 FMT, 37 controls | Mild- severe, [Mayo 4–12] | Unrelated | Enema | 50g stool in 50 ml infusion | 6 [weekly] | Frozen 21, fresh 15, combination fresh & frozen 1 | No | No | Clinical and endoscopic remission Mayo < 3 with endoscopic Mayo 0 9/38 [24%] vs 2/37 [5%], p = 0.03 | 9/38 [24%] vs 2/37 [5%], p = 0.03 [Mayo < 3] | 15/38 [39%] vs 9/37 [24%], p = 0.16 [Mayo drop ≥ 3] | 9/38 [24%] vs 2/37 [5%], p = 0.03 [Mayo endoscopy subscore 0] | NR | 7 FMT, 1 placebo | 7 weeks |
| DBRCT | Rossen et al., 201510 | 48: 23 FMT, 25 control autologous stool | Mild- moderate [SCCAI 4–11] | Unrelated & related | Nasoduodenal | Minimum 60 g stool in 500 ml | 2 [3 weeks apart] | Fresh | No | Yes | Clinical remission and endoscopic improvement SCCAI ≤ 2 in combination with ≥ 1 point drop in combined Mayo endoscopic score [rectum & sigmoid], 7/23 [30%] vs 5/25 [20%], p = 0.51 | 7/23 [30%] vs 8/25 [32%], p = NS [SCCAI ≤ 2] | 11/23 [48%] vs 13/25 [52%], p = NS [SCCAI drop ≥ 1.5] | NR | 8/23 [35%] vs 9/25 [36%], p = NS (≥ 1 point drop in combined Mayo endoscopic score [rectum & sigmoid]) | NR | 12 weeks |
| DBRCT | Paramsothy et al., 201711 | 81: 41 FMT, 40 controls | Mild- moderate [Mayo 4–10] | Unrelated multi- donor [3–7 donors/ infusion] | Colonoscopy followed by enemas | 37.5 g stool in 150 ml saline infusion | 40 [5/week for 8 weeks] | Frozen | No | Yes | Steroid-free clinical remission and endoscopic improvement, Mayo ≤ 2, all subscores ≤ 1, ≥ 1 point drop in endoscopy subscore, off steroids 11/41 [27%] vs 3/40 [8%], p = 0.02 | 18/41 [44%] vs 8/40 [20%], p = 0.02 [steroid- free Mayo subscore ≤ 1 for bleeding & stool frequency combined] | 22/41 [54%] vs 9/40 [23%], p = 0.01 [steroid- free drop in combined Mayo subscore for bleeding & stool frequency of ≥ 3 or 50%] | 5/41 [12%] vs 3/40 [8%], p = NS [steroid- free Mayo endoscopy subscore 0] | 13/41 [32%] vs 4/40 [10%], p = 0.02 [steroid- free Mayo endoscopy subscore ≤ 1 with drop ≥ 1] | NR | 8 weeks post FMT [total 16 weeks] |
| DBRCT | Costello et al., 201712 | 73: 38 FMT, 35 control autologous stools | Mild- moderate [Mayo 3–10] | Unrelated multi- donor [3–4 donors/ infusion] | Colonoscopy followed by enemas | NR | 3 [3/week] | Frozen | No | Yes | Steroid-free remission, Mayo ≤ 2, endoscopic subscore ≤ 1, off steroids 12/38 [32%] vs 3/35 [9%], p = 0.02 | 19/38 [50%] vs 6/35 [17%], p < 0.01 [steroid-free SCCAI ≤ 2] | 21/38 [55%] vs 7/35 [20%], p < 0.01 [steroid- free Mayo drop ≥ 3] | 21/38 [55%] vs 6/35 [17%], p < 0.01 [steroid- free Mayo endoscopy subscore ≤ 1] | NR | NR | 8 weeks |
DBRCT, double-blind ramdomised placebo-controlled trial; NR, not recorded; SCCAI, Simple Clinical Colitis Activity Index; NS, not significant.
Case Reports and Cohort Studies of FMT in Crohn’s Disease
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow-up . | NOS total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Borody et al., 198919 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | - | NR | NR | 4 months | - |
| Case report | Swaminath et al., 201455 | 1 | Patchy colitis, severe from 11 to 22 cm | Partner | Enema | NR | 5 [daily for 5 days] | Fresh | NR | NR | 0 [worsening of colitis symptoms with FMT] | 0 [worsening of colitis symptoms with FMT] | NR | NR | 3 weeks, near resolution of bleeding and diarrhoea with topical 5-ASA | - |
| Case report | Gordon et al., 201456 | 1 | Severe, HBI 30 | partner | NR | NR | Daily, number not specified | Fresh | Vancomycin for previous Clostridium difficile infection | NR | 0 | 1 [HBI drop 30 to 7] | NR | NR | Relapse at 6 months, commenced azathioprine | - |
| Case report | Kao et al., 201457 | 1 | Moderate-severe, HBI 12 | Unrelated | Colonoscopy | 400 ml of 1:4 stool: saline | Single | Fresh | 7-day course of ciprofloxacin & metronidazole till 2 days before FMT | Yes | 1 [HBI 0] | - | 1 complete mucosal healing | 1 | 4 weeks | - |
| Cohort | Kahn et al., 201458 | 8 | Active, HBI > 6 | Unrelated | Colonoscopy | NR | Single | NR | NR | Yes | NR [safety study] | NR | NR | NR | 1 week | 4 |
| Cohort | Cui et al., 201559 | 30 | Moderate- severe, HBI > 6 | Unrelated & related | Midgut through gastroscope | 150-200 ml infusion | Single | Fresh or frozen | No | yes | 23/30 [76.7%] [HBI < 5] | 26/30 [86.7%] [HBI drop > 3] | NR | NR | 6–15 months | 4 |
| Cohort | Suskind et al., 201560 | 9 [paediatric] | Mild- moderate [PCDAI 10–29] | Related [parent] | Nasogastric | 30 g stool in 100-200 ml saline | Single | Fresh | Rifaximin 200 mg tds for 3 days, omeprazole day before and day of FMT | Yes | Week 2: 7/9 [78%], Weeks 6 & 12: 5/9 [56%] [PCDAI < 10] | NR | NR | NR | 12 weeks | 6 |
| Cohort | Vermeire et al., 201642 | 6 | Moderate- severe | Unrelated & related | Nasogastric | 200 g stool in 400 ml saline | 2 [daily for 2 days] | Fresh | No | Yes | 0 | NR | 0 | NR | 8 weeks | 5 |
| Cohort | Wei et al., 201543 | 3 | Active, CDAI 150–400 | Unrelated | Nasogastric [2] colonoscopy [1] | 60 g stool in 350 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days beforeFMT | Yes | 0 | Mean CDAI drop from 345 to 135 | NR | NR | 4 weeks | 5 |
| Cohort | Vaughn et al., 201661 | 19 | Active, HBI ≥ 5 | Unrelated | Colonoscopy | 50 g stool in 250 ml solution | Single | Frozen | No | Yes | 10/19 [53%] [HBI < 5 at Week 4] | 11/19 [58%] [HBI drop ≥ 3 at Week 4] | NR | NR | 26 weeks | 6 |
| Cohort | Goyal et al., 201646 | 4 [paediatric] | Mild- moderate, PCDAI < 40 | Recipient- identified [related & unrelated] | Both duodenoscopy and jejunoscopy [20–30 ml] and colonoscopy [200-250 ml] | 150 g stool in 250 ml saline | Single | Fresh | Metronidazole/vancomycin for 5 days, ceasing 48 h before FMT | yes | 2/4 [50%] PCDAI < 10 or | normalisation of lactoferrin/ calprotectin | 3/4 [75%] PCDAI drop ≥ 12.5 | NR | NR | 6 months | 4 |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow-up . | NOS total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Borody et al., 198919 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | - | NR | NR | 4 months | - |
| Case report | Swaminath et al., 201455 | 1 | Patchy colitis, severe from 11 to 22 cm | Partner | Enema | NR | 5 [daily for 5 days] | Fresh | NR | NR | 0 [worsening of colitis symptoms with FMT] | 0 [worsening of colitis symptoms with FMT] | NR | NR | 3 weeks, near resolution of bleeding and diarrhoea with topical 5-ASA | - |
