-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel Ahlert, Andrew R J Mitchell, Delayed presentation of right ventricular lead perforation following defibrillator implantation: a case report, European Heart Journal - Case Reports, Volume 3, Issue 3, September 2019, ytz121, https://doi.org/10.1093/ehjcr/ytz121
Close - Share Icon Share
Abstract
Perforation of a device lead through the myocardium is a recognized complication of cardiac device implantation. The associated morbidity and mortality are significant, even though it is a relatively rare complication. Therefore, it is vital for acute clinicians to be aware of the diagnosis and subsequent management of myocardial perforation.
We present the case of a 48-year-old woman who presented to the emergency department 1 month following implantable cardioverter-defibrillator implantation with chest and shoulder pain. Initial assessment revealed bilateral pleural effusions and anaemia. Computerized tomography of her chest and abdomen demonstrated a pericardial effusion, but it was transthoracic echocardiography that confirmed the diagnosis of right ventricular perforation. Urgent system revision was undertaken.
This case highlights the importance of clinical suspicion and the use of diagnostic echocardiography as an important diagnostic tool in symptomatic patient’s post-cardiac device implantation.
Myocardial perforation after device implantation carries significant morbidity and mortality and patients may present late.
Echocardiography as an imaging modality for myocardial perforation can be diagnostic, is widely available, and should be used in addition to other imaging modalities such as computerized tomography.
Risks and benefits for implantable cardioverter-defibrillator implantation for primary prevention have to be carefully considered.
Introduction
The complication rate of cardiac device implantation is estimated to be between 7 and 9.5%, and the female sex appears to be an independent predictor of risk.1–3 Lead perforation was observed in 0.4% of patients undergoing device implantation in a Danish nationwide cohort study in 2010–113 but was found to occur in just 0.14% of procedures in the United States National Cardiovascular Data Registry for first time implantable cardioverter-defibrillator (ICD) recipients.4
Lead perforation may be asymptomatic and the diagnosis incidental. Symptoms of chest, neck, and shoulder pain with pericardial effusion however, are more typical. The diagnosis has been reported via echocardiography, computerized tomography (CT), X-ray, and video fluoroscopy.5,6 Lead failure detected on device interrogation aids the diagnosis.
Timeline
| Day 1 | Single lead implantable cardioverter-defibrillator (ICD) implantation. |
| Until presentation | Building symptoms of chest pain and fatigue. |
| Day 29 | Presentation to the emergency department whilst on holiday. |
| Day 30 | Diagnosis of myocardial perforation, transfer to tertiary centre with subsequent pericardial drain and lead removal. |
| Day 35 | Discharge from hospital. |
| Day 60 | Energy levels improving, considering return to work. |
| Future | Consideration of further ICD implantation. |
| Day 1 | Single lead implantable cardioverter-defibrillator (ICD) implantation. |
| Until presentation | Building symptoms of chest pain and fatigue. |
| Day 29 | Presentation to the emergency department whilst on holiday. |
| Day 30 | Diagnosis of myocardial perforation, transfer to tertiary centre with subsequent pericardial drain and lead removal. |
| Day 35 | Discharge from hospital. |
| Day 60 | Energy levels improving, considering return to work. |
| Future | Consideration of further ICD implantation. |
| Day 1 | Single lead implantable cardioverter-defibrillator (ICD) implantation. |
| Until presentation | Building symptoms of chest pain and fatigue. |
| Day 29 | Presentation to the emergency department whilst on holiday. |
| Day 30 | Diagnosis of myocardial perforation, transfer to tertiary centre with subsequent pericardial drain and lead removal. |
| Day 35 | Discharge from hospital. |
| Day 60 | Energy levels improving, considering return to work. |
| Future | Consideration of further ICD implantation. |
| Day 1 | Single lead implantable cardioverter-defibrillator (ICD) implantation. |
| Until presentation | Building symptoms of chest pain and fatigue. |
| Day 29 | Presentation to the emergency department whilst on holiday. |
| Day 30 | Diagnosis of myocardial perforation, transfer to tertiary centre with subsequent pericardial drain and lead removal. |
| Day 35 | Discharge from hospital. |
| Day 60 | Energy levels improving, considering return to work. |
| Future | Consideration of further ICD implantation. |
Case presentation
A 48-year-old woman presented to our local emergency department whilst on holiday. She had been experiencing a month of increasing exertional dyspnoea associated with left-sided chest pain radiating to her shoulder, neck, and arm culminating in symptoms at rest. She had also experienced 48 h of vomiting and rigors.
