-
PDF
- Split View
-
Views
-
Cite
Cite
Alessandro Brunelli, Egidio Beretta, Stephen D. Cassivi, Robert J. Cerfolio, Frank Detterbeck, Thomas Kiefer, Giuseppe Miserocchi, Joseph Shrager, Sunil Singhal, Dirk Van Raemdonck, Gonzalo Varela, Consensus definitions to promote an evidence-based approach to management of the pleural space. A collaborative proposal by ESTS, AATS, STS, and GTSC, European Journal of Cardio-Thoracic Surgery, Volume 40, Issue 2, August 2011, Pages 291–297, https://doi.org/10.1016/j.ejcts.2011.05.020
Close - Share Icon Share
Abstract
The present project involved a collective effort agreed by the European Society of Thoracic Surgeons, the American Association for Thoracic Surgery, the Society of Thoracic Surgeons, and the General Thoracic Surgery Club to assemble a joint panel of experts to review the available data and address ambiguous aspects of chest tube definitions and nomenclature. The task force was composed of 11 invited participants, identified for their expertise in the area of chest tube management. The subject was divided in different topics, which were in turn assigned to at least two experts. The draft reports written by the experts on each topic were distributed to the entire expert panel, and comments solicited in advance of the meetings. During the meetings, the drafts were reviewed, discussed, and agreed on by the entire panel. Standardized definitions and nomenclature were proposed for the following topics related to chest tube management: pleural and respiratory mechanics after pulmonary resection; external suction versus no external suction; fixed versus variable suction; objective air leak evaluation; objective fluid drainage evaluation; and chest drain: type, number, and size. A standardized set of definitions and nomenclature were proposed to set a scientifically based framework with which to evaluate existing studies and to more clearly formulate questions, parameters, and outcomes for future studies.
1 Introduction
After a thoracic surgical procedure, the duration of chest tube drainage for either air or fluid is a major factor in length of stay (LOS), cost, and morbidity. However, chest tube management is determined primarily by habit and personal experience rather than a scientifically valid foundation. Several works have tried to shed light on this subject, but synthesis is difficult due to incomplete understanding of the basic physiology and inconsistent definitions and terminology. The field is poised for additional research, given a better definition of pleural physiology than was previously available and the recent introduction of new systems capable to objectively quantify air leak rates. This creates a need to establish a consistent starting point for parameters and terminology, so that future studies can be synergistic and lead to evidence-based guidelines and recommendations.
The present project involved a collective effort agreed by the European Society of Thoracic Surgeons, the American Association for Thoracic Surgery, the Society of Thoracic Surgeons, and the General Thoracic Surgery Club to assemble a joint panel of experts to review the available data and address ambiguous aspects of this topic. The objective is to develop a sound scientifically based framework with which to evaluate existing studies and to more clearly formulate questions, parameters, and outcomes for future studies.
The subjects to be considered in this position paper are the followings:
pleural and respiratory mechanics after pulmonary resection;
external suction versus no external suction;
fixed versus variable suction;
objective air leak evaluation;
objective fluid drainage evaluation; and
chest drain: type, number, and size
2 Methods
The task force was composed of 11 invited participants, identified by an initial core group (AB, FD, RJC, and TK) for their expertise in the area of chest tube management. The project and composition of the panel were endorsed by the four organizations. The subject was divided into different topics, which were in turn assigned to at least two experts. The draft reports written by the experts on each topic were distributed to the entire expert panel, and comments solicited in advance of the meetings. During the meetings (held at the 2010 ESTS and at the 2011 STS Congresses), the drafts were reviewed, discussed, and agreed upon by the entire panel.
