-
PDF
- Split View
-
Views
-
Cite
Cite
Kathrine B. Sondergaard, Peter Weeke, Mads Wissenberg, Anne-Marie Schjerning Olsen, Emil L. Fosbol, Freddy K. Lippert, Christian Torp-Pedersen, Gunnar H. Gislason, Fredrik Folke, Non-steroidal anti-inflammatory drug use is associated with increased risk of out-of-hospital cardiac arrest: a nationwide case–time–control study, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 3, Issue 2, April 2017, Pages 100–107, https://doi.org/10.1093/ehjcvp/pvw041
Close - Share Icon Share
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used and have been associated with increased cardiovascular risk. Nonetheless, it remains unknown whether use of NSAIDs is associated with out-of-hospital cardiac arrest (OHCA).
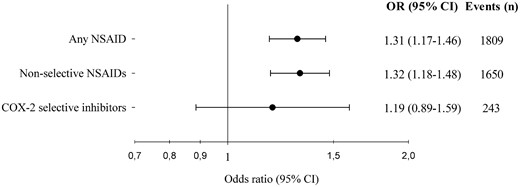
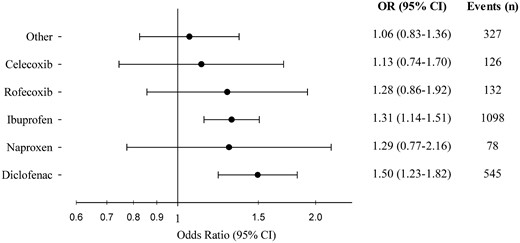
From the nationwide Danish Cardiac Arrest Registry, all persons with OHCA during 2001–10 were identified. NSAID use 30 days before OHCA was categorized as follows: diclofenac, naproxen, ibuprofen, rofecoxib, celecoxib, and other. Risk of OHCA associated with use of NSAIDs was analysed by conditional logistic regression in case–time–control models matching four controls on sex and age per case to account for variation in drug utilization over time. We identified 28 947 persons with OHCA of whom 3376 were treated with an NSAID up to 30 days before OHCA. Ibuprofen and diclofenac were the most commonly used NSAIDs and represented 51.0% and 21.8% of total NSAID use, respectively. Use of diclofenac (odds ratio [OR], 1.50 [95% confidence interval (CI) 1.23–1.82]) and ibuprofen [OR, 1.31 (95% CI 1.14–1.51)] was associated with a significantly increased risk of OHCA. Use of naproxen [OR, 1.29 (95% CI 0.77–2.16)], celecoxib [OR, 1.13 (95% CI 0.74–1.70)], and rofecoxib (OR, 1.28 [95% CI 0.74–1.70)] was not significantly associated with increased risk of OHCA; however, these groups were characterized by few events.
Use of non-selective NSAIDs was associated with an increased early risk of OHCA. The result was driven by an increased risk of OHCA in ibuprofen and diclofenac users.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly used drugs worldwide. More than 50% of the adult Danish population redeemed at least one prescription of an NSAID during 1997–2005 and 29.6 million adults in the USA used NSAIDs on a regular basis in 2010.1,2
Over the last decade, evidence of a cardiovascular risk associated with use of NSAIDs has emerged. The selective cyclooxygenase-2 (COX-2) inhibitors, and to a lesser extent the non-selective NSAIDs, have been associated with cardiovascular adverse events, such as myocardial infarction, stroke, and heart failure in randomized and observational studies.3–11 Cardiac arrest is the ultimate adverse drug event, and though it is closely linked to ischaemic disease and cardiovascular morbidity, the association between NSAIDs and cardiac arrest has never been investigated.12–14 Currently, there is no research pertaining to NSAIDs pro-arrhythmic effects regarding ventricular arrhythmias as isolated events; however, an association with NSAIDs and sudden cardiac death as part of a composite endpoint with myocardial infarction has been observed.5,15 Consequently, it remains to be established, whether use of NSAIDs is associated with cardiac arrest.
This prompted us to conduct an observational case–time–control study using data from the Danish nationwide registries to investigate the association between use of NSAIDs and risk of out-of-hospital cardiac arrest (OHCA).
Methods
Study design
The association between NSAID use and cardiac arrest was explored in the case–time–control design, an acknowledged pharmacoepidemiologic method designed to evaluate brief exposures with acute effects.16 The design has been validated and used in several previous studies.16–19 The case–time–control design is an extended case-crossover design where each individual serves as both case and their own control in two different time-periods. The ‘case period’ precedes outcome, and the ‘control period’ of same length precedes the case period. Exposure to NSAIDs 30 days before cardiac arrest (case period) was assessed and compared with exposure to NSAIDs in a preceding 30-day period where the individual did not experience an event (control period). A 30-day washout period separated case and control period to eliminate possible carry-over effects. A major advantage of the case-crossover design is the elimination of unmeasured confounding from characteristics that remain stable over time (e.g. comorbidity).
Although a case-crossover design is vulnerable to changes in prevalence of exposure over time, a case–time–control design incorporates data from a control group selected from the general population to adjust for possible time-variant biases related to exposure. The prevalence of exposure to NSAIDs in Denmark could have been influenced by changes in prescription patterns over time due to safety alerts regarding the COX-2 selective inhibitors. Consequently, we performed a case–time–control analysis, consisting of two separate case-crossover analyses for cardiac arrest cases and the control group, respectively. Thus, the control group is used only to adjust for changes in prescribing patterns in the background population. Controls were assigned a fictive date of cardiac arrest according to the case they were matched upon. Only individuals with differing exposure in case and control period contributed to the final analysis. Because median treatment duration in Denmark for all types of NSAIDs is between 13 and 29 days and previous studies have observed enhanced cardiovascular risk associated with less than 30 days of treatment with NSAIDs, the case period was defined to 30 days.1,20,21 Using the odds ratio (OR) from the case-crossover analysis of the control group as a measurement of time trends in exposure, the case–time–control OR is calculated by dividing the OR from cases by the OR from controls.22
Data sources
All residents in Denmark have a unique and permanent civil registration number that enables individual-level linkage between the national Danish registries. To identify patients with OHCA, we used data from the Danish Cardiac Arrest Registry, which contains information about all OHCAs in Denmark since June 2001. Data are close to complete, as all ambulance personnel in Denmark are required to fill out a form every time they encounter a cardiac arrest, defined as a clinical condition of cardiac arrest where resuscitative efforts are initiated. Persons with obvious late signs of death where no resuscitation is attempted are not included in the registry.23
The Danish Patient Registry holds information about all admissions in Denmark from 1978. Each admission is registered by one primary diagnose and one or more secondary diagnoses according to the International Classification of Diagnoses. Information includes date and duration of admissions, operations, treatments, and discharge diagnoses. Information about cause and time of death is registered in The National Causes of Death Registry.