| Case report | Gordon et al., 201456 | 1 | Severe, HBI 30 | partner | NR | NR | Daily, number not specified | Fresh | Vancomycin for previous Clostridium difficile infection | NR | 0 | 1 [HBI drop 30 to 7] | NR | NR | Relapse at 6 months, commenced azathioprine | - |
| Case report | Kao et al., 201457 | 1 | Moderate-severe, HBI 12 | Unrelated | Colonoscopy | 400 ml of 1:4 stool: saline | Single | Fresh | 7-day course of ciprofloxacin & metronidazole till 2 days before FMT | Yes | 1 [HBI 0] | - | 1 complete mucosal healing | 1 | 4 weeks | - |
| Cohort | Kahn et al., 201458 | 8 | Active, HBI > 6 | Unrelated | Colonoscopy | NR | Single | NR | NR | Yes | NR [safety study] | NR | NR | NR | 1 week | 4 |
| Cohort | Cui et al., 201559 | 30 | Moderate- severe, HBI > 6 | Unrelated & related | Midgut through gastroscope | 150-200 ml infusion | Single | Fresh or frozen | No | yes | 23/30 [76.7%] [HBI < 5] | 26/30 [86.7%] [HBI drop > 3] | NR | NR | 6–15 months | 4 |
| Cohort | Suskind et al., 201560 | 9 [paediatric] | Mild- moderate [PCDAI 10–29] | Related [parent] | Nasogastric | 30 g stool in 100-200 ml saline | Single | Fresh | Rifaximin 200 mg tds for 3 days, omeprazole day before and day of FMT | Yes | Week 2: 7/9 [78%], Weeks 6 & 12: 5/9 [56%] [PCDAI < 10] | NR | NR | NR | 12 weeks | 6 |
| Cohort | Vermeire et al., 201642 | 6 | Moderate- severe | Unrelated & related | Nasogastric | 200 g stool in 400 ml saline | 2 [daily for 2 days] | Fresh | No | Yes | 0 | NR | 0 | NR | 8 weeks | 5 |
| Cohort | Wei et al., 201543 | 3 | Active, CDAI 150–400 | Unrelated | Nasogastric [2] colonoscopy [1] | 60 g stool in 350 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days beforeFMT | Yes | 0 | Mean CDAI drop from 345 to 135 | NR | NR | 4 weeks | 5 |
| Cohort | Vaughn et al., 201661 | 19 | Active, HBI ≥ 5 | Unrelated | Colonoscopy | 50 g stool in 250 ml solution | Single | Frozen | No | Yes | 10/19 [53%] [HBI < 5 at Week 4] | 11/19 [58%] [HBI drop ≥ 3 at Week 4] | NR | NR | 26 weeks | 6 |
| Cohort | Goyal et al., 201646 | 4 [paediatric] | Mild- moderate, PCDAI < 40 | Recipient- identified [related & unrelated] | Both duodenoscopy and jejunoscopy [20–30 ml] and colonoscopy [200-250 ml] | 150 g stool in 250 ml saline | Single | Fresh | Metronidazole/vancomycin for 5 days, ceasing 48 h before FMT | yes | 2/4 [50%] PCDAI < 10 or | normalisation of lactoferrin/ calprotectin | 3/4 [75%] PCDAI drop ≥ 12.5 | NR | NR | 6 months | 4 |
NOS, Newcastle-Ottawa Scale; 5-ASA, 5-aminosalicylic acid; NR, not recorded; HBI, Harvey-Bradshaw Index; PCDAI, Paediatric Crohn’s Disease Activity Index; tds, three times daily; bd, twice daily.
Case Reports and Cohort Studies of FMT in Crohn’s Disease
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow-up . | NOS total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Borody et al., 198919 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | - | NR | NR | 4 months | - |
| Case report | Swaminath et al., 201455 | 1 | Patchy colitis, severe from 11 to 22 cm | Partner | Enema | NR | 5 [daily for 5 days] | Fresh | NR | NR | 0 [worsening of colitis symptoms with FMT] | 0 [worsening of colitis symptoms with FMT] | NR | NR | 3 weeks, near resolution of bleeding and diarrhoea with topical 5-ASA | - |
| Case report | Gordon et al., 201456 | 1 | Severe, HBI 30 | partner | NR | NR | Daily, number not specified | Fresh | Vancomycin for previous Clostridium difficile infection | NR | 0 | 1 [HBI drop 30 to 7] | NR | NR | Relapse at 6 months, commenced azathioprine | - |
| Case report | Kao et al., 201457 | 1 | Moderate-severe, HBI 12 | Unrelated | Colonoscopy | 400 ml of 1:4 stool: saline | Single | Fresh | 7-day course of ciprofloxacin & metronidazole till 2 days before FMT | Yes | 1 [HBI 0] | - | 1 complete mucosal healing | 1 | 4 weeks | - |
| Cohort | Kahn et al., 201458 | 8 | Active, HBI > 6 | Unrelated | Colonoscopy | NR | Single | NR | NR | Yes | NR [safety study] | NR | NR | NR | 1 week | 4 |
| Cohort | Cui et al., 201559 | 30 | Moderate- severe, HBI > 6 | Unrelated & related | Midgut through gastroscope | 150-200 ml infusion | Single | Fresh or frozen | No | yes | 23/30 [76.7%] [HBI < 5] | 26/30 [86.7%] [HBI drop > 3] | NR | NR | 6–15 months | 4 |
| Cohort | Suskind et al., 201560 | 9 [paediatric] | Mild- moderate [PCDAI 10–29] | Related [parent] | Nasogastric | 30 g stool in 100-200 ml saline | Single | Fresh | Rifaximin 200 mg tds for 3 days, omeprazole day before and day of FMT | Yes | Week 2: 7/9 [78%], Weeks 6 & 12: 5/9 [56%] [PCDAI < 10] | NR | NR | NR | 12 weeks | 6 |
| Cohort | Vermeire et al., 201642 | 6 | Moderate- severe | Unrelated & related | Nasogastric | 200 g stool in 400 ml saline | 2 [daily for 2 days] | Fresh | No | Yes | 0 | NR | 0 | NR | 8 weeks | 5 |
| Cohort | Wei et al., 201543 | 3 | Active, CDAI 150–400 | Unrelated | Nasogastric [2] colonoscopy [1] | 60 g stool in 350 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days beforeFMT | Yes | 0 | Mean CDAI drop from 345 to 135 | NR | NR | 4 weeks | 5 |
| Cohort | Vaughn et al., 201661 | 19 | Active, HBI ≥ 5 | Unrelated | Colonoscopy | 50 g stool in 250 ml solution | Single | Frozen | No | Yes | 10/19 [53%] [HBI < 5 at Week 4] | 11/19 [58%] [HBI drop ≥ 3 at Week 4] | NR | NR | 26 weeks | 6 |
| Cohort | Goyal et al., 201646 | 4 [paediatric] | Mild- moderate, PCDAI < 40 | Recipient- identified [related & unrelated] | Both duodenoscopy and jejunoscopy [20–30 ml] and colonoscopy [200-250 ml] | 150 g stool in 250 ml saline | Single | Fresh | Metronidazole/vancomycin for 5 days, ceasing 48 h before FMT | yes | 2/4 [50%] PCDAI < 10 or | normalisation of lactoferrin/ calprotectin | 3/4 [75%] PCDAI drop ≥ 12.5 | NR | NR | 6 months | 4 |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic remission . | Histologic remission . | Follow-up . | NOS total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Borody et al., 198919 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 1 | - | NR | NR | 4 months | - |