3 Pleural and respiratory mechanics after pulmonary resection
3.1 Pleural fluid turnover and lung mechanics
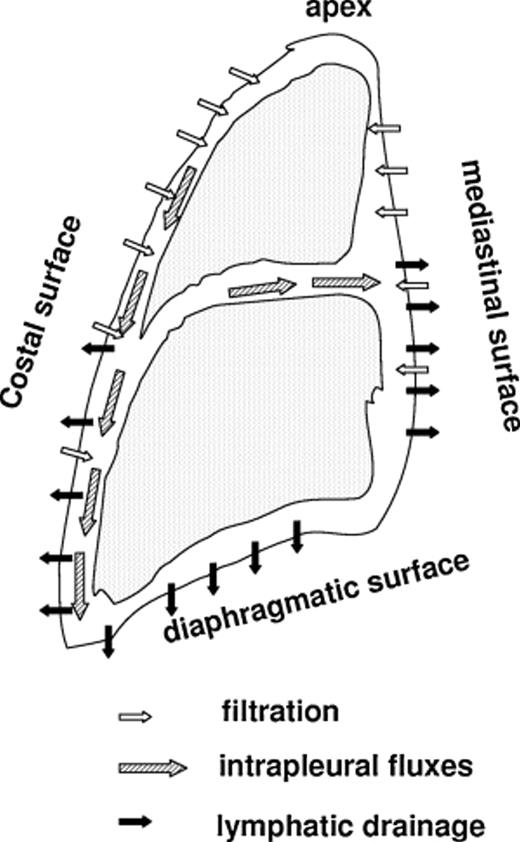
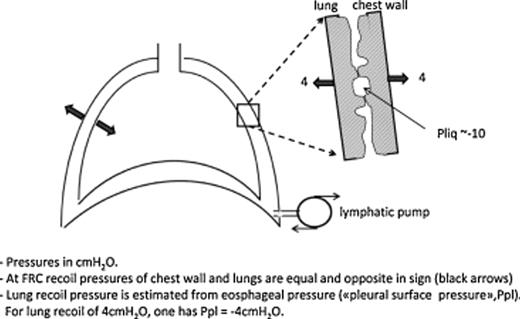
Pleural fluid turnover occurs at parietal pleural level in physiological conditions: fluid filters from the capillaries of the parietal pleura and is drained by the lymphatic network that connects directly to the pleural cavity through the lymphatic stomata [1]. In a gravitational field, the pressure of the pleural liquid (Pliq) becomes more subatmospheric with increasing height in the cavity, being about zero at the bottom and ∼−10 cmH2O at mid-heart level both in supine and head up posture. Pliq reflects the absorption pressure of the lymphatics that act as a draining pump (‘sump’) and, at the same time, sets the pressure gradient for fluid filtration. Fig. 1 highlights schematically pleural fluid dynamics: filtration mostly occurs in less-dependent regions and pleural fluid is mostly drained toward preferential absorption sites at the bottom of the cavity and in the mediastinal region. Pliq forces the lung and chest wall to match each other closely; in doing so, both structures develop an elastic recoil that tends to pull them apart (Fig. 2, red arrows ). Fig. 2 also shows on enlarged scale that, since the absolute value of Pliq is more negative than the elastic recoil of lung and chest wall, the visceral and parietal pleura actually push one against the other, though efficient sliding is assured by phospholipid molecules adsorbed on pleural surfaces [2]. The recoil pressure of the lung can be estimated from esophageal pressure and is commonly referred to as ‘pleural surface pressure’ (Ppl), being equal in modulus but opposite in sign relative to recoil pressure. At the functional residual capacity (FRC), Ppl is ∼−4 cmH2O at mid-heart level. However, Ppl reflects the elastic properties of the lung; for an emphysematous lung Ppl is remarkably less negative than −4 cmH2O, while for a fibrotic lung it is more negative than −4 cmH2O.
Regional distribution of pleural fluid filtration (secretion), drainage (absorption) and intrapleural fluxes in the pleural cavity.
Pleural mechanical coupling between lung and chest wall. On enlarged scale the difference between pleural liquid pressure (Pliq) and pressure generated by elastic recoil of lung and chest wall (Ppl).
In summary, the role of lymphatics is to: (1) set a Pliq that holds the lung and chest wall together, (2) maintain pleural fluid at a negligible volume, (3) act as regulators of pleural fluid volume by adjusting draining flow to match increased filtration [1–3]. In the absence of an efficient lymphatic pleural drainage, fluid would accumulate in the chest, causing lung to collapse and chest to expand.
3.2 Postoperative drainage of the pleural cavity
The mechanical characteristics of the pleural space are altered by thoracic surgery even after closure of the chest. The most immediate problem is evacuation of air from the cavity. The compliance (ΔV/ΔP) of the remaining part of the lung is decreased in proportion to the amount of lung resected. For example, the compliance of the remaining part of the lung is halved in case 50% of the lung tissue is removed. Therefore, re-expansion of the remaining lung to fully match the original chest volume would require considerable more subatmospheric Ppl, as well as a remarkable deformation of its natural shape.