Information on use of drugs is obtained from the Danish Prescription Registry. The registry contains information about dosage, type, date of delivery, and classification according to the Anatomical Therapeutic Chemical Classification System. All pharmacies in Denmark are required to register all redeemed prescriptions in the Danish Prescription Registry, which ensures completeness of the registry.24
Study patients
Via the Danish Cardiac Arrest Registry, we identified all persons with OHCA during 2001–10, who on 1 January 1997 were aged ≥ 10 years. From the Danish Patient Registry, four controls matched on age and sex were selected for every case by using the greedy matching algorithm, a method used and described previously.17 As previously done, information on comorbidity in the cardiac arrest population was obtained via discharge diagnoses from admissions up to 5 years before event, and concomitant pharmacotherapy was identified from redeemed prescriptions up to 90 days before event.17 As the diagnosis code of diabetes has a low sensitivity in the Danish registries, diabetes was identified based on redemption of a prescription for a glucose-lowering drug within 180 days before cardiac arrest, as done previously.25
Exposure
We detected all redeemed prescriptions for NSAIDs divided into the most commonly used NSAIDs in Denmark: non-selective NSAIDs (diclofenac, naproxen, and ibuprofen), COX-2 selective inhibitors (rofecoxib and celecoxib), and other.
To determine whether a patient was using NSAIDs during the case or control period, we identified dates when prescriptions were redeemed and then calculated duration of treatment by dividing the number of tablets in the prescription of interest by daily dosage. As information about daily dosage is not included in the registries, daily dosage was estimated by calculating mean dosages from up to five consecutive prescriptions before the prescription of interest. If only one prescription was identified, the minimal recommended daily dosage was used as daily dosage. This method has been used in several papers and has been described in detail previously.1,26 We did not have information on over-the-counter medication; however, the only NSAID available in Denmark without prescription is ibuprofen sold only in low dosage (200 mg) and small packages (max 20 tablets).
For further details regarding Anatomical Therapeutic Chemical Classification codes, see Supplementary material online, Table S1.
Outcome
Our primary endpoint was OHCA. As cause of cardiac arrest is not registered in the Danish Cardiac Arrest Registry, presumed cause of cardiac arrest was classified using discharge diagnoses from The Danish Patient Registry and death certificates from The National Causes of Death Registry. A presumed cardiac cause of cardiac arrest was defined as an event with diagnosis code covering a known cardiac disease, unknown disease, or unexpected collapse according to the Utstein template.23,27 Other medical disorders were considered non-cardiac causes, as was trauma, drug overdose, attempted suicide, and violent attack.23 Cardiac arrest of presumed cardiac cause represented about 75% of all OHCA recorded in the Danish Cardiac Arrest Registry during 2001–10.23
Statistical methods
Differences in baseline characteristics in the cardiac arrest population between NSAID users versus non-users and among those using specific types of NSAIDs were tested using the χ2 test for binary variables and the Wilcoxon–Mann–Whitney test for continuous variables. Trends in drug use over time were tested with the Cochran–Armitage trend test.
The association between NSAID use and cardiac arrest was tested by conditional logistic regression in case–time–control models. We tested for confounding by acute disease exacerbation by excluding individuals admitted up to 60 days before cardiac arrest and excluding individuals with cancer, respectively. This was done since the case–time–control method does not eliminate confounding from characteristics that change between case and control period and lack of clinical data in the Danish registries makes control for time-varying variables impossible.
All statistical analyses were performed using SAS version 9.2 (SAS institute Inc., NC, USA). A two-sided P-value < 0.05 was considered significant.
Ethics
The project was approved by the Danish Data Protection Agency (Ref.no. 2007-58-0015, local ref.no. GEH-2014-017, I-Suite.nr. 02735). No ethical approval is required for register-based studies performed on anonymized data in Denmark.
Results
We identified 28 947 persons with OHCA during 2001–10 and matched 115 788 controls with cases on age and sex. Within the 30-day case period, 3376 (11.7%) persons were treated with an NSAID. Compared with non-users, NSAID users were more often women, had generally less cardiovascular diseases, such as ischaemic heart disease, myocardial infarction, and heart failure, but were more likely to have cancer and rheumatic diseases (P for all <0.05, Table 1). Moreover, NSAID users were more often treated with morphine, diuretics, and psychiatric medication (P for all < 0.05).