| Case report | Swaminath et al., 201455 | 1 | Patchy colitis, severe from 11 to 22 cm | Partner | Enema | NR | 5 [daily for 5 days] | Fresh | NR | NR | 0 [worsening of colitis symptoms with FMT] | 0 [worsening of colitis symptoms with FMT] | NR | NR | 3 weeks, near resolution of bleeding and diarrhoea with topical 5-ASA | - |
| Case report | Gordon et al., 201456 | 1 | Severe, HBI 30 | partner | NR | NR | Daily, number not specified | Fresh | Vancomycin for previous Clostridium difficile infection | NR | 0 | 1 [HBI drop 30 to 7] | NR | NR | Relapse at 6 months, commenced azathioprine | - |
| Case report | Kao et al., 201457 | 1 | Moderate-severe, HBI 12 | Unrelated | Colonoscopy | 400 ml of 1:4 stool: saline | Single | Fresh | 7-day course of ciprofloxacin & metronidazole till 2 days before FMT | Yes | 1 [HBI 0] | - | 1 complete mucosal healing | 1 | 4 weeks | - |
| Cohort | Kahn et al., 201458 | 8 | Active, HBI > 6 | Unrelated | Colonoscopy | NR | Single | NR | NR | Yes | NR [safety study] | NR | NR | NR | 1 week | 4 |
| Cohort | Cui et al., 201559 | 30 | Moderate- severe, HBI > 6 | Unrelated & related | Midgut through gastroscope | 150-200 ml infusion | Single | Fresh or frozen | No | yes | 23/30 [76.7%] [HBI < 5] | 26/30 [86.7%] [HBI drop > 3] | NR | NR | 6–15 months | 4 |
| Cohort | Suskind et al., 201560 | 9 [paediatric] | Mild- moderate [PCDAI 10–29] | Related [parent] | Nasogastric | 30 g stool in 100-200 ml saline | Single | Fresh | Rifaximin 200 mg tds for 3 days, omeprazole day before and day of FMT | Yes | Week 2: 7/9 [78%], Weeks 6 & 12: 5/9 [56%] [PCDAI < 10] | NR | NR | NR | 12 weeks | 6 |
| Cohort | Vermeire et al., 201642 | 6 | Moderate- severe | Unrelated & related | Nasogastric | 200 g stool in 400 ml saline | 2 [daily for 2 days] | Fresh | No | Yes | 0 | NR | 0 | NR | 8 weeks | 5 |
| Cohort | Wei et al., 201543 | 3 | Active, CDAI 150–400 | Unrelated | Nasogastric [2] colonoscopy [1] | 60 g stool in 350 ml saline | Single | Fresh | Vancomycin 500 mg bd for 3 days beforeFMT | Yes | 0 | Mean CDAI drop from 345 to 135 | NR | NR | 4 weeks | 5 |
| Cohort | Vaughn et al., 201661 | 19 | Active, HBI ≥ 5 | Unrelated | Colonoscopy | 50 g stool in 250 ml solution | Single | Frozen | No | Yes | 10/19 [53%] [HBI < 5 at Week 4] | 11/19 [58%] [HBI drop ≥ 3 at Week 4] | NR | NR | 26 weeks | 6 |
| Cohort | Goyal et al., 201646 | 4 [paediatric] | Mild- moderate, PCDAI < 40 | Recipient- identified [related & unrelated] | Both duodenoscopy and jejunoscopy [20–30 ml] and colonoscopy [200-250 ml] | 150 g stool in 250 ml saline | Single | Fresh | Metronidazole/vancomycin for 5 days, ceasing 48 h before FMT | yes | 2/4 [50%] PCDAI < 10 or | normalisation of lactoferrin/ calprotectin | 3/4 [75%] PCDAI drop ≥ 12.5 | NR | NR | 6 months | 4 |
NOS, Newcastle-Ottawa Scale; 5-ASA, 5-aminosalicylic acid; NR, not recorded; HBI, Harvey-Bradshaw Index; PCDAI, Paediatric Crohn’s Disease Activity Index; tds, three times daily; bd, twice daily.
Case Reports and Cohort Studies of FMT in Pouchitis
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic outcomes . | Histologic outcomes . | Follow-up . | NOS total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Fang et al., 201662 | 1 [primary diagnosis: UC] | Chronic antibiotic- refractory pouchitis [mPDAI 10; clinical mPDAI 6] | NR | Pouchoscopy | Stool diluted in 250 ml saline | Single | Fresh | Antibiotics ceased 48 h before FMT | NR | 1 [clinical PDAI 0] | - | NR | NR | 6 months | - |
| Cohort | Landy et al., 201563 | 8 [primary diagnosis: UC] | Chronic pouchitis [PDAI > 7] | Unrelated & related | Nasogastric | 30 g stool in 50 ml saline | Single | Fresh | No, antibiotic- free for 2 weeks before FMT | NR | 0 | 2/8 [25%] [PDAI drop ≥ 3] | NR | NR | 4 weeks | 6 |
| Cohort | El-Nachef et al., 201664 | 9 [4 weeks’ data on 7] [primary diagnosis: NR] | NR | Unrelated | Pouchoscopy | NR | Single | Frozen | NR | NR | NR | 5/7 [71%] [global symptom improvement] | NR | NR | 4 weeks | 5 |
| Cohort | Stallmach et al., 201665 | 5 [primary diagnosis: NR] | Chronic antibiotic-refractory pouchitis [PDAI 9–14] | Unrelated | UGI [jejunum] via endoscopy | 75 g stool in 200 ml saline | 1–7 [at 3–4-week intervals] | Fresh for initial; either frozen or fresh for subsequent infusions | Not part of FMT protocol. All patients failed ≥ 3 cycles of metronidazole and ciprofloxacin +/- rifaximin | NR | 4/5 [80%] | 5/5 [100%] | Endoscopic remission: 1/5 [20%] endoscopic response: 5/5 [100%] [based on endoscopy subscore on PDAI] | 0 [histology subscore of 0 on PDAI] | 3 months | 6 |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic outcomes . | Histologic outcomes . | Follow-up . | NOS total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Fang et al., 201662 | 1 [primary diagnosis: UC] | Chronic antibiotic- refractory pouchitis [mPDAI 10; clinical mPDAI 6] | NR | Pouchoscopy | Stool diluted in 250 ml saline | Single | Fresh | Antibiotics ceased 48 h before FMT | NR | 1 [clinical PDAI 0] | - | NR | NR | 6 months | - |
| Cohort | Landy et al., 201563 | 8 [primary diagnosis: UC] | Chronic pouchitis [PDAI > 7] | Unrelated & related | Nasogastric | 30 g stool in 50 ml saline | Single | Fresh | No, antibiotic- free for 2 weeks before FMT | NR | 0 | 2/8 [25%] [PDAI drop ≥ 3] | NR | NR | 4 weeks | 6 |
| Cohort | El-Nachef et al., 201664 | 9 [4 weeks’ data on 7] [primary diagnosis: NR] | NR | Unrelated | Pouchoscopy | NR | Single | Frozen | NR | NR | NR | 5/7 [71%] [global symptom improvement] | NR | NR | 4 weeks | 5 |
| Cohort | Stallmach et al., 201665 | 5 [primary diagnosis: NR] | Chronic antibiotic-refractory pouchitis [PDAI 9–14] | Unrelated | UGI [jejunum] via endoscopy | 75 g stool in 200 ml saline | 1–7 [at 3–4-week intervals] | Fresh for initial; either frozen or fresh for subsequent infusions | Not part of FMT protocol. All patients failed ≥ 3 cycles of metronidazole and ciprofloxacin +/- rifaximin | NR | 4/5 [80%] | 5/5 [100%] | Endoscopic remission: 1/5 [20%] endoscopic response: 5/5 [100%] [based on endoscopy subscore on PDAI] | 0 [histology subscore of 0 on PDAI] | 3 months | 6 |
NOS, Newcastle-Ottawa Scale; PDAI, Perianal Disease Activity Index; UC, ulcerative colitis; NR, not recorded; UGI, upper gastrointestinal.