It is customary to set the post-operative draining pressure in the gas phase (Ppl) at a level comparable to the preoperative one. To avoid lung overdistension, gas volume has to remain in the chest in the immediate postoperative period (roughly equal to the volume occupied by the resected tissue at FRC) but offset by the amount of mediastinal shift and diaphragmatic elevation that occurs. The risk of overdistension obviously increases with increasing the volume of resected lung. From a purely physiological point of view, air collects in the retrosternal region (the less-dependent portion of the chest in supine posture), whereas pleural fluid collects in the lowermost part of the pleural space (dorsal costodiaphragmatic sinus, both in supine and head up posture [4,5]). Yet, many surgeons use only a single drain (probably oriented differently by each surgeon) as, based on experience, this works fine in draining both air and pleural fluid to reach a new steady-state condition.
After the initial gas drainage, gas will be slowly reabsorbed, ∼1%/day [6], being progressively replaced by pleural fluid. Hydrothorax can develop in this phase due to increase in permeability of the mesothelial membranes (surgical insult) and/or to rather subatmospehric pressure of the suction line favoring fluid filtration. Liquid drainage is ideally better performed by having the tube draining from the dorsal costodiaphragmatic sinus where Pliq in physiological conditions is close to 0 cmH2O and may become positive with increasing liquid pooling. Recommended suction pressure from bottom of the cavity is slightly subatmospheric. Too much negative suction pressure (as by lowering the collecting flask on the floor with the patient lying in bed) may increase pleural liquid filtration.
Recovery from pleural effusion is slow, ranging from weeks to months [7]. A useful strategy is to insert in the lower chest a single tube and advance it to retrosternal regions. The tube must have two openings, one at the top to drain gas and the other at the bottom to drain fluid [8]. This setting allows some recirculation of pleural fluid: whenever the pressure in the gas bubble becomes subatmospheric on inspiration, fluid might be sucked up from the lowermost part of the chest and then outflow from the top opening down to the bottom of the cavity again [4,5]. This method allows one to control the volume of fluid drained from the cavity and also the postoperative pain [8] that appears the main factor limiting post-surgery respiratory activity.
Pulmonary complications represent the major cause of morbidity after lung resection surgery; furthermore, despite different clinical manifestation (‘idiopatic edema’, ALI, atelectasis, ARDS), the common patho-physiological mechanism is a severe perturbation in lung water balance [9]. Several cofactors may acutely induce an increase in microvascular filtration:
Overinflation due to an aggressive drainage and/or due to prolonged mechanical ventilation with excessive tidal volume [10,11]. Stretching of lung parenchyma due to overinflation results in a marked subatmospheric interstitial pressure that, in turn, favors microvascular filtration [9].
Greater blood flow, flow velocity, capillary recruitment, and endothelial shear stress result in increase in microvascular permeability in the remaining lung [12].
Fragmentation of extracellular matrix [9], lack of clearance of the fragments, neutrophil and macrophage activation [15], production of reactive oxygen species, diffuse alveolar damage, and inhibition of the active alveolar fluid reabsorption [16].
Large amounts of intraoperative fluid administration [17,18], particularly when coupled to increased microvascular permeability, as clearly shown by experimental models of lung edema [9]. It is important to remark that the rigidity (high elastance) of the extracellular matrix represents the main line of defense of the lung against severe edema [3,9] that, in fact, develops through an ‘accelerated phase’ when the process of fragmentation proceeds beyond a critical threshold [9].
As much as in physiological conditions, also after lung resection, the absorption pressure of the pleural lymphatics will determine the final ‘postoperative residual pleural space’, reflecting the modified chest wall–lung mechanical coupling. Due to decrease in compliance and deformation, the over-extended remaining lung will unlikely occupy the volume left free by the resected portion that will be shared among pleural fluid and mediastinal and diaphragmatic displacement. The suction pressure of the draining tube should only serve to help in reaching the new mechanical and fluid dynamic equilibrium at pleural level.
4 External suction versus no external suction
The terms ‘active suction’ and ‘passive suction’ have been and continue to be terms, which are prone to misunderstanding and misinterpretation.