Baseline characteristics of subjects with out-of-hospital cardiac arrest divided into NSAID users and non-users
| . | Any NSAIDa . | No NSAID . | P for difference . |
|---|---|---|---|
| n (%) | 3376 (11.7) | 25 571 (88.3) | |
| Age, years, median (IQR) | 69.0 (58.7–78.5) | 70.7 (59.2–80 .0) | |
| Women, n (%) | 1389 (41.1) | 8659 (33.9) | |
| Age, years, median (IQR) | 71.1 (59.9–80 .3) | 74.0 (62.3–83.0) | |
| Men, n (%) | 1987 (58.9) | 16 912 (66.1) | |
| Age, years, median (IQR) | 67.7 (57.5–76.9) | 68.9 (57.9–78.4) | |
| Comorbidity, n (%) | |||
| Diabetes | 392 (11.6) | 3160 (12.4) | 0 .21 |
| Peripheral vascular disease | 143 (4.2) | 1257 (4.9) | 0 .084 |
| Cerebral vascular disease | 236 (7.0) | 2291 (9.0) | 0 .0001 |
| Ischaemic heart disease | 466 (13.8) | 4106 (16.1) | 0 .0007 |
| Previous myocardial infarction | 343 (10 .2) | 3179 (12.4) | 0 .0001 |
| Chronic heart failure | 377 (11.2) | 3704 (14.5) | <0 .0001 |
| Cardiac arrythmias | 382 (11.3) | 4020 (15.7) | <0 .0001 |
| COPD | 410 (12.1) | 2979 (11.7) | 0 .40 |
| Cancer | 391 (11.6) | 2034 (8.0) | <0 .0001 |
| Rheumatic diseases | 56 (1.7) | 263 (1.0) | 0 .0010 |
| Renal diseases | 87 (2.6) | 854 (3.3) | 0 .019 |
| Peptic ulcers | 122 (3.6) | 1087 (4.3) | 0 .082 |
| Concomitant pharmacotherapy | |||
| ASA | 807 (23.9) | 6329 (24.8) | 0 .28 |
| Warfarin | 151 (4.5) | 1816 (7.1) | <0 .0001 |
| ACE inhibitors | 834 (24.7) | 6579 (25.7) | 0 .20 |
| Loop diuretics | 851 (25.2) | 6211 (21.5) | 0 .24 |
| β-Blockers | 622 (18.4) | 4911 (19.2) | 0 .28 |
| Spironolactone | 209 (6.2) | 1743 (6.8) | 0 .17 |
| Thiazides | 420 (12.4) | 2726 (10 .7) | 0 .0018 |
| Calcium channel blockers | 484 (14.3) | 3492 (13.7) | 0 .28 |
| Antidepressants | 662 (19.6) | 4254 (16.6) | <0 .0001 |
| Anxiolytics | 1067 (31.6) | 5879 (23.0) | <0 .0001 |
| Morphine | 448 (13.3) | 1716 (6.7) | <0 .0001 |
| . | Any NSAIDa . | No NSAID . | P for difference . |
|---|---|---|---|
| n (%) | 3376 (11.7) | 25 571 (88.3) | |
| Age, years, median (IQR) | 69.0 (58.7–78.5) | 70.7 (59.2–80 .0) | |
| Women, n (%) | 1389 (41.1) | 8659 (33.9) | |
| Age, years, median (IQR) | 71.1 (59.9–80 .3) | 74.0 (62.3–83.0) | |
| Men, n (%) | 1987 (58.9) | 16 912 (66.1) | |
| Age, years, median (IQR) | 67.7 (57.5–76.9) | 68.9 (57.9–78.4) | |
| Comorbidity, n (%) | |||
| Diabetes | 392 (11.6) | 3160 (12.4) | 0 .21 |
| Peripheral vascular disease | 143 (4.2) | 1257 (4.9) | 0 .084 |
| Cerebral vascular disease | 236 (7.0) | 2291 (9.0) | 0 .0001 |
| Ischaemic heart disease | 466 (13.8) | 4106 (16.1) | 0 .0007 |
| Previous myocardial infarction | 343 (10 .2) | 3179 (12.4) | 0 .0001 |
| Chronic heart failure | 377 (11.2) | 3704 (14.5) | <0 .0001 |
| Cardiac arrythmias | 382 (11.3) | 4020 (15.7) | <0 .0001 |
| COPD | 410 (12.1) | 2979 (11.7) | 0 .40 |
| Cancer | 391 (11.6) | 2034 (8.0) | <0 .0001 |
| Rheumatic diseases | 56 (1.7) | 263 (1.0) | 0 .0010 |
| Renal diseases | 87 (2.6) | 854 (3.3) | 0 .019 |
| Peptic ulcers | 122 (3.6) | 1087 (4.3) | 0 .082 |
| Concomitant pharmacotherapy | |||
| ASA | 807 (23.9) | 6329 (24.8) | 0 .28 |
| Warfarin | 151 (4.5) | 1816 (7.1) | <0 .0001 |
| ACE inhibitors | 834 (24.7) | 6579 (25.7) | 0 .20 |
| Loop diuretics | 851 (25.2) | 6211 (21.5) | 0 .24 |
| β-Blockers | 622 (18.4) | 4911 (19.2) | 0 .28 |
| Spironolactone | 209 (6.2) | 1743 (6.8) | 0 .17 |
| Thiazides | 420 (12.4) | 2726 (10 .7) | 0 .0018 |
| Calcium channel blockers | 484 (14.3) | 3492 (13.7) | 0 .28 |
| Antidepressants | 662 (19.6) | 4254 (16.6) | <0 .0001 |
| Anxiolytics | 1067 (31.6) | 5879 (23.0) | <0 .0001 |
| Morphine | 448 (13.3) | 1716 (6.7) | <0 .0001 |
ACE, angiotensin converting enzyme; ASA, acetylsalicylic acid; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug.
Use of any NSAID up to 30 days before out-of-hospital cardiac arrest.
Baseline characteristics of subjects with out-of-hospital cardiac arrest divided into NSAID users and non-users
| . | Any NSAIDa . | No NSAID . | P for difference . |
|---|---|---|---|
| n (%) | 3376 (11.7) | 25 571 (88.3) | |
| Age, years, median (IQR) | 69.0 (58.7–78.5) | 70.7 (59.2–80 .0) | |
| Women, n (%) | 1389 (41.1) | 8659 (33.9) | |
| Age, years, median (IQR) | 71.1 (59.9–80 .3) | 74.0 (62.3–83.0) | |
| Men, n (%) | 1987 (58.9) | 16 912 (66.1) | |
| Age, years, median (IQR) | 67.7 (57.5–76.9) | 68.9 (57.9–78.4) | |
| Comorbidity, n (%) | |||
| Diabetes | 392 (11.6) | 3160 (12.4) | 0 .21 |
| Peripheral vascular disease | 143 (4.2) | 1257 (4.9) | 0 .084 |
| Cerebral vascular disease | 236 (7.0) | 2291 (9.0) | 0 .0001 |
| Ischaemic heart disease | 466 (13.8) | 4106 (16.1) | 0 .0007 |
| Previous myocardial infarction | 343 (10 .2) | 3179 (12.4) | 0 .0001 |
| Chronic heart failure | 377 (11.2) | 3704 (14.5) | <0 .0001 |