Following the recent sudden death of a first-degree relative, the patient was diagnosed with Brugada syndrome. She subsequently received a single lead ICD indicated for the primary prevention of ventricular arrhythmias (see Timeline section). Admission medications were mirtazapine 30 mg once a day, lactulose, paracetamol, ibuprofen, and codeine when required for post-operative pain. She has never smoked and alcohol intake was negligible.
On examination, the patient was pale and deep inspiration was reduced due to pleuritic pain. She was apyrexial with a regular pulse of 69 b.p.m., blood pressure of 103/70 mmHg, and normal oxygen saturation on room air. Auscultation revealed bi-basal reduced air entry. Her heart sounds were normal with no additional murmur and jugular venous pressure did not appear elevated. A pericardial rub was not documented. There was no peripheral oedema and no signs of deep vein thrombosis. Abdominal examination was unremarkable. It was noted that power in the left arm was reduced due to pain.
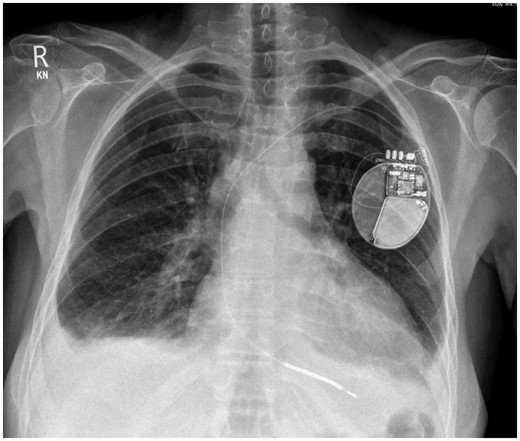
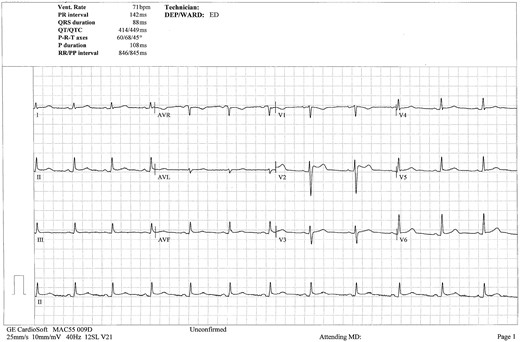
Chest X-ray showed increased cardiothoracic ratio with bi-basal pleural effusions and patchy bilateral bronchopneumonic changes suspicious of congestive cardiac failure. Implantable cardioverter-defibrillator lead position appeared low (Figure 1). The 12-lead electrocardiogram revealed normal sinus rhythm at a rate of 96 b.p.m., there was T-wave inversion noted in leads III and aVF, with isolated 3 mm ST-elevation in lead V2, consistent with a Brugada pattern (Figure 2). Serial electrocardiograms did not demonstrate dynamic changes.
Initial chest X-ray demonstrating bilateral pleural effusions and patchy bilateral bronchopneumonic changes. Single lead implantable cardioverter-defibrillator in situ.
Electrocardiogram demonstrating sinus rhythm with J-point elevation in lead V2 consistent with a Brugada pattern.