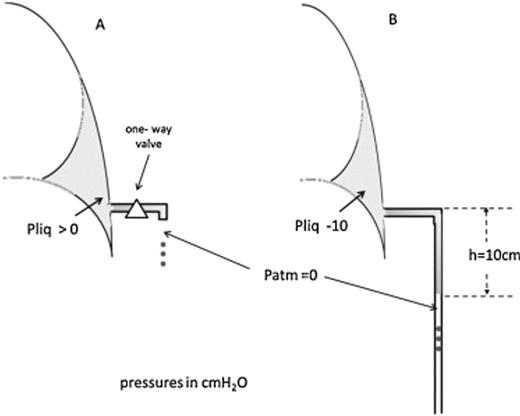
The term ‘passive suction’ is, in and of itself, intrinsically ambiguous. We will speak of ‘passive’ and ‘active’ drainage of fluid. Fig. 3A shows a ‘passive’ drainage setup: fluid will drain whenever the pressure in the hydrothorax will exceed atmospheric pressure (reported zero at tube outlet). To avoid suction of liquid/air back into the pleural cavity when a subatmospheric pleural pressure is developed on inspiration, a one-way valve should be placed on the tube.
(A) A ‘passive’ drainage set up: pleural liquid pressure is above atmospheric. (B) An ‘active’ drainage is generated when fluid proceeds downward the tube and a subatmospheric pressure is applied to the pleural liquid.
Fig. 3B shows the concept of ‘active’ drainage as well as its functional consequences. As fluid advances down the tube (in this case by 10 cm), a subatmospheric pressure is generated at the tip of chest tube in the pleural space: if the fluid stops moving, the pressure at the tip is exactly −10 cmH2O; if fluid still moves down, the pressure at the tip will be less negative than −10 cmH2O (say, −9 cmH2O). Clearly, this condition speeds up drainage. The same reasoning now applies for further downward progression of the fluid. Consider that for a pressure at the tip of the order of ∼−60 cmH2O (a fluid column from patient bed down to the floor), the pressure gradient for fluid filtration across the pleurae is increased by about 10 times! As a result such pressure will contribute to increased fluid filtration. Interestingly, the negative pressure generated at the tip remains basically confined to the fluid pool and is not transmitted to the rest of the pleural space due to the extremely high flow resistance of the pleural space once the visceral pleura adheres to the parietal one [19]. In summary, a ‘passive drainage’ takes place when intrapleural pressure rises above atmospheric pressure, whereas an ‘active drainage’ occurs when a subatmospheric pressure is applied to the pleural space either by a suction device or by creating a liquid column within the chest tube that extends below the level of the pleural space (as in Fig. 3B).
In a measure to simplify terminology and understanding of this latter form of drainage of the pleural space, we propose to define the situation with the application of a subatmospheric pressure by an external suction device as ‘external suction applied.’ In all other cases (previously referred as ‘passive suction’ or ‘water-seal’), we propose the definition ‘no external suction applied’.
5 Variable suction versus fixed suction
A suction source that delivers ‘regulated suction’ is one that adjusts its activity according to the need or situation in the chest cavity. In the case of regulated suction, the suction source is able to detect a need to increase the flow provided to achieve a preset intrapleural pressure. An example of this would be the situation of a parenchymal air leak where the lung is not able to remain expanded and maintain negative intrapleural pressure. A collection device with a so-called regulated suction system will apply active suction to the pleural space at a variable level only when needed to maintain a preset intrapleural pressure. We therefore propose to call this kind of suction ‘variable’ instead of ‘regulated’. ‘Unregulated suction’ or ‘fixed suction’ is delivered by all fixed suction sources such as wall suction. In this situation, there is no feedback from the chest cavity to the suction source and no ability to react according to the actual needs inside the chest cavity and provides a constant, fixed level of suction to the pleural space. Since this type of suction is controlled but fixed in its amount, we therefore propose to call this mode of suction ‘fixed’ rather than ‘unregulated’.
6 Objective air leak evaluation
By using traditional chest drainage systems, air leak is evaluated by detecting bubbles of air in the air leak chamber during forced expiratory maneuvers or cough.