| Cardiac arrythmias | 382 (11.3) | 4020 (15.7) | <0 .0001 |
| COPD | 410 (12.1) | 2979 (11.7) | 0 .40 |
| Cancer | 391 (11.6) | 2034 (8.0) | <0 .0001 |
| Rheumatic diseases | 56 (1.7) | 263 (1.0) | 0 .0010 |
| Renal diseases | 87 (2.6) | 854 (3.3) | 0 .019 |
| Peptic ulcers | 122 (3.6) | 1087 (4.3) | 0 .082 |
| Concomitant pharmacotherapy | |||
| ASA | 807 (23.9) | 6329 (24.8) | 0 .28 |
| Warfarin | 151 (4.5) | 1816 (7.1) | <0 .0001 |
| ACE inhibitors | 834 (24.7) | 6579 (25.7) | 0 .20 |
| Loop diuretics | 851 (25.2) | 6211 (21.5) | 0 .24 |
| β-Blockers | 622 (18.4) | 4911 (19.2) | 0 .28 |
| Spironolactone | 209 (6.2) | 1743 (6.8) | 0 .17 |
| Thiazides | 420 (12.4) | 2726 (10 .7) | 0 .0018 |
| Calcium channel blockers | 484 (14.3) | 3492 (13.7) | 0 .28 |
| Antidepressants | 662 (19.6) | 4254 (16.6) | <0 .0001 |
| Anxiolytics | 1067 (31.6) | 5879 (23.0) | <0 .0001 |
| Morphine | 448 (13.3) | 1716 (6.7) | <0 .0001 |
| . | Any NSAIDa . | No NSAID . | P for difference . |
|---|---|---|---|
| n (%) | 3376 (11.7) | 25 571 (88.3) | |
| Age, years, median (IQR) | 69.0 (58.7–78.5) | 70.7 (59.2–80 .0) | |
| Women, n (%) | 1389 (41.1) | 8659 (33.9) | |
| Age, years, median (IQR) | 71.1 (59.9–80 .3) | 74.0 (62.3–83.0) | |
| Men, n (%) | 1987 (58.9) | 16 912 (66.1) | |
| Age, years, median (IQR) | 67.7 (57.5–76.9) | 68.9 (57.9–78.4) | |
| Comorbidity, n (%) | |||
| Diabetes | 392 (11.6) | 3160 (12.4) | 0 .21 |
| Peripheral vascular disease | 143 (4.2) | 1257 (4.9) | 0 .084 |
| Cerebral vascular disease | 236 (7.0) | 2291 (9.0) | 0 .0001 |
| Ischaemic heart disease | 466 (13.8) | 4106 (16.1) | 0 .0007 |
| Previous myocardial infarction | 343 (10 .2) | 3179 (12.4) | 0 .0001 |
| Chronic heart failure | 377 (11.2) | 3704 (14.5) | <0 .0001 |
| Cardiac arrythmias | 382 (11.3) | 4020 (15.7) | <0 .0001 |
| COPD | 410 (12.1) | 2979 (11.7) | 0 .40 |
| Cancer | 391 (11.6) | 2034 (8.0) | <0 .0001 |
| Rheumatic diseases | 56 (1.7) | 263 (1.0) | 0 .0010 |
| Renal diseases | 87 (2.6) | 854 (3.3) | 0 .019 |
| Peptic ulcers | 122 (3.6) | 1087 (4.3) | 0 .082 |
| Concomitant pharmacotherapy | |||
| ASA | 807 (23.9) | 6329 (24.8) | 0 .28 |
| Warfarin | 151 (4.5) | 1816 (7.1) | <0 .0001 |
| ACE inhibitors | 834 (24.7) | 6579 (25.7) | 0 .20 |
| Loop diuretics | 851 (25.2) | 6211 (21.5) | 0 .24 |
| β-Blockers | 622 (18.4) | 4911 (19.2) | 0 .28 |
| Spironolactone | 209 (6.2) | 1743 (6.8) | 0 .17 |
| Thiazides | 420 (12.4) | 2726 (10 .7) | 0 .0018 |
| Calcium channel blockers | 484 (14.3) | 3492 (13.7) | 0 .28 |
| Antidepressants | 662 (19.6) | 4254 (16.6) | <0 .0001 |
| Anxiolytics | 1067 (31.6) | 5879 (23.0) | <0 .0001 |
| Morphine | 448 (13.3) | 1716 (6.7) | <0 .0001 |
ACE, angiotensin converting enzyme; ASA, acetylsalicylic acid; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug.
Use of any NSAID up to 30 days before out-of-hospital cardiac arrest.
Ibuprofen was the most commonly prescribed NSAID followed by diclofenac comprising 51.0% and 21.8% of total NSAIDs, respectively (Table 2). Overall, baseline characteristics did not differ substantially within groups of users of non-selective NSAIDs (ibuprofen, diclofenac, and naproxen) and COX-2 selective inhibitors (rofecoxib and celecoxib), respectively. However, baseline characteristics did differ between groups; non-selective NSAIDs were associated with younger age, male gender, and lower prevalence of heart failure and cardiac arrhythmias in comparison with users of the COX-2 selective inhibitors.
Baseline characteristics of subjects with out-of-hospital cardiac arrest according to use of the most common types of NSAIDs
| . | Non-selective NSAIDs . | COX-2 selective inhibitors . | Other NSAIDa . | |||
|---|---|---|---|---|---|---|
| Diclofenaca . | Naproxena . | Ibuprofena . | Rofecoxiba . | Celecoxiba . | ||
| n (%) | 737 (21.8) | 103 (3.1) | 1721 (51.0) | 153 (4.5) | 189 (5.6) | 473 (14.0) |
| Age, years, median (IQR) | 68.4 (58.8–77.8) | 67.9 (55.1–78.2) | 67.2 (56.5–76.7) | 73.0 (65.7–82.5) | 75.9 (64.6–83.2) | 72.4 (63.0–81.5) |
| Women, n (%) | 270 (36.6) | 38 (36.9) | 687 (39.9) | 80 (52.3) | 94 (49.7) | 220 (46.5) |
| Age, years, median (IQR) | 70.8 (59.4–80.3) | 69.9 (52.0–78.8) | 69.1 (57.9–78.5) | 74.7 (66.2–85.1) | 75.8 (65.2–81.6) | 75.8 (64.0–83.9) |
| Men, n (%) | 467 (63.4) | 65 (63.1) | 1034 (60.1) | 73 (47.7) | 95 (50.3) | 253 (53.5) |
| Age, years, median (IQR) | 67.5 (57.8–76.1) | 67.8 (56.6–78.2) | 66.6 (55.6–75.6) | 72.0 (63.2–80.1) | 75.9 (63.1–84.2) | 70.1 (61.4–79.5) |
| Comorbidity, n (%) | ||||||
| Diabetes | 82 (11.1) | 11 (10.7) | 199 (11.6) | 23 (15.0) | 25 (13.2) | 52 (11.0) |
| Peripheral vascular disease | 27 (3.7) | 5 (4.9) | 72 (4.2) | 10 (6.5) | 9 (4.8) | 20 (4.2) |
| Cerebral vascular disease | 51 (6.9) | 5 (4.9) | 121 (7.0) | 11 (7.2) | 20 (10.6) | 28 (5.9) |
| Ischaemic heart disease | 86 (11.7) | 13 12.6) | 228 (13.3) | 31 (20.3) | 49 (25.9) | 59 (12.5) |
| Previous myocardial infarction | 81 (11.0) | 5 (4.9) | 168 (9.8) | 10 (6.5) | 25 (13.2) | 54 (11.4) |