Case Reports and Cohort Studies of FMT in Pouchitis
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic outcomes . | Histologic outcomes . | Follow-up . | NOS total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Fang et al., 201662 | 1 [primary diagnosis: UC] | Chronic antibiotic- refractory pouchitis [mPDAI 10; clinical mPDAI 6] | NR | Pouchoscopy | Stool diluted in 250 ml saline | Single | Fresh | Antibiotics ceased 48 h before FMT | NR | 1 [clinical PDAI 0] | - | NR | NR | 6 months | - |
| Cohort | Landy et al., 201563 | 8 [primary diagnosis: UC] | Chronic pouchitis [PDAI > 7] | Unrelated & related | Nasogastric | 30 g stool in 50 ml saline | Single | Fresh | No, antibiotic- free for 2 weeks before FMT | NR | 0 | 2/8 [25%] [PDAI drop ≥ 3] | NR | NR | 4 weeks | 6 |
| Cohort | El-Nachef et al., 201664 | 9 [4 weeks’ data on 7] [primary diagnosis: NR] | NR | Unrelated | Pouchoscopy | NR | Single | Frozen | NR | NR | NR | 5/7 [71%] [global symptom improvement] | NR | NR | 4 weeks | 5 |
| Cohort | Stallmach et al., 201665 | 5 [primary diagnosis: NR] | Chronic antibiotic-refractory pouchitis [PDAI 9–14] | Unrelated | UGI [jejunum] via endoscopy | 75 g stool in 200 ml saline | 1–7 [at 3–4-week intervals] | Fresh for initial; either frozen or fresh for subsequent infusions | Not part of FMT protocol. All patients failed ≥ 3 cycles of metronidazole and ciprofloxacin +/- rifaximin | NR | 4/5 [80%] | 5/5 [100%] | Endoscopic remission: 1/5 [20%] endoscopic response: 5/5 [100%] [based on endoscopy subscore on PDAI] | 0 [histology subscore of 0 on PDAI] | 3 months | 6 |
| Study type . | Author . | Patients . | Severity . | Donor . | Route . | Dosage [volume] . | Frequency [number of infusions] . | Fresh vs frozen . | Pre-antibiotic . | Bowel lavage . | Clinical remission . | Clinical response . | Endoscopic outcomes . | Histologic outcomes . | Follow-up . | NOS total . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case report | Fang et al., 201662 | 1 [primary diagnosis: UC] | Chronic antibiotic- refractory pouchitis [mPDAI 10; clinical mPDAI 6] | NR | Pouchoscopy | Stool diluted in 250 ml saline | Single | Fresh | Antibiotics ceased 48 h before FMT | NR | 1 [clinical PDAI 0] | - | NR | NR | 6 months | - |
| Cohort | Landy et al., 201563 | 8 [primary diagnosis: UC] | Chronic pouchitis [PDAI > 7] | Unrelated & related | Nasogastric | 30 g stool in 50 ml saline | Single | Fresh | No, antibiotic- free for 2 weeks before FMT | NR | 0 | 2/8 [25%] [PDAI drop ≥ 3] | NR | NR | 4 weeks | 6 |
| Cohort | El-Nachef et al., 201664 | 9 [4 weeks’ data on 7] [primary diagnosis: NR] | NR | Unrelated | Pouchoscopy | NR | Single | Frozen | NR | NR | NR | 5/7 [71%] [global symptom improvement] | NR | NR | 4 weeks | 5 |
| Cohort | Stallmach et al., 201665 | 5 [primary diagnosis: NR] | Chronic antibiotic-refractory pouchitis [PDAI 9–14] | Unrelated | UGI [jejunum] via endoscopy | 75 g stool in 200 ml saline | 1–7 [at 3–4-week intervals] | Fresh for initial; either frozen or fresh for subsequent infusions | Not part of FMT protocol. All patients failed ≥ 3 cycles of metronidazole and ciprofloxacin +/- rifaximin | NR | 4/5 [80%] | 5/5 [100%] | Endoscopic remission: 1/5 [20%] endoscopic response: 5/5 [100%] [based on endoscopy subscore on PDAI] | 0 [histology subscore of 0 on PDAI] | 3 months | 6 |
NOS, Newcastle-Ottawa Scale; PDAI, Perianal Disease Activity Index; UC, ulcerative colitis; NR, not recorded; UGI, upper gastrointestinal.
2.3. Data extraction
References were imported into a bibliographic database [Microsoft Excel 2015]. Two authors [SP, RP] independently reviewed all articles, initially by title and abstract, then by full text review where relevant, to determine eligibility. Duplicate studies/data were removed manually; when multiple publications related to the same patient group, the most complete data set was included. Eligible studies were categorised based on FMT indication. Data related to the study design and characteristics, treatment groups, and outcome measures were recorded. Where there was disagreement on study eligibility or data extraction, consensus was achieved through discussion [SP, RP, NCR].
2.4. Study quality assessment
For eligible cohort studies, the methodological quality was assessed using the Newcastle-Ottawa Scale [NOS]16 on the standard 9-point scale. Included RCTs were assessed using the Cochrane risk of bias score17 incorporating random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting.
2.5. Statistical analysis
Descriptive statistics were performed on data extracted from all included studies. The efficacy of treatment [clinical remission and/or response] was compared across studies per IBD subtype. For disease subtypes where three or more cohort or RCT studies were included, a meta-analysis was performed. The pooled effect sizes, as well as 95% confidence intervals [CIs], were calculated using both fixed and random effects models. However, the random effects model was the preferred option as it assumes that there is a distribution of true effect sizes rather than one true effect, and it assigns a more balanced weight to each study. For meta-analyses including cohort studies, the effect size refers to the pooled estimate proportion of patients that achieved efficacy. For meta-analyses including RCTs, pooled odd ratios [P-ORs] were calculated by weighting individual ORs by the inverse of their variance. p-values < 0.05 were considered significant. Heterogeneity was assessed using Cochran′s Q test [p-value < 0.10 is indicative of heterogeneity] and Higgins′ test [I2] [low heterogeneity: < 25%, moderate heterogeneity: 25–75%, and high heterogeneity: > 75%].18 Moderator variables including disease severity [mild vs moderate vs severe], route of administration [upper vs lower gastrointestinal FMT infusion], number of infusions (low [1 infusion] vs medium [2–4 infusions] vs high [5–10] vs very high [> 10]), population [paediatric vs adult], preparation of inoculum [fresh vs frozen], FMT donor source [related vs unrelated donor], antibiotic pre-treatment, and bowel lavage, were used to perform subgroup analyses. Sensitivity [leave-one-out] analyses were also conducted to assess statistical robustness. Publication bias was assessed using the Egger’s regression asymmetry test as well as funnel plots. Statistical analyses were performed using the Comprehensive Meta-Analysis software V. 3.0 [Biostat, Englewood, NJ, 2004].