Initial laboratory results were significantly abnormal. There was evidence of a microcytic anaemia with a haemoglobin of 76 g/L (110–150 g/L), mean corpuscular volume of 77.2 fL (83–100 fL), elevated C-reactive protein of 63 mg/L (0–10 mg/L). Liver function tests were deranged with albumin 33 g/L (35–50 g/L), bilirubin 30 µmol/L (0–21 µmol/L), gamma-glutamyl transferase 61 IU/L (12–43 IU/L), and alanine aminotransferase 122 IU/L (<35 IU/L). Iron and transferrin saturations were low [2 µmol/L (7–30 mol/L), 2.8% (20–50%), respectively]. N-terminal pro b-type natriuretic peptide was 234 pg/mL (<400 pg/mL). Prothrombin time was prolonged at 15.7 s (<13 s).
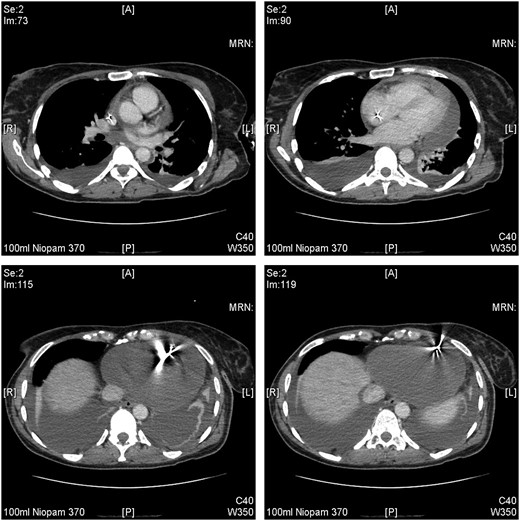
Diagnostic thoracocentesis demonstrated blood-stained fluid. A CT scan of her chest, abdomen, and pelvis then confirmed bilateral pleural effusions accompanied by predominantly left-sided subsegmental atelectasis of both lower lung lobes. A moderate pericardial effusion was seen. The ICD lead position was not commented on in the initial CT report (Figure 3).
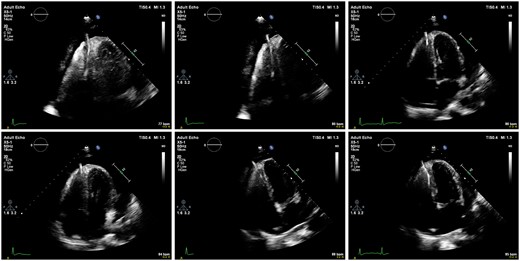
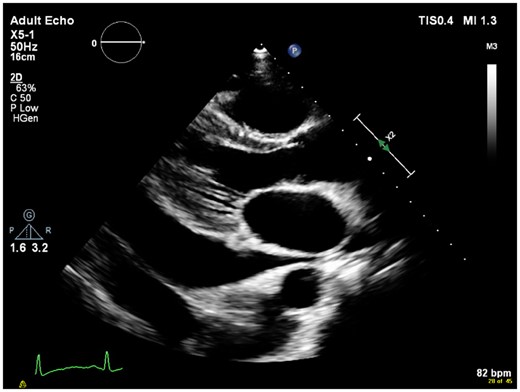
Implantable cardioverter-defibrillator interrogation revealed no shocks and that the lead was failing to sense appropriately. Transthoracic echocardiography demonstrated penetration of the right ventricular lead through the right ventricular apex into the pericardium (Figure 4). A large global pericardial effusion was present measuring ∼3.5 cm in diameter without evidence of cardiac tamponade. Left ventricular function was normal (Figure 5 and Supplementary material).
Series of echocardiographic images demonstrating perforation of lead through the right ventricular apex with associated global pericardial effusion in the four-chamber view.
Early interventions included the administration of intravenous fluids, amoxicillin, analgaesia, and the transfusion of red blood cells. Once the diagnosis was confirmed, aeromedical transfer to the linked tertiary cardiac centre was performed without complication. There, the patient underwent immediate pericardiocentesis and right ventricular lead explant under general anaesthesia guided by transoesophageal echocardiography and with cardiothoracic surgical stand-by. The patient opted against the implantation of a new lead at the time of the procedure. A total volume of 1000 mL of blood-stained fluid was drained from the pericardium. Post-procedure echocardiography confirmed resolution of the pericardial effusion. Local cardiology follow-up was planned at the time of discharge. At the time of writing, the patient-reported resolution of symptoms and is planning on discussing the implantation of a new system with her cardiologist.