| Chronic heart failure | 77 (10.5) | 11 (10.7) | 183 (10.6) | 28 (18.3) | 31 (16.4) | 47 (9.9) |
| Cardiac arrythmias | 74 (10.0) | 7 (6.8) | 194 (11.3) | 23 (15.0) | 37 (19.6) | 47 (9.9) |
| COPD | 79 (10.7) | 12 (11.7) | 209 (12.1) | 30 (19.6) | 33 (17.5) | 47 (9.9) |
| Cancer | 107 (14.5) | 11 (10.7) | 180 (10.5) | 21 (13.7) | 30 (15.9) | 42 (8.9) |
| Rheumatic diseases | 9 (1.2) | 3 (2.9) | 18 (1.1) | 5 (3.3) | 9 (4.8) | 12 (2.5) |
| Renal diseases | 20 (2.7) | 2 (1.9) | 41 (2.4) | 3 (2.0) | 6 (3.2) | 15 (3.2) |
| Peptic ulcers | 25 (3.4) | 2 (1.9) | 51 (3.0) | 9 (5.9) | 11 (5.8) | 24 (5.1) |
| Concomitant pharmacotherapy | ||||||
| ASA | 155 (21.0) | 18 (17.5) | 406 (24.0) | 38 (24.8) | 60 (31.8) | 130 (27.5) |
| Warfarin | 37 (5.0) | 4 (3.9) | 70 (4.1) | 10 (6.5) | 13 (6.9) | 17 (3.6) |
| ACE inhibitors | 198 (26.9) | 26 (25.2) | 412 (23.9) | 35 (22.9) | 44 (23.3) | 119 (25.2) |
| Loop diuretics | 167 (22.7) | 29 (28.2) | 385 (22.4) | 66 (43.1) | 73 (38.6) | 131 (27.7) |
| β-Blockers | 125 (17.0) | 24 (23.3) | 294 (17.1) | 29 (19.0) | 41 (21.7) | 109 (23.0) |
| Spironolactone | 44 (6.0) | 9 (8.7) | 105 (6.1) | 11 (7.2) | 19 (10.1) | 21 (4.4) |
| Thiazides | 95 (12.9) | 15 (14.6) | 209 (12.1) | 15 (9.8) | 27 (14.3) | 59 (12.5) |
| Calcium channel blockers | 107 (14.5) | 13 (12.6) | 226 (13.1) | 20 (13.1) | 40 (21.2) | 78 (16.5) |
| Antidepressants | 120 (16.3) | 12 (11.7) | 321 (18.7) | 37 (24.2) | 63 (33.3) | 109 (23.0) |
| Anxiolytics | 208 (28.2) | 27 (26.2) | 513 (29.8) | 65 (42.5) | 82 (43.4) | 172 (36.4) |
| Morphine | 116 (15.7) | 13 (12.6) | 193 (11.2) | 28 (18.3) | 37 (19.6) | 61 (12.9) |
| . | Non-selective NSAIDs . | COX-2 selective inhibitors . | Other NSAIDa . | |||
|---|---|---|---|---|---|---|
| Diclofenaca . | Naproxena . | Ibuprofena . | Rofecoxiba . | Celecoxiba . | ||
| n (%) | 737 (21.8) | 103 (3.1) | 1721 (51.0) | 153 (4.5) | 189 (5.6) | 473 (14.0) |
| Age, years, median (IQR) | 68.4 (58.8–77.8) | 67.9 (55.1–78.2) | 67.2 (56.5–76.7) | 73.0 (65.7–82.5) | 75.9 (64.6–83.2) | 72.4 (63.0–81.5) |
| Women, n (%) | 270 (36.6) | 38 (36.9) | 687 (39.9) | 80 (52.3) | 94 (49.7) | 220 (46.5) |
| Age, years, median (IQR) | 70.8 (59.4–80.3) | 69.9 (52.0–78.8) | 69.1 (57.9–78.5) | 74.7 (66.2–85.1) | 75.8 (65.2–81.6) | 75.8 (64.0–83.9) |
| Men, n (%) | 467 (63.4) | 65 (63.1) | 1034 (60.1) | 73 (47.7) | 95 (50.3) | 253 (53.5) |
| Age, years, median (IQR) | 67.5 (57.8–76.1) | 67.8 (56.6–78.2) | 66.6 (55.6–75.6) | 72.0 (63.2–80.1) | 75.9 (63.1–84.2) | 70.1 (61.4–79.5) |
| Comorbidity, n (%) | ||||||
| Diabetes | 82 (11.1) | 11 (10.7) | 199 (11.6) | 23 (15.0) | 25 (13.2) | 52 (11.0) |
| Peripheral vascular disease | 27 (3.7) | 5 (4.9) | 72 (4.2) | 10 (6.5) | 9 (4.8) | 20 (4.2) |
| Cerebral vascular disease | 51 (6.9) | 5 (4.9) | 121 (7.0) | 11 (7.2) | 20 (10.6) | 28 (5.9) |
| Ischaemic heart disease | 86 (11.7) | 13 12.6) | 228 (13.3) | 31 (20.3) | 49 (25.9) | 59 (12.5) |
| Previous myocardial infarction | 81 (11.0) | 5 (4.9) | 168 (9.8) | 10 (6.5) | 25 (13.2) | 54 (11.4) |
| Chronic heart failure | 77 (10.5) | 11 (10.7) | 183 (10.6) | 28 (18.3) | 31 (16.4) | 47 (9.9) |
| Cardiac arrythmias | 74 (10.0) | 7 (6.8) | 194 (11.3) | 23 (15.0) | 37 (19.6) | 47 (9.9) |
| COPD | 79 (10.7) | 12 (11.7) | 209 (12.1) | 30 (19.6) | 33 (17.5) | 47 (9.9) |
| Cancer | 107 (14.5) | 11 (10.7) | 180 (10.5) | 21 (13.7) | 30 (15.9) | 42 (8.9) |
| Rheumatic diseases | 9 (1.2) | 3 (2.9) | 18 (1.1) | 5 (3.3) | 9 (4.8) | 12 (2.5) |
| Renal diseases | 20 (2.7) | 2 (1.9) | 41 (2.4) | 3 (2.0) | 6 (3.2) | 15 (3.2) |
| Peptic ulcers | 25 (3.4) | 2 (1.9) | 51 (3.0) | 9 (5.9) | 11 (5.8) | 24 (5.1) |
| Concomitant pharmacotherapy | ||||||
| ASA | 155 (21.0) | 18 (17.5) | 406 (24.0) | 38 (24.8) | 60 (31.8) | 130 (27.5) |
| Warfarin | 37 (5.0) | 4 (3.9) | 70 (4.1) | 10 (6.5) | 13 (6.9) | 17 (3.6) |
| ACE inhibitors | 198 (26.9) | 26 (25.2) | 412 (23.9) | 35 (22.9) | 44 (23.3) | 119 (25.2) |
| Loop diuretics | 167 (22.7) | 29 (28.2) | 385 (22.4) | 66 (43.1) | 73 (38.6) | 131 (27.7) |
| β-Blockers | 125 (17.0) | 24 (23.3) | 294 (17.1) | 29 (19.0) | 41 (21.7) | 109 (23.0) |
| Spironolactone | 44 (6.0) | 9 (8.7) | 105 (6.1) | 11 (7.2) | 19 (10.1) | 21 (4.4) |
| Thiazides | 95 (12.9) | 15 (14.6) | 209 (12.1) | 15 (9.8) | 27 (14.3) | 59 (12.5) |
| Calcium channel blockers | 107 (14.5) | 13 (12.6) | 226 (13.1) | 20 (13.1) | 40 (21.2) | 78 (16.5) |
| Antidepressants | 120 (16.3) | 12 (11.7) | 321 (18.7) | 37 (24.2) | 63 (33.3) | 109 (23.0) |
| Anxiolytics | 208 (28.2) | 27 (26.2) | 513 (29.8) | 65 (42.5) | 82 (43.4) | 172 (36.4) |
| Morphine | 116 (15.7) | 13 (12.6) | 193 (11.2) | 28 (18.3) | 37 (19.6) | 61 (12.9) |
ACE, angiotensin converting enzyme; ASA, acetylsalicylic acid; COPD, chronic obstructive pulmonary disease; COX-2, cyclooxygenase-2; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug.
Use of a NSAID up to 30 days before out-of-hospital cardiac arrest.