3. Results
3.1. Study characteristics
A total of 6806 articles were identified in the search, which included 261 internal and external duplicates [Figure 1]. Titles and abstracts of 6545 articles were screened and only 109 were deemed potentially eligible, of which 107 were available for review. A total of 53 articles or abstracts of FMT in IBD were deemed to satisfy the study selection criteria and were included in the final analysis, of which three included more than one IBD subtype. This included 41 articles or abstracts assessing FMT in UC and reporting on 555 UC patients, 11 in CD reporting on 83 CD patients, and four in pouchitis reporting on 23 patients.
3.2. Study quality
The methodological quality of the included cohort studies and RCTs are outlined in the Appendix [Tables A2, A3, available as Supplementary data at ECCO-JCC online]. Only four cohort studies included a control group, with a mean NOS score of 5 [range 3 to 9] out of 9. The risk of bias in the included randomized trials was low [Costello et al., 2017,12 presented in abstract form but yet to undergo full publication peer review]. All significant results obtained through the meta-analyses remained significant in sensitivity analyses, inferring statistical robustness.
3.3. Ulcerative colitis
A total of 41 studies were identified assessing FMT in UC (nine case reports, five case series, 24 prospective cohort studies [20 uncontrolled, four controlled] and four RCTs), reporting on 555 UC patients [Tables 1-3].
Overall, 36% [201/555] of UC patients achieved clinical remission during follow-up. Among the 24 cohort studies included in the meta-analysis [Figure 2], which comprised 307 individuals, the pooled proportion of patients that achieved clinical remission was 33% [95% CI = 23–43%] for UC, with a moderate risk of heterogeneity [Cochran’s Q, p = 0.001; I2 = 54%] [Table A4, available as Supplementary data at ECCO-JCC online] and no publication bias [Table A5, available as Supplementary data at ECCO-JCC online]. The pooled proportion of patients that achieved clinical response was 52% [95% CI = 40–64%] in a meta-analysis that included 234 individuals from 20 cohort studies [Figure A1, available as Supplementary data at ECCO-JCC online]; a moderate level of heterogeneity [Cochran’s Q, p = 0.001; I2 = 58%] and no publication bias was observed in this meta-analysis [Table A5].
Forest plot of the meta-analysis of clinical remission and faecal microbiota transplantation [FMT] in ulcerative colitis including available cohort studies to date. The pooled proportions with 95% confidence intervals [CIs] were calculated using the random effects model [diamond]. The filled squares represent the studies in relation to their weights. In this meta-analysis, four case-control studies [Kump et al. 2015, Scaldaferri et al. 2015, Pai et al. 2016, and Ishikawa et al. 2017] were included as cohorts [data from controls was removed] as the software did not allow the combination of one and two groups comparison analyses.
Meta-analysis including four RCTs of FMT in UC [Figure 3], which comprised a total of 140 FMT-treated individuals, showed that FMT was significantly associated with clinical remission in these patients [P-OR = 2.89, 95% CI = 1.36–6.13, p = 0.006]. Heterogeneity was moderate in this meta-analysis [Cochran’s Q, p = 0.188; I2 = 37%] with no publication bias [Table A5]. A significant association was also found between FMT and clinical response in UC patients [P-OR = 2.48, 95% CI = 1.18-5.21, p = 0.016] [Figure A2, available as Supplementary data at ECCO-JCC online], with a moderate level of heterogeneity [Cochran’s Q, p = 0.102; I2 = 52%] and no publication bias [Table A5].
Forest plot of the meta-analysis of clinical remission and faecal microbiota transplantation [FMT] in ulcerative colitis including four randomised controlled trials [RCTs] available to date. The pooled odds ratios [ORs] with 95% confidence intervals [CIs] were calculated using the random effects model [diamond]. The filled squares represent the studies in relation to their weights.
Interestingly, sensitivity analyses showed that on removal of the RCT by Rossen et al.10 [which in contrast to the other studies used only two infusions and administered them via an upper gastrointestinal infusion] the association between FMT and clinical remission in UC patients was highly significant [P-OR of 4.05, 95% CI = 2.08–7.89, p = < 0.001; Cochran’s Q, p = 0.783; I2 = 0%] [Figure A3, available as Supplementary data at ECCO-JCC online]. Similarly, the association between FMT and clinical response in these patients when the RCT by Rossen et al.10 was removed showed a higher P-OR of 3.39 [95% CI = 1.90–6.04, p = < 0.001; Cochran’s Q, p = 0.442; I2 = 0%] [Figure A4, available as Supplementary data at ECCO-JCC online].
3.4. Crohn’s disease
Eleven studies in CD [four case reports, seven prospective uncontrolled cohort studies] reporting on 83 CD patients were included [Table 4]. Overall 50.5% [42/83] of CD patients achieved clinical remission during follow-up. Among the six cohort studies included in the meta-analysis [Figure 4], comprising 71 individuals, the pooled proportion of CD patients that achieved clinical remission was 52% [95% CI = 31–72%] with a moderate risk of heterogeneity [Cochran’s Q, p = 0.063; I2 = 52%]; however, publications bias was observed in this meta-analysis [Table A5]. A meta-analysis including four cohort studies [Figure A5, available as Supplementary data at ECCO-JCC online], which comprised 59 individuals, showed that the pooled estimate proportion of patients that achieved clinical response was 63% [95% CI = 30–88%]. Moderate heterogeneity was observed in this meta-analysis [Cochran’s Q, p = 0.016, I2 = 71%]; no publication bias was detected [Table A5].
Forest plot of the meta-analysis of clinical remission and faecal microbiota transplantation [FMT] in Crohn’s disease including available cohort studies to date. The pooled proportions with 95% confidence intervals [CIs] were calculated using the random effects model [diamond]. The filled squares represent the studies in relation to their weights.
3.5. Pouchitis
Three prospective uncontrolled cohort studies and one case report assessing FMT in pouchitis were identified [Table 5], reporting on 23 patients. Two of the cohort studies used a single FMT infusion; no patients achieved clinical remission, with 2/8 [25%] achieving clinical response in one study63 and, in the other study, 5/7 [71%] had global symptom improvement [not defined] at 1 month.64 In the only study that allowed for multiple FMT infusions, 4/5 patients achieved clinical remission [with the other patient achieving clinical response].65 We did not perform a pouchitis meta-analysis as only three small cohort studies were identified which had differing endpoints and conflicting outcomes.
