-
PDF
- Split View
-
Views
-
Cite
Cite
Horacio Bach, Greg Rosenfeld, Brian Bressler, Treatment of Crohn's disease patients with infliximab is detrimental for the survival of Mycobacterium avium ssp. paratuberculosis within macrophages and shows a remarkable decrease in the immunogenicity of mycobacterial proteins , Journal of Crohn's and Colitis, Volume 6, Issue 5, June 2012, Pages 628–629, https://doi.org/10.1016/j.crohns.2012.01.011
Close - Share Icon Share
Dear Sir,
The association between Mycobacterium avium ssp. paratuberculosis (MAP) and Crohn's disease (CD) although debatable, is supported by several studies 1 which have reported the detection or isolation of MAP from human tissues 2 including serum, 3 body fluids (breast milk), 4 and high levels of TNF-α was found secreted by the gut mucosa in MAP-associated CD patients. 5 Infliximab is a monoclonal antibody that specifically inhibits TNF-α and is used as a current therapy for CD.
Recently, Nakase et al. 6 demonstrated that THP-1 cells infected with MAP induced the production of a higher amount of TNF-α when compared to macrophages infected with either Mycobacterium avium or Mycobacterium smegmatis , suggesting that MAP is directly involved in the upregulation of this cytokine.
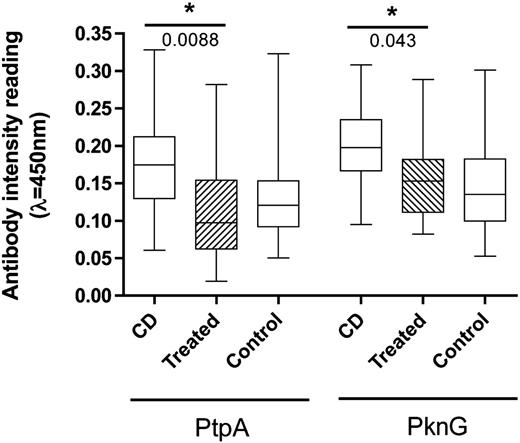
Previously, we have reported that MAP is able to infect, reside, and multiply intracellularly in human macrophages, 7 , 8 suggesting that the pathogen is able to subvert the host's immune response to avoid its own demise even at the earliest stage of the infection. Moreover, we have reported that CD patients, 8 but not healthy controls, have a significantly higher level of antibodies against two MAP proteins, a Protein tyrosine phosphatase (PtpA), and a Protein kinase (PknG). Both proteins are part of the signal transduction system of the bacterium, have been shown to be secreted within the host, and are essential for the intracellular survival and the establishment of a successful infection of the MAP's close relative Mycobacterium tuberculosis . 9 , 10 Therefore, to persist within the host, both proteins have to be secreted in a regular manner by the pathogen, in order to manipulate the immunological response elicited by macrophages. Recently, we have shown that CD patients possess a higher titer of antibodies against PtpA and PknG when compared to healthy controls, 8 and we suggested that both proteins can be used to determine the status of MAP in CD patients.
We report our analysis of the impact that infliximab upon the presence of antibodies against PtpA and PknG in sera of CD patients. A cohort of 20 CD patients treated with infliximab, 20 CD patients not treated with infliximab, and 20 healthy controls were enrolled in this study. 43.3% of the subjects were male with average ages for CD, infliximab, and healthy control groups being 41 ± 14, 33 ± 12, and 46 ± 18 years, respectively. In the CD and infliximab groups, the mean Harvey Bradshaw Index score was 5.2 ± 5.0 and 4.6 ± 4.5 and the mean time since diagnosis of CD was 10.4 ± 10.2 and 10.4 ± 8.2 years, respectively. The Research Ethics Board of the University of British Columbia, Vancouver, Canada, approved the protocol for this study. Protocols for blood collection, serum processing, antigen production and ELISA were followed as published. 8
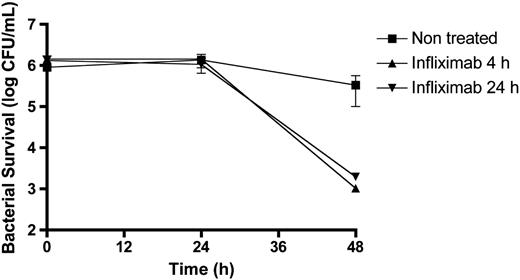
Patients treated with infliximab show a significant decrease in the level of antibodies against both MAP proteins, and had levels similar to the negative control ( Fig. 1 ), suggesting that inhibition of TNF-α has an effect in the secretion of both mycobacterial proteins within CD patients. Next, we evaluated the survival of MAP in THP-1 cells treated with infliximab. Prior to the infection with MAP, 8 THP-1 cells were differentiated with phorbol 12-myristate 13-acetate and exposed to infliximab (5 μg/mL) for 4 and 24 h. Macrophages infected with MAP were collected and processed as published. 8 Interestingly, we observed a significant decrease in the survival of MAP when macrophages were exposed to infliximab ( Fig. 2 ), suggesting that the suppression of TNF-α was detrimental to the intracellular survival of MAP. We found no difference in the survival of MAP between different times of infliximab exposure to macrophages prior to the infection.
In conclusion, our findings suggest that infliximab treatment results in: (i) a decrease in the antibody titer of two mycobacterial antigens that are essential for the establishment of an infection, and (ii) a decrease in the survival of the bacterium within human macrophages. Both observations imply that in CD patients, a suppression of TNF-α leads to the activation of other immunological pathways in macrophages, which suppresses the growth of MAP in these patients. Our findings together with the study reported by Nakase et al. 6 demonstrate that the regulation of specific cytokines, such as TNF-α, is critical for the survival of MAP within macrophages. Based on the accumulated information related to the increase of TNF-α production in infections associated with MAP, a remaining question is: why is it beneficial for MAP to induce TNF-α production upon infection? More studies related to the balance of cytokines and their link to MAP survival are necessary to understand the pathophysiology of CD.
Conflict of interest
None.
Acknowledgments
We thank Jeffrey Helm for helpful discussions.
References
Figures
Distribution of the antibody intensity readings. A boxplot analysis shows the distribution of the antibody intensity readings obtained by ELISA in CD patients, CD patients under treatment with infliximab, and healthy controls ( n = 20 individuals in each category). *P-value obtained using Wilconox rank sum test. CD = Crohn's disease; PtpA = protein tyrosine phosphatase; PknG = protein kinase G. Experiments were performed in triplicate.
Survival of MAP in infected human macrophages THP-1 cell lines treated with infliximab (5 μg/mL) for 4 h (triangle) and 24 h (inverted triangle). Non-treated cells (square) were used as control. Shown are the mean values (± SD) of three independent experiments. CFU = Colony Forming Unit.