Discussion
Most myocardial perforations occur at the time of device implantation with the lead exits the right ventricle into the pericardium. Delayed presentation is unusual and as many as two-thirds of patients with perforation can develop cardiac tamponade.7 In the US registry, cardiac perforation was associated with 7.4% incidence of markers of morbidity including cardiac arrest, myocardial infarction, or infection, compared with 0.3% incidence without cardiac perforation.4 The same study claims a 16-fold increased risk of prolonged hospital stay as well as 15-fold increased risk of in-hospital death.4
The clinical symptoms of neck, chest, and shoulder pain in a relatively young patient in the week’s post-device procedure can be mistaken for musculoskeletal or post-operative pain without further investigations. This case demonstrated that vigilance must be exercised as symptoms were attributable to the (sub-)acute presentation and sequelae of device lead myocardial perforation, fortunately without immediate haemodynamic compromise or cardiac tamponade. The finding of pleural effusions and significant anaemia prompted cross-sectional CT imaging. A lateral view chest X-ray was not performed but is also recommended when assessing lead position. A review of the CT images suggested that the tip may have misplaced inferiorly, pointing towards the antero-lateral chest wall (Figure 3). This was not featured in the initial report, which may be due to motion artefact of non-electrocardiogram gated imaging. This diagnostic challenge has also been demonstrated in other cases, illustrating the importance of echocardiography.8 Other reports concluded that cardiac CT is the preferred diagnostic modality.9 The early use of a portable echocardiography device could have aided diagnosis early on.
Considering the degree of anaemia and free fluid detected on cross-sectional imaging, it is likely that the perforation had been present for a considerable time before presentation. Had there been evidence of cardiac tamponade, it would have been necessary to perform pericardiocentesis prior to aeromedical transfer to stabilize the patient.
Transfer to a cardiac centre was critical for this patient’s management. This is because device lead removal carries considerable risk of infection, bleeding, and damage to cardiac structures such as the tricuspid valve and myocardial wall, including cardiac avulsion. Whilst lead extraction has been shown to be safe in high-volume centres, even with experienced operators, cardiac surgery including cardiopulmonary bypass may be required for complications.10 If the operator is not a cardiac surgeon, surgical stand-by is recommended by the European Heart Rhythm Association.11
Finally, there may be other underlying pathology. Therefore, local follow-up is recommended to ensure resolution of symptoms and the abnormal haematological/biochemical findings.
Conclusions
Myocardial device lead perforation is a rare but significant complication of device implantation for all clinicians who perform acute assessments to be aware of. The symptoms in our patient were classic for this presentation. A high index of suspicion and an appropriate combination of imaging modalities including echocardiography are vital for diagnosis.
Lead author biography
Dr. Daniel Ahlert is a cardiac surgery trainee at the University Hospital Zurich, Switzerland. After completing his Master of Research in Cardiovascular Science in 2014 with research in endothelial dysfunction, he graduated with a Bachelor of Medicine and Bachelor of Surgery from the University of Newcastle in 2015. He completed the UK Foundation Training in the North East. With a keen interest in Cardiology, he became a Clinical Fellow in Cardiology in 2017 at Jersey General Hospital gaining experience in the diagnosis and management of cardiac patients including aeromedical transfer. Dr. Ahlert publishes in the fields of cardiovascular and renal medicine.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- anemia
- myocardium
- pericardial effusion
- echocardiography
- computed tomography
- pleural effusion
- implantable defibrillators
- emergency service, hospital
- heart ventricle
- shoulder pain
- abdomen
- diagnosis
- heart
- morbidity
- mortality
- chest
- echocardiography, transthoracic
- implantable defibrillator insertion
- clinical diagnostic instrument
- medical devices





Comments