3.6. Endoscopic data
Specific endoscopic outcomes were reported in the four RCTs and six of the 24 cohort studies of FMT in UC [Table 2]. Accounting for differing definitions of endoscopic outcomes, endoscopic remission or endoscopic response rates of 24–55% with allogeneic FMT vs 5–17% with control [placebo or autologous FMT] [mean difference 26.3% ± 9.9, p-value: 0.057] were noted in the RCTs involving multiple lower gastrointestinal FMT infusions,9,11,12 whereas no difference was noted in endoscopic response between allogenic or autologous FMT administered by two nasoduodenal infusions [35% vs 36%]10 [Table 3]. Only one study in CD reported endoscopic outcomes,42 with none of six patients achieving endoscopic remission. In the one pouchitis study that reported endoscopic outcomes, all five patients had an endoscopic response and one patient [20%] achieved endoscopic remission after 1–7 FMT infusions.65
3.7. Histologic data
Only a small number of studies in UC reported histologic outcomes. Post hoc analysis of one RCT identified that 7/38 patients in the FMT arm and 1/37 in the placebo arm achieved histologic remission.9 Only two of the 24 identified cohort studies of FMT in UC reported histologic data.36,38 Only one case report of FMT in CD provided histologic outcomes.57 In the one pouchitis study that reported histologic outcomes, none of five patients achieved a PDAI histologic subscore of 0.65
3.8. Paediatric vs adult populations
Subgroup analyses were performed for a number of variables thought to be of importance [Table A6, available as Supplementary data at ECCO-JCC online], including population age [paediatric vs adult]. There were six cohort studies assessing 34 patients in paediatric UC and only two cohort studies assessing 13 patients in paediatric CD. The pooled estimate proportion of patients that achieved clinical remission was 23% [95% CI = 7–51%; Cochran’s Q, p = 0.171; I2 = 35%] for paediatric UC and 34% [95% CI = 24–46%; Cochran’s Q, p = 0.001; I2 = 58%] for adult UC. For CD, the pooled estimate of clinical remission was 54% [95% CI = 28–78%; Cochran’s Q, p = 0.853; I2 = 0%] in paediatric CD patients and 46% [95% CI = 18–77%; Cochran’s Q, p = 0.017; I2 = 71%] in adult CD patients. No completed randomised controlled trials have been published assessing FMT in paediatric IBD.
3.9. FMT methodology
The included studies varied substantially in FMT infusion methodology/protocol, including route of administration, number and frequency of infusions, dosage of stool per infusion, preparation of inoculum [fresh or frozen], antibiotic pre-treatment, bowel lavage, and FMT donor source [related or unrelated].
Subgroup analyses of the cohort studies [Table A6] showed that route of administration might play a significant role in clinical remission among UC patients, as the pooled proportion of UC patients receiving upper gastrointestinal infusions was 17% [95% CI = 8–32%; Cochran’s Q, p = 0.604; I2 = 0%] whereas the pooled proportion of UC patients receiving lower gastrointestinal infusions was 36% [95% CI = 24–50%; Cochran’s Q, p = 0.004; I2 = 57%]. Further subgroup analyses by number of infusions showed that the pooled proportion of UC patients receiving a high number of infusions [> 10 infusions] that achieved clinical remission was 49% [95% CI = 21–77%; Cochran’s Q, p = 0.246; I2 = 29%], which was considerably higher than in those UC patients who received ≤ 10 infusions [pooled proportion = 27%, 95% CI = 17–40%; Cochran’s Q, p = 0.001; I2 = 58%]. Although the pooled proportion of UC patients receiving fresh infusions that achieved clinical remission [28%, 95% CI = 15–46%; Cochran’s Q, p = 0.001; I2 = 63%] was less than with frozen infusions [36%, 95% CI = 13–67%; Cochran’s Q, p = 0.045; I2 = 63%], this was likely confounded by association with an increased number of infusions. Further, the pooled proportion of UC patients who received an antibiotic course before FMT and achieved clinical remission was 33% [95% CI = 17–54%; Cochran’s Q, p = 0.026; I2 = 58%], whereas the proportion in UC patients who did not receive an antibiotic course pre-FMT was 28% [95% CI = 16–44%; Cochran’s Q, p = 0.002; I2 = 61%]. The relevance of the other subgroup analyses findings is uncertain, given the small number of studies and patients. Only a few studies used a multi-donor infusion,11,12,50 but all reported some degree of clinical and endoscopic benefit [clinical remission rates 15–50%, endoscopic remission or response rates 10–55%] despite varying number of infusions [Tables 2, 3].
3.10. Safety
The majority of studies did not report major adverse events or serious adverse events that were deemed clinically related to FMT therapy. Most reported adverse events were transient minor gastrointestinal complaints [bloating, diarrhoea, flatulence, abdominal pain/cramping, borborygmus] and/or fever.31-35,44,46–48,52,54,58–61,63 The lack of a control arm in most of the studies makes it difficult to determine to what degree symptoms are specifically attributable to FMT. Nasogastric FMT infusion was associated with aspiration pneumonia in one study,42 prompting a switch to lower gastrointestinal [GI] administration. A few reports of disease worsening40,49,55 were identified, including one of cytomegalovirus [CMV] colitis in a patient who self-administered unscreened FMT.21 One death due to toxic megacolon and sepsis was reported.53
The RCTs found no difference between FMT and control arms in terms of minor or serious adverse events or disease worsening [Table 6], though it must be noted that these studies were not powered to specifically assess for safety.
Adverse event data of faecal microbiota transplant [FMT] in ulcerative colitis randomised controlled trials [RCTs].
| Author . | Minor adverse events . | Serious adverse event . |
|---|---|---|
| Moayeddi et al., 20159 | Not specified | 3/38 [8%] vs 2 /37 [5%] [p = 1.0]: instead of, 2 FMT patients had change in diagnosis to Crohn’s colitis, 1 FMT patient had Clostridium difficile infection |
| Rossen et al., 201510 | 78.3% of donor arm vs 64% placebo [p = 0.28], most commonly transient borborygmus or increase in stool frequency; 2 patients in FMT arm vomited, 2 patients in FMT arm had transient fever | 2 FMT, 2 control: instead of, 1 admitted for suspicion of small bowel perforation [noted to have small bowel CD], 1 with severe cytomegalovirus [autologous arm], 1 with cervical cancer requiring surgery, 1 severe abdominal pain requiring admission with spontaneous recovery |
| Paramsothy et al., 201711 | 78% in FMT arm vs 83% in placebo [p = NS], mostly self-limiting GI complaints [abdominal pain, bloating, flatulence] | 3 worsening colitis requiring hospitalisation [2 FMT including 1 colectomy, 1 placebo] |
| Costello et al., 201712 | Not specified | 3 FMT group, 2 control: instead of, 3 worsening colitis [2 autologous arm], 1 Clostridium difficile colitis requiring colectomy, 1 pneumonia |
| Author . | Minor adverse events . | Serious adverse event . |
|---|---|---|
| Moayeddi et al., 20159 | Not specified | 3/38 [8%] vs 2 /37 [5%] [p = 1.0]: instead of, 2 FMT patients had change in diagnosis to Crohn’s colitis, 1 FMT patient had Clostridium difficile infection |
| Rossen et al., 201510 | 78.3% of donor arm vs 64% placebo [p = 0.28], most commonly transient borborygmus or increase in stool frequency; 2 patients in FMT arm vomited, 2 patients in FMT arm had transient fever | 2 FMT, 2 control: instead of, 1 admitted for suspicion of small bowel perforation [noted to have small bowel CD], 1 with severe cytomegalovirus [autologous arm], 1 with cervical cancer requiring surgery, 1 severe abdominal pain requiring admission with spontaneous recovery |
| Paramsothy et al., 201711 | 78% in FMT arm vs 83% in placebo [p = NS], mostly self-limiting GI complaints [abdominal pain, bloating, flatulence] | 3 worsening colitis requiring hospitalisation [2 FMT including 1 colectomy, 1 placebo] |
| Costello et al., 201712 | Not specified | 3 FMT group, 2 control: instead of, 3 worsening colitis [2 autologous arm], 1 Clostridium difficile colitis requiring colectomy, 1 pneumonia |
NS, not significant; GI, gastrointestinal.