With these devices it can sometimes be difficult to differentiate a true air leak versus the appearance of an air leak resulting from the momentum in the fluid column within the drainage device that sometimes results from the changes in the intrapleural pressure that occur with coughing. Particularly in large, muscular patients who are able to generate an unusually strong cough, and in patients who have a residual air space that may or may not be visible on their chest radiogram, a few bubbles in the air leak indicator chamber may simply represent a ‘momentum leak’ rather than a true leak and may not be a reason to keep a chest tube in place. These ‘momentum leaks’ will typically be present with coughing but not with normal tidal breathing, and they will often be observed only during the first two or three coughs a patient is asked to generate.
Despite a severity score has been proposed by using one of these traditional systems [20], this practice is vexed by a high degree of inter-observer variability [21]. Recently, different companies have produced objective systems capable to precisely measure the airflow through the chest tube. These systems have been shown to markedly reduce the interobserver variability in deciding when to remove a chest tube [21] and to shorten the duration of chest tube and hospital stay in randomized trials [22–24].
The expression of air leak in ml min−1 rather than bubbles and the capability to record and retrieve the information makes possible to standardize chest tube management across different surgeons and institution. This translates in important clinical and research benefits.
The use of different electronic systems, however, has introduced a new variability factor. These systems in fact may use different technologies and software to measure or estimate the airflow. Some of them use air flow meter to directly measure the airflow through the chest tube; others derive these data from an algorithm based on the intrapleural pressure maintained by a suction pump and measured through a pressure sensor. This means that a flow of 50 ml min−1 detected by using a system may not exactly correspond to the same value detected by using another system. Future research is needed to compare findings obtained by different technologies.
Several studies have shown that with one system, which used a flow sensor to measure air leak, an average airflow of less than 5 ml min−1 during the last 6 h was a safe threshold for removing the chest tube [23].
Another available system is a portable pump, which works to maintain the intrapleural pressure to a pre-set level. The pump works to compensate less negative intrapleural pressure levels, and an algorithm is used to estimate the amount of airflow from the work applied (Table 1 ).
Clinical experience from different centers, which regularly use this system, has shown that a flow of less than 40 ml min−1 for the last 6–8 h with a plateau or a sloping down trend is safe for removing a chest tube.
There are other electronic or volumetric systems capable to objectively measure the airflow but both scientific evidence and clinical experience with these devices are still scarce.
One of the most important features of the digital devices is their capability to store the information and retrieve it either in a graphical mode or in a excel format for analysis. The graphical mode is particularly useful and can be used directly at the bedside of the patient to deduce the trend of the air leak. This trend information may be even more important than the absolute value of an instantaneously detected air leak and may be the information that should be used to decide to remove the chest tube.
Whenever an electronic or a volumetric device is used, we recommend that the amount of air leak in ml min−1 is reported. The qualitative trend over time (>6 h) and details of the system used should be provided as well (i.e., preset level of external suction, type and position of sensors, algorithms, etc.).
7 Objective fluid drainage evaluation
The management of chest tubes and threshold for their removal remains controversial, and is based primarily on tradition and dogma more than data. Many surgeons use the threshold of 50 cm3/shift or 200 cm3/day; some use 300 cm3/day. Recently, several authors suggested that the removal of chest tubes with 400–450 cm3 of fluid drainage/day or less is safe [24–26]. These figures appear reasonable as they are in the range of physiological daily pleural fluid filtration (an estimated value of 350 ml day−1[1]), on the assumption that most of the drainage occurs through the chest tube whose flow resistance is much lower compared to that of lymphatics.
Further research would benefit from consistent reporting of several details. Obviously, the amount of fluid drainage per day or part of a day (e.g., 8 h) should be reported in future studies. It would also be useful to know the time course of pleural liquid/plasma protein ratio since closure of the chest, as this would provide indications on the permeability of the pleural membranes [27]. Furthermore, unusual fluid characteristics should be reported separately (e.g., blood, chyle, or CSF). The type of pulmonary resection should be reported (e.g., sublobar, lobectomy, or greater). The extent of mediastinal node dissection might not appear to be important [28]. Patient characteristics that may contribute to pleural effusion should be noted (e.g., renal failure, congestive heart failure, or ascites).