Baseline characteristics of subjects with out-of-hospital cardiac arrest according to use of the most common types of NSAIDs
| . | Non-selective NSAIDs . | COX-2 selective inhibitors . | Other NSAIDa . | |||
|---|---|---|---|---|---|---|
| Diclofenaca . | Naproxena . | Ibuprofena . | Rofecoxiba . | Celecoxiba . | ||
| n (%) | 737 (21.8) | 103 (3.1) | 1721 (51.0) | 153 (4.5) | 189 (5.6) | 473 (14.0) |
| Age, years, median (IQR) | 68.4 (58.8–77.8) | 67.9 (55.1–78.2) | 67.2 (56.5–76.7) | 73.0 (65.7–82.5) | 75.9 (64.6–83.2) | 72.4 (63.0–81.5) |
| Women, n (%) | 270 (36.6) | 38 (36.9) | 687 (39.9) | 80 (52.3) | 94 (49.7) | 220 (46.5) |
| Age, years, median (IQR) | 70.8 (59.4–80.3) | 69.9 (52.0–78.8) | 69.1 (57.9–78.5) | 74.7 (66.2–85.1) | 75.8 (65.2–81.6) | 75.8 (64.0–83.9) |
| Men, n (%) | 467 (63.4) | 65 (63.1) | 1034 (60.1) | 73 (47.7) | 95 (50.3) | 253 (53.5) |
| Age, years, median (IQR) | 67.5 (57.8–76.1) | 67.8 (56.6–78.2) | 66.6 (55.6–75.6) | 72.0 (63.2–80.1) | 75.9 (63.1–84.2) | 70.1 (61.4–79.5) |
| Comorbidity, n (%) | ||||||
| Diabetes | 82 (11.1) | 11 (10.7) | 199 (11.6) | 23 (15.0) | 25 (13.2) | 52 (11.0) |
| Peripheral vascular disease | 27 (3.7) | 5 (4.9) | 72 (4.2) | 10 (6.5) | 9 (4.8) | 20 (4.2) |
| Cerebral vascular disease | 51 (6.9) | 5 (4.9) | 121 (7.0) | 11 (7.2) | 20 (10.6) | 28 (5.9) |
| Ischaemic heart disease | 86 (11.7) | 13 12.6) | 228 (13.3) | 31 (20.3) | 49 (25.9) | 59 (12.5) |
| Previous myocardial infarction | 81 (11.0) | 5 (4.9) | 168 (9.8) | 10 (6.5) | 25 (13.2) | 54 (11.4) |
| Chronic heart failure | 77 (10.5) | 11 (10.7) | 183 (10.6) | 28 (18.3) | 31 (16.4) | 47 (9.9) |
| Cardiac arrythmias | 74 (10.0) | 7 (6.8) | 194 (11.3) | 23 (15.0) | 37 (19.6) | 47 (9.9) |
| COPD | 79 (10.7) | 12 (11.7) | 209 (12.1) | 30 (19.6) | 33 (17.5) | 47 (9.9) |
| Cancer | 107 (14.5) | 11 (10.7) | 180 (10.5) | 21 (13.7) | 30 (15.9) | 42 (8.9) |
| Rheumatic diseases | 9 (1.2) | 3 (2.9) | 18 (1.1) | 5 (3.3) | 9 (4.8) | 12 (2.5) |
| Renal diseases | 20 (2.7) | 2 (1.9) | 41 (2.4) | 3 (2.0) | 6 (3.2) | 15 (3.2) |
| Peptic ulcers | 25 (3.4) | 2 (1.9) | 51 (3.0) | 9 (5.9) | 11 (5.8) | 24 (5.1) |
| Concomitant pharmacotherapy | ||||||
| ASA | 155 (21.0) | 18 (17.5) | 406 (24.0) | 38 (24.8) | 60 (31.8) | 130 (27.5) |
| Warfarin | 37 (5.0) | 4 (3.9) | 70 (4.1) | 10 (6.5) | 13 (6.9) | 17 (3.6) |
| ACE inhibitors | 198 (26.9) | 26 (25.2) | 412 (23.9) | 35 (22.9) | 44 (23.3) | 119 (25.2) |
| Loop diuretics | 167 (22.7) | 29 (28.2) | 385 (22.4) | 66 (43.1) | 73 (38.6) | 131 (27.7) |
| β-Blockers | 125 (17.0) | 24 (23.3) | 294 (17.1) | 29 (19.0) | 41 (21.7) | 109 (23.0) |
| Spironolactone | 44 (6.0) | 9 (8.7) | 105 (6.1) | 11 (7.2) | 19 (10.1) | 21 (4.4) |
| Thiazides | 95 (12.9) | 15 (14.6) | 209 (12.1) | 15 (9.8) | 27 (14.3) | 59 (12.5) |
| Calcium channel blockers | 107 (14.5) | 13 (12.6) | 226 (13.1) | 20 (13.1) | 40 (21.2) | 78 (16.5) |
| Antidepressants | 120 (16.3) | 12 (11.7) | 321 (18.7) | 37 (24.2) | 63 (33.3) | 109 (23.0) |
| Anxiolytics | 208 (28.2) | 27 (26.2) | 513 (29.8) | 65 (42.5) | 82 (43.4) | 172 (36.4) |
| Morphine | 116 (15.7) | 13 (12.6) | 193 (11.2) | 28 (18.3) | 37 (19.6) | 61 (12.9) |
| . | Non-selective NSAIDs . | COX-2 selective inhibitors . | Other NSAIDa . | |||
|---|---|---|---|---|---|---|
| Diclofenaca . | Naproxena . | Ibuprofena . | Rofecoxiba . | Celecoxiba . | ||
| n (%) | 737 (21.8) | 103 (3.1) | 1721 (51.0) | 153 (4.5) | 189 (5.6) | 473 (14.0) |
| Age, years, median (IQR) | 68.4 (58.8–77.8) | 67.9 (55.1–78.2) | 67.2 (56.5–76.7) | 73.0 (65.7–82.5) | 75.9 (64.6–83.2) | 72.4 (63.0–81.5) |
| Women, n (%) | 270 (36.6) | 38 (36.9) | 687 (39.9) | 80 (52.3) | 94 (49.7) | 220 (46.5) |
| Age, years, median (IQR) | 70.8 (59.4–80.3) | 69.9 (52.0–78.8) | 69.1 (57.9–78.5) | 74.7 (66.2–85.1) | 75.8 (65.2–81.6) | 75.8 (64.0–83.9) |
| Men, n (%) | 467 (63.4) | 65 (63.1) | 1034 (60.1) | 73 (47.7) | 95 (50.3) | 253 (53.5) |
| Age, years, median (IQR) | 67.5 (57.8–76.1) | 67.8 (56.6–78.2) | 66.6 (55.6–75.6) | 72.0 (63.2–80.1) | 75.9 (63.1–84.2) | 70.1 (61.4–79.5) |
| Comorbidity, n (%) | ||||||
| Diabetes | 82 (11.1) | 11 (10.7) | 199 (11.6) | 23 (15.0) | 25 (13.2) | 52 (11.0) |
| Peripheral vascular disease | 27 (3.7) | 5 (4.9) | 72 (4.2) | 10 (6.5) | 9 (4.8) | 20 (4.2) |
| Cerebral vascular disease | 51 (6.9) | 5 (4.9) | 121 (7.0) | 11 (7.2) | 20 (10.6) | 28 (5.9) |
| Ischaemic heart disease | 86 (11.7) | 13 12.6) | 228 (13.3) | 31 (20.3) | 49 (25.9) | 59 (12.5) |
| Previous myocardial infarction | 81 (11.0) | 5 (4.9) | 168 (9.8) | 10 (6.5) | 25 (13.2) | 54 (11.4) |
| Chronic heart failure | 77 (10.5) | 11 (10.7) | 183 (10.6) | 28 (18.3) | 31 (16.4) | 47 (9.9) |