Adverse event data of faecal microbiota transplant [FMT] in ulcerative colitis randomised controlled trials [RCTs].
| Author . | Minor adverse events . | Serious adverse event . |
|---|---|---|
| Moayeddi et al., 20159 | Not specified | 3/38 [8%] vs 2 /37 [5%] [p = 1.0]: instead of, 2 FMT patients had change in diagnosis to Crohn’s colitis, 1 FMT patient had Clostridium difficile infection |
| Rossen et al., 201510 | 78.3% of donor arm vs 64% placebo [p = 0.28], most commonly transient borborygmus or increase in stool frequency; 2 patients in FMT arm vomited, 2 patients in FMT arm had transient fever | 2 FMT, 2 control: instead of, 1 admitted for suspicion of small bowel perforation [noted to have small bowel CD], 1 with severe cytomegalovirus [autologous arm], 1 with cervical cancer requiring surgery, 1 severe abdominal pain requiring admission with spontaneous recovery |
| Paramsothy et al., 201711 | 78% in FMT arm vs 83% in placebo [p = NS], mostly self-limiting GI complaints [abdominal pain, bloating, flatulence] | 3 worsening colitis requiring hospitalisation [2 FMT including 1 colectomy, 1 placebo] |
| Costello et al., 201712 | Not specified | 3 FMT group, 2 control: instead of, 3 worsening colitis [2 autologous arm], 1 Clostridium difficile colitis requiring colectomy, 1 pneumonia |
| Author . | Minor adverse events . | Serious adverse event . |
|---|---|---|
| Moayeddi et al., 20159 | Not specified | 3/38 [8%] vs 2 /37 [5%] [p = 1.0]: instead of, 2 FMT patients had change in diagnosis to Crohn’s colitis, 1 FMT patient had Clostridium difficile infection |
| Rossen et al., 201510 | 78.3% of donor arm vs 64% placebo [p = 0.28], most commonly transient borborygmus or increase in stool frequency; 2 patients in FMT arm vomited, 2 patients in FMT arm had transient fever | 2 FMT, 2 control: instead of, 1 admitted for suspicion of small bowel perforation [noted to have small bowel CD], 1 with severe cytomegalovirus [autologous arm], 1 with cervical cancer requiring surgery, 1 severe abdominal pain requiring admission with spontaneous recovery |
| Paramsothy et al., 201711 | 78% in FMT arm vs 83% in placebo [p = NS], mostly self-limiting GI complaints [abdominal pain, bloating, flatulence] | 3 worsening colitis requiring hospitalisation [2 FMT including 1 colectomy, 1 placebo] |
| Costello et al., 201712 | Not specified | 3 FMT group, 2 control: instead of, 3 worsening colitis [2 autologous arm], 1 Clostridium difficile colitis requiring colectomy, 1 pneumonia |
NS, not significant; GI, gastrointestinal.
3.11. Microbiological analyses
Microbiota analysis was performed in 17/41 UC, 4/11 CD, and 3/4 pouchitis studies [Table A7, available as Supplementary data at ECCO-JCC online]. Most studies assessed luminal [faecal] samples with only a limited number analysing mucosal [biopsy] samples.33,63,66,67 A range of studies commented on recipient microbiota changes after FMT, with increased α-diversity or richness9,11,38,42,48,50,61,66 and a shift towards the donor profile, which in some cases was associated with colonisation by donor-derived taxa, though this was reported in patients both with clinical benefit10,26,32,35,57,60,65 and without improvement.33,36 Some studies did report that the increase in recipient microbial diversity after FMT was greater in responders relative to non-responders.11,42,61,66 In particular, the study by Paramsothy et al.11, 66 found that recipient microbial diversity at baseline predicted response to FMT, that microbial diversity increased with FMT, and that this persisted for 8 weeks following FMT. In this study, the multi-donor FMT batches used for the FMT infusions had substantially greater microbial diversity relative to the individual donors. A correlation between increased donor microbial diversity and therapeutic success of FMT in UC has been identified in some studies42,68 but not others.51 In the RCT by Moayeddi et al.,9 there was a trend towards a difference in recipient outcomes based on particular donor, with improved outcomes noted in patients receiving infusions derived from donor B [p = 0.06]. A variety of taxa were reported to be associated with both FMT in IBD in general, and more specifically with therapeutic outcomes in IBD patients, across the identified studies.
4. Discussion
This paper represents an up-to-date systematic review and meta-analysis of FMT in IBD, incorporating both full text and abstract studies. There are almost three times as many studies included in this paper compared with previous systematic reviews and/or meta-analyses on the topic,7,8,69,70 illustrating the rapid growth in global research interest and activity with regards to FMT for IBD, including the first randomised trials of FMT in IBD.9–12 However, the overall quality of the studies remains low, primarily consisting of either case reports/series or small cohort studies of limited duration. Additionally, there remains considerable heterogeneity among the studies in terms of design, with conflicting treatment protocols [route of administration, number and frequency of infusions, antibiotic pre-treatment, bowel lavage] along with differing and often highly variable and/or poorly defined efficacy endpoints.
FMT in UC appears very promising, especially with multiple infusions administered via the lower gastrointestinal tract. An earlier meta-analysis,8 assessing only UC cohort studies, identified 79 patients with a pooled proportion achieving clinical remission of 22% [95% CI = 10.4%–40.8%]. The current meta-analysis identified 24 UC cohort studies assessing 307 patients, with a pooled proportion of patients that achieved clinical remission of 33% [95% CI = 23–43%]. Furthermore, four RCTs reporting on 140 FMT-treated UC patients were analysed. Meta-analysis of all four studies produced a significant association [P-OR = 2.89, 95% CI = 1.36–6.13, p = 0.006] between FMT and UC clinical remission induction [Figure 3]. Further sensitivity analyses showed that removal of the smallest study10 [which used only two infusions and administered them via an upper gastrointestinal infusion, as opposed to the other studies] resulted in an even more highly significant association between FMT and clinical remission in UC patients [P-OR of 4.05, 95% CI = 2.08–7.89, p = < 0.001] [Figure A3]. This, along with subgroup analyses of the UC cohort studies, suggests that multiple infusions [and possibly lower gastrointestinal administration] increases the likelihood of remission in UC patients treated with FMT, though the precise number required varied substantially between studies, remains to be defined, and likely is donor-and recipient-dependent.