It is important to define end points for further study. A mere radiographic visualization of fluid or a specific volume of pleural fluid accumulating after tube removal is not sufficiently clinically relevant, and dependent on other factors (such as the presence of a residual space that cannot be filled by the remaining lung parenchyma). We propose that the most relevant measure is the development of a symptomatic pleural effusion that requires and responds to intervention within 1 month of tube removal. Because dyspnea is common after thoracic surgery, the presence of dyspnea should be correlated with the presence of an effusion, and should be correlated with relief after intervention to remove the fluid. If the degrees of symptoms and/or the amount of fluid are not sufficient to warrant intervention, it should be viewed as not clinically relevant. It is acknowledged that there is still a subjective component on the part of the physician and the patient whether to perform an intervention and whether this affected the symptoms. However, the performance of an intervention is concrete enough to be a relevant clinical end point, whatever the subjective decision-making process involved. Furthermore, the fluid accumulation and intervention should have occurred within 1 month of tube removal to be reasonably related to the thoracic surgery and tube management.
8 Chest drain: size, number, and type
Although most of the clinical practice related to postoperative pleural drainage is based on personal preferences of the surgeon [29], some evidences can be drawn from the literature to clarify the size, number, and type of chest tubes needed for an uneventful recovery after lung resection.
8.1 Size of the chest tubes
Probably, the critical point is that the chest tube drains together fluid and air bubbles; furthermore, while fluid collects downward, air bubbles move in opposite direction, thus preventing a smooth fluid drainage. On purely fluid mechanics basis, the most important factor is the diameter of the pipe, so a large bore chest tube (28–32F) is frequently advocated after thoracotomy. However, conclusive scientific data on the practical effects of chest tube diameter in a clinical situation are not available; furthermore, the pleural cavity is not comparable to a fluid container due to the complex interaction between the chest wall and the lung and the absorption of fluid through the parietal pleura. Small caliber catheters (16F) are successfully used for spontaneous pneumothorax and pleural effusion [30] but no evidences of their effectiveness in patients after thoracotomy have been published. Due to the reliability of these small tubes in non-surgical patients and the low clinical impact of chest tube clearance on postoperative morbidity, a trial comparing small versus large bore chest drainage tubes seems to be advisable (but is not expected to be popular among surgeons).
Clearance through spiral silicone tubes is not regulated by the aforementioned physical principles since capillarity plays an important role in these tubes. Their effectiveness in lung resection patients is discussed below.
Studies on air leak and chest tube management should always report the size of the chest tube used.
8.2 Number of pleural drainages
Based on postoperative pleural dynamics, the use of two pleural tubes (one placed in the apex of the pleural cavity and the other over the diaphragm) is frequently recommended in the medical literature and textbooks [31]. We are not aware of any scientific paper demonstrating better clinical result using two chest tubes. There is available information on the usefulness of a single pleural drainage after small wedge resections and after lobectomy [32–35], which apparently causes less postoperative pain and less pleural fluid loss.
According to the previously cited clinical papers [32–35], the use of one single chest tube after lobectomy seems to offer the same clinical results compared to the conventional practice of two tubes, one apical and one basal; further studies on this topic are probably unnecessary.
Studies on air leak and chest tube management should always report the number of chest tube used in every patient and their position.
8.3 Type
Small bore spiral silicone catheters have been proposed instead of conventional ones to be used after lung resection.
In an animal model, the drainage capacity of small spiral silicone chest drains (Blake tubes) has been found almost identical to that of the conventional chest tubes [36]. On the contrary, in clinical settings, suction is required for Blake drainages to obtain fluid drainage performance comparable to that of the water-sealed conventional tubes. When air leakage occurs, air evacuation by the Blake tubes tends to be insufficient, irrespective of suction conditions [37]. In clinical practice, one [38] or two [39,40] spiral drains proved to be at least as safe and effective as conventional tubes after lung surgery; they allowed for evacuation of large amounts of blood/fluid as well as air, and were associated with minimal discomfort. Nevertheless, because the evidence on this topic is weak and due to the limitations found in the laboratory, more scientifically sound data are needed regarding their use, especially in patients with postoperative air leak.
Studies on air leak and chest tube management should always detail the type of chest tube used.
Disclosures
Dr Brunelli is a consultant for Medela Healthcare; Dr Cerfolio is a consultant for Medela Healthcare and Atmos Inc.; Dr Kiefer is a consultant for Medela Healthcare; Dr Varela is a consultant for Atrium Medical.