| Cardiac arrythmias | 74 (10.0) | 7 (6.8) | 194 (11.3) | 23 (15.0) | 37 (19.6) | 47 (9.9) |
| COPD | 79 (10.7) | 12 (11.7) | 209 (12.1) | 30 (19.6) | 33 (17.5) | 47 (9.9) |
| Cancer | 107 (14.5) | 11 (10.7) | 180 (10.5) | 21 (13.7) | 30 (15.9) | 42 (8.9) |
| Rheumatic diseases | 9 (1.2) | 3 (2.9) | 18 (1.1) | 5 (3.3) | 9 (4.8) | 12 (2.5) |
| Renal diseases | 20 (2.7) | 2 (1.9) | 41 (2.4) | 3 (2.0) | 6 (3.2) | 15 (3.2) |
| Peptic ulcers | 25 (3.4) | 2 (1.9) | 51 (3.0) | 9 (5.9) | 11 (5.8) | 24 (5.1) |
| Concomitant pharmacotherapy | ||||||
| ASA | 155 (21.0) | 18 (17.5) | 406 (24.0) | 38 (24.8) | 60 (31.8) | 130 (27.5) |
| Warfarin | 37 (5.0) | 4 (3.9) | 70 (4.1) | 10 (6.5) | 13 (6.9) | 17 (3.6) |
| ACE inhibitors | 198 (26.9) | 26 (25.2) | 412 (23.9) | 35 (22.9) | 44 (23.3) | 119 (25.2) |
| Loop diuretics | 167 (22.7) | 29 (28.2) | 385 (22.4) | 66 (43.1) | 73 (38.6) | 131 (27.7) |
| β-Blockers | 125 (17.0) | 24 (23.3) | 294 (17.1) | 29 (19.0) | 41 (21.7) | 109 (23.0) |
| Spironolactone | 44 (6.0) | 9 (8.7) | 105 (6.1) | 11 (7.2) | 19 (10.1) | 21 (4.4) |
| Thiazides | 95 (12.9) | 15 (14.6) | 209 (12.1) | 15 (9.8) | 27 (14.3) | 59 (12.5) |
| Calcium channel blockers | 107 (14.5) | 13 (12.6) | 226 (13.1) | 20 (13.1) | 40 (21.2) | 78 (16.5) |
| Antidepressants | 120 (16.3) | 12 (11.7) | 321 (18.7) | 37 (24.2) | 63 (33.3) | 109 (23.0) |
| Anxiolytics | 208 (28.2) | 27 (26.2) | 513 (29.8) | 65 (42.5) | 82 (43.4) | 172 (36.4) |
| Morphine | 116 (15.7) | 13 (12.6) | 193 (11.2) | 28 (18.3) | 37 (19.6) | 61 (12.9) |
ACE, angiotensin converting enzyme; ASA, acetylsalicylic acid; COPD, chronic obstructive pulmonary disease; COX-2, cyclooxygenase-2; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug.
Use of a NSAID up to 30 days before out-of-hospital cardiac arrest.
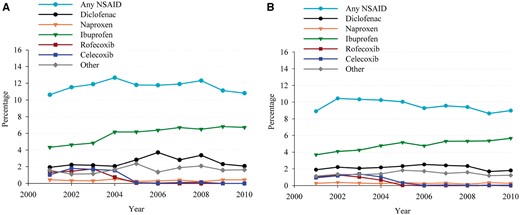
Trends in non-steroidal anti-inflammatory drug (NSAID) use within 30 days before event in percentages during 2001–10. (A) Out-of-hospital cardiac arrest population, P for trend = 0.65. (B) Controls, P for trend < 0.0001. Light blue circle: any NSAID; black circle: diclofenac; orange triangle: Naproxen; green triangle: Ibuprofen; red square: Rofecoxib; blue square: Celecoxib; grey diamond: other.
Risk of out-of-hospital cardiac arrest following treatment with non-steroidal anti-inflammatory drugs (NSAIDs). Odds ratios derive from the conditional logistic regression analyses on case–time–control models. ‘Events’ comprises only persons with discordant exposure history, thus contributing to the analyses. OR, odds ratio; CI, confidence interval; COX-2, cyclooxygenase-2.
Risk of out-of-hospital cardiac arrest following treatment with the most common types of non-steroidal anti-inflammatory drugs (NSAIDs). Odds ratios derive from the conditional logistic regression analyses on case–time–control models. ‘Events’ comprises only persons with discordant exposure history, thus contributing to the analyses. OR, odds ratio; CI, confidence interval.
Effect modification by age and sex was tested by stratified analyses, see Supplementary material online, Figures S1 and S2.
For more details regarding the distribution of cardiac arrest subjects in case and control period, respectively, and respective OR from cases and control group, see Supplementary material online, Table S2.
Sensitivity analyses
The robustness of our results was confirmed by performing the case–time control analysis on different time windows of 7, 14, 20, and 40 days that yielded similar results: ibuprofen, OR 1.57 (95% CI 1.24–2.00), OR 1.53 (95% CI 1.27–1.83), OR 1.40 (95% CI 1.19–1.64), OR 1.24 (95% CI 1.09–1.41), respectively, and diclofenac, OR 1.62 (95% CI 1.16–2.26), OR 1.42 (95% CI 1.10–1.83), OR 1.30 (95% CI 1.04–1.63), and OR 1.49 (95% CI 1.24–1.79), respectively.
Excluding 253 subjects < 18 years yielded the exact same results.
The results were unaltered excluding all subjects with a hospital admission 60 days before cardiac arrest: ibuprofen OR 1.32 (95% CI 1.13–1.55) and diclofenac OR 1.45 (95% CI 1.15–1.83), Supplementary material online, Figure S3. Including only subjects with a presumed cardiac cause of cardiac arrest did not change the results either: ibuprofen OR 1.29 (95% CI 1.10–1.51) and diclofenac OR 1.45 (95% CI 1.18–1.87), Supplementary material online, Figure S4. Lastly, exclusion of subjects with cancer produced similar results; ibuprofen OR 1.33 (95% CI 1.15–1.54); diclofenac OR 1.50 (1.21–1.85). The association between cardiac arrest and naproxen, celecoxib, and rofecoxib remained insignificant in all sensitivity analyses.