Regarding the role of FMT in CD, the pooled proportion of patients that achieved clinical remission presented in the current meta-analysis [52%] is slightly lower than the figure reported in the previous meta-analysis8 [pooled proportion = 60.5%, 95% CI = 28.4%–85.6%]. As previously highlighted by these authors, however, the CD results should be interpreted with caution, as the confidence intervals remain wide and the pooled effect size may be inflated due to the variability of methodology among individual studies and the still limited data. This is further supported by the publications bias observed in the current meta-analysis on clinical remission and FMT in CD patients. Furthermore, it is known that clinical remission does not correlate with endoscopic outcomes in CD. Of note, in the only CD cohort study to report endoscopic outcomes, no patient experienced endoscopic remission.42
There remain major limitations in the available literature of this developing field. There are insufficient data to support FMT for other indications besides CDI,71,72 with no randomised trials published or presented to date outside UC. Even within UC, the existing studies are relatively small in size [largest 81 patients], and where FMT would be best placed in the therapeutic algorithm is unclear given the growing number of biologics73 and emerging targeted small molecule therapies.74,75 Long-term follow-up data regarding FMT efficacy/durability and safety in IBD are lacking. The available data suggest that disease relapse will invariably occur [though the durability and impact of number of infusions are poorly defined] and some form of maintenance therapy is required. However, almost all studies performed to date have assessed the role of FMT in remission induction for IBD, with a paucity of literature on the potential of FMT as a maintenance therapy38 once remission is established. The safety data from the available literature are reassuring though limited by study size and follow-up period. There have been reports from the FMT in IBD and CDI co-infection literature, of disease flare following FMT.76,77 However, these must be considered in the context of an absence of a control arm [to account for gastrointestinal symptoms after FMT in non-IBD patients], difficulty in distinguishing colitis symptoms attributable to IBD as opposed to CDI, along with variable endoscopic mucosal activity assessment. In this context, Fischer et al.77 reported improvement in clinician assessment of IBD activity after FMT for CDI in 31/67 [46%] and worsening in 12/67 [18%]. Additionally, there are few well-conducted microbiological studies on the effect of FMT on the intestinal microbiota in IBD. These are clearly required if we are to better understand the underlying mechanism of action and microbial predictors of therapeutic outcome, both beneficial and detrimental. Most studies to date have included small numbers of patients and focused primarily on microbial composition and not functional/metabolic consequences. Taxanomic changes identified to date associated with FMT and therapeutic benefit, are variable and inconsistent [Table A7]. There exist inherent differences among donors, regardless of whether they are related or unrelated/anonymous, and the clinical and microbiological factors that are of importance in donor outcomes remain largely undefined.
The American Gastroenterological Association [AGA] has recently set up an FMT registry [http://www.gastro.org/patient-care/registries-studies/fmt-registry] to help characterise long-term outcomes of FMT [though this is primarily directed towards Clostridium difficile infection], and there are many new studies of FMT in IBD in progress [clinicaltrials.gov] that will hopefully address these issues. Future directions should also include more specific and targeted allied microbiological studies to try to identify donor and recipient factors of importance, which may potentially facilitate progress to donor-recipient matching, and ultimately defined microbial consortia based on recipient phenotype, along with ongoing development of capsule therapy with directed small bowel or colonic release.
Funding
No funding source was associated with the production of this manuscript. SP is supported by a Postgraduate Scholarship from the National Health and Medical Research Council [NHMRC], Australia. NCR is supported by an Early Career Fellowship from the NHMRC. NOK is supported by a Career Development Fellowship from the Cancer Institute NSW, Australia.
Conflict of Interest
The authors have no financial or potential competing interest or affiliation with any institution, organisation, or company relating to the manuscript.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
References
Author notes
Conference Presentations: 12th Congress of ECCO, Barcelona, 2017; Digestive Disease Week, Chicago, 2017.



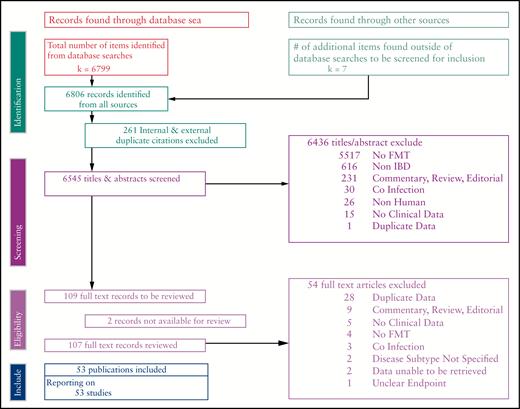
![Forest plot of the meta-analysis of clinical remission and faecal microbiota transplantation [FMT] in ulcerative colitis including available cohort studies to date. The pooled proportions with 95% confidence intervals [CIs] were calculated using the random effects model [diamond]. The filled squares represent the studies in relation to their weights. In this meta-analysis, four case-control studies [Kump et al. 2015, Scaldaferri et al. 2015, Pai et al. 2016, and Ishikawa et al. 2017] were included as cohorts [data from controls was removed] as the software did not allow the combination of one and two groups comparison analyses.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ecco-jcc/11/10/10.1093_ecco-jcc_jjx063/1/m_jjx06302.jpeg?Expires=1716372918&Signature=17yrokq81Aa~dJIQO1PnTqC9tKcJmiJAh6DET0MQqddqlMwQI1rU2y-Tt827OdTmzHnDuDfveDdFIGhtWEiRWNOGiDJW~0fO5bZhfZtzGtO5FFPt~UW9~ZcPTPcTB-uhgh8GMpkhTOxOvcDvH33ll7wk-CxBg1DvXsZOGsfPKvN-NUsxpU6YxrB~jhLk7xS5zLn9jjsVnp1quV--gBahz0WyPaNjYDvwqKTMhOl1mQRufZ6C7kqjzEX6jQrb-Zm4~2EeLJK3QCtBkFPMi1UJRZ61M563uGCkgOqZscRGwQfnJZHSzIfxOYgaR3FQkuw9fMCAgVC8UUHThF1BmEkfuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Forest plot of the meta-analysis of clinical remission and faecal microbiota transplantation [FMT] in ulcerative colitis including four randomised controlled trials [RCTs] available to date. The pooled odds ratios [ORs] with 95% confidence intervals [CIs] were calculated using the random effects model [diamond]. The filled squares represent the studies in relation to their weights.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ecco-jcc/11/10/10.1093_ecco-jcc_jjx063/1/m_jjx06303.jpeg?Expires=1716372918&Signature=vRICj7ldReGx4FQj9REB1grdVqQQ1pkEQGE3W56J7CSXkBQBTDoIXpCiCJyMh6DxXpXmL9z8-fbK4GsIOd0wxlzzGxexIog9ghHwFF6S~qKGWYwSaQrfMUiKuGJuRaui9fzK9eUQ5B4c7O0tf8ZtBZg6dSvas74Yee2qB8RPkSr7I2-lrdewzMFpbQqcyf67gxRZREffWPP6ZKyK03ffBLap0y3Rc7Un4ArOy9d7dUpaWgwTw7KJtdXMURwcAkEb2gsqKHWqgRd7jeNFxd77bqra0ON5KlIiPFJ0QM-f0mXA~rLiakcMGNTTKrSmfVXV-eHti1zwIhYRTYl3RiGedw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Forest plot of the meta-analysis of clinical remission and faecal microbiota transplantation [FMT] in Crohn’s disease including available cohort studies to date. The pooled proportions with 95% confidence intervals [CIs] were calculated using the random effects model [diamond]. The filled squares represent the studies in relation to their weights.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ecco-jcc/11/10/10.1093_ecco-jcc_jjx063/1/m_jjx06304.jpeg?Expires=1716372918&Signature=JQzq3Y2nqFZ3lLQOaZm2rExV2HyeiKiU1ql7tK5rb4Fz9qSlz24djG6WiQ267SrbtlzGOCCAvsH9ZV-BjaOebDEXIn2Y52KBcZuj5t6ige4CyUSVIBrfUu0zwKtFIGR1FFTMRtHZAB2wvZLM3Z-MQXZ1wZTRcMIJneEKedZznPSKdPuEeMJ~5i3TBFkHvpuaBdZnlOZdmYZNpeT~o-tsjvEWpTpa4uTYvOOg49s-sf-VP2a8pkW9bB31mj8FywSKxcZbyz~gd~LDysFKOTf3Y00JKTUPCUc8Y1iMa4doalvU1OJ0f7eWlp7rnxvRTvlqmh4NSHUGVRShm3hhk51ycw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