Discussion
In this nationwide case–time control study, we found that use of non-selective NSAIDs was associated with an increased risk of OHCA. The result was primarily driven by an increased risk of cardiac arrest in ibuprofen and diclofenac users. We found no significant association between cardiac arrest and use of the COX-2 selective inhibitors, rofecoxib and celecoxib, nor with the unselective NSAID naproxen. The results were verified in multiple sensitivity analyses, including the exclusion of presumed non-cardiac causes of cardiac arrest, and using different definitions of exposure periods.
To our knowledge, this is the first study to investigate the association between NSAIDs and OHCA. Currently, no research relating to NSAIDs’ pro-arrhythmic effects with regard to ventricular arrhythmias has been published; consequently, we compared our findings in relation to studies with other cardiovascular endpoints, such as myocardial infarction and cardiovascular death. However, although cardiac arrest is a combined endpoint, it is closely related to cardiovascular morbidity, particularly ischaemic heart disease, which has been observed in more than 60% of all OHCA and 80% of sudden cardiac deaths.12,–14,28
In this study, short-term use of ibuprofen was associated with an increased risk of cardiac arrest, OR 1.31. In two recent meta-analyses of randomized trials, ibuprofen was also reported to be associated with a substantial cardiovascular hazard: Bhala et al.4 reported a two-fold increased risk of major coronary events when using ibuprofen compared with placebo and a non-significant increased risk of vascular death and death from any cause; Trelle et al.7 reported a more than 30% increased risk of several cardiovascular endpoints, such as myocardial infarction, cardiovascular death, and death from any cause, comparing ibuprofen with placebo. As few randomized trials of ibuprofen exist, the meta-analyses lack statistical power regarding ibuprofen. Moreover, the trials were investigating high-dose treatment only with daily use of 2400 mg ibuprofen, which is considerably higher than dosages used in daily practice.1 Even though the case–time–control method eliminates confounding from stable characteristics, such as cardiac comorbidity and concomitant pharmacotherapy, we cannot fully exclude confounding by indication related to fluctuations in disease severity that could lead to a change in NSAID use. Nonetheless, our findings indicate a harmful association between ibuprofen and cardiac arrest and support the emerging evidence of an unfavourable association between ibuprofen and cardiovascular safety.
We found that diclofenac was associated with an increased risk of cardiac arrest, OR 1.50, which concurs with findings in several other studies: Bhala et al. found a significantly increased risk of major coronary events and vascular death of 70% and 65%, respectively, and Trelle et al.4,7 reported a significantly increased risk of cardiovascular death with a rate ratio of four. Again, the composite nature of cardiac arrest entails careful interpretation, yet our results add to the accumulating evidence that diclofenac is associated with increased cardiovascular risk.
Notably, we found no significant association between cardiac arrest and the COX-2 selective inhibitors, rofecoxib and celecoxib. Several randomized clinical trials found a dose-dependent increased cardiovascular hazard with rofecoxib treatment, which led to a complete withdrawal of rofecoxib from the market in 2004.3,9,10 Studies on celecoxib have had more ambiguous findings, and although a large randomized clinical trial found a dose-related risk of death from cardiovascular causes, acute myocardial infarction, stroke, and heart failure, other studies, including the meta-analysis by Trelle et al.,6,7 found no such risk. However, when grouping the COX-2 selective inhibitors, Bhala et al.4 found a significant increased risk of vascular death and death from any cause. Rofecoxib and celecoxib are seldom used in Denmark, especially after 2006 when rofecoxib was withdrawn from the market, and this combined with a hard endpoint (cardiac arrest) entailed relatively few events in our study and possibly low statistical power. Thus, in light of previous research, our findings of an apparent safety regarding cardiac arrest and use of COX-2 selective inhibitors should be interpreted with caution.
Lack of statistical power applies to naproxen as well. As naproxen is rarely used in Denmark, there were few events and a wide CI. Though the OR point estimate suggests an association with cardiac arrest similar to what we find in ibuprofen and diclofenac users, the study has insufficient power to examine the importance of naproxen.
Limitations
The main limitation of the study is inherent in the observational nature of the analyses. The treatment allocation is not randomized and the study reports only associations and therefore any conclusion on causality should be made with caution.
The case–time–control method accounts for fixed confounders but could be influenced by transient factors, such as acute disease exacerbation or fluctuation in disease severity. Although, the exclusion of patients with admissions 60 days before index date did not change our results, we cannot fully eliminate the risk of confounding by indication, as we do not have access to possibly confounding clinical covariables, such as blood samples, electrocardiographic parameters, or treatment indication, e.g. chest pain.
Information on drug use originates from the Danish pharmacies and we have no information regarding compliance; however, as drug expenses are only partially reimbursed by Danish Health Care authorities, we assume that non-compliance had little influence on our results. In addition, non-compliance would affect the results towards null and thus reduce the association between exposure and outcome. Moreover, we do not have information on use of over-the-counter drugs. In Denmark, ibuprofen is the only NSAID sold as an over-the-counter drug (since 1 November 2001) and accounts for 30% of total ibuprofen sales during 1999–2012. It is sold only in 200 mg dosages and in small packages (maximum 30 tablets per package).29 Using prescription data without information on over-the-counter ibuprofen entails a risk of underestimating the true exposure prevalence. However, in our study, the exposure misclassification should be equally distributed in both case- and control period. Moreover, a study quantifying the effect of missing information on over-the-counter NSAID found the effect on study validity to be almost negligable.30 Acetylsalicylic acid is also sold as an over-the-counter drug in Denmark, but since 2002 more than 80% of low-dose acetylsalicylic acid has been dispensed by prescription and the influence on our results should be minimal.29 Lastly, the determination of treatment duration is an approximation, however, used in numerous papers before.1,11
Conclusion
In a nationwide cohort of persons with OHCA, we found that short-term treatment with non-selective NSAIDs, particularly ibuprofen and diclofenac, was associated with an increased early risk of cardiac arrest. We found no association between cardiac arrest and use of the COX-2 selective inhibitors, rofecoxib and celecoxib, nor the non-selective NSAID naproxen.
Our findings support the accumulating evidence of an unfavourable cardiovascular risk profile associated with use of the non-selective NSAIDs. This calls for special awareness in order to balance risks against benefits in treatment with NSAIDs.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Acknowledgements
We thank the Danish Emergency Medical Services personnel who completed the case report forms for the Danish Cardiac Arrest Registry.
Funding
The European Regional Development Fund through the Interreg IV A OKS programme (NYPS ID:167157); independent research scholarship from the Novo Nordisk Foundation (to G.H.G.). TrygFonden supports the Danish Cardiac Arrest Registry with no commercial interest in the field of cardiac arrest.
Conflict of interest: none